Abstract
Environmental conditions can dramatically influence the behavioral and neurochemical effects of drugs of abuse. For example, stress increases the reinforcing effects of drugs and plays an important role in determining the vulnerability to develop drug addiction. On the other hand, positive conditions, such as environmental enrichment, can reduce the reinforcing effects of psychostimulants and may provide protection against the development of drug addiction. However, whether environmental enrichment can be used to “treat” drug addiction has not been investigated. In this study, we first exposed mice to drugs and induced addiction-related behaviors and only afterward exposed them to enriched environments. We found that 30 days of environmental enrichment completely eliminates behavioral sensitization and conditioned place preference to cocaine. In addition, housing mice in enriched environments after the development of conditioned place preference prevents cocaine-induced reinstatement of conditioned place preference and reduces activation of the brain circuitry involved in cocaine-induced reinstatement. Altogether, these results demonstrate that environmental enrichment can eliminate already established addiction-related behaviors in mice and suggest that environmental stimulation may be a fundamental factor in facilitating abstinence and preventing relapse to cocaine addiction.
Keywords: dopamine, drug abuse, environment, stress, treatment
Notwithstanding public campaigns to inform the general population about the risks of addiction, the use of addictive drugs has increased in the last decades and represents a serious problem for our societies. Among drugs of abuse, cocaine represents a major concern because its use is spreading among the young population and because it has powerful addictive properties. Unfortunately, although a great deal of information has been collected on the mechanisms of cocaine addiction, effective therapies are still very limited (1). Thus, the discovery of pharmacologic and nonpharmacologic strategies that can help cocaine addicts in their recovery efforts is a pressing necessity.
Environmental conditions can dramatically influence the behavioral and neurochemical effects of drugs of abuse (2–4). In laboratory animals, manipulations of environments generally consist of providing negative conditions, such as exposure to several types of stress, or positive conditions, such as enriched environments. Enriched environments are combinations of complex inanimate and social stimulation that aim at enhancing sensory, cognitive, and motor functions (5). Such complex environments have been shown to produce beneficial effects on several brain functions (6, 7) and to prevent or reverse several pathologic conditions (8–10). Stressful conditions, such as social isolation or food restriction, increase the activating and reinforcing effects of drugs and are considered to play an important role in determining vulnerability to the development of drug addiction (2–4), whereas enriched environments reduce the activating and reinforcing effects of psychostimulants and may provide protection against the development of drug addiction (11–13). For example, we have recently shown that exposing mice to enriched environments during adolescence produces dramatic molecular changes in the striatum that result in reduced reactivity to the rewarding and activating effects of cocaine (12–14). These preclinical findings are in agreement with the notion that drug addiction is highly influenced by environmental conditions and life experience (15) and underscore the importance of providing positive environments, especially during crucial periods of development, to prevent addiction. Although there is a substantial amount of data describing the influences of housing conditions on the effects of subsequent administration of drugs of abuse, no study has investigated whether exposure to environmental enrichment during periods of abstinence after the development of addiction-related behaviors could reduce the enhanced reactivity of animals to the motivational effects of drugs and drug-associated environmental stimuli that leads to relapse.
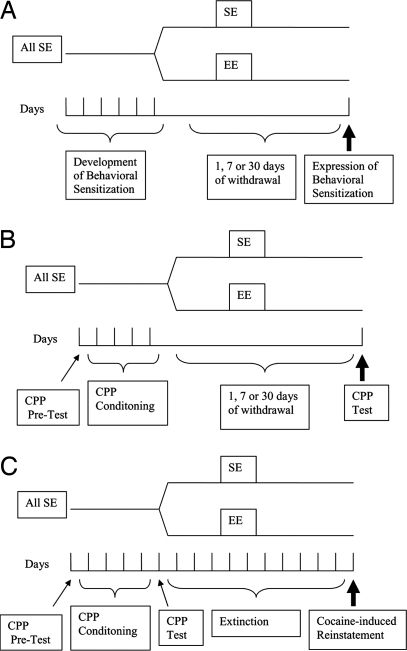
In this study, we first exposed animals to drugs and obtained addiction-related behavioral phenotypes and only afterward exposed them to enriched environments. We investigated the effects of enriched environments on cocaine addiction using three established animal models (Fig. 1): behavioral sensitization, which measures the progressive increase in the activating effects of cocaine following repeated administration (16); conditioned place preference, which measures the ability of contexts previously associated with drugs to elicit approach behaviors (17); and drug-induced reinstatement of extinguished place preference (18), an animal model of relapse that measures the ability of cocaine to elicit drug seeking that was previously lost. In addition, we used functional neuroanatomy techniques (19) to investigate the brain mechanisms underlying the protective effects of environmental enrichment on cocaine-induced reinstatement.
Fig. 1.
Graphic representation of the protocols used for (A) experiment 1 (behavioral sensitization), (B) experiment 2 (conditioned place preference [CPP]), and (C) experiment 3 (reinstatement of extinguished CPP). Note that at the beginning of experiments all mice were housed in standard environments (SE). Only after establishment of behavioral sensitization or CPP were mice divided into SE or enriched environments (EE). They were then kept in these conditions for 1, 7, or 30 days before being further tested for the presence of addiction-related behaviors.
Results
Reversal of Behavioral Sensitization by Environmental Enrichment.
Adult C57BL6 mice were injected six times with cocaine (15 mg/kg) and developed behavioral sensitization (two-way ANOVA for repeated measures: Time effect, F5,215 = 24.97, P < 0.0001) (Fig. 2A). After the last injection of cocaine, mice were randomly divided into two groups: one group was kept in standard housing conditions, whereas the other group was housed in an enriched environment; both groups remained in the animal facility until they were tested for expression of behavioral sensitization (Fig. 1A). At 1, 7, or 30 days after the last cocaine injection, the reactivity to cocaine (10 mg/kg) was measured in independent groups of mice. Behavioral sensitization to cocaine was long lasting (up to 30 days) in mice housed in the standard environment, as demonstrated by an enhanced behavioral response (approximately 200%) to cocaine (10 mg/kg) compared with cocaine-naïve control mice (Fig. 2 B and C) (Mann–Whitney test, P = 0.0003). In contrast, mice switched to enriched environments were sensitized to cocaine after 1 day of enrichment, but after 30 days their response to cocaine was reduced (approximately 65%) compared with sensitized mice housed in standard environments (Fig. 2B) (two-way ANOVA: Time effect, F2,61 = 9.94, P < 0.0001; Time × Environment interaction, F2,61 = 6.77, P < 0.01). Importantly, after 30 days of environmental enrichment, the reactivity to cocaine of sensitized mice was similar to that of the nonsensitized mice that were housed in standard or enriched environments (Fig. 2C).
Fig. 2.
Environmental enrichment eliminates behavioral sensitization. (A) Locomotor activity after injections of cocaine (15 mg/kg) during the development of behavioral sensitization in mice that will later be assigned to standard environment (SE, full symbols) or enriched environment (EE, empty symbols) housing. The two groups develop similar sensitization to the activating effects of cocaine. Results represent the means ± SEM from 32–34 mice. (B) Locomotor activity after a challenge of 10 mg/kg of cocaine in sensitized and (C) in cocaine-naïve control mice that were housed in SE (full bars) or EE (empty bars) for 1, 7, or 30 days after the development of behavioral sensitization. Locomotor responses to cocaine remained sensitized in mice housed in SE, even after 30 days of withdrawal. In contrast, mice housed in EE for the 30 days of withdrawal show no sign of sensitization and respond to cocaine similarly to cocaine-naïve mice. Results represent the means ± SEM from 10–12 mice. Two-way ANOVA followed by post hoc Student-Newman-Keuls's test: **, P < 0.01 vs. respective SE control. Comparison between cocaine-induced locomotion after 30 days of withdrawal in cocaine-treated and cocaine-naïve mice was carried out by Mann–Whitney test: ##, P < 0.01 vs. cocaine-treated mice at day 30.
To rule out the possibility that change in environments per se could be responsible for the reversal of behavioral sensitization, two groups of mice were sensitized to cocaine as described above and, after development of sensitization, were housed in either standard or isolated environments for 30 days. When challenged with cocaine (10 mg/kg), mice switched to isolated environments showed significantly higher locomotor activity than mice kept in standard environments (standard environments = 303 ± 39, isolated environments = 441 ± 55; Mann–Whitney test, P = 0.01).
Elimination of Established Conditioned Place Preferences by Environmental Enrichment.
Addiction is believed to result not only from a higher sensitivity to the effects of cocaine but also from a robust control of drug seeking by stimuli and contexts associated with the drug's effects (20–22). Therefore, we used a conditioned place preference paradigm (17) to assess whether the appetitive effects of cocaine and the incentive values acquired by contexts associated with cocaine would also be eliminated by environmental enrichment (Fig. 1B). Mice were conditioned to cocaine (10 mg/kg) and, at the end of the last session of conditioning, were randomly divided into standard and enriched housing conditions. The expression of conditioned place preferences was measured in independent groups of mice 1, 7, or 30 days after the last conditioning session. Mice housed in standard environments showed significant preferences for the compartment previously associated with cocaine after 1, 7, or 30 days (Fig. 3A) indicating that, as previously described (23, 24), the ability of contextual stimuli to elicit drug-seeking behavior is not lost spontaneously (without repeatedly exposing animals to the conditioned place preference apparatus in the absence of the drug) and persists for long periods. In contrast, the preferences for cocaine-paired compartments were completely abolished after 30 days of environmental enrichment (two-way ANOVA: Environment effect, F1,66 = 6.53, P < 0.05) (Fig. 3A).
Fig. 3.
Environmental enrichment eliminates cocaine-conditioned place preference. Expression of (A) cocaine conditioned place preference and (B) lithium-conditioned place aversion in mice housed, after development of place conditioning, in standard environment (SE, full bars) or enriched environment (EE, empty bars) housing for 1, 7, or 30 days. Cocaine-induced conditioned place preference persists for up to 30 days after conditioning in SE mice. In contrast, mice housed in EE during the 30 days of withdrawal show no preference for the cocaine-paired compartment. Lithium-conditioned place aversion persists for up to 30 days after conditioning in both SE and EE mice, indicating that the ability to retain motivational memories was not affected in EE mice. Results represent the means ± SEM from 11 to 12 mice. Two-way ANOVA followed by post hoc Student-Newman-Keuls's test: *, P < 0.05 vs. SE mice at day 30.
Although environmental enrichment is generally believed to increase learning and memory (6), we wanted to exclude the possibility that loss of conditioned place preference in mice housed in enriched environments might be due to memory impairment or to a general reduction in motivation. Thus, we conditioned mice to the aversive effects of lithium (3 mEq/kg), separated them randomly into standard or enriched housing conditions, and measured the expression of conditioned place aversion 1, 7, or 30 days after the last conditioning session. As shown in Fig. 3B, lithium induced long-lasting place aversions in mice housed in both standard and enriched conditions, indicating that memory of aversive motivational stimuli is preserved in mice housed in enriched environments.
Prevention of Cocaine-Induced Reinstatement by Environmental Enrichment.
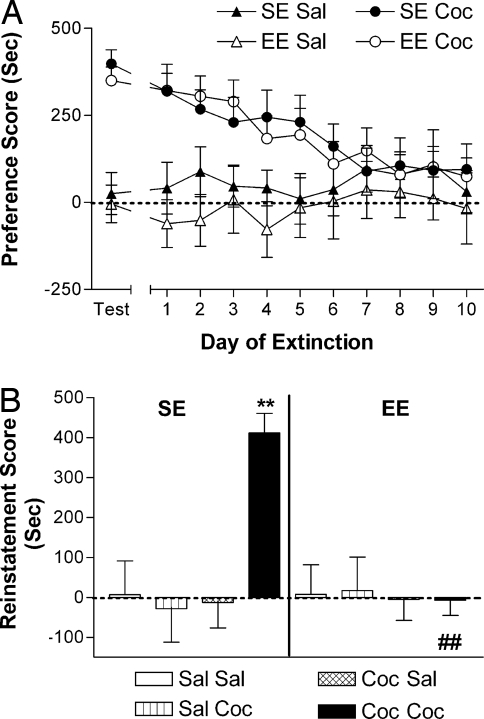
One of the hallmarks of addiction and one of its most troubling aspects is relapse. Relapse can be studied in laboratory animals by reinstatement procedures that can be applied both to drug self-administration and conditioned place preference (18). In the latter case, conditioned place preferences can be eliminated by repeatedly exposing mice to the conditioning apparatus in the absence of drug injections and can then be reinstated by injections of the conditioning drug (24, 25). In our experiments (Fig. 1C) mice were conditioned to cocaine (10 mg/kg) and then tested for conditioned place preference. After this test, mice that showed a preference for the cocaine-paired compartment were assigned to either the enriched or standard housing condition, ensuring that similar levels of preferences were present in the two groups (Fig. 4A). Starting the next day, mice were exposed each day for 10 days to the place preference apparatus and tested for extinction. As previously described (24, 25), mice housed in standard environments lost their preference for the compartment previously paired with cocaine (Fig. 4A) (two-way ANOVA for repeated measures: Time effect, F9,279 = 5.96, P < 0.0001). In addition, environmental enrichment did not significantly alter the rate of extinction (Fig. 4A). Control groups of mice that received only saline during conditioning showed no preference on the test day, and their preference score remained stable during the 10 days of extinction (Fig. 4A). After extinction, mice were injected with cocaine, and reinstatement of conditioned place preferences was measured. Although cocaine (10 mg/kg) readily reinstated extinguished conditioned place preferences in mice housed in standard environments after conditioning, mice housed in enriched environments after conditioning did not show any cocaine-induced reinstatement of preference for the cocaine-paired compartment (Fig. 4B) (three-way ANOVA: Environment effect, F1,56 = 4.08, P < 0.05; Challenge effect, F1,56 = 4.36, P < 0.05; Environment × Conditioning interaction, F1,56 = 5.82, P < 0.05; Environment × Challenge interaction, F1,56 = 4.07, P < 0.05; Conditioning × Challenge interaction, F1,56 = 5.57, < 0.05; Environment × Conditioning × Challenge interaction, F1,56 = 6.16, < 0.05).
Fig. 4.
Environmental enrichment prevents cocaine-induced reinstatement of conditioned place preferences. (A) Expression of conditioned place preferences in mice before being assigned to either standard environment (SE, full symbols) or enriched environment (EE, empty symbols) housing and extinction of established conditioned place preference tested in the conditioned place preference apparatus for 10 consecutive days in mice housed in SE (full symbols) or switched to EE (empty symbols). Mice that received cocaine during conditioning (SE Coc and EE Coc), but not those that received saline (SE Sal and EE Sal), show significant place preference on the day of test. Basal place preference and extinction did not differ between SE and EE mice. (B) Reinstatement of extinguished conditioned place preferences by a saline or cocaine (10 mg/kg) challenge in mice housed in SE (Left) or EE (Right) after development of conditioned place preferences and during extinction. Cocaine reinstated drug-seeking in mice kept in SE but not in mice switched to EE after development of conditioned place preferences. Cocaine did not reinstate drug seeking in mice that received only saline during conditioning sessions. Saline did not reinstate cocaine seeking in any group. Results represent the means ± SEM from 28–36 mice for extinction and 6–12 mice for reinstatement experiments. Three-way ANOVA followed by post hoc Student-Newman-Keuls's test: **, P < 0.01 vs. respective saline-challenge control; ##, P < 0.01 vs. respective SE control.
Environmental Enrichment Decreases Activation of Several Brain Areas Involved in Cocaine-Induced Reinstatement.
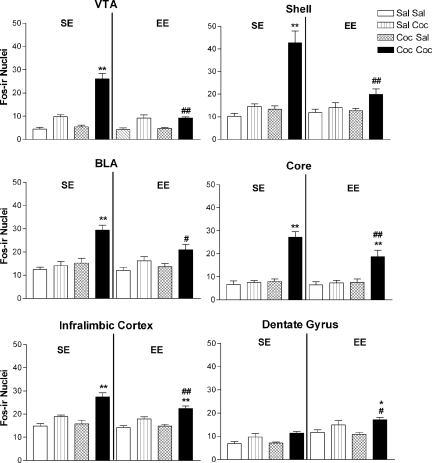
Because relapse represents one of the most important and intriguing aspects of drug addiction, we investigated the mechanisms underlying the ability of environmental enrichment to prevent cocaine-induced reinstatement. Thus, in brain tissues obtained from animals used in the reinstatement experiments (Fig. 4B), we evaluated the degree of c-FOS expression (19) in several brain areas involved in drug addiction and cocaine-induced reinstatement [see supporting information (SI)] (26–28). In cocaine-naïve mice, injections of cocaine (10 mg/kg i.p.) induced significant increases in c-FOS expression only in the orbitofrontal cortex and did so to a similar extent in mice housed in standard or enriched environments (see SI). This indicates that, as previously reported (29), this dose of cocaine is low and may be insufficient to induce c-FOS expression in other areas, such as the striatum of cocaine-naïve mice. On the other hand, in mice conditioned to cocaine, challenge injections of cocaine induced similar significant increases in c-FOS expression in mice housed in standard or enriched environments in the dorsal caudate putamen, the central amygdala, the ventral pallidum, the prelimbic cortex, the anterior cingulate cortex, and the orbitofrontal cortex. However, in mice conditioned to cocaine and then housed in enriched environments, specific reductions in cocaine-induced c-FOS expression were found in the infralimbic cortex, the shell and core of the nucleus accumbens, the basolateral amygdala, and the ventral tegmental area (Fig. 5), suggesting that these regions may be involved in the protective effects of environmental enrichment on cocaine-induced reinstatement. Interestingly, given the known effects of environmental enrichment on the hippocampus (6), mice housed in enriched environments showed higher levels of c-FOS expression in the dentate gyrus than mice housed in standard environments regardless of the treatment, with cocaine inducing similar increases in c-FOS expression when basal levels are taken into account (Fig. 5). However, the activation of the dentate gyrus does not seem to be specific to the reinstatement condition, suggesting that this region does not play a direct role in the protective effects of environmental enrichment on cocaine-induced reinstatement. Analysis of locomotor activity in the conditioned place preference apparatus during reinstatement tests (see SI) demonstrated that cocaine-induced increases in locomotor activity did not differ significantly between mice housed in enriched and standard environments, ruling out the possibility that differences in motor behaviors could account for the differences in c-FOS expression.
Fig. 5.
Environmental enrichment decreases activation of brain circuitry involved in cocaine-induced reinstatement. In mice kept in standard environments (SE, Left), administration of cocaine (10 mg/kg i.p.) significantly increased c-FOS expression only in the group that was previously conditioned to cocaine. In contrast, in mice switched to enriched environments (EE, Right) after conditioning to cocaine, no significant induction in c-FOS expression was found in the shell of the nucleus accumbens (Shell), the ventral tegmental area (VTA), and the basolateral amygdala (BLA) after administration of cocaine (10 mg/kg i.p.). In addition, cocaine-induced expression of c-FOS was significantly reduced in the core of the nucleus accumbens and in the infralimbic cortex of EE mice. In the dentate gyrus, both environmental enrichment and cocaine increased c-FOS expression. However, the profile of c-FOS expression in this region suggests that the dentate gyrus is not involved in the protective effects of environmental enrichment on cocaine-induced reinstatement. Results represent the means ± SEM from six to eight mice. Three-way ANOVA followed by post hoc Student-Newman-Keuls's test: **, P < 0.01 vs. respective saline-challenge control; #, P < 0.05, ##, P < 0.01 vs. respective SE control.
Discussion
Previous studies, including ours (12, 13), have mostly focused on the ability of environmental manipulations provided before any contact with the psychostimulant drugs to subsequently alter drug effects (2–4, 11–13). Thus, the information from those studies concerns the role of environment in determining vulnerability to addiction and suggests that positive environments reduce the risks of becoming addicted, whereas negative environments increase the risks. This is indeed in agreement with clinical data supporting an important influence of environmental conditions on the development of drug abuse and addiction (30). The present study provided environmental stimulation after discontinuation of drug administration, when addiction-related phenotypes are already present and, thus, suggests that environmental conditions can not only protect against or prevent drug addiction but can also reduce the behavioral and neurochemical consequences of repeated administration of drugs.
Many studies have shown that acute negative environmental manipulations, such as exposure to stressful events, can reinstate extinguished drug-seeking behavior in rats (4). For example, intermittent foot-shock or physical restraint given acutely just before the reinstatement test can powerfully reinstate extinguished cocaine self-administration (4). In contrast to these studies, we provided a positive environmental housing condition for an extended period and in contexts completely separate from those where exposure to drug had taken place. Thus, our results demonstrate that environmental housing conditions, by themselves, are capable of influencing the effects of drugs and of drug-related stimuli even after establishment of addiction-related behaviors.
Whereas environmental enrichment eliminates addiction-related behaviors, 30 days of social isolation, a negative environmental condition for social animals such as rodents (31), led to an exacerbation of behavioral sensitization. These results not only suggest that a change in housing condition per se does not reverse behavioral sensitization but furthermore suggest that the quality of environments (positive or negative) in which individuals live during periods of abstinence may determine whether addiction-related behaviors are ameliorated or aggravated. In addition, because social isolation is a form of chronic stress (31), these results also suggest that environmental enrichment may act as a functional opposite of stress.
Addiction is often considered a form of aberrant learning whereby drugs and drug-related stimuli acquire excessive motivational values leading to abnormal behaviors (21). An interesting aspect of our study is that not only the direct activating effects of cocaine and its ability to induce reinstatement were blunted but also the motivational effects of places associated with cocaine administration. In fact, the place preference experiments (Fig. 3) show that the compartments associated with cocaine lose their appetitive value after 30 days of exposure to environmental enrichment. These results indicate that environmental stimulation can reduce the craving induced by cues and contexts associated to cocaine use, which play a pivotal role in relapse to drug seeking (21, 22). Importantly, environmental stimulation did not prevent mice from avoiding places previously paired with aversive stimuli, such as administration of lithium, indicating that deficits in memory or blunting of all types of motivational stimuli do not account for the lack of expression of cocaine-induced place preferences and that the effects of environmental enrichment are selective for cocaine-associated stimuli.
Cocaine is believed to induce reinstatement by activating specific brain circuitries (26–28). Here, we found that the effects of enriched environments are paralleled by reductions of cocaine-induced effects in 5 of the 12 regions investigated: the infralimbic cortex, the shell and the core of the nucleus accumbens, the ventral tegmental area, and the basolateral amygdala. The most striking effects were found in the mesolimbic dopaminergic circuit, namely in the ventral tegmental area, that contains the cell bodies of dopaminergic neurons, and the shell of the nucleus accumbens, one of the main terminal regions of these neurons, where cocaine-induced activation was almost completely abolished in mice housed in an enriched environment. In the other three regions, cocaine was still able to increase c-FOS expression in mice housed in an enriched environment but at a level significantly lower than in mice housed in a standard environment. These five regions have been previously shown to be part of a network that controls cocaine-induced drug-seeking behavior (26–28, 32). For example, c-FOS expression is increased in these regions during cocaine- (33), cue- (34), or context-induced (35) reinstatement of self-administration. Activation the core of the nucleus accumbens, the ventral tegmental area, or the basolateral amygdala has been shown to be necessary to promote drug-seeking behavior, because inactivation of these regions blocks cocaine-induced reinstatement (36). On the other hand, activation of the infralimbic cortex seems to inhibit drug-seeking behavior: its inactivation produces drug seeking in rats undergoing extinction, whereas its activation inhibits cocaine-induced reinstatement (32). Concerning the shell of the nucleus accumbens, it has been shown that its activation can both facilitate (37) or inhibit cocaine-induced reinstatement (32), depending on the experimental conditions. c-FOS expression allows for the identification of brain regions where neurotransmitters are likely released and second-messenger cascades activated (19), but it does not allow determination of whether activating neurotransmitters, such as glutamate, or inhibiting neurotransmitters, such as GABA, are released. Although future studies are needed to further characterize the role of the regions we identified in the protective effects of environmental enrichment on cocaine seeking, our study suggests that environmental enrichment prevents cocaine-induced reinstatement by modifying the reactivity to cocaine in several brain areas known to play critical roles in drug-seeking behavior.
Behavioral therapies have proven to be relatively successful in treating addicts (38). Cognitive behavioral therapies consist of learning to recognize and avoid situations that trigger cravings and developing strategies to cope with craving (38). Although precautions should be used in extrapolating our results to human conditions, this study suggests that, in addition to cognitive and behavioral therapies targeted to specific craving-inducing situations, positive and stimulating environmental conditions could per se reduce craving and facilitate abstinence from drug use. This indicates that general life conditions of drug addicts should be considered as part of their therapy and that positive environments and environmental stimulation could be key factors in the long-term treatment of addicts.
Materials and Methods
Subjects.
Adult male C57/BL6 mice (Elevage Janvier), 10–12 weeks old, experimentally naïve at the start of the study, were housed in a temperature- and humidity-controlled room and maintained on a 12 h light/dark cycle with the lights on from 7:00 a.m. to 7:00 p.m. and had ad libitum access to food and water. All experimentation was conducted during the light period. Experiments were carried out in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC) for the care of laboratory animals.
Housing Environmental Conditions.
After arrival and during the development of behavioral sensitization or conditioned place preference (see following sections), all mice were housed in standard environments (i.e., were housed in groups of four in common housing cages, 25 × 20 × 15 cm). After being subjected to experimental manipulations, half of the mice were kept in standard environments, whereas the other half was switched to enriched environments, which consisted of larger (60 × 38 × 20 cm) cages containing constantly a running wheel and a small house and four to five toys that were changed once per week with new toys of different shapes and colors. To avoid stress, all mice in each cage were assigned to either standard environment or enriched environment, and littermates were not changed during the course of the study.
Behavioral Procedures.
Behavioral sensitization and conditioned place preference or conditioned place aversion were conducted as previously described (13). At the end of the last sensitization or conditioning session, half the mice were kept in standard environments, and the other half were switched to enriched environments before being tested for expression of addiction-related behaviors (for detailed description, see SI).
For the reinstatement procedure, mice were assigned to the enriched environment or standard environment condition after being tested for expression of conditioned place preference. The next day and for 10 subsequent days, conditioned place preferences were extinguished by running 30-min test sessions similar to the initial test session. On the 11th day, mice were injected with either saline or 10 mg/kg of cocaine, and reinstatement of extinguished conditioned place preference was studied.
Immunohistochemistry.
Fifty-two mice from experiment 3 (n = 6–8 per group) were used for immunohistochemistry analysis. General procedures for c-FOS immunostaining and counting were based on previous publications (33). Twelve regions were quantified for c-FOS expression: (i) prelimbic cortex cortex, (ii) infralimbic cortex, (iii) orbitofrontal cortex, (iv) anterior cingulate cortex, (v) the dorsolateral caudate putamen, (vi) the shell of the nucleus accumbens, (vii) the core of the nucleus accumbens, (viii) the ventral pallidum, (ix) the dentate gyrus of the hippocampus, (x) the central amygdala, (xi) the basolateral amygdala, and (xii) the ventral tegmental area.
Drugs.
Cocaine HCl was obtained from the Research Triangle Institute. Lithium chloride was purchased from Sigma-Aldrich (France). Drugs were dissolved in sterile saline solutions (0.9%) and administered i.p. in a volume of 1 ml/100 g.
Statistics.
All results are presented as group means (± SEM). Differences in locomotor activity or preference scores between standard environment and enriched environment groups were assessed by two-way or three-way ANOVA. Results showing significant overall changes were subjected to Student–Newman–Keuls post hoc test. For comparison between two sets of values, a Mann–Whitney nonparametric test was used. Differences were considered significant when P < 0.05.
Supplementary Material
Acknowledgments.
We thank S.R. Goldberg, F. Becq, and D. Belin for comments on the paper, A. Gaillard for help with immunohistochemistry procedures, and V. Lardeux for technical assistance. This work was supported by Centre National de la Recherche Scientifique (CNRS), University of Poitiers, Mission Interministérielle de Lutte contre la Drogue et la Toxicomanie, and Région Poitou-Charentes. R.E. R. is a recipient of a CNRS Ph.D. fellowship (BDI-PED, 2005–2008).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 16829.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806889105/DCSupplemental.
References
- 1.O'Brien CP. Anticraving medications for relapse prevention: A possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 2.Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- 3.Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- 4.Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: A review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 5.Rosenzweig MR. Environmental complexity, cerebral change, and behavior. Am Psychol. 1966;21:321–332. doi: 10.1037/h0023555. [DOI] [PubMed] [Google Scholar]
- 6.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 7.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 8.Laviola G, Hannan AJ, Macrì S, Solinas M, Jaber M. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol Dis. 2008;31:159–168. doi: 10.1016/j.nbd.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 10.Pizzorusso T, Berardi N, Maffei L. A richness that cures. Neuron. 2007;54:508–510. doi: 10.1016/j.neuron.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- 12.Bezard E, et al. Enriched environment confers resistance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and cocaine: Involvement of dopamine transporter and trophic factors. J Neurosci. 2003;23:10999–11007. doi: 10.1523/JNEUROSCI.23-35-10999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solinas M, Thiriet N, Rawas RE, Lardeux V, Jaber M. Environmental enrichment during early stages of life reduces the behavioral, neurochemical, and molecular effects of cocaine. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.51. [DOI] [PubMed] [Google Scholar]
- 14.Thiriet N, et al. Environmental enrichment during adolescence regulates gene expression in the striatum of mice. Brain Res. 2008;1222:31–41. doi: 10.1016/j.brainres.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Compton WM, Thomas YF, Conway KP, Colliver JD. Developments in the epidemiology of drug use and drug use disorders. Am J Psychiatry. 2005;162:1494–1502. doi: 10.1176/appi.ajp.162.8.1494. [DOI] [PubMed] [Google Scholar]
- 16.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: A critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- 18.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: An assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 20.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 21.Hyman SE. Addiction: A disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 22.See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 23.Itzhak Y, Martin JL. Cocaine-induced conditioned place preference in mice: Induction, extinction and reinstatement by related psychostimulants. Neuropsychopharmacology. 2002;26:130–134. doi: 10.1016/S0893-133X(01)00303-7. [DOI] [PubMed] [Google Scholar]
- 24.Mueller D, Stewart J. Cocaine-induced conditioned place preference: Reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- 25.Szumlinski KK, Price KL, Frys KA, Middaugh LD. Unconditioned and conditioned factors contribute to the ‘reinstatement’ of cocaine place conditioning following extinction in C57BL/6 mice. Behav Brain Res. 2002;136:151–160. doi: 10.1016/s0166-4328(02)00102-x. [DOI] [PubMed] [Google Scholar]
- 26.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 27.Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: An update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Crombag HS, Jedynak JP, Redmond K, Robinson TE, Hope BT. Locomotor sensitization to cocaine is associated with increased Fos expression in the accumbens, but not in the caudate. Behav Brain Res. 2002;136:455–462. doi: 10.1016/s0166-4328(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 30.Kodjo CM, Klein JD. Prevention and risk of adolescent substance abuse. The role of adolescents, families, and communities. Pediatr Clin North Am. 2002;49:257–268. doi: 10.1016/s0031-3955(01)00003-7. [DOI] [PubMed] [Google Scholar]
- 31.Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- 32.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neisewander JL, et al. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145:438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 36.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- 38.Carroll KM, Onken LS. Behavioral therapies for drug abuse. Am J Psychiatry. 2005;162:1452–1460. doi: 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.