Abstract
Neuronal adaptations in striatal dopamine signaling have been implicated in enhanced responses to addictive drugs. Cyclin-dependent kinase 5 (Cdk5) regulates striatal dopamine signaling and is a downstream target gene of the transcription factor ΔFosB, which accumulates in striatal neurons after chronic cocaine exposure. Here we investigated the role of Cdk5 activity in the nucleus accumbens (NAc) on cocaine-induced locomotor sensitization, responding for reward-associated stimuli (conditioned reinforcement), and cocaine self-administration under a progressive ratio schedule. Repeated infusions of the Cdk5 inhibitor roscovitine into the NAc before cocaine injections augmented both the development and expression of cocaine sensitization without having any intrinsic stimulant actions of its own. Additionally, repeated intra-NAc infusions of roscovitine to saline-injected rats enhanced locomotor responses to a subsequent cocaine challenge. Similar effects were found after infusions of another Cdk5 inhibitor, olomoucine, but not its inactive congener, iso-olomoucine. Repeated inhibition of Cdk5 within the NAc also robustly enhanced the incentive-motivational effects of cocaine, similar to the effect of prior repeated cocaine exposure. The enhanced responding with conditioned reinforcement induced by cocaine persisted at least 2 weeks after the final roscovitine infusion. NAc infusions of olomoucine also produced acute and enduring increases in “breakpoints” achieved on a progressive ratio schedule for cocaine reinforcement. These results demonstrate profound and persistent effects of NAc Cdk5 inhibition on locomotor sensitization and incentive-motivational processes and provide direct evidence for a role for striatal Cdk5-induced alterations in the brain's long-term adaptations to cocaine.
Keywords: addiction, dopamine, conditioned reinforcement
Repeated cocaine exposure produces long-lasting functional and structural alterations in cortico–limbic–striatal circuits. Such neuroadaptations may contribute to changes in synaptic plasticity underlying aspects of addiction (1–5). Specifically, maladaptive incentive-motivational processes may be relevant to addiction because progressive enhancements in the incentive qualities of drugs and drug-associated stimuli may contribute to compulsive drug-seeking behavior (6–8). Nevertheless, the role for persistent drug-induced neuroadaptations in the behavioral responses to cocaine and incentive-motivational processes has yet to be fully investigated.
A number of alterations in intracellular signaling molecules associated with repeated exposure to cocaine have been identified (3, 9). Chronic cocaine administration increases the activity of PKA-regulated signaling pathways in dopaminoceptive medium spiny neurons of the nucleus accumbens (NAc). Moreover, chronic cocaine increases PKA-regulated gene expression (10). One of the most stable and persistent proteins expressed in striatal regions after cessation of repeated cocaine is ΔFosB (11). Inducible transgenic overexpression of ΔFosB in striatum enhances, or sensitizes, behavioral responses to cocaine (12–14). Notably, the neuronal protein kinase cyclin-dependent kinase 5 (Cdk5) serves as a downstream target gene of ΔFosB: chronic cocaine exposure increases striatal Cdk5 levels and activity via a ΔFosB-dependent process (15, 16). Importantly, intra-NAc infusions of Cdk5 inhibitors given daily before cocaine injections enhanced cocaine's locomotor stimulant actions. These findings led to the hypothesis that Cdk5 negatively regulates biochemical and behavioral effects associated with chronic cocaine exposure (15). Cdk5 also regulates striatal proteins involved in both dopamine signaling (15, 17, 18) and glutamate neurotransmission (17, 19, 20), and it is becoming increasingly clear that this kinase plays complex roles in both inhibiting and facilitating several molecular, cellular, and behavioral mechanisms (21). However, evidence for the direct involvement of Cdk5 in the brain's persistent responses to repeated cocaine exposure or, in particular, in incentive-motivational processes and compulsive aspects of addiction (6) has not yet been demonstrated.
Results
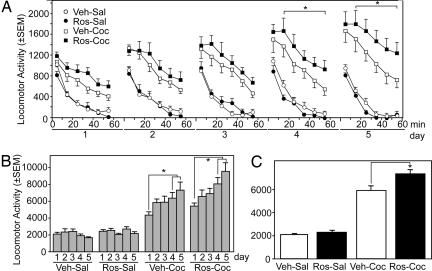
We hypothesized that repeated inhibition of Cdk5 within the NAc would mimic the ability of prior drug exposure to augment the effects of cocaine. Therefore, the behavioral effects of prior repeated intra-NAc infusions of Cdk5 inhibitors on cocaine-induced locomotor activity were examined over 5 consecutive days in 10-min bins for 60 min. Cocaine produced progressively greater increases in locomotor activity over the 5 days in comparison to saline. Intra-NAc roscovitine markedly potentiated these behavioral effects of cocaine (Fig. 1). Locomotor activity rates differed in the experimental groups over the 60-min test periods [F(15,165) = 6.44; P < 0.001]. Significant differences occurred between the treatment groups over the 5 testing days [F(12,165) = 1.81; P < 0.049], and rates generally increased over the testing days in both groups (Fig. 1B) [F(4,60) = 3.08; P < 0.02], with overall group differences in total activity rates (Fig. 1C).
Fig. 1.
Effects of intra-NAc infusions of the Cdk5 inhibitor roscovitine on the development of cocaine sensitization. Locomotor activity for animals bilaterally infused with roscovitine (40 nmol per 0.5 μl) or vehicle into the NAc 20 min before i.p. injection of 15 mg/kg cocaine or saline for 5 days is shown (A) with the total activity counts for the 60-min session for each of the 5 test days (B) and overall group differences in locomotor activity counts averaged for the five test sessions (C). Data are presented as mean locomotor activity counts (± SEM). The four experimental groups were Veh-Sal (n = 6), Ros-Sal (n = 11), Veh-Coc (n = 9), and Ros-Coc (n = 11). Asterisks denote significant differences between Veh-Coc and Ros-Coc at 20–60 min (A), between day 4 or 5 and day 1 (B), and between Veh-Coc and Ros-Coc (C). See text for additional details of statistical comparisons.
Intra-NAc infusions of roscovitine increased cocaine-induced activity compared with vehicle (Ros-Coc vs. Veh-Coc, P < 0.01), as well as controls where animals were infused with either roscovitine or vehicle and given saline injections (Fig. 1B) (Ros-Coc vs. Ros-Sal or Veh-Sal, P < 0.001). In contrast, no differences in locomotor activity occurred between rats in the two control groups (i.e., Ros-Sal or Veh-Sal). These data indicate that roscovitine selectively enhances the locomotor stimulant effects of cocaine. There was also an increase in the effects of cocaine over days irrespective of group, such that activity rates were increased more on days 4 and 5 than on day 1 (P < 0.001). Similarly, there was also a difference between days 2 and 3 compared with day 5 (P < 0.02). An interaction between treatment group and day [F(12,165) = 1.81; P = 0.05] was most evident in the cocaine-injected animals (Ros-Coc and Veh-Coc). Together, these data confirm that intra-NAc infusions of roscovitine enhance the development of locomotor sensitization to cocaine.
Examination of the temporal features of the locomotor responses revealed clear differences between the groups during each of the 10-min periods over each of the 5 days (Fig. 1A) [F(3,165) = 20.67, 52.49, 55.82, 79.74, 74.79, 61.15; P < 0.001]. Roscovitine significantly enhanced cocaine-induced locomotor activity throughout all 10-min intervals during the 5 days of testing, except for the first 10-min interval after injection, and progressively enhanced the effects of cocaine over the 5 days of testing during the 10-min intervals. The maximal effects of intra-NAc infusions of roscovitine in potentiating the effects of cocaine occurred at 50–60 min after the injection. Although there were differences between the four groups at this time period for each of the days [F(3,33) = 10.04, 10.14, 14.16, 13.09, 16.31; P < 0.001], it was not until day 3 of injections that a trend occurred for differences between the roscovitine/cocaine and vehicle/cocaine groups (Ros-Coc vs. Veh-Coc). By day 4, significant differences were observed (P < 0.02), and by day 5, mean cocaine-induced activity rates for roscovitine-infused animals were almost double that measured for vehicle-infused animals during the 50–60 min after the injection (P < 0.01).
Although roscovitine infusions enhanced the locomotor-activating effects of cocaine at these later time periods, the effects of roscovitine were so robust that there were also differences between the groups when total activity rates over the 60 min were examined irrespective of the day (Fig. 1C) [F(3,165) = 77.74; P < 0.02]. Thus, there were overall differences in total activity (i.e., 0–60 min combined) between all of the groups except for the Ros-Sal vs. Veh-Sal treated groups; notably, roscovitine progressively enhanced cocaine-induced locomotor activity over the 5 days to a greater degree than that observed in cocaine-injected animals infused with vehicle (Fig. 1C).
There were also significant differences (P < 0.001) between the cocaine-injected and saline-injected animals for each of the 10-min time periods over each of the 5 days of testing regardless of whether intra-NAc roscovitine or vehicle infusions were given, confirming that cocaine resulted in sensitization. In no cases were there significant differences between the saline-exposed rats given intra-NAc roscovitine vs. vehicle infusions at any of the 10-min time periods. Together these data show that cocaine causes locomotor sensitization and that intra-NAc roscovitine infusions enhanced this sensitization without itself affecting locomotor activity.
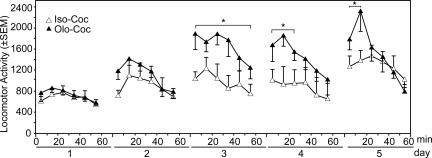
Infusions of another less selective Cdk5 inhibitor, olomoucine, produced similar effects to those of roscovitine, with the exception that the maximal behavioral effects during the session occurred earlier, during day 3 of infusions (Fig. 2). The potentiation of cocaine-induced increases in locomotor activity by repeated intra-NAc infusions of olomoucine (Olo-Coc) compared with its inactive isomer iso-olomoucine (Iso-Coc) were confirmed by group differences [F(1,12) = 8.36; P < 0.01] over the 60-min test session [F(4,30) = 16.64; P < 0.001]. There were also differences between the groups over the 5 days of testing during the 10-, 20-, 30-, and 40-min periods [F(1,30) = 16.63, 10.37, P < 0.001; 6.57, P < 0.01; 4.34; P < 0.05]. By day 3, olomoucine significantly increased the locomotor response to cocaine, effects that were evident throughout the session. By the final test day, animals given intra-NAc olomoucine and cocaine initially showed significant and robust augmented locomotor responses to cocaine but subsequently began to exhibit stereotypy. Stereotypy can compete with increases in locomotor activity, but its development is consistent with sensitization to cocaine. Consequently, there were no differences between animals given intra-NAc infusions of olomoucine or iso-olomoucine after cocaine infusions at these later time points, in contrast to the earlier robust increases in the olomoucine group.
Fig. 2.
Effects of intra-NAc infusions of the Cdk5 inhibitor olomoucine on the development of cocaine sensitization. Locomotor activity for animals receiving intra-NAc bilateral infusions of olomoucine (40 nmol per 0.5 μl) or its inactive cogener iso-olomoucine is shown. Data are presented as mean locomotor activity counts (± SEM). Groups are denoted as intra-NAc iso-olomoucine (Iso) or olomoucine (Olo) infusions and cocaine (Coc) for the two experimental groups as Iso-Coc (n = 6) and Olo-Coc (n = 8). Asterisks denote significant differences between Iso-Coc and Olo-Coc on day 3 (10–60 min), day 4 (10–20 min), and day 5 (10–20 min) after cocaine.
Further evidence for the ability of intra-NAc Cdk5 inhibitors to enhance the locomotor-activating effects of cocaine was obtained in the cocaine challenge test in which the expression of sensitization to cocaine (15 mg/kg) was evaluated 10 days later. Sensitized cocaine-induced locomotor activity was again observed in animals previously treated with cocaine. Rats that had previously received intra-NAc roscovitine infusions showed an augmented locomotor response to the cocaine challenge (Fig. 3A). These effects were group- and time-dependent [F(24,240) = 3.66; P < 0.001], with significant differences between the treatment groups at 10 [F(3,30) = 3.92; P < 0.02], 20 [F(3,30) = 7.57; P < 0.01], 30 [F(3,30) = 5.32; P < 0.01], a trend at 50 [F(3,30) = 2.72; P < 0.06], and 60 [F(3,30) = 2.86; P < 0.05] min, respectively, after the cocaine challenge (Fig. 3A). Specifically, intra-NAc roscovitine infusions significantly potentiated the effects of cocaine at the 20-min (P < 0.001) and 30-min (P < 0.05) time periods when maximal responses to cocaine are observed, rather than prolonging the behavioral activating effects of cocaine at later time periods. Prior infusions of intra-NAc Cdk5 inhibitors nearly doubled locomotor activity rates at these times, compared with vehicle-infused animals after the cocaine challenge (Ros-Coc vs. Veh-Sal), and activity levels were consistently elevated throughout the 60-min test compared with control rats (Ros-Coc vs. Veh-Sal P < 0.01, 10–60 min). Overall group differences in activity were found to occur only after the cocaine challenge and not during the baseline period [before vs. after and group interaction; F(3,30) = 5.11; P < 0.01] (Fig. 3B); this confirms that behavior was selectively altered only after the cocaine challenge [F(3,30) = 5.54; P < 0.001]. Notably, prior roscovitine treatment increased activity rates in cocaine-exposed animals compared with vehicle-infused animals given cocaine (P < 0.03, Ros-Coc vs. Veh-Coc) or those given saline (P < 0.007, Ros-Coc vs. Veh-Sal) and increased the response to cocaine challenge in rats never having been exposed to cocaine (P < 0.003, Ros-Coc vs. Ros-Sal). Similar behavioral effects were observed after intra-NAc infusions of olomoucine compared with the inactive congener, iso-olomoucine (data not shown). Of particular note was that animals previously given repeated intra-NAc infusions of roscovitine, but that did not receive cocaine, showed evidence of sensitization to a challenge dose of cocaine (Fig. 3B). Here activity rates were not different in animals given intra-NAc roscovitine and saline-injections compared with those given intra-NAc vehicle and cocaine-injections (i.e., Ros-Sal vs. Veh-Coc) at any time point after the cocaine challenge.
Fig. 3.
Effects of prior intra-NAc infusions of roscovitine on the expression of cocaine sensitization. (A) Locomotor activity for animals challenged with 15 mg/kg cocaine 10 days after the last repeated cocaine or saline injection and intra-NAc infusions of roscovitine or vehicle. Groups are denoted as intra-NAc vehicle (Veh) or roscovitine (Ros) infusions and saline (Sal) or cocaine (Coc) for the four experimental groups as follows: Veh-Sal (n = 6), Ros-Sal (n = 11), Veh-Coc (n = 8), and Ros-Coc (n = 9). Data are presented as mean locomotor activity counts (± SEM) during the test session measured in 10-min time periods before (−30 to −10 min) and after (10–60 min) the cocaine challenge. Activity is also shown for the groups during the 30 min before and 60 min after the cocaine challenge (B). Asterisks in A denote significant differences between Veh-Coc and Ros-Coc at 20 and 30 min after the cocaine challenge, confirming that roscovitine enhanced the expression of cocaine sensitization. Asterisks in B denote significant differences between Ros-Coc and all other groups, and between Veh-Sal and Ros-Sal, indicating that prior roscovitine enhanced subsequent responses to cocaine.
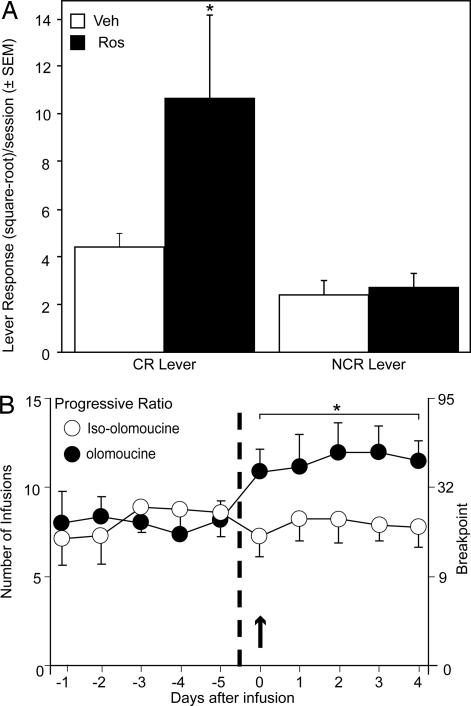
The conditioned reinforcement (CR) paradigm provides a measure of incentive motivation (22), and prior cocaine exposure enhances CR in rats (23). To determine whether inhibition of Cdk5 in the NAc also can enhance incentive motivation, we first examined the effect of repeated intra-NAc infusions of roscovitine on subsequent cocaine-induced responding with CR. After training on Pavlovian approach behavior, rats were given infusions of roscovitine or vehicle into the NAc for 5 consecutive days. Ten days after the last infusion animals were tested on responding for CR after a cocaine challenge (15 mg/kg cocaine, i.p.). Roscovitine markedly enhanced responding with CR compared with those previously given vehicle infusions, as suggested by an interaction between treatment group and lever (Fig. 4A) [F(1,14) = 5.45; P < 0.03]. The selective increase in responding with CR after cocaine administration in roscovitine-infused, compared with vehicle-infused, animals was confirmed by a significant difference between the groups for the CR (P < 0.01) but not control [no conditioned reinforcement (NCR)] lever responses.
Fig. 4.
Effects of intra-NAc Cdk5 inhibition on CR response and cocaine self-administration. (A) Effects of prior intra-NAc infusions of the Cdk5 inhibitor roscovitine on postcocaine challenge CR response. Lever press activity is shown for animals given five bilateral intra-NAc roscovitine (40 nmol per 0.5 μl) or vehicle (0.5 μl) infusions and conditioned stimulus–unconditioned stimulus pairings followed 10 days later by the acquisition of a new response test with CR and a challenge dose of cocaine (15 mg/kg). Data are shown for both a CR and NCR (control) lever pressing in animals given intra-NAc infusions of roscovitine (n = 7) compared with those given intra-NAc infusions of vehicle (n = 9). Data were square-root-transformed and are expressed as mean lever responses (± SEM) during the test session. The asterisk denotes significant differences in cocaine responding on the CR lever after roscovitine, and also compared with the NCR lever. (B) Effects of intra-NAc infusions of the Cdk5 inhibitor olomoucine on cocaine self-administration under a PR schedule. Data show the mean (± SEM) number of self-administered infusions (Left) and the corresponding final ratios (breakpoints, Right) for animals receiving control bilateral intra-NAc infusions of olomoucine (open circles, n = 7) or iso-olomoucine (filled circles, n = 7, 40 nmol per 0.5 μl). PR data are shown as days after infusions, where PR responses are depicted over the five sessions before infusions (−1 to −5), the day of the infusion (0, arrow), and 4 days after the infusions (1 to 4). The asterisk denotes significant differences at all days of testing after the infusions of olomoucine.
One week later, the effects of systemic cocaine (15 mg/kg) were again evaluated. The responding with CR after cocaine continued to be elevated in animals that had previously received intra-NAc roscovitine infusions, demonstrating persistent effects of these treatments (data not shown). There was a trend for significant interaction between treatment group and lever responses [F(1,12) = 4.27; P < 0.06]. Responses on the CR lever in roscovitine-infused animals were slightly reduced compared with the initial cocaine challenge, probably because of a low level of extinction, as no reinforcement is given during the CR test. Nevertheless, prior intra-NAc roscovitine significantly enhanced cocaine-induced responding selectively on the CR lever in roscovitine-infused compared with vehicle-infused animals (P < 0.05).
To further explore the influence of Cdk5 inhibition in the NAc on motivation for drug rather than food, we examined cocaine self-administration under a progressive ratio (PR) schedule, which provides a measure of how hard an animal is willing to work to obtain cocaine reinforcement (24). Animals were given acute bilateral intra-NAc infusions of olomoucine or its inactive isomer, iso-olomoucine, and their PR responding for cocaine was examined on the day of infusion and for an additional four sessions to determine whether Cdk5 inhibition would produce persistent motivational changes (Fig. 4B). Bilateral intra-NAc infusion of olomoucine, but not iso-olomoucine, produced a robust increase in breakpoints maintained for cocaine under the PR schedule that began on the day of administration. Comparing the number of infusions obtained at baseline to those obtained on the day that either olomoucine or iso-olomoucine was administered by repeated-measures ANOVA revealed a significant effect of time [F(1,12) = 8.1; P < 0.05], a significant interaction of time by treatment group [F(1,12) = 30.2; P < 0.0001], and a nonsignificant effect of group (P > 0.05). The effect of olomoucine on PR responding for cocaine was persistent: after one bilateral infusion of olomoucine, the breakpoints maintained under the PR schedule were increased from baseline on the day of infusion and remained elevated for the duration of the 5 days of testing (i.e., 4 days after infusion). A repeated-measures ANOVA, comparing the effect of olomoucine and iso-olomoucine on percent change in the number of infusions obtained at baseline to those obtained on the day of infusion and on the four sessions that followed, showed a significant effect of treatment group [F(1,12) = 17.6; P < 0.01], but nonsignificant effects of time (P > 0.05) or an interaction of time by treatment group (P > 0.05). Notably, the number of inactive lever responses did not differ significantly between groups at baseline (P > 0.05) or as a function of session post infusion (P > 0.05). Thus, the effects of olomoucine were robust, persistent, and appeared to be specific to cocaine-reinforced responding. These results further suggest that Cdk5 activity may function to ameliorate some of the motivational effects associated with cocaine self-administration, although the effects of Cdk5 inhibition may also indicate enhanced perseveration and/or resistance to extinction.
Discussion
Drug addiction is characterized by persistent and enhanced behavioral control exerted by drugs and drug-associated stimuli. Details of the biochemical processes underlying these behavioral effects are incomplete but are hypothesized to result from neuroadaptations in dopamine-regulated signaling. The present study demonstrates that inhibition of Cdk5 within the NAc enhances responses to cocaine in multiple behavioral models relevant to the incentive qualities of drugs and reward-associated stimuli. Infusions of Cdk5 inhibitors before daily cocaine injections enhanced the development of locomotor sensitization and the expression of sensitization after a withdrawal period. Additionally, although repeated NAc infusions of Cdk5 inhibitors alone had no effect on locomotor activity, animals displayed enhanced cocaine-induced locomotor stimulation when tested after a 10-day withdrawal period. Prior repeated NAc infusions of Cdk5 inhibitors in drug-naive animals subsequently increased cocaine-induced enhancement of responding with CR. This suggests that repeated exposure to roscovitine may result in adaptations that mimic the behavioral effects of prior cocaine exposure. Inhibition of NAc Cdk5 produced a persistent enhancement of PR responding for cocaine reinforcement in animals trained to self-administer cocaine. This was not attributable to motor abnormalities or increased arousal because Cdk5 inhibitors did not affect locomotor responses to saline and enhancements were selective for CR and PR responding. These observations suggest that Cdk5 acts as an inhibitory constraint on the expression of cocaine-regulated behaviors, including alterations in incentive-motivational processes.
The importance of enhanced incentive-motivational processes, as a form of behavioral plasticity, to addiction has previously been described (6–8, 25). Our findings offer strong evidence that Cdk5-regulated signaling plays an important role in these processes because Cdk5 inhibition enhanced psychostimulant sensitization and the control over behavior by drugs (i.e., PR self-administration) and reward-associated cues (i.e., CR). Our findings add to the growing body of evidence implicating cocaine-induced neuroadaptations in processes involved in compulsive drug-seeking and -taking. It has been established that repeated psychostimulant exposure promotes drug self-administration under fixed and PR schedules (26–28) and enhances responding for drug-associated stimuli (1, 29–32). The expression of sensitization is also associated with enhanced incentive-motivational processes (23, 33–36), and the incentive value of drug-associated stimuli appears to progressively increase over time (37–39). Although the neurocircuitry and precise cellular alterations that underlie these phenomena are not understood, a critical role for dopamine-regulated signaling pathways is likely involved (4, 40, 41).
The cAMP–PKA pathway is thought to mediate reward-related learning in the NAc (42, 43) and cue-induced cocaine-seeking (44–49). Cdk5 appears to be positioned to interfere with dopamine signaling and multiple downstream targets including PKA and DARPP-32 (50, 51). In the brain, Cdk5 has been implicated in diverse biochemical processes involved in both functional and structural synaptic plasticity (21, 52, 53). Striatal Cdk5 levels are increased in response to chronic exposure to cocaine (15) and may be altered in response to voluntary alcohol drinking (54). Although the present study focuses on the role of Cdk5 in NAc, cocaine also induces increased expression of the kinase in dorsal striatum (15), which may also influence cocaine's behavioral effects. Furthermore, neuroadaptive changes in spine density induced by chronic exposure to cocaine may be dependent on Cdk5 (3, 55). However, the precise postsynaptic molecular mechanisms by which Cdk5 inhibition augments the behavioral effects of cocaine reported here is the subject of ongoing studies. Cdk5 may also exert an inhibitory effect on dopamine neurotransmission presynaptically, as it phosphorylates and regulates tyrosine hydroxylase (56), and Cdk5 inhibition increases stimulus-evoked dopamine release (19). Through these actions it may further enhance the effects of cocaine. Conversely, activation of Cdk5 in transgenic mice by overexpression of Cdk5 or its coactivator p35 attenuates cocaine-mediated dopamine signaling in vivo (18). Together with the current data, these observations suggest that Cdk5 activity has unique actions as an endogenous negative regulator of dopamine transmission, both pre- and postsynaptically, and can thereby potently modulate striatal function and the actions of cocaine.
Materials and Methods
The effects of intra-NAc Cdk5 inhibitors on the development of cocaine-induced locomotor activity and the expression of sensitization were measured in adult male Sprague–Dawley rats according to published procedures for surgery, drug infusion, apparatus, and locomotor behavioral methods (23, 42). One week after lateral shell NAc cannulation and 2 days after habituation to the locomotor chambers, animals received five daily injections of cocaine (15 mg/kg, i.p.) or saline given 20 min after bilateral intra-NAc infusions of 0.5 μl of roscovitine, olomoucine, or iso-olomoucine (40 nmol in PBS/50% DMSO) or vehicle. Rats were then given a 10-day drug-free interim period before a 15 mg/kg cocaine challenge after being placed into the chambers for 30 min. Histological analyses to ensure accurate cannulae placement were conducted on all animals. Data were analyzed by repeated-measures ANOVA using time as the repeated variable. Significant behavioral effects were further analyzed by factorial ANOVA with Scheffé's F test for post hoc analyses. Data are expressed as mean locomotor activity counts ± SEM. A more detailed description of the behavioral methodology is provided in supporting information (SI) Text.
Evaluation of the effects of intra-NAc Cdk5 inhibition upon cocaine-enhanced CR was conducted by using naive cannulated rats as previously described (23, 57). After a 14-day period when animals were trained to associate a compound conditioned stimulus with water as the unconditioned stimulus, roscovitine or vehicle was infused daily for 5 days. Ten days after the last infusion all animals were tested on CR after an acute injection of cocaine (15 mg/kg, i.p.). Lever presses on the active (CR) and inactive (NCR) lever were analyzed by ANOVA after square-root transformation to preserve homogeneity of variance (22). A more detailed description of the methodology used in this paradigm is provided as SI Text.
To assess the effects of intra-NAc Cdk5 inhibitors on cocaine self-administration under a PR schedule, animals were implanted with a chronic indwelling catheter into the right jugular vein and allowed at least 7 days to recover (58). Rats were trained to self-administer cocaine infusions (0.5 mg/kg, i.v.) as described (59), and responding was assessed under a PR schedule of reinforcement designed so that the breakpoint serves as a sensitive measure of motivation to obtain cocaine (24, 60). Once responding stabilized under the PR schedule (defined as five consecutive sessions with no increasing of decreasing trend in breakpoint), the effect of NAc infusions of olomoucine or iso-olomoucine on PR responding for cocaine was determined. Responding was assessed for an additional four sessions after the test session because pilot studies showed persistent changes in PR responding for cocaine after an intra-NAc infusion of olomoucine. The mean number of infusions obtained during the five baseline PR sessions was obtained for each rat and compared with those obtained on the day an infusion was administered (test session) by using repeated-measures ANOVA. The percent change in the mean number of infusions obtained at baseline to the number of infusions obtained on a test session and after four sessions was determined for each rat and compared with repeated-measures ANOVA. Identical analyses were used to investigate the effects of olomoucine and iso-olomoucine on inactive lever responding. Subsequent a priori pairwise comparisons with baseline were made with the t test. A more detailed description of the methodology used for these experiments is provided in SI Text.
Supplementary Material
Acknowledgments
We thank V. Phantharangsy for technical assistance, J. D. Jentsch and A. Nairn for discussions, and L. Meijer for roscovitine. This work was supported by grants from the National Institute on Drug Abuse (to J.R.T., E.J.N., and J.A.B.) and the National Institute of Mental Health (to E.J.N. and J.A.B.), and by the Abraham Ribicoff Research Facilities, Connecticut Mental Health Center, Connecticut Department of Mental Health and Addiction Services (J.R.T.).
Abbreviations
- Cdk5
cyclin-dependent kinase 5
- NAc
nucleus accumbens
- PR
progressive ratio
- CR
conditioned reinforcement
- NCR
no conditioned reinforcement.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610288104/DC1.
References
- 1.Di Ciano P, Everitt BJ. Neuropharmacology. 2004;47:202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Hyman SE, Malenka RC. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 3.Nestler EJ. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 4.Vanderschuren LJ, Kalivas PW. Psychopharmacology (Berlin) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 5.White FJ, Kalivas PW. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 6.Jentsch JD, Taylor JR. Psychopharmacology (Berlin) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 7.Robbins TW, Everitt BJ. Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- 8.Robinson TE, Berridge KC. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 9.Nestler EJ. Neuropharmacology. 2004;47(Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 10.McClung CA, Nestler EJ. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- 11.Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 12.Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. J Neurosci. 2003;23:2488–2493. doi: 10.1523/JNEUROSCI.23-06-02488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, et al. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- 14.Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR. J Neurosci. 2006;26:9196–9204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, et al. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, et al. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Nishi A, Bibb JA, Matsuyama S, Hamada M, Higashi H, Nairn AC, Greengard P. J Neurochem. 2002;81:832–841. doi: 10.1046/j.1471-4159.2002.00876.x. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi S, Ohshima T, Cho A, Sreenath T, Iadarola MJ, Pant HC, Kim Y, Nairn AC, Brady RO, Greengard P, Kulkarni AB. Proc Natl Acad Sci USA. 2005;102:1737–1742. doi: 10.1073/pnas.0409456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chergui K, Svenningsson P, Greengard P. Proc Natl Acad Sci USA. 2004;101:2191–2196. doi: 10.1073/pnas.0308652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei FY, Tomizawa K, Ohshima T, Asada A, Saito T, Nguyen C, Bibb JA, Ishiguro K, Kulkarni AB, Pant HC, et al. J Neurochem. 2005;93:502–512. doi: 10.1111/j.1471-4159.2005.03058.x. [DOI] [PubMed] [Google Scholar]
- 21.Angelo M, Plattner F, Giese KP. J Neurochem. 2006;99:353–370. doi: 10.1111/j.1471-4159.2006.04040.x. [DOI] [PubMed] [Google Scholar]
- 22.Taylor JR, Robbins TW. Psychopharmacology (Berlin) 1984;84:405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- 23.Taylor JR, Horger BA. Psychopharmacology (Berlin) 1999;142:31–40. doi: 10.1007/s002130050859. [DOI] [PubMed] [Google Scholar]
- 24.Stafford D, LeSage MG, Glowa JR. Psychopharmacology (Berlin) 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- 25.Robinson TE, Berridge KC. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 26.Kruzich PJ, Congleton KM, See RE. Behav Neurosci. 2001;115:1086–1092. doi: 10.1037//0735-7044.115.5.1086. [DOI] [PubMed] [Google Scholar]
- 27.Lorrain DS, Arnold GM, Vezina P. Behav Brain Res. 2000;107:9–19. doi: 10.1016/s0166-4328(99)00109-6. [DOI] [PubMed] [Google Scholar]
- 28.Mendrek A, Blaha CD, Phillips AG. Psychopharmacology (Berlin) 1998;135:416–422. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- 29.Arroyo M, Markou A, Robbins TW, Everitt BJ. Psychopharmacology (Berlin) 1998;140:331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- 30.De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Eur J Neurosci. 1998;10:3565–3571. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- 31.Morgan D, Brebner K, Lynch WJ, Roberts DC. Behav Pharmacol. 2002;13:389–396. doi: 10.1097/00008877-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O. Neuropsychopharmacology. 2001;25:361–372. doi: 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]
- 33.Harmer CJ, Phillips GD. Behav Pharmacol. 1998;9:299–308. [PubMed] [Google Scholar]
- 34.Shippenberg TS, Heidbreder C. J Pharmacol Exp Ther. 1995;273:808–815. [PubMed] [Google Scholar]
- 35.Taylor JR, Jentsch JD. Biol Psychiatry. 2001;50:137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- 36.Wyvell CL, Berridge KC. J Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grimm JW, Hope BT, Wise RA, Shaham Y. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu L, Grimm JW, Hope BT, Shaham Y. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 39.Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Everitt BJ, Wolf ME. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Neuropharmacology. 2004;47(Suppl 1):61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jentsch JD, Olausson P, Nestler EJ, Taylor JR. Biol Psychiatry. 2002;52:111–118. doi: 10.1016/s0006-3223(02)01358-6. [DOI] [PubMed] [Google Scholar]
- 44.Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu L, Dempsey J, Shaham Y, Hope BT. J Neurochem. 2005;94:161–168. doi: 10.1111/j.1471-4159.2005.03178.x. [DOI] [PubMed] [Google Scholar]
- 47.Lu L, Grimm JW, Shaham Y, Hope BT. J Neurochem. 2003;85:1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- 48.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 49.Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. J Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnsten AF, Ramos BP, Birnbaum SG, Taylor JR. Trends Mol Med. 2005;11:121–128. doi: 10.1016/j.molmed.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Bibb JA. Neurosignals. 2003;12:191–199. doi: 10.1159/000074620. [DOI] [PubMed] [Google Scholar]
- 52.Cheung ZH, Fu AK, Ip NY. Neuron. 2006;50:13–18. doi: 10.1016/j.neuron.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 53.Kim Y, Sung JY, Ceglia I, Lee K-W, Ahn J-H, Halford JM, Kim AM, Kwak SP, Park JB, Ryu SH, et al. Nature. 2006;442:814–817. doi: 10.1038/nature04976. [DOI] [PubMed] [Google Scholar]
- 54.Camp MC, Mayfield RD, McCracken M, McCracken L, Alcantara AA. Alcohol Clin Exp Res. 2006;30:1322–1335. doi: 10.1111/j.1530-0277.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- 55.Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kansy J, Daubner SC, Nishi A, Sotogaku N, Lloyd MD, Nguyen C, Lu L, Haycock J, Hope BT, Fitzpatrick PF, Bibb JA. J Neurochem. 2004;91:374–384. doi: 10.1111/j.1471-4159.2004.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor JR, Robbins TW. Psychopharmacology (Berlin) 1986;90:390–397. doi: 10.1007/BF00179197. [DOI] [PubMed] [Google Scholar]
- 58.Lynch WJ, Carroll ME. Psychopharmacology (Berlin) 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- 59.Lynch WJ, Taylor JR. Neuropsychopharmacology. 2005;30:927–935. doi: 10.1038/sj.npp.1300656. [DOI] [PubMed] [Google Scholar]
- 60.Richardson NR, Roberts DC. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.