Blaise reaction: Difference between revisions
Appearance
Content deleted Content added
m r2.7.1) (robot Adding: ar:تفاعل بليز |
m r2.7.1) (Robot: Adding nl:Blaise-reactie |
||
| Line 29: | Line 29: | ||
[[ar:تفاعل بليز]] |
[[ar:تفاعل بليز]] |
||
[[es:Reacción de Blaise]] |
[[es:Reacción de Blaise]] |
||
[[nl:Blaise-reactie]] |
|||
[[zh:布莱斯反应]] |
[[zh:布莱斯反应]] |
||
Revision as of 15:21, 23 December 2011
The Blaise reaction is an organic reaction that forms a β-ketoester from the reaction of zinc metal with a α-bromoester and a nitrile.[1] [2] [3] The final intermediate is a metaloimine, which is hydrolyzed to give the desired β-ketoester.[4]
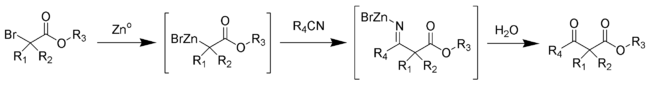
Bulky aliphatic esters tend to give higher yields. Steven Hannick and Yoshito Kishi have developed an improved procedure.[5]
It has been noted [6][7] that free hydroxyl groups can be tolerated in the course of this reaction, which is surprising for reactions of organometal halides.
References
- ^ Edmond E. Blaise; Compt. Rend. 1901, 132, 478.
- ^ Rinehart, K. L., Jr. Organic Syntheses, Coll. Vol. 4, p.120 (1963); Vol. 35, p.15 (1955). (Article)
- ^ Rao, H. S. P.; Rafi, S.; Padmavathy, K. Tetrahedron 2008, 64, 8037-8043. (Review)
- ^ Cason, J.; Rinehart, K. L., Jr.; Thorston, S. D., Jr. J. Org. Chem. 1953, 18, 1594. (doi:10.1021/jo50017a022)
- ^ Hannick, S. M.; Kishi, Y. J. Org. Chem. 1983, 48, 3833. (doi:10.1021/jo00169a053)
- [8] Marko, I.E. J. Am. Chem. Soc. 2007, ASAP doi:10.1021/ja0691728
- [9] Wang, D.; Yue, J.-M. Synlett 2005, 2077-2079.