Blaise reaction: Difference between revisions
Clean up using AWB |
m link [hH]ydrochloric acid |
||
| (27 intermediate revisions by 21 users not shown) | |||
| Line 1: | Line 1: | ||
{{Reactionbox |
|||
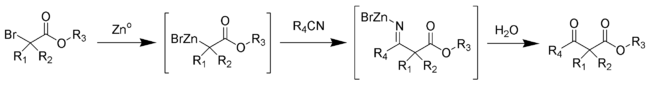
| ⚫ | The '''Blaise reaction''' is an [[organic reaction]] that forms a β-ketoester from the reaction of [[zinc]] metal with a [[haloketone|α-bromoester]] and a [[nitrile]].{{Ref|Blaise1901}} |
||
|Name = Blaise reaction |
|||
|Type = Coupling reaction |
|||
|NamedAfter = Edmond E. Blaise |
|||
|Section3 = {{Reactionbox Identifiers |
|||
| OrganicChemistryNamed = blaise-reaction |
|||
| RSC_ontology_id = 0000237 |
|||
}} |
|||
}} |
|||
| ⚫ | The '''Blaise reaction''' is an [[organic reaction]] that forms a β-ketoester from the reaction of [[zinc]] metal with a [[haloketone|α-bromoester]] and a [[nitrile]].{{Ref|Blaise1901}}{{Ref|OS1955}}{{Ref|Rao2008}} The reaction was first reported by '''Edmond Blaise''' (1872–1939) in 1901. The final intermediate is a metaloimine, which is then [[hydrolyzed]] to give the desired β-ketoester.{{Ref|Cason1953}} |
||
[[Image: |
[[Image:Blaise Reaction Scheme.png|center|650px|The Blaise reaction]] |
||
Bulky [[aliphatic]] esters tend to give higher yields. Hannick and Kishi have developed an improved procedure.{{Ref|Hannick1983}} |
Bulky [[aliphatic]] esters tend to give higher yields. Steven Hannick and [[Yoshito Kishi]] have developed an improved procedure.{{Ref|Hannick1983}} |
||
It has been noted{{Ref|Hannick1983}}{{Ref|Wang2005}} that free hydroxyl groups can be tolerated in the course of this reaction, which is surprising for reactions of organometallic halides. |
|||
| ⚫ | |||
| ⚫ | |||
==Mechanism== |
|||
| ⚫ | |||
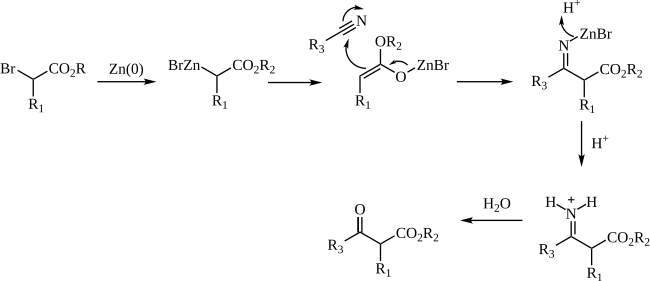
The mechanism of the Blaise reaction involves the formation of an organozinc complex with the bromine alpha to the ester carbonyl. This makes the alpha carbon nucleophilic, allowing it to attack the electrophilic carbon of the nitrile. The negative nitrile nitrogen resulting from this attack complexes with the zinc monobromide cation. The β-enamino ester (tautomer of the imine intermediate pictured above) product is revealed by work-up with 50% K<sub>2</sub>CO<sub>3</sub> aq. If the β-ketoester is the desired product, addition of 1 M [[hydrochloric acid]] hydrolyzes the β-enamino ester to turn the enamino into a ketone, forming the β-ketoester. |
|||
| ⚫ | |||
| ⚫ | |||
[[File:BlaiseRxnMech.svg|center|650px|Blaise Rxn Mechanism]] |
|||
==See also== |
==See also== |
||
*[[Blaise ketone synthesis]] |
*[[Blaise ketone synthesis]] |
||
*[[Reformatsky reaction]] |
*[[Reformatsky reaction]] |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
# {{Note|Rao2008}} Rao, H. S. P.; Rafi, S.; Padmavathy, K. ''[[Tetrahedron (journal)|Tetrahedron]]'' '''2008''', ''64'', 8037-8043. (Review) |
|||
| ⚫ | |||
| ⚫ | |||
# {{Ref|Marko2007}} Marko, I.E. ''[[J. Am. Chem. Soc.]]'' '''2007''', ASAP {{doi|10.1021/ja0691728}} |
|||
# {{Ref|Wang2005}} Wang, D.; Yue, J.-M. ''[[Synlett]]'' '''2005''', 2077-2079. |
|||
== External links == |
|||
* [https://www.organic-chemistry.org/namedreactions/blaise-reaction.shtm Blaise Reaction - Details and Recent Literature] |
|||
{{Organic reactions}} |
|||
[[Category:Addition reactions]] |
[[Category:Addition reactions]] |
||
[[Category:Carbon-carbon bond forming reactions]] |
[[Category:Carbon-carbon bond forming reactions]] |
||
[[Category:Name reactions]] |
|||
Latest revision as of 18:29, 26 October 2023
| Blaise reaction | |
|---|---|
| Named after | Edmond E. Blaise |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | blaise-reaction |
| RSC ontology ID | RXNO:0000237 |
The Blaise reaction is an organic reaction that forms a β-ketoester from the reaction of zinc metal with a α-bromoester and a nitrile.[1][2][3] The reaction was first reported by Edmond Blaise (1872–1939) in 1901. The final intermediate is a metaloimine, which is then hydrolyzed to give the desired β-ketoester.[4]
Bulky aliphatic esters tend to give higher yields. Steven Hannick and Yoshito Kishi have developed an improved procedure.[5]
It has been noted[6][7] that free hydroxyl groups can be tolerated in the course of this reaction, which is surprising for reactions of organometallic halides.
Mechanism
[edit]The mechanism of the Blaise reaction involves the formation of an organozinc complex with the bromine alpha to the ester carbonyl. This makes the alpha carbon nucleophilic, allowing it to attack the electrophilic carbon of the nitrile. The negative nitrile nitrogen resulting from this attack complexes with the zinc monobromide cation. The β-enamino ester (tautomer of the imine intermediate pictured above) product is revealed by work-up with 50% K2CO3 aq. If the β-ketoester is the desired product, addition of 1 M hydrochloric acid hydrolyzes the β-enamino ester to turn the enamino into a ketone, forming the β-ketoester.
See also
[edit]References
[edit]- ^ Edmond E. Blaise; Compt. Rend. 1901, 132, 478.
- ^ Rinehart, K. L., Jr. Organic Syntheses, Coll. Vol. 4, p. 120 (1963); Vol. 35, p. 15 (1955). (Article)
- ^ Rao, H. S. P.; Rafi, S.; Padmavathy, K. Tetrahedron 2008, 64, 8037-8043. (Review)
- ^ Cason, J.; Rinehart, K. L., Jr.; Thorston, S. D., Jr. J. Org. Chem. 1953, 18, 1594. (doi:10.1021/jo50017a022)
- ^ Hannick, S. M.; Kishi, Y. J. Org. Chem. 1983, 48, 3833. (doi:10.1021/jo00169a053)
- [8] Marko, I.E. J. Am. Chem. Soc. 2007, ASAP doi:10.1021/ja0691728
- [9] Wang, D.; Yue, J.-M. Synlett 2005, 2077-2079.