Summary
Elimination of regulatory T lymphocytes may provide a way to break self-tolerance and unleash the anti-tumor properties of circulating lymphocytes. The use of fusion proteins, which link cytotoxic molecules to receptor targets, provides one approach to this problem. This study examined the ability of a fusion protein of interleukin-2 (IL-2) and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes based on their expression of high-affinity IL-2 receptors. Thirteen patients (12 with metastatic melanoma, 1 with metastatic renal cell carcinoma) were treated at one of the two Food and Drug Administration–approved doses of Denileukin Diftitox (seven patients at 9 μg/kg, six patients at 18 μg/kg). None of the patients experienced an objective clinical response. Foxp3 expression did not decrease significantly overall, although it did decrease minimally among patients receiving 18 μg/kg (−2.01 ± 0.618 copies of Foxp3/103 copies of β-actin; P = 0.031). Denileukin Diftitox did not decrease the suppressive ability of CD4+CD25+ cells as quantified by an in vitro co-culture suppression assay. Furthermore, the increased numbers of lymphocytes in patients resulting from treatment with IL-2 were not susceptible to Denileukin Diftitox. Administration of Denileukin Diftitox does not appear to eliminate regulatory T lymphocytes or cause regression of metastatic melanoma.
Keywords: oncology, regulatory T cells, melanoma
Regulatory T lymphocytes, a subset of CD4+ lymphocytes, defined by their ability to suppress proliferative and effector functions of T lymphocytes, have become a recent focus of intense investigation in the field of immunology.1–7 In 1981, the observation that mouse thymectomy on neonatal day 3 led to the development of multiorgan autoimmune disease8 paved the way for future seminal experiments by Sakaguchi and colleagues that demonstrated that it was the depletion of the CD4+CD25+ subset of cells in these mice that led to their autoimmunity.9,10 Furthermore, restoration of this subset to the thymectomized mice prevented the development of such autoimmune disease.11
The recent identification of the forkhead transcription factor, Foxp3, which is specifically expressed in CD4+CD25+ regulatory T cells and is required for their development,12,13 in conjunction with the retrospective observation that the immune dysregulation polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) in humans is caused by mutations of Foxp3,14–16 have allowed a link between the murine autoimmune model, murine regulatory T cells, and human autoimmunity.
Recent demonstrations of how breaking self-tolerance by inhibiting cytotoxic T lymphocyte antigen 4 (CTLA-4) have led to durable regressions of established tumors in humans17 have prompted interest in targeting other molecules unique to resting inhibitory or regulatory T cells. It appears that inhibition of regulatory T cells may play a role in breaking tolerance to self-antigens and enabling effector T cells to unleash their ability to recognize and destroy tumor-bearing cells. These clinical results have prompted a search for other means by which regulatory T cells may be inhibited or temporarily eliminated.
One potential method for eliminating regulatory T cells exploits the notion that they are the only resting circulating T cells expressing the high-affinity interleukin-2 (IL-2) receptor (IL-2R) consisting of the IL-2Rα, β, and γ chains. Denileukin Diftitox was designed to direct the cytocidal action of the diphtheria toxin (DT) by binding to cells that express the IL-2R and can then mediate the internalization of the toxin.
The predecessor of Denileukin Diftitox, DAB486IL-2, was the first recombinant ligand fusion toxin tested both in vitro and in animals. It encoded a 68-kDa protein containing the first 486 amino acids of DT and the full-length sequence for IL-2.18,19
It was extensively studied in the late 1980s and early 1990s and had modest success in phase I and II clinical trials against various lymphoid malignancies (intermediate- and low-grade non-Hodgkin lymphoma, Hodgkin disease, cutaneous T cell leukemia (CTCL)).20 However, an exceptionally short in vivo half-life (related in large part to its hydrophobicity and tendency to aggregate in solution) prompted efforts to improve on the original design.21,22
Successive truncations between amino acids 370 and 480 of DT were constructed and tested. Ultimately, the identification of amino acid 389 as the optimal site for truncation and fusion to the IL-2 molecule was confirmed by x-ray crystallography, which demonstrated that amino acid 389 was positioned at the end of a random coil separating the transmembrane domain from the native receptor-binding domain.23
Thus, Denileukin Diftitox (DAB389IL-2, ONTAK) is a recombinant DNA-derived cytotoxic fusion protein composed of the amino acid sequences for DT fragments A and B (Met1-Thr387)-His followed by the sequences for IL-2 (Ala1-Thr133). Native DT is a 535-amino acid protein consisting of three domains: an enzymatically active domain (fragment A), a hydrophobic domain (N-terminal portion of fragment B), and the receptor-binding domain (C-terminal portion of fragment B). DT intoxicates sensitive eukaryotic cells by a receptor-mediated endocytosis.
DAB389IL-2 is capable of intoxicating cells bearing the high-affinity (α-β-γ) isoform of the IL-2R at a 100 times lower concentration than cells expressing the intermediate-affinity (β-γ) isoform. In vitro, an initial signaling event is seen that mimics that of stimulation by IL-2, including increases in mRNA for IL-2, IL-2R, and interferon-γ.24
Internalization occurs within approximately 11 minutes of receptor binding; once the fusion protein is within the endocytic vesicle, the low pH leads to cleavage of the catalytic domain, which is liberated into the cytosol. Whereas the exact details are currently unknown, this leads to ADP-ribosylation of elongation factor 2. Protein synthesis is inhibited within 4 to 6 hours, and morphologic evidence of cell death is observed within 40 to 72 hours.21,22,25–27
Recent published reports have documented the success of Denileukin Diftitox in treating patients with cutaneous T-cell leukemia,28–31 follicular non-Hodgkin lymphoma,29,32 refractory B-cell non-Hodgkin lymphoma,33,34 and acute steroid-refractory graft-versus-host disease.35
Because resting regulatory T cells express CD25 and because the in vivo half-life of Denileukin Diftitox is relatively short (approximately 70–80 minutes), we hypothesized that treating patients with metastatic cancer with Denileukin Diftitox might eliminate regulatory T cells and ultimately allow activated (possibly by vaccination or adoptive cell transfer), newly expressing CD25, effector T cells to target cancer-bearing cells in an uninhibited manner. The first step is this sequence, however, would be to determine if treatment of patients with Denileukin Diftitox could eliminate regulatory T cells.
MATERIALS AND METHODS
Patients and Treatment
All patients had stage IV metastatic melanoma or renal cell carcinoma, with Karnofsky Performance Status score of ≥60%, no evidence of autoimmune or immunodeficiency disease, and ≥3 weeks elapsed since any previous systemic cancer therapy. All patients signed an informed consent prior to protocol enrollment and were treated in the Surgery Branch, National Institutes of Health, Bethesda, MD, USA. The treatment, Denileukin Diftitox (DAB389IL-2, ONTAK; Ligand Pharmaceuticals, San Diego, CA, USA), was administered intravenously over 1 hour for 5 consecutive days every 21 days. Each 5-day treatment and 16-day rest period constituted one treatment cycle. This study was approved by the Institutional Review Board of the National Cancer Institute and consisted of two cohorts: seven patients treated at the Food and Drug Administration–approved low dose (9 μg/kg/day) and six patients treated at the Food and Drug Administration–approved high dose (18 μg/kg/day).
When possible, patients underwent apheresis to collect peripheral blood mononuclear cells (PBMCs) before treatment and within 1–2 days following treatment. PBMCs were isolated by Ficoll–Hypaque separation and cryopreserved at 108 cells/vial in heat-inactivated human AB serum with 10% dimethyl sulfoxide and stored at −180°C until further use.
Clinical Response Evaluation
All patients underwent computed tomography of the chest, abdomen, and pelvis and magnetic resonance imaging of the brain within 4 weeks before treatment and subsequently after every two cycles of therapy; other radiologic modalities were used as needed to evaluate disease sites. For every patient, the sum of the longest diameters of all tumors before and after treatment was calculated, as per Response Evaluation Criteria In Solid Tumors (RECIST) criteria.36 A partial response was defined as the reduction of ≥30% (but <100%) of the sum of the longest diameters of all evaluable metastases lasting at least 1 month with no new or enlarging tumors; a complete response was the disappearance of all evaluable tumors for at least 1 month. Patients not having either a partial or a complete response were deemed nonresponders.
Flow Cytometry
Flow cytometry was used to assess the surface expression of selected T cell markers and was performed as previously described.17 In brief, cryopreserved PBMCs were thawed into ice-cold buffer and washed, and Fc receptor was blocked with mouse IgG (Caltag Laboratories, Burlingame, CA, USA). CD25 staining was performed using a PE-conjugated 4A3 clone (Miltenyi Biotec, Auburn, CA, USA). Cells were then incubated with appropriate fluorochrome-labeled antibodies (BD Biosciences, San Diego, CA, USA) and relevant isotype controls and washed twice subsequently. FACS-Calibur and CellQuest software (BD Biosciences) was used for acquisition and analysis.
Atac4 Cell Line
Atac4 is a cell line derived from A431 (vulvar carcinoma) transfected with CD25 and was obtained from Ira Pastan (Laboratory of Molecular Biology, Bethesda, MD, USA).
Cell Viability
Cell viability was assessed using the WST-1 calorimetric test (Roche, Basle, Switzerland) according to the manufacturer’s instructions. Plates were read on a Micro-Titer (Wallac 1420 multilabel counter) using a wavelength of 450 nm, with a reference of 650 nm.
DT IgG Measurement
Measurement of DT IgG was carried out by Mayo Medical Laboratories (Rochester, MN, USA) using an enzyme-linked immunosorbent assay.
Real-Time Polymerase Chain Reaction
Levels of mRNA Foxp3 were analyzed using quantitative real-time polymerase chain reaction (PCR) with the TaqMan Gene Expression Assay (Applied Biosystems, Foster City, CA, USA). In each experiment, 500 ng of total RNA was isolated from CD4 purified lymphocytes (see below) using the RNeasy mini kit (Qiagen, Valencia, CA, USA) and reverse-transcribed to prepare cDNA using the ThermoScript RT-PCR system (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The TaqMan Universal Master Mix (Applied Biosystems) was also used as a template. For β-actin, the forward primer used was as follows: 5′-GCGA GAAGATGACCCAGATC-3′; the reverse primer used was as follows: 5′-CCAGTAGGTACGGCCAGAGG-3′; and the probe used was as follows: 5′-FAM-CCAGCCATGTACGT TGCTATCCAGGC-TAMRA-3′. For Foxp3, the combined primer and probe reagent was used (Assay-on-demand gene expression assay; Applied Biosystems). The ABI Prism 7700 detection system (Applied Biosystems) was used with the following settings: 50°C for 2 minutes followed by 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute.
Lymphocyte Separation
CD4+ cells were separated from whole PBMCs using the Dynal CD4 negative isolation kit (Dynal Biotech, Invitrogen) according to the manufacturer’s instructions. For suppression assays, CD4+ cells were further purified into CD25− and CD25+ fractions using the Dynal Treg kit according to the manufacturer’s instructions. Separations were performed in phosphate-buffered saline with 0.1% bovine serum albumin.
Suppression Assay
Autologous Antigen Presenting Cells (APCs) were isolated from fresh PBMCs that were T cell depleted using the Dynal CD3+ depletion kit according to the manufacturer’s instructions and then irradiated with 3000 rad. The APCs were plated in 96-well round-bottom plates at 5 × 104 cells/well. CD4+CD25− cells, which served as the effector cells, were plated in various ratios with CD4+CD25+ cells. Ratios of 1:0 (104 CD4+CD25−:0 CD4+CD25+), 1:0.5 (104:5 × 103), 1:1 (104:104), and 0:1 (0:104) were added to each well. Replicates were performed in sextuplets. Each well was treated with anti-CD3 at a concentration of 1 μg/mL. On the fifth day of culture, 1 μCi of [3H]thymidine was added to each well, and incorporation was determined 18 hours later. Percentage suppression was calculated using the following formula:
RESULTS
In Vitro Studies
Ability of Denileukin Diftitox to Eliminate CD25 Expressing Cells in Vitro
The Atac4 cell line, a highly expressing CD25 line, was treated with Denileukin Diftitox at four concentrations (0, 100, 500, and 1000 ng/mL) for 72 hours. WST-1 assay was performed after 72 hours and demonstrated 77%, 96%, and 100% reduction in metabolic activity at concentrations of 100, 500, and 1000 ng/mL, respectively, compared with the activity of the untreated group (Fig. 1A).
FIGURE 1.

A, WST-1 assay on Atac4 cell line that expressed high levels of CD25 following treatment with Denileukin Diftitox in vitro for 72 hours. Numbers in parentheses above columns indicate the percentage decrease in optical density relative to untreated group. Uptake of a blank well (medium alone) is shown for comparison. In vitro, Denileukin Diftitox eliminated cells expressing CD25. B, PBMCs treated with 50 ng/mL anti-CD3 for 72 hours, prior to 72-hour in vitro exposure to Denileukin Diftitox. Viability was assessed by failure to take up PI stain. The percentage of cells expressing CD25 decreased with increasing doses of Denileukin Diftitox, and this was paralleled by cell viability, demonstrating that Denileukin Diftitox could eliminate activated PBMCs in vitro.
PBMCs were isolated from normal donors and stimulated with soluble anti-CD3 at 50 ng/mL for 72 hours. Following anti-CD3-induced up-regulation of CD25, the PBMCs were treated with Denileukin Diftitox at four concentrations (0, 100, 500, and 1000 ng/mL) for an additional 72 hours. Flow cytometry revealed a decrease in cell viability, as measured by increased uptake of propidium iodide (PI), and a decrease in CD25 surface expression by both frequency (see Fig. 1B) and mean fluorescence intensity (MFI) (data not shown). These studies demonstrated that Denileukin Diftitox was capable of eliminating cells whose CD25 was up-regulated by anti-CD3 antibodies.
Inability of Denileukin Diftitox to Eliminate CD4+CD25+ Regulatory T Cells In Vitro
Whole PBMCs, CD4+, CD4+CD25−, and CD4+CD25+ purified cells from two normal donors were treated with Denileukin Diftitox at 0, 100, and 500 ng/mL for 72 hours and evaluated for change of surface CD25 expression by flow cytometry based on frequency and MFI (Table 1). PBMCs from patient 1 demonstrated a slight decrease in the frequency and MFI of CD25+ expressing cells with increasing Denileukin Diftitox dose, whereas CD4 purified cells experienced a decrease in CD25 frequency with an increase in MFI. The CD25 expressing cells in the CD4+CD25− purified fraction were also susceptible to Denileukin Diftitox in frequency; however, the remaining cells did show an increase in MFI. CD4+CD25+ enriched cells treated with 500 ng/mL Denileukin Diftitox had a 29% decrease in overall viability (data not shown), no change in CD25 frequency, but an increase in CD25 MFI. In contrast to this, patient 2 experienced a uniform increase in CD25 frequency with escalating doses of Denileukin Diftitox with minimal impact on MFI. Thus, when testing the phenotype of whole or purified subsets of PBMCs, little, if any, consistent impact of treatment with Denileukin Diftitox was seen.
TABLE 1.
Changes in Frequency and Mean Fluorescence Intensity (MFI) of CD25+ Expression for Different Cell Populations Treated With Denileukin Diftitox In Vitro for 48 Hours
|
Concentration of ONTAK |
|||
|---|---|---|---|
| 0 ng/mL | 100 ng/mL | 500 ng/mL | |
|
%CD25+ (MFI) |
|||
| Patient I | |||
| PBMC | 33.8% (487) | 21.56% (401) | 23.4% (337) |
| CD4+ | 47.3% (68) | 24.5% (180) | 23.5% (178) |
| CD4+CD25− | 12.8% (64) | 10.3% (98) | 8.4% (88) |
| CD4+CD25+ | 99.4% (197) | NT | 98.2% (344) |
| Patient II | |||
| PBMC | 9.0% (38) | 19.7% (40) | 19.1% (46) |
| CD4 | 28.7% (61) | 35.5% (54) | 37% (60) |
| CD4+CD25− | 8.6% (42) | 10.6% (45) | 14.2% (44) |
Little consistent impact was seen on phenotype following in vitro treatment with Denileukin Diftitox.
NT, not treated.
Foxp3 Expression for Assessment of Impact of Denileukin Diftitox on Regulatory T Cells
CD4+ cells were isolated by negative selection from normal donor PBMCs and treated with Denileukin Diftitox at four concentrations (0, 15, 150, and 750 ng/mL) for 48 hours (Fig. 2A). Foxp3 expression increased fourfold with the smallest dose and was virtually unchanged with further increasing doses. This experiment was repeated on a different normal donor at different doses (0, 100, and 500 ng/mL) and time of exposure (72 hours; see Fig. 2B). Again, there was no evidence of reduced Foxp3 expression in purified CD4+ cells.
FIGURE 2.

A, Foxp3 expression in purified CD4+ cells following 48-hour in vitro exposure to Denileukin Diftitox did not demonstrate evidence of Denileukin Diftitox–mediated elimination of Foxp3 expressing regulatory T cells. B, Foxp3 expression in purified CD4+ cells following 72-hour in vitro exposure to Denileukin Diftitox also failed to demonstrate elimination of Foxp3 expressing regulatory T cells.
Next, CD4+CD25+ cells were isolated and treated for 72 hours with two doses: either 0 or 500 ng/mL of Denileukin Diftitox. Following this treatment, suppression assays were carried out to examine the ability of Denileukin Diftitox to eliminate CD4+CD25+ cells with regulatory properties. The untreated CD4+CD25+ cells demonstrated 89.1% suppression at a 1:1 ratio with CD4+CD25− cells (Fig. 3A), whereas the treated CD4+CD25+ cells demonstrated 91.7% suppression (see Fig. 3B). Taken together, these results suggested that in vitro, Denileukin Diftitox failed to eliminate Foxp3 expressing cells and cells capable of exerting suppressive or regulatory function.
FIGURE 3.

Suppressive capacity of untreated CD4+CD25+ purified T cells (A) and Denileukin Diftitox–treated CD4+CD25+ purified T cells (500 ng/mL × 72 hours) (B). Percent suppression reflects suppression for 1:1 treatment group (10,000 CD25−:10,000 CD25+ cells). Treatment with Denileukin Diftitox did not reduce suppressive abilities of CD4+CD25+ in vitro. ACC, T cell–depleted irradiated accessory cell.
In Vivo Studies
Patient Characteristics
Thirteen patients were evaluated in the clinical trial: 12 with metastatic melanoma and 1 with metastatic renal cell cancer. All patients had previously undergone resection of their primary tumor. Eleven (85%) had previously received an immune-based therapy, and four (31%) had previously received chemotherapy (Table 2). None of the patients experienced an objective clinical response.
TABLE 2.
Patient Characteristics, Clinical Response, and Toxicity
| Patient | Age/Sex | Disease Site (s) | Prior Therapy | Dose (mcg/kg) | Clinical Response** | No. Cycles Received* | Grade III/IV Toxicities |
|---|---|---|---|---|---|---|---|
| 1 | 52/M | Brain, lung, liver, adrenal, axillary, RP, SQ | C, I, R, S | 9 | NR | 2 | Lymphopenia |
| 2 | 22/M | Lung | I, S | 9 | NR | 11 | Lymphopenia |
| 3 | 40/F | Brain, lung | I, S | 9 | NR | 4 | Elevated AST |
| 4 | 51/M | Lung, RP, SQ | I, S | 9 | NR | 4 | Lymphopenia |
| 5 | 43/F | Axillary LN, neck | I, S | 9 | NR | 2 | |
| 6 | 50/M | Lung | I, S | 9 | NR | 4 | Lymphopenia |
| 7‡ | 47/M | Lung, mediastinal LN | S | 9 | NR | 4 | Lymphopenia |
| 8 | 28/F | Axillary LN, bone, liver | C, I, S | 18 | NR | 2 | Lymphopenia |
| 9 | 38/F | Lung, liver, right ventricle | S, I | 18 | NR | 1 | Thrombocytopenia |
| 10 | 34/M | Brain, axillary LN, lung | S, I | 18 | NR | 2 | |
| 11 | 24/F | Lung, liver, breast, bone, abdominal wall, SQ | C, I, R, S | 18 | NR | 2 | Lymphopenia |
| 12 | 40/M | Lung, mediastinal LN, neck | C, I, R, S | 18 | NR | 3 | Lymphopenia, Prolonged aPTT |
| 13 | 22/M | RP | S | 18 | NR | 2 | Lymphopenia |
aPTT, activated partial thromboplastin time; AST, Aspartate aminotransferase; C, chemotherapy; F, female; I, immunotherapy; LN, lymph node; M, male; NR, no response; R, radiation therapy; RP, retroperitoneal; S, surgery; SQ, subcutaneous.
One cycle consisted of once daily infusion with 9 or 18 mcg/kg for 5 days.
Responses as of March 1, 2005.
Renal Cell Cancer.
Hematologic Impact on Denileukin Diftitox
Within 12 hours of Denileukin Diftitox administration, patients experienced sudden lymphopenia. In nine patients (69%), the nadir of the lymphocyte count reached the threshold for grade III toxicity (absolute lymphocyte count [ALC] ≤500 cells/μL; see Table 2). Figure 4A depicts representative changes in ALC and absolute neutrophil count (ANC) during the first two to three cycles. The rise in ANC essentially mirrored the fall in ALC in proportion, such that the total white blood cell count (WBC) actually rose slightly with each initial dose in the cycle (of five doses). An evaluation of cell subsets found that both CD4+ and CD8+ cell counts decreased during this time, with no selective difference (P = 0.108, two-tailed paired t test). With successive treatments, however, the magnitude of these changes in peripheral blood counts dampened (see Fig. 4B).
FIGURE 4.
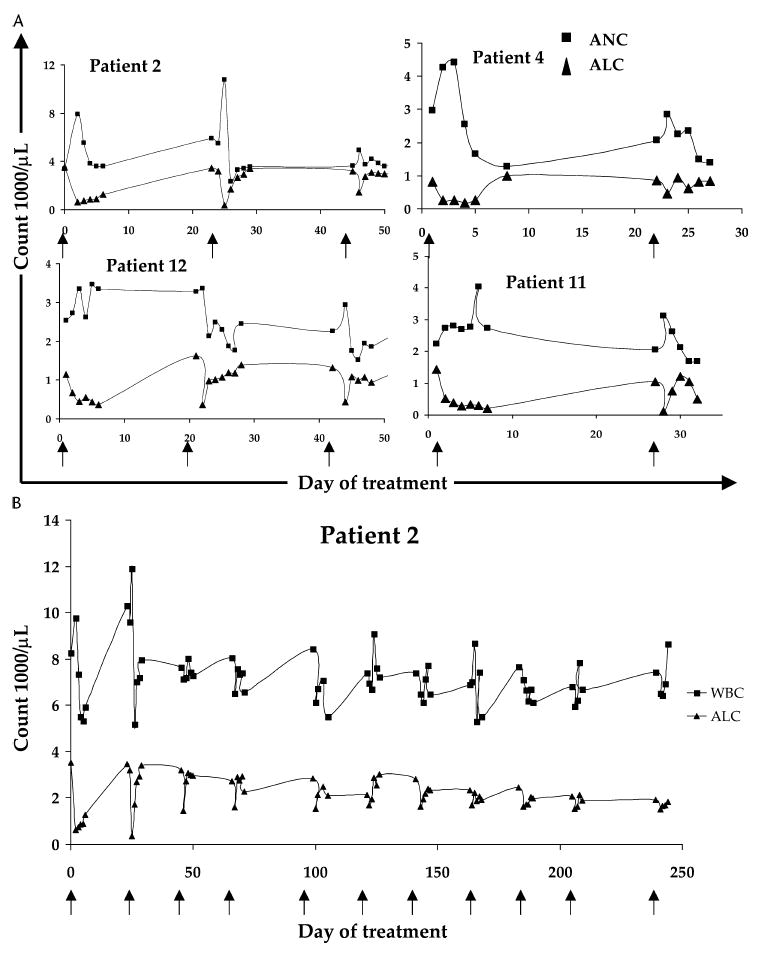
A, Peripheral blood counts from four patients during the first two to three treatment cycles. Arrows along x axis denote the first day of treatment of a given 5-day cycle. Treatment with Denileukin Diftitox caused an abrupt fall in ALC and rise in ANC, both of which were transient changes. B, Peripheral blood counts for patient 2 over the entire course of his 11 cycles. Arrows along x axis denote the first day of treatment of a given 5-day cycle. With successive treatments, the magnitude of change of both the ALC and the total WBC decreased.
Serum samples from four patients in the 9-μg/kg cohort were evaluated for presence of IgG antibody to the DT in longitudinal samples over the course of their treatments (Table 3). In each patient, pretreatment levels suggested successful previous vaccination (or exposure). However, following two cycles (10 doses), each patient had generated enough IgG antibody to saturate the assay (>3 IU/mL), suggesting that repeated exposure to Denileukin Diftitox generated antibodies directed against the DT.
TABLE 3.
DT IgG Antibody Titer* for Selected Patients Treated With Denileukin Diftitox At 9 μg/kg
| Pre | Post C1 | Post C2 | Post C3 | Post C4 | Post C5 | |
|---|---|---|---|---|---|---|
|
(IU/mL) |
||||||
| Patient 2 | 0.36 | 0.27 | >3.0 | >3.0 | >3.0 | >3.0 |
| Patient 4 | 1.17 | >3.0 | >3.0 | >3.0 | >3.0 | n/a |
| Patient 6 | 0.14 | 0.14 | >3.0 | >3.0 | >3.0 | n/a |
| Patient 7 | 0.18 | 0.11 | >3.0 | >3.0 | >3.0 | n/a |
With progressive treatment, each patient experienced increasing titers of IgG directed against the DT.
>0.10 IU/mL reflects immunization and/or exposure.
Phenotypic Changes
With use of flow cytometry, pre- and posttreatment peripheral blood leukocyte samples were examined for changes in surface expression of human leukocyte antigen (HLA)-DR and CD25 before and after the first cycle of treatment (Table 4). HLA-DR expression, a surrogate for activation of cells, was evaluated on both CD4+ and CD8+ cells. In patients treated with 9 μg/kg, there was no significant change in HLA-DR expression on CD4+ cells (+0.1%; P = 0.987) or on CD8+ cells (+3.2%; P = 0.350). Among patients treated with 18 μg/kg, there was a trend toward an increase in HLA-DR expression on CD4+ cells (+13.8%; P = 0.135) and CD8+ cells (+13.3%; P = 0.046).
TABLE 4.
Phenotypic Changes on CD4+ and CD8+ Cells Before and After One Cycle of Denileukin Diftitox Treatment
|
HLA-DR+ |
CD25+ |
||||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| Patient | Dose (mcg/kg) | (% of CD3+CD4+) | (% of CD3+CD4+) | ||
| 1 | 9 | 12.6% | 10.8% | 74.6% | 61.8% |
| 2 | 9 | 36.9% | 15.7% | 51.6% | 43.6% |
| 4 | 9 | 18.4% | 23.8% | 68.7% | 55.1% |
| 6 | 9 | 10.9% | 17.3% | 68.0% | 56.5% |
| 7 | 9 | 19.1% | 30.8% | 61.9% | 54.1% |
| 8 | 18 | 5.5% | 42.3% | 62.4% | 54.2% |
| 9 | 18 | 7.2% | 6.8% | 62.0% | 44.1% |
| 10 | 18 | 19.7% | 47.2% | 51.2% | 69.7% |
| 11 | 18 | 16.1% | 44.3% | 71.1% | 81.1% |
| 12 | 18 | 45.3% | 42.2% | 43.3% | 41.5% |
| 13 | 18 | 13.2% | 7.1% | 45.3% | 39.4% |
| Mean | Total | +7.6% (±5.2%; P = 0.176) | −5.4% (±3.2%; P = 0.129) | ||
| 9 mcg/kg | +0.1% (P = 0.987) | −10.7% (P = 0.0009) | |||
| 18 mcg/kg | +13.8% (P = 0.135) | −0.9% (P = 0.876) | |||
|
HLA-DR+ |
CD25+ |
||||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| Patient | Dose (mcg/kg) | (% of CD3+CD4−) | (% of CD3+CD4−) | ||
| 1 | 9 | 27.9% | 31.6% | 19.8% | 18.3% |
| 2 | 9 | 34.8% | 27.1% | 12.1% | 15.9% |
| 4 | 9 | 26.9% | 37.8% | 20.6% | 14.8% |
| 6 | 9 | 10.3% | 13.6% | 13.0% | 10.6% |
| 7 | 9 | 57.5% | 63.4% | 7.5% | 16.0% |
| 8 | 18 | 21.8% | 46.4% | 16.7% | 19.6% |
| 9 | 18 | 4.5% | 9.2% | 12.9% | 12.3% |
| 10 | 18 | 30.4% | 56.7% | 18.8% | 45.2% |
| 11 | 18 | 18.8% | 40.0% | 12.2% | 14.6% |
| 12 | 18 | 71.2% | 67.8% | 6.0% | 18.4% |
| 13 | 18 | 20.6% | 27.0% | 8.5% | 29.5% |
| Mean | Total | +8.7% (±3.3%; P = 0.026) | +6.1% (±3.2%; P = 0.073) | ||
| 9 mcg/kg | +3.2% (P = 0.350) | +0.5% (P = 0.844) | |||
| 18 mcg/kg | +13.3% (P = 0.046) | +10.8% (P = 0.063) | |||
Mean ± SEM changes are shown. P values are shown for two-tailed paired t test.
Patients treated at 9 μg/kg demonstrated a small but significant decrease in CD25 expression on CD4+ cells (−10.7%; P = 0.0009) but no change in CD25 expression on CD8+ cells (+0.5%; P = 0.844). Conversely, patients treated with 18 μg/kg experienced virtually no change in CD25 expression on CD4+ cells (−0.9%; P = 0.876) but a slight increase in CD25 expression on CD8+ cells (+10.8%; P = 0.063).
Changes in Foxp3 Expression
Nine patients (four patients at 9 μg/kg, five patients at 18 μg/kg) were evaluated for change in Foxp3 expression in purified CD4+ cells following their first cycle of Denileukin Diftitox (Fig. 5A). Overall, patients experienced minimal change in Foxp3 expression of −1.68 copies/103 copies of β-actin (±1.11; P = 0.167). Patients in the 9-μg/kg group experienced a change of −1.27 copies/103 copies of β-actin (±2.57; P = 0.656), whereas patients in the 18-μg/kg group experienced a minimal but significant change of −2.01 copies/103 copies of β-actin (±0.618; P = 0.031).
FIGURE 5.
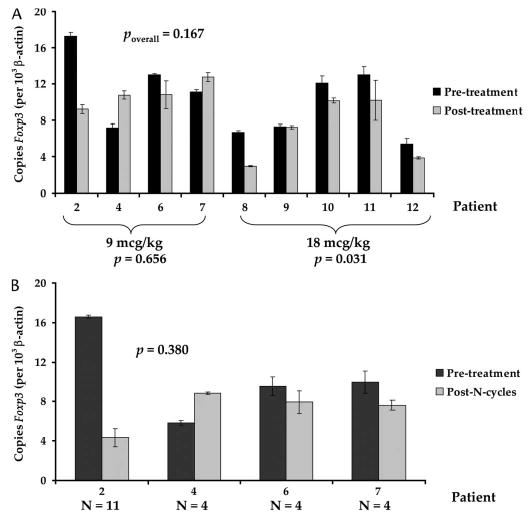
A, Change in Foxp3 expression in purified CD4+ cells following in vivo administration of Denileukin Diftitox after the first cycle of treatment of nine patients. The four patients (2, 4, 6, 7) who received 9 μg/kg did not demonstrate a significant change in Foxp3 expression, whereas the five patients (8, 9, 10, 11, 12) who received 18 μg/kg experienced a small but statistically significant decrease in Foxp3 expression, suggesting that Denileukin Diftitox had minimal impact on Foxp3 expression in vivo. P values reflect two-tailed paired t test. B, Change in Foxp3 expression in purified CD4+ cells following multiple cycles of Denileukin Diftitox. Foxp3 expression did not change significantly following multiple treatments. N = number of cycles. P value reflects two-tailed paired t test.
Four patients who received four or more cycles were evaluated for changes in Foxp3 expression in purified CD4+ cells obtained before treatment compared with those obtained after the last dose of Denileukin Diftitox (see Fig. 5B). The mean difference in Foxp3 expression was −3.30 copies/103 copies of β-actin (±3.21; P = 0.380). Thus, no evidence of loss of Foxp3 expression in CD4+ cells was seen in patients receiving the two different approved doses of Denileukin Diftitox.
Impact of Denileukin Diftitox on Regulatory T-Cell Function
An in vitro suppression assay was performed on cryopreserved pretreatment and posttreatment samples from patient 6 (data not shown). Pretreatment suppression at the 1:1 ratio was 62.3%, whereas posttreatment (post cycle 4) suppression was 65.2%. The suppression assay was also carried out on fresh cells taken from the posttreatment (post cycle 2) apheresis samples from patients 6, 7, 10, 11, and 12 (Fig. 6). Following a second cycle of treatment, the levels of suppression for these five patients were 67.9%, 59.7%, 50.6%, 79.4%, and 98.2%, respectively.
FIGURE 6.

Suppressive ability of CD4+CD25+ freshly isolated T cells from five patients following two cycles of Denileukin Diftitox. In each patient, at least 50% suppressive activity was measured following treatment, suggesting that Denileukin Diftitox failed to eliminate T cells capable of mediating suppression.
Thus, following administration of Denileukin Diftitox, PBMCs of treated patients contained significant levels of T cells with regulatory activity.
IL-2 Susceptibility
IL-2 administration can lead to an increase in the number of regulatory T cells in the circulation of patients. To test the hypothesis that these regulatory T cells generated in vivo to IL-2 may be more sensitive to Denileukin Diftitox, fresh PBMCs from two patients (A and B) with metastatic melanoma being treated with bolus IL-2 (720,000 IU/kg every 8 hours as tolerated) were collected at the completion of their treatment, during the time of rebound lymphocytosis, and CD4+ cells were isolated.
The CD4+ cells were treated with three doses of Denileukin Diftitox (0, 100, and 1000 ng/mL) for 72 hours, and Foxp3 expression was quantified (Fig. 7). In both patients, there was an increase in Foxp3 expression following in vitro Denileukin Diftitox administration, suggesting that lymphocytes from patients receiving IL-2 were not more susceptible to Denileukin Diftitox.
FIGURE 7.

Foxp3 expression in purified CD4+ cells from two patients treated with high-dose IL-2. Cells were harvested 3 days following last dose of IL-2, during rebound lymphocytosis, and treated with 0, 100, or 1000 ng/mL Denileukin Diftitox in vitro. These IL-2-activated regulatory T cells were not more sensitive to Denileukin Diftitox treatment in vitro.
DISCUSSION
Our previous work demonstrated that CTLA-4 blockade17 could result in durable regression of established metastatic melanoma that was highly associated with the induction of autoimmune destruction of normal tissue (P. Attia et al, unpublished). Because many cancer antigens are derived from nonmutated proteins,37 this work suggested that the mechanism governing tolerance to normal self tissues was similar to that mediating tolerance to melanoma antigens. Whereas the exact mechanism of CTLA-4 blockade is unknown, the possibility remains that inhibition of regulatory T cells, which at rest constitutively express CTLA-4, was in part responsible for the loss of self-tolerance that led to immune-mediated tumor regression.38 These results have prompted investigation into strategies to eliminate or inhibit regulatory T cells.39,40 One method of targeting regulatory T cells involves the use of fusion proteins such as Denileukin Diftitox, which fuses the enzymatically active portion of the DT (fragment A) to the binding portion of IL-2. Because regulatory T cells at rest express high-affinity IL-2Rs (CD25, CD122, and CD132), they should be particularly susceptible to toxin-mediated destruction.
The elimination of regulatory T cells has been shown to increase cancer immunity in murine models.41–44 Immunization of mice with tumor cells in the absence of CD4+CD25+ regulatory cells induces immunity against a variety of different tumor cell lines,45 suggesting that effector T cells, which possess anti-tumor capabilities, can be kept at bay by regulatory T cells. Furthermore, transfer of CD4+ T cells that contained a mixture of CD4+CD25− and CD4+CD25+ cells or transfer of CD4+CD25+ cells alone prevented effective adoptive immunotherapy.41
Quantification of regulatory T cells in the human is difficult, and most methods are indirect. Regulatory CD4+ T cells in resting PBMCs express high-affinity IL-2Rs, including CD25. Evaluating surface expression of CD25 is of limited utility, however, as activated cells also express CD25. Functional analysis, using the suppression co-culture assay,46,47 has provided another means to quantify suppressor function. However, the assay is difficult to perform on cryopreserved specimens48 and thus not practical for comparing pre- and posttreatment patient samples. Foxp3 expression, high levels of which have recently found to be associated with regulatory T cells,12,13 has provided another means to identify levels of regulatory T cells. Each of these methods was used in the current study and provided similar results.
The elimination of regulatory T cells is a goal in the studies of cancer immunology because of the enhanced anti-tumor effects resulting from their depletion. Several strategies have emerged, including nonmyeloablative lymphodepleting chemotherapy prior to adoptive cell transfer,37,49 whole-body irradiation prior to adoptive cell transfer,50 and the use of anti-CD25 antibodies.51 Whereas CTLA-4 blockade has emerged as an effective means for breaking self-tolerance and inducing tumor regression, extensive in vitro and in vivo analyses of patients treated with anti-CTLA-4 have suggested that T-cell activation, rather than inhibition of regulatory T cells, may play a larger role in anti-CTLA-4-mediated tumor regression (A.V. Maker, personal communication). In the solid organ transplant setting, daclizumab (anti-Tac) has emerged as a potent induction immunosuppressive agent.52 However, the in vivo half-life of daclizumab is >4 weeks, and this limits its suitability for applications to cancer immunotherapy. Therapeutic depletion of regulatory T cells is likely to be followed by activation of remaining CD4+CD25− cells, and the presence of an antibody directed toward CD25 would disarm the effector cells, as well. Denileukin Diftitox has an in vivo half-life of 70–80 minutes, which makes it an attractive candidate to remove regulatory T cells as part of a cancer immunotherapy approach.
Our preliminary data showed that in vitro, Denileukin Diftitox could kill cells bearing only the α-subunit of the IL-2R (CD25) (see Fig. 1A), suggesting that resting regulatory T cells, expressing CD25, might be susceptible to intoxication by this fusion protein. In addition, anti-CD3-activated PBMCs that expressed high levels of CD25 were also sensitive to intoxication by Denileukin Diftitox in vitro. However, in vitro treatment with Denileukin Diftitox failed to reduce Foxp3 in CD4+ cells and may have increased it (see Fig. 2). Because IL-2 exposure is known to increase Foxp3 expression,53–57 even in CD4+CD25− cells,58 these results could have been explained by stimulation by the IL-2 portion of the fusion protein. Functional in vitro studies revealed treatment of PBMCs with Denileukin Diftitox failed to eliminate T cells capable of mediating suppression (see Fig. 3). Thus, although there was evidence that Denileukin Diftitox could eliminate CD25 expressing cells in vitro, there was no evidence that it could eliminate regulatory T cells. Nevertheless, the possibility that Denileukin Diftitox, in vivo, might impact regulatory T cells was investigated.
None of the 13 patients receiving high doses of Denileukin Diftitox in vivo experienced an objective clinical response (see Table 2). Eleven (85%) patients experienced transient grade III/IV toxicity, 10 of them hematologic. None of the patients required any intervention for their toxicities, and all returned to baseline before the end of the treatment cycle. Lymphopenia was the most frequent toxicity noted, and it was noted among both CD4+ and CD8+ subpopulations. Recovery of circulating lymphocytes was faster with successive cycles. Furthermore, loss of circulating lymphocytes with the initial dose in each treatment cycle decreased with successive cycles. This pattern suggested a tolerance to Denileukin Diftitox, and data presented in Table 3 demonstrated that following the second cycle of Denileukin Diftitox, each of the four patients tested had developed levels of antibody to the DT that saturated the assay.
The phenotypic changes on the cells of treated patients underscored the two components of Denileukin Diftitox (see Table 4). The IL-2 portion of the fusion protein tended to activate both CD4+ and CD8+ cells, although not statistically significantly on the CD4+ cells. In contrast, the CD4+ cells of the patients treated with 9 μg/kg demonstrated a small but statistically significant decrease in CD4+CD25+ frequency. This decrease was not noted in the CD8+CD25+ cells.
Real-time PCR measurements of mRNA encoding Foxp3 was performed on RNA isolated from PBMCs (data not shown), CD4+ purified cells, and CD4+CD25+ purified cells (data not shown). CD4+CD25+ cells expressed 5- to 10-fold greater Foxp3 per β-actin than purified CD4+ cells. PBMCs displayed the lowest amount of Foxp3 expression of the three cell isolates. However, the changes between pre- and posttreatment samples were consistent between cell populations, although the magnitude of change varied. Figure 5A depicts the change in Foxp3 expression that occurred following one cycle of Denileukin Diftitox for nine patients. The four evaluated patients who received 9 μg/kg did not experience a statistically significant change in Foxp3 expression. However, the five evaluated patients who received 18 μg/kg experienced a small but statistically significant decrease in Foxp3 expression, although it does not appear that this decrease was clinically significant.
To determine if Denileukin Diftitox had a functional impact on regulatory T cells, suppression assays were performed on selected patients (see Fig. 6). Initially, suppression assays were attempted on cryopreserved pre- and posttreatment samples simultaneously. However, it was technically difficult to purify CD4+CD25+ cells from cryopreserved PBMCs. Thus, we examined the suppressive capacity of posttreatment CD4+CD25+ fresh cells acquired by apheresis the day following the final dose on Denileukin Diftitox. In five patients (patients 6, 7, 10, 11, and 12), at least 50% suppression was present following Denileukin Diftitox administration. Thus, because neither the frequency of CD4+CD25+ cells nor the content of Foxp3 among CD4+ cells had decreased in a clinically significant manner, and suppressor cells were present in PBMCs post treatment, we concluded that treatment of patients with Denileukin Diftitox failed to eliminate regulatory T cells.
Our final attempt to explore the ability of Denileukin Diftitox to eliminate regulatory T cells was based on the fact that IL-2 is a growth factor for regulatory T cells. We hypothesized that treating patients first with IL-2, to up-regulate the regulatory T cells, may render them more susceptible to Denileukin Diftitox. To test this hypothesis, we treated, in vitro, rebound samples from two patients receiving bolus IL-2 (720,000 IU/kg every 8 hours), generally collected 2–3 days following their final dose of IL-2, at a time when the maximum number of circulating lymphocytes and regulatory T cells would be present.59–61 Treatment of CD4+ purified samples from these patients with Denileukin Diftitox had little impact (Fig. 7) and produced similar results to that seen in resting cells (see Fig. 2).
It is therefore our conclusion that Denileukin Diftitox failed to eliminate regulatory T cells in patients with metastatic melanoma. Current efforts are under way to explore other immune-based fusion proteins that can specifically target these cells.
References
- 1.Kawaida H, Kono K, Takahashi A, et al. Distribution of CD4+CD25 high regulatory T-cells in tumor-draining lymph nodes in patients with gastric cancer. J Surg Res. 2005;124:151–157. doi: 10.1016/j.jss.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Lundgren A, Stromberg E, Sjoling A, et al. Mucosal Foxp3-expressing CD4+ CD25 high regulatory T cells in Helicobacter pylori-infected patients. Infect Immun. 2005;73:523–531. doi: 10.1128/IAI.73.1.523-531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonuleit H, Schmitt E. Regulatory T-cells in antitumor therapy: Isolation and functional testing of CD4+CD25+ regulatory T-cells. Methods Mol Med. 2005;109:285–296. doi: 10.1385/1-59259-862-5:285. [DOI] [PubMed] [Google Scholar]
- 4.Karube K, Ohshima K, Tsuchiya T, et al. Expression of Foxp3, a key molecule in CD4CD25 regulatory T cells, in adult T-cell leukaemia/lymphoma cells. Br J Haematol. 2004;126:81–84. doi: 10.1111/j.1365-2141.2004.04999.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang HY, Lee DA, Peng G, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: Implications for immunotherapy. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 6.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 7.Waldmann TA, Broder S, Krakauer R, et al. The role of suppressor cells in the pathogenesis of common variable hypogammaglobulinemia and the immunodeficiency associated with myeloma. Fed Proc. 1976;35:2067–2072. [PubMed] [Google Scholar]
- 8.Kojima A, Prehn RT. Genetic susceptibility to postthymectomy autoimmune diseases in mice. Immunogenetics. 1981;14:15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- 9.Asano M, Toda M, Sakaguchi N, et al. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Sakaguchi N. Organ-specific autoimmune disease induced in mice by elimination of T cell subsets. V. Neonatal administration of cyclosporin A causes autoimmune disease. J Immunol. 1989;142:471–480. [PubMed] [Google Scholar]
- 11.Suri-Payer E, Amar AZ, Thornton AM, et al. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- 12.Fontenot JD, Rudensky AY. Molecular aspects of regulatory T cell development. Semin Immunol. 2004;16:73–80. doi: 10.1016/j.smim.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 14.Bennett CL, Ochs HD. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr Opin Pediatr. 2001;13:533–538. doi: 10.1097/00008480-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Bennett CL, Brunkow ME, Ramsdell F, et al. A rare polyadenylation signal mutation of the Foxp3 gene (AAUAAA→AAUGAA) leads to the IPEX syndrome. Immunogenetics. 2001;53:435–439. doi: 10.1007/s002510100358. [DOI] [PubMed] [Google Scholar]
- 16.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of Foxp3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 17.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams DP, Parker K, Bacha P, et al. Diphtheria toxin receptor binding domain substitution with interleukin-2: Genetic construction and properties of a diphtheria toxin-related interleukin-2 fusion protein. Protein Eng. 1987;1:493–498. doi: 10.1093/protein/1.6.493. [DOI] [PubMed] [Google Scholar]
- 19.Bacha P, Williams DP, Waters C, et al. Interleukin 2 receptor-targeted cytotoxicity. Interleukin 2 receptor-mediated action of a diphtheria toxin-related interleukin 2 fusion protein. J Exp Med. 1988;167:612–622. doi: 10.1084/jem.167.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sweeney EB, Murphy JR. Diphtheria toxin-based receptor-specific chi-maeric toxins as targeted therapies. Essays Biochem. 1995;30:119–131. [PubMed] [Google Scholar]
- 21.Foss FM. DAB(389)IL-2 (Denileukin Diftitox, ONTAK): a new fusion protein technology. Clin Lymphoma. 2000;1(Suppl 1):S27–S31. [PubMed] [Google Scholar]
- 22.Foss FM, Saleh MN, Krueger JG, et al. Diphtheria toxin fusion proteins. Curr Top Microbiol Immunol. 1998;234:63–81. doi: 10.1007/978-3-642-72153-3_5. [DOI] [PubMed] [Google Scholar]
- 23.Choe S, Bennett MJ, Fujii G, et al. The crystal structure of diphtheria toxin. Nature. 1992;357:216–222. doi: 10.1038/357216a0. [DOI] [PubMed] [Google Scholar]
- 24.Walz G, Zanker B, Brand K, et al. Sequential effects of interleukin 2-diphtheria toxin fusion protein on T-cell activation. Proc Natl Acad Sci USA. 1989;86:9485–9488. doi: 10.1073/pnas.86.23.9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frankel AE, Fleming DR, Powell BL, et al. DAB389IL2 (ONTAK) fusion protein therapy of chronic lymphocytic leukaemia. Expert Opin Biol Ther. 2003;3:179–186. doi: 10.1517/14712598.3.1.179. [DOI] [PubMed] [Google Scholar]
- 26.Frankel AE, Powell BL, Lilly MB. Diphtheria toxin conjugate therapy of cancer. Cancer Chemother Biol Response Modif. 2002;20:301–313. [PubMed] [Google Scholar]
- 27.Frankel AE, Fleming DR, Hall PD, et al. A phase II study of DT fusion protein denileukin diftitox in patients with fludarabine-refractory chronic lymphocytic leukemia. Clin Cancer Res. 2003;9:3555–3561. [PubMed] [Google Scholar]
- 28.Olsen E, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19:376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- 29.LeMaistre CF, Saleh MN, Kuzel TM, et al. Phase I trial of a ligand fusion-protein (DAB389IL-2) in lymphomas expressing the receptor for interleukin-2. Blood. 1998;91:399–405. [PubMed] [Google Scholar]
- 30.Rook AH, Junkins-Hopkins JM, McGinnis KS, et al. Cytokines and other biologic agents as immunotherapeutics for cutaneous T-cell lymphoma. Adv Dermatol. 2002;18:29–43. [PubMed] [Google Scholar]
- 31.Duvic M, Cather J, Maize J, et al. DAB389IL2 diphtheria fusion toxin produces clinical responses in tumor stage cutaneous T cell lymphoma. Am J Hematol. 1998;58:87–90. doi: 10.1002/(sici)1096-8652(199805)58:1<87::aid-ajh18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Paschal BR. Remission of follicular non-Hodgkins lymphoma with denileukin diftitox (ONTAK) after progression during rituximab, CHOP and fludarabine therapy. Leukemia Lymphoma. 2003;44:731–733. doi: 10.1080/1042819031000063854. [DOI] [PubMed] [Google Scholar]
- 33.Dang NH, Hagemeister FB, Pro B, et al. Phase II study of denileukin diftitox for relapsed/refractory B-cell non-Hodgkins lymphoma. J Clin Oncol. 2004;22:4095–4102. doi: 10.1200/JCO.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 34.Foss FM. DAB(389)IL-2 (ONTAK): A novel fusion toxin therapy for lymphoma. Clin Lymphoma. 2000;1:110–116. doi: 10.3816/clm.2000.n.009. [DOI] [PubMed] [Google Scholar]
- 35.Ho VT, Zahrieh D, Hochberg E, et al. Safety and efficacy of denileukin diftitox in patients with steroid-refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:1224–1226. doi: 10.1182/blood-2004-01-0028. [DOI] [PubMed] [Google Scholar]
- 36.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. JNCI. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 37.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishimura E, Sakihama T, Setoguchi R, et al. Induction of antigen-specific immunologic tolerance by in vivo and in vitro antigen-specific expansion of naturally arising Foxp3+CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1189–1201. doi: 10.1093/intimm/dxh122. [DOI] [PubMed] [Google Scholar]
- 40.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 41.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antony PA, Restifo NP. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother. 2005;28:120–128. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones E, Dahm-Vicker M, Simon AK, et al. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun. 2002;2:1. [PubMed] [Google Scholar]
- 44.Golgher D, Jones E, Powrie F, et al. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32:3267–3275. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 45.Jones E, Golgher D, Simon AK, et al. The influence of CD25+ cells on the generation of immunity to tumour cell lines in mice. Novartis Found Symp. 2004;256:149–152. [PubMed] [Google Scholar]
- 46.Baecher-Allan C, Viglietta V, Hafler DA. Inhibition of human CD4(+)CD25(+high) regulatory T cell function. J Immunol. 2002;169:6210–6217. doi: 10.4049/jimmunol.169.11.6210. [DOI] [PubMed] [Google Scholar]
- 47.Baecher-Allan C, Brown JA, Freeman GJ, et al. CD4+CD25 high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 48.Javia LR, Rosenberg SA. CD4+CD25+ suppressor lymphocytes in the circulation of patients immunized against melanoma antigens. J Immunother. 2003;26:85–93. doi: 10.1097/00002371-200301000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dummer W, Niethammer AG, Baccala R, et al. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasad SJ, Farrand KJ, Matthews SA, et al. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J Immunol. 2005;174:90–98. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 52.Ingle GR, Moudgil A, Vo A, et al. Cyclosporine-sparing effects of daclizumab in renal allograft recipients. Am J Health Syst Pharm. 2005;62:391–396. doi: 10.1093/ajhp/62.4.0391. [DOI] [PubMed] [Google Scholar]
- 53.Zheng SG, Wang JH, Gray JD, et al. Natural and induced CD4+CD25+ cells educate CD4+ J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 54.Horwitz DA, Zheng SG, Gray JD, et al. Regulatory T cells generated ex vivo as an approach for the therapy of autoimmune disease. Semin Immunol. 2004;16:135–143. doi: 10.1016/j.smim.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Zheng SG, Wang JH, Koss MN, et al. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol. 2004;172:1531–1539. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 56.Horwitz DA, Zheng SG, Gray JD. The role of the combination of IL-2 and TGF-beta or IL-10 in the generation and function of CD4+ CD25+ and CD8+ regulatory T cell subsets. J Leukocyte Biol. 2003;74:471–478. doi: 10.1189/jlb.0503228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horwitz DA, Gray JD, Zheng SG. The potential of human regulatory T cells generated ex vivo as a treatment for lupus and other chronic inflammatory diseases. Arthritis Res. 2002;4:241–246. doi: 10.1186/ar414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng SG, Gray JD, Ohtsuka K, et al. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 59.Rosenberg SA, Yang JC, White DE, et al. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: Identification of the antigens mediating response. Ann Surg. 1998;228:307–319. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenberg SA, Lotze MT, Yang JC, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–484. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phan GQ, Attia P, Steinberg SM, et al. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001;19:3477–3482. doi: 10.1200/JCO.2001.19.15.3477. [DOI] [PubMed] [Google Scholar]
