Abstract
The novel phosphatidylinositol-3-kinase (PI-3-kinase) inhibitor PX-866 was tested against 13 experimental human tumor xenografts derived from cell lines of various tissue origins. Mutant PI-3-kinase (PIK3CA) and loss of PTEN activity were sufficient but not necessary as predictors of sensitivity to the antitumor activity of the PI-3-K inhibitor PX-866 in the presence of wild type Ras, while mutant oncogenic Ras was a dominant determinant of resistance, even in tumors with coexisting mutations in PIK3CA. The level of activation of PI-3-kinase signaling measured by tumor phospho-Ser473-Akt was insufficient to predict in vivo antitumor response to PX-866. Reverse phase protein array (RPPA) revealed that the Ras dependent down stream targets c-Myc and cyclin B were elevated in cell lines resistant to PX-866 in vivo. Studies using an H-Ras construct to constitutively and preferentially activate the three best defined downstream targets of Ras, namely Raf, RalGDS, and PI-3-kinase, showed that mutant Ras mediates resistance through its ability to utilize multiple pathways for tumorigenesis. The identification of Ras and downstream signaling pathways driving resistance to PI-3-kinase inhibition may serve as an important guide for patient selection as inhibitors enter clinical trials, and for the development of rational combinations with other molecularly targeted agents.
Keywords: PX-866, PI-3-K, Akt, Ras, response
INTRODUCTION
An important cell survival mechanism for many cancers is mediated by the phosphatidylinositol-3-kinase (PI-3-kinase)/Akt (protein kinase B) signaling pathway (1). Class I PI-3-kinases phosphorylate membrane phosphatidylinositols to give PI(3,4,5)P3, which then binds and recruits the serine/threonine kinase Akt through its N-terminal pleckstrin homology (PH) domain, in a process reversed by PTEN phosphatase. The membrane associated Akt is activated by Thr308 phosphorylation by membrane associated phosphoinositide dependent kinase-1 (PDK1) (2) and Ser473 phosphorylation most likely through the TORC2 complex (3). Activated Akt detaches from the plasma membrane and moves to the cytoplasm and the nucleus where it phosphorylates a battery of targets leading to changes in cellular functions (4). PI-3-kinase activity has been found to be aberrantly activated in many human cancers including breast, glioma, prostate, non small cell lung, ovarian, head and neck, urinary tract, colon and cervical cancer (5–7). Activation of the PI-3-kinase/Akt pathway can occur due to upstream inputs including deregulated growth factor signaling (8), activating mutations in the proto-oncogene Ras (9), point mutations or overexpression of the PI-3-kinase alpha catalytic subunit (PIK3CA) (7, 10), mutation or loss of PTEN (11), and activating mutations in the PH domain of Akt (12).
The PI-3-kinase/Akt signaling pathway offers several targets for therapeutic intervention and a number of agents are now in or entering clinical trials (13). Much early work on the anticancer potential of PI-3-kinase inhibitors was conducted with LY294002 a chromene of low potency and selectivity, that inhibits multiple kinases both related and unrelated to PI-3-kinase (14). The other frequently utilized PI-3-kinase inhibitor is the fungal metabolite wortmannin that shows a much higher specificity for the Class 1 PI-3-kinases compared with other related family members. However, wortmannin has proved to be too biologically unstable and toxic for use as an antitumor agent (15). In our studies we utilized PX-866, a semisynthetic viridin which has been found to be a specific, irreversible inhibitor of the Class 1 PI3-kinases with a mechanism similar to wortmannin. Due to its chemically modified tetracyclic core, PX-866 possesses a greatly increased metabolic stability, decreased toxicity and an increased specificity relative to other PI-3-kinase family members (16) and has entered clinical trial as an anticancer agent. PX-866 has been characterized against the Class 1 isoforms and found to selectively inhibit the alpha, delta and gamma isoforms with IC50s of 5 nM, 9 nM, and 2 nM respectively (17). PX-866 is a selective inhibitor of PI-3-kinase and against a panel of 235 unrelated kinases PX-866 at 1 μM inhibited only 2 kinases more than 30%, Lck by 32% and LOK by 40%. PX-866 has antitumor efficacy in experimental cancer models as a single agent and in combination with both conventional chemotherapy and targeted agents (17, 18). As PX-866 and other specific inhibitors of PI-3-kinase/Akt signaling move through clinical development, the identification of positive and negative predictors of response to identify individuals most likely to receive the maximum therapeutic benefit is critical. Predictors for PI-3-kinase/Akt inhibitor sensitivity so far reported include PI-3-kinase pathway -specific mutations (7, 10–12) increased insulin like growth factor binding protein-2 (IGFBP-2) in glioma (19), stathmin in breast cancer (20) and acidic ribosomal phosphoprotein P2 (21).
In the present study, we tested PX-866 against a panel of experimental human tumor xenografts derived from cell lines of various tissue origins showing that mutant PIK3CA and loss of PTEN activity were sufficient but not necessary predictors of sensitivity to PX-866 in the presence of wild type Ras, while mutant oncogenic predicted resistance, even in tumors with coexisting mutations in PIK3CA. Thus, mutant oncogenic Ras as a primary determinant of resistance to the antitumor activity of PX-866. Further studies used an H-Ras construct to constitutively and preferentially activate the three best-defined downstream targets of Ras: Raf, RalGDS, and PI-3-kinase. These constructs revealed that activated Ras mediates resistance through its ability to utilize these pathways concurrently. The identification of signaling pathways driving resistance to PI-3-kinase inhibitors will aid patient selection and reveals a need for the development of rational combinations with other molecularly targeted agents.
MATERIALS AND METHODS
Cells
A-549, H460 and H1299 human non small cell (NSC) lung cancer, HT-29, HCT-15 and HCT-116 human colon cancer, MDA-MB-361 human breast cancer, Panc-1, BxPC-3 and MiaPaCa-2 pancreatic cancer, PC-3 prostate cancer, Skov-3 ovarian cancer, and RPMA-8226 multiple myeloma cancer cells were obtained from the American Tissue Type Collection (ATTC, Rockville, MD). The cell lines were grown in humidified 95% air, 5% CO2 at 37°C in their ATCC recommended media with 10% fetal bovine serum (FBS). All cell lines were tested to be mycoplasma free using a PCR ELISA assay (Roche Diagnostics Inc., Indianapolis). HCT116 K-Ras-deleted cells generated by homologous deletion of the mutant K-Ras allele [20, 21] were transfected by electroporation at 600 V for 60 milliseconds using a Multiporator Eppendorf (Hamburg, Germany) with G418 selectable plasmids expressing mutant active H-Ras (H-Ras V12), and the selective effectors H-Ras V12S35, H-Ras V12G37, or H-Ras V12C40, which preferentially activate Raf, RalGDS, and PI-3-kinase enzymes, respectively, and individual colonies isolated. The plasmids were generously provided by Dr. M. Wigler (Cold Spring Harbor, NY). These Ras mutations have been previously characterized in a number of models (22–26)
Antitumor studies
Approximately 107 cells in log cell growth were injected subcutaneously in 0.1 ml sterile 0.9% NaCl into the flanks of severe combined immunodeficient (SCID) mice. Cell lines with H-Ras constructs were removed from G418 one passage before injection. When the tumors reached 200 mm2, the mice were stratified into groups of 8–10 animals having approximately equal mean tumor volumes and oral administration of 2.5 to 3 mg/kg of PX-866 begun every other day for 1 to 3 weeks. For oral (po) administration to mice PX-866 (4S,4aR,5R,6aS,9aR,E)-1-((diallylamino)methylene)-11-hydroxy-4-(methoxymethyl)-4a,6a-dimethyl-2,7,10-trioxo-1,2,4,4a,5,6,6a,7,8,9,9a,10-dodecahydroindeno[4,5-h]isochromen-5-yl acetate) was dissolved at 0.3 to 0.5 mg/ml in 5% ethanol in water and dosed by oral gavage At the end of the study antitumor activity was expressed as percent test/control (T/C%) determined by dividing the increase in volume of the PX-866 treated tumors by the increase in volume of the control tumors, from the start of treatment. Information on mutations in the cell lines was obtained from the Sanger Institute data base (http://www.sanger.ac.uk) and confirmed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometric sequencing.
Tumor PI-3-kinase activity
Mice were killed 24 hr after the last PX-866 treatment, the tumors excised and immediately the tumors were homogenized in lysis buffer containing 50 mM frozen in liquid N2. For assay, HEPES buffer, pH 7.5, 50 mM NaCl, 0.2 mM NaF, 0.2 mM sodium orthovanadate, 1 mM, phenylmethylsulfonyl fluoride, 20 μg/ml aprotinin, 20 μg/ml leupeptin, 1% NP-40, and 0.25% sodium deoxycholate. Protein concentration was determined by BCA assay (Pierce Biotechnology, Rockford, IL) and 50 μg of cell lysate protein was boiled for 5 minutes with denaturing buffer containing 0.25 M Tris, pH 6.8, 35% glycerol, 8% sodium dodecyl sulfate, and 10% 2-mercaptoethanol, loaded on a 10% acrylamide/bisacrylamide gel, and separated by electrophoresis at 150 V for 40 minutes. Proteins were electrophoretically transferred to a polyvinylidene fluoride membrane, preincubated with a blocking buffer of 137 mM NaCl, 2.7 mM KCl, 897 mM CaCl2, 491 mM MgCl2, 3.4 mM Na2HPO4, 593 mM KH2PO4, and 5% bovine serum albumin, and incubated overnight with anti-phospho-Ser473-Akt, anti-Akt, anti-phospho-Ser338-Raf, anti-Raf, anti-cyclin B, anti-cMyc polyclonal antibodies (Cell Signaling, Beverly, MA) or anti-β-actin (Santa Cruz, Santa Cruz, CA). Detection used donkey anti-rabbit IgG peroxidase-coupled secondary antibody (GE Healthcare, Buckinghamshire, UK). Band density was measured using the Renaissance chemiluminescence system on Kodak X-Omat Blue ML films (Eastman Kodak, New Haven, CT).
Reverse phase protein array (RPPA)
Cells were lysed with buffer containing 150 mM NaCl, 50 mM HEPES, pH 7.4, 1.5 mM MgCl2, 1 mM EGTA, 100 mM NaF, 10 mM sodium pyrophosphate, 10% glycerol, 1% Triton X-100 supplemented with Complete Protease Inhibitor Cocktail Tablets (Roche Applied Science, Indianapolis, IN) and cleared by centrifugation at 15,000 rpm for 10 min at 4°C. Samples were denatured by the addition of 1 part denaturing buffer to 3 parts cell lysate and boiling for 5 min. Sample concentrations were adjusted to 1mg/ml with dilution buffer (1 part denaturing buffer and 3 parts cell lysis buffer) and printed as serial dilutions on glass slides, and specific proteins quantified using 52 validated antibodies as previously described (27, 28).
Apoptosis measurement
Cells were treated with 0.5 μM PX866 for 48 hr and harvested by 10 min exposure to trypsin/EDTA at 37 °C. Apoptotic cells that detached from the culture surface were collected by centrifugation of the medium at 1,500 rpm for 5 min. The pooled cell pellets were resuspended and mixed with trypan blue dye. Dye incorporation into non-viable cells was measured by counting 500 cells from randomly chosen fields with a light microscope and a hemocytometer, and expressed as a percentage of the total number of cells counted. For confirmatory purposes the extent of apoptosis was evaluated by assessing Hoechst and TUNEL stained cytospin slides under fluorescent light microscopy and scoring the number of cells exhibiting the “classic” morphological features of apoptosis and necrosis. For each condition, 10 randomly selected fields per slide were evaluated, encompassing at least 1500 cells. Alternatively, the Annexin V/propidium iodide assay was carried to determine cell viability out as per the manufacturer’s instructions (BD PharMingen) using a Becton Dickinson FACScan flow cytometer. (Mansfield, MA).
Colony formation
For studies of the effects of PX-866 on cell survival 250 to 2,000 cells were plated in a 60 mm dish and 12 hr later treated with 0.5 μM PX-866 for 4 hr. The media was changed and the cells grown for 10–14 days. After fixation and staining with crystal violet colonies of more than 50 cells were counted using a ColCount™ colony counter (Oxford Optronics, Oxford, England). Individual assays were performed at multiple dilutions with a total of six plates per data point.
Data analysis
Comparison of the effects of treatments and comparison of protein levels in vitro used a two-tailed t test. Differences with a p < 0.05 were considered statistically significant. A two tailed t test and a Pearson correlation was performed between normalized data obtained from the RPPA analysis. For studies of the in vivo antitumor activity of PX-866, a Whitney Mann U test was performed using SPSS software. (SPSS Inc. Chicago, Il)
RESULTS
In vivo activity of PX-866
The antitumor activity of PX-866 was measured in 13 human tumor cell line-derived xenografts in SCID mice. These tumors were then classified into three groups: resistant tumors that showed minimal or no response (No Response, T/C >70%); tumors that displayed a slowed but continued growth through treatment (Low Response, T/C 35–69%) and sensitive tumors that displayed a antitumor response to PX-866 (Antitumor, T/C <35%), which included two that showed a cytostatic response and one that showed a regression. The mutation status of the cell lines was obtained from the Sanger Institute database and confirmed by mass spectral sequencing (Figure 1A).
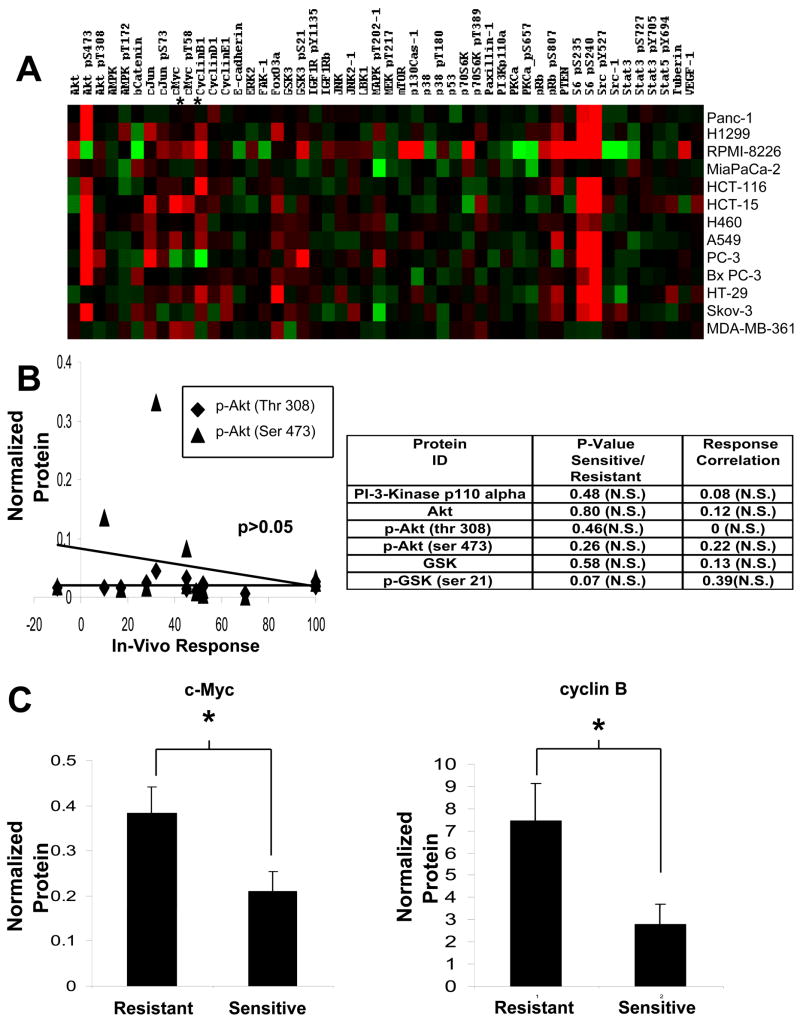
Figure 1. Effect of PX-866 on cell line derived xenografts.
A, Cell line derived xenografts were grown subcutaneously in female SCID mice. Upon reaching 200mm3 the mice were treated with PX-866, 2.5–3.0 mg/kg every other day administered po. At the end of treatment the tumor volume was expressed as a percentage of the increase in the vehicle alone treated tumor volume (T/C%). * p <0.05. Tumor responses were characterized as No response (T/C >70%), Low response (T/C 35–69%), or Antitumor (T/C <35%). The Ras, Raf, PIK3A, LKB1 and PTEN mutation status of the tumors is shown: B, phospho-Ser473-Akt levels measured in representative tumors removed from mice treated with PX-866 2.5–3 mg/kg po at the end of treatment, Images were taken from different fields on the same film.
PC-3 prostate, BxPC3 pancreatic, HT-29 colon, Skov-3 ovarian and MDA-MB-361 breast cancer were all sensitive to PX-866. PC-3 prostate cancer is PTEN null while HT-29 colon, Skov-3 ovarian and MDA-MB-361 breast cancer all have activating mutations in PIK3CA. HT-29 colon cancer has a coexisting activating mutation in B-Raf, but this was insufficient to cause resistance to PX-866 antitumor activity. Of the sensitive tumors only BxPC3 has no reported mutation in the PI-3-kinase/Akt pathway.
All tumors that have an activating mutation in Ras displayed moderate to marked resistance to the antitumor activity of PX-866. This includes HCT-116, HCT-15, and H460 colon cancer which have an activating PIK3CA mutation as well as an activating Ras mutation. The resistant lines A549 and H460 NSC lung cancer also have a mutation resulting in a dysfunctional LKB1 concurrent with an activating mutation in Ras. LKB1 is a known tumor suppressor that downregulates mTor mediated protein translation in the presence of low energy conditions in the cell (29). How LKB1 contributes to the sensitivity of the tumors to PI-3-kinase inhibition has not been determined, but in the two xenografts studied the intermediate response to PX-866 observed was similar to tumors with a PIK3CA mutation together with oncogenic Ras. PX-866 was tested for its ability to inhibit tumor PI-3-kinase activity measured by the phosphorylation of Akt at Ser473 by Western blotting, which was found to be equally inhibited in tumors that showed varying degrees of sensitivity to PX-866 treatment (Figure 1B). Thus RAS mutation appears to be an indicator of resistance to PX-866, dominant over the sensitizing effects of PI-3-kinase pathway mutations.
PX-866 resistant cells display characteristics of Ras transformed cells
We next used RPPA technology to address whether response to PX-886 was dependent on the level of expression or activation of proteins of the PI-3-kinase/Akt pathway (Figure 2A). Neither PI-3-kinase protein levels, nor AKT activation measured by Thr308 or Ser473 phosphorylation, nor the phosphorylation of the downstream Akt target GSK-3 were significantly altered in sensitive compared to resistant lines, or when correlated with in vivo antitumor response (Figure 2B). Two proteins on the array did show a significant difference between sensitive and resistant lines, with levels of c-Myc and cyclin B being significantly higher in lines resistant to PX-866 in vivo. (Figure 2C). It is noteworthy that an increase in both of these proteins has been reported as a result of ras induced transformation (30–32).
Figure 2. Protein analysis of sensitive and resistant cell lines.
A, Cell lines were analyzed by 52 validated antibodies in a reverse phase protein array (RPPA). Protein levels were quantified and arranged in a heat map, red indicating high expression, black median and green low expression. B, Analysis of expression of components of the PI-3-kinase/Akt pathway and correlation with in vivo antitumor response. The lower panel shows a plot of phospho-Akt levels against in vivo antitumor response. C, Levels of c-Myc and cyclin B proteins showing differences between sensitive and resistant cell lines. * p < 0.05
H-Ras or H-Ras mutants preferentially activating Raf or RalGDS, but not RAS mutants linked to PI-3-kinase, are resistant to PX-866 in vitro and in vivo
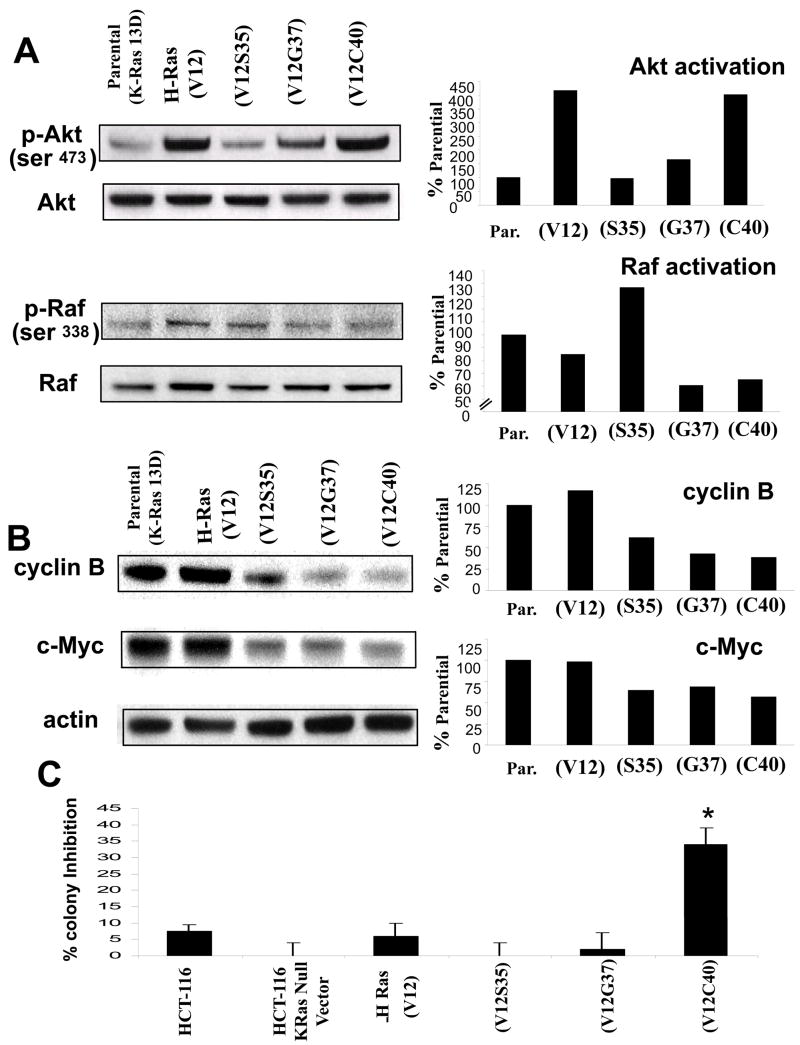
Wild type HCT-116 K-Ras positive cells, HCT-116 K-Ras-null cells and K-Ras-null cells constitutively expressing an active H-Ras, an H-Ras modified to preferentially activate Raf (H-Ras V12S35), RalGDS (H-Ras V12G37), or PI-3-kinase (H-Ras V12C40) were used as a model for the simultaneous and individual activation of proteins effected by Ras signaling as previously described (33). This model was used to determine p-AKT and p-Raf activation (Figure 3A). HCT-116 cells with activated H-Ras, and PI-3-kinase activating H-Ras showed a robust activation of PI-3-kinase signaling measured by phospho-Ser473-Akt. H-Ras V12 and H-Ras V12C40 displayed a similar levels of Akt activation. H-Ras activating RalGDS also retained the ability to activate Akt which has been recently reported (34). Wild type HCT-116, H-Ras V12 and H-Ras activating Raf, all showed similar levels of Raf activation. H-Ras activating Ral and H-Ras activating PI-3-kinase showed around a 40% decrease in Raf signaling. These cell lines were then probed for cyclin B and c-Myc, which had been shown to to be associated with resistance to PI-3-kinase inhibition through our RPPA experiment. Both wild type HCT-116 and the K- Ras null HCT-116 transfected with activated H-Ras showed a robust expression of cyclin B, as did the H-Ras activating Raf, while the H-Ras linked to RalGDS and PI-3-kinase showed lower levels of activation (Figure 3B). Wild type HCT-116 and the K-Ras null HCT-116 transfected with activated H-Ras showed high levels of c-Myc protein, while the three conditional Ras lines all showed lower levels. Despite slight differences, all the selective H-Ras constructs retained the ability to activate c-Myc or cyclin B to some extent. The lines were also studied for their sensitivity to PX-866 measured by colony formation (Figure 3C). K-Ras null cells, H-Ras, Raf, and RalGDS activated cell lines behaved similar to the wild type HCT-116 (mutant K-Ras, mutant PIK3CA) line when treated with PX-866. In contrast, an H-Ras mutant that preferentially activates PI-3-kinase without activating RalGDS or Raf showed significant inhibition of colony formation by PX-866.
Figure 3. Signaling and clonogenic potential of HCT-116 H- Ras construct cells.
A, Measurement of cellular PI3-kinase activity and Raf actvation by on the left Western blot analysis using phospho-Ser473-Akt and phospho-Ser338-Raf The panel on the right showns densiometric data from the blots. B, On the left Western blot analysis for cellular cyclin B and c-Myc normalized against actin The panel on the right showns densiometric data from the blots.. C, Colony formation assay performed on H-Ras construct HCT-116 cells treated with 0.5 μM PX-866. * p<0.05 compared to wild type cells.
Apoptosis was measured in the cell lines both by trypan blue assay and flow cytometry, Cells with active Raf and RalGDS lines showed levels of apoptosis similar to wild type HCT-116 cells while H-Ras cells showed a moderate but significant increase in apoptosis. In contrast H-Ras cells with active PI-3-kinase, but not Raf or RalGDS activation, showed a large and significant increase in apoptosis.(Figure 4A).
Figure 4. Apoptosis and in vivo effects of PX-866 on HCT-116 H- Ras construct cells.
A. Trypan blue and flow cytometry analysis of annexin positive cells treated with 0.5 μM PX-866. * p<0.05 compared to wild type cells. B, Comparison of final volumes of vehicle (white bars) or PX-866 (black bars) treated tumors, * p<0.05 of treated compared to control.
Tumors lacking the mutant K-Ras allele have previously been shown to be non-tumorigenic (22) indicating that ras is a dominant tumorigenic factor in this cell line. Tumors derived from implanted ras driven cell lines treated with vehicle or 2.5 mg/kg PX-866 were measured at the final day of treatment (Figure 4B). The T/C percentage for the wild type HCT-116 cells was 49%, for K-Ras null, H-Ras tumors 54% and for Raf driven tumors 53% (p = 0.05) RalGDS driven tumors showed a significant decrease in T/C to 37% (p = 0.01) and PI-3-kinase driven tumors showed the greatest sensitivity with a T/C value of 31% (p = 0.02).
DISCUSSION
The PI-3-kinase/Akt signaling pathway is critical for cancer cell growth and survival (1) and a number of inhibitors of PI-3-kinase or Akt have been, or will soon be introduced into clinical trial as antitumor agents (13). Determining which patients respond to these drugs will play a role in how, and at what pace they move through clinical development (35). Our in vivo antitumor studies in a panel of 13 molecularly characterized human tumor cell line-derived xenografts found that tumors with mutant PIK3CA or PTEN null, but without mutant Ras were sensitive to PX-866. The three most sensitive lines, which displayed a cytostatic or regression response had activating mutations in PI-3-kinase. It is noteworthy that an activating mutation in Raf in the HT-29 derived xenograft together with a PIK3CA activating mutation was insufficient to reverse the cytostatic effects of PX-866. The BxPC3 pancreatic cancer cell line with no reported mutations in the PI-3-kinase/Akt pathway also showed a cytostatic response to PX-866. This cell line has been characterized as having an inactivating mutation in Smad 4, which has recently been shown to allow these cells to downregulate PTEN and thus activate PI-3-kinase signaling (36). This illustrates that while individual mutations or deletions in the PI-3-kinase/Akt/PTEN signaling pathway may be sufficient to predict response to PX-866, they are not be necessary for response as multiple inputs can influence this pathway. Most importantly we found that mutant oncogenic Ras is a negative predictor of response to the PI-3-kinase inhibitor PX-866 in xenografts, even those with concurrent activating mutations in PI-3-kinase, thus PI-3-Kinase mutation cannot be used as individual marker for sensitivity.
RPPA technology was used to measure protein levels and activation in the cell lines in vivo. Several proteins known to be directly involved in PI-3-kinase/Akt signaling were studied, including PIK3CA, Akt, and GSK. Neither total nor phosphoprotein levels were significantly different between sensitive and resistant lines nor was there a significant association with the in vivo antitumor response. Thus, the expression level and activation of these PI-3-kinase/Akt pathway proteins in vitro under basal conditions does not translate into in vivo tumor sensitivity. Although this finding excludes the level of Akt Thr308 or Ser473 phosphorylation as a predictive biomarker, AKT phosphorylation remains a surrogate endpoint for measuring the efficacy of target inhibition as phosphorylation of Akt was inhibited by PX-866 in vivo, independent of the sensitivity or resistance of the tumor to PX-866 in terms of its growth. Cyclin B and c-Myc were shown to be significantly overexpressed in cell lines forming PX-866 resistant xenografts compared to cell lines showing sensitivity to PX-866. In previous studies, oncogenic Ras has been shown to have the ability to upregulate total c-Myc levels both through an increase in mRNA levels (31) and increases in protein stability (32). Cyclin B was increased in cells resistant to PX-866 treatment and showed a significant negative association with antitumor response. Cyclin B has been shown to be upregulated during Ras induced transformation and is associated with an increased mitotic rate. Whether cyclin B and c-Myc act are factors contributing to the resistance of mutant Ras tumors to PX-866, or only serve as markers of mutant Ras is not known. Additionally, the data derived from the H-Ras lines possessing the ability to activate specific downstream pathways showed that all three pathways studied are capable of activating cyclin B and c-Myc. Therefore it is possible that the robust expression of these proteins in the mutant Ras lines in the RPPA may come from a cooperative effect between the pathways.
In colony formation assays the HCT-116 H-Ras line with specific activation of PI-3-Kinase was found to be the only line sensitive to the effects of PI-3-kinase inhibition, indicating this line had been made more dependent on PI-3-Kinase signaling. Cells with the wild type H-Ras showed greater amounts of apoptosis than cells with mutant K-Ras, which may reflect differences between the two Ras isoforms in their utilization of downstream signaling (37), or the ability of parental K-Ras to utilize less characterized pathways downstream of Ras. This also suggests the slowing of growth seen in mutant Ras tumors in vivo following PX-866 treatment may be a result increased apoptosis. The highest apoptosis was seen in the H-Ras cell line selectively activating PI-3-K, as it lacked the resistance provided by parallel signaling pathways. Of interest, HCT-116 K-Ras null cells which retain a mutant PI-3-Kinase were not sensitized to PX-866 suggesting that in cell lines arising from a Ras mutation, in contrast to tumors with exclusive PI-3-Kinase mutations, the input from Ras may be essential for PI-3-Kinase signaling to be utilized in tumorigenic processes. This observation agrees with previous studies that found that despite this PI-3-kinase heterozygous mutation, to sensitize HCT-116 to the effects of PI-3-Kinase inhibitors in colony formation and growth inhibition assays, a homozygous PIK3CA variant need to be created and the assays performed in low serum conditions, additionally HCT-116 cells retaining mutant K-Ras but with only a homozygous wild type PI-3-kinase were able to form tumors (38). In contrast, K-Ras null HCT-116 cells have been shown to lack the ability to form tumors (22).
When injected into mice the oncogenic H-Ras and selective H-Ras cells all retained the ability to form tumors. Despite both having increased Akt activity compared with the parental HCT-116 line, the cells with an active H-Ras responded similarly to PX-866 as the parental HCT-116, while PI-3-kinase activated H-Ras cells showed a 23% greater response to PX-866 than control cells. H-Ras cells displaying low levels of Akt activity than the other constructs showed activity similar to the parental HCT-116 or HCT-116 H-Ras lines. The H-Ras line specific for RalGDS signaling showed an intermediate response, with a 17% increase in activity of PX-866 against the tumor. Together these results show that the magnitude of Akt activation does not determine response to PI-3-kinase inhibition but that activation of pathways downstream of oncogenic Ras, parallel or compensatory for active PI-3-kinase, can rescue tumors from inhibition.
Mutant active Ras has recently been described to predict resistance to small molecule inhibitors and antibodies against the EGF receptor (39, 40) presumably due to the down-stream location of Ras in EGF receptor signaling (41). Additionally, Ras-driven cell lines exhibit a modest response to MEK inhibitors, showing a growth delay when grown as xenografts, while B-Raf driven xenografts display a cytostatic response (42). This is similar to our observation with PX-866 in the context of PI-3-kinase signaling and derive from the ability of Raf and PI-3-kinase to converge on redundant downstream mediators, “funnel factors”, of translation, such as eiF4e (43), survival factors such as Bad (44), and cyclin D for cell cycle progression (42). Ultimately, treatment of Ras-driven tumors may lie in the direct inhibition of the active Ras protein itself, or the combination of PI-3-kinase inhibitors, including PX-866, with other agents targeting endpoints of the Ras pathway.
The relationships of the signaling pathways we have studied are shown in Figure 5. Oncogenic Ras effectively negates the effects of other potential predictors such as mutant PIK3CA which has been proposed to be a positive marker of response to inhibitors of PI-3-kinase/Akt signaling and decreases the reliance of the tumor on PI3-kinase signaling, in favor of other Ras dependent signaling pathways. Thus, it may be necessary to know the mutational status of K-Ras, PIK3CA and PTEN as markers for predicting response to PI-3-kinase inhibition. This information may have considerable significance in tumor types, such as ovarian, endometrial, and colon where mutations in PIK3CA or PTEN and Ras have been found to coexist at relevant rates (45, 46).
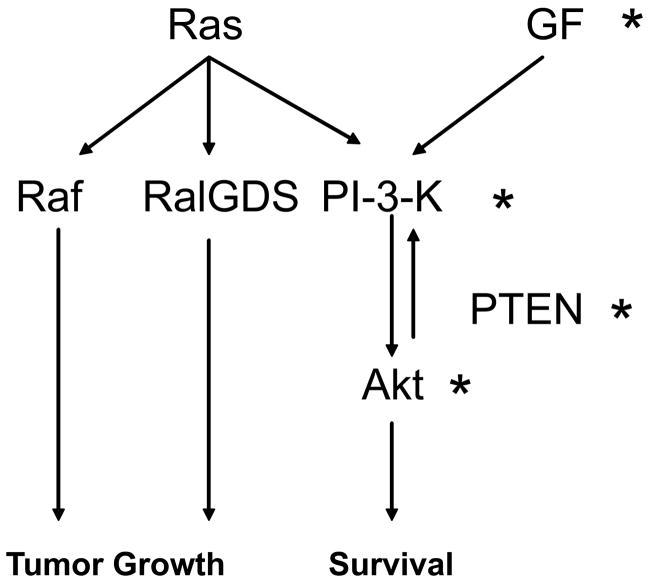
Figure 5. Signaling in resistant and sensitive lines.
Diagram showing the interactions of the cell signaling studied in sensitive and resistant lines. Signaling in PX-866 sensitive tumors comes from an increased reliance on the PI-3-kinase pathway, arising from aberrant activation through growth factors (GF) or mutated components of the pathway itself (*). Tumors with an activated Ras protein show a minimal response to inhibition of the PI-3-kinase pathway due to a shared reliance on alternate signaling pathways including the Raf and RalGDS pathways.
In summary, we have studied the activity of the PI-3-kinase inhibitor PX-866 in a panel of tumor cell line-derived xenografts and shown mutant oncogenic Ras to be a negative predictor of response to PX-866, both independently and in the presence of positive prognostic indictors such as PI3lCA and loss of PTEN activity. Known effects of Ras-induced transformation such as increased cyclin B and c-Myc were also negatively associated with antitumor response to PX-866 The level of activation of PI-3-kinase signaling measured with phospho-Akt was not sufficient to predict in vivo antitumor response to PX-866. Ras constructs modified to activate specific components of the Ras signaling pathway showed that multiple pathways are utilized for growth and survival both in vitro and in vivo in Ras dependent signaling.
Acknowledgments
Supported by NIH grants CA52995, CA090821, CA17094, CA95060 (GP) and CA99031 (GBM).
References
- 1.Cantley LC. The phosphoinositide 3-kinase pathway. Science (New York, NY. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 3.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science (New York, NY. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 4.Meier R, Alessi DR, Cron P, Andjelkovic M, Hemmings BA. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bbeta. The Journal of biological chemistry. 1997;272:30491–7. doi: 10.1074/jbc.272.48.30491. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cellular signalling. 2002;14:381–95. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 6.Moore SM, Rintoul RC, Walker TR, Chilvers ER, Haslett C, Sethi T. The presence of a constitutively active phosphoinositide 3-kinase in small cell lung cancer cells mediates anchorage-independent proliferation via a protein kinase B and p70s6k-dependent pathway. Cancer research. 1998;58:5239–47. [PubMed] [Google Scholar]
- 7.Shayesteh L, Lu Y, Kuo WL, et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 8.Soltoff SP, Rabin SL, Cantley LC, Kaplan DR. Nerve growth factor promotes the activation of phosphatidylinositol 3-kinase and its association with the trk tyrosine kinase. The Journal of biological chemistry. 1992;267:17472–7. [PubMed] [Google Scholar]
- 9.Campbell PM, Groehler AL, Lee KM, Ouellette MM, Khazak V, Der CJ. K-Ras promotes growth transformation and invasion of immortalized human pancreatic cells by Raf and phosphatidylinositol 3-kinase signaling. Cancer research. 2007;67:2098–106. doi: 10.1158/0008-5472.CAN-06-3752. [DOI] [PubMed] [Google Scholar]
- 10.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15587–91. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–44. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 13.Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene. 2005;24:7482–92. doi: 10.1038/sj.onc.1209088. [DOI] [PubMed] [Google Scholar]
- 14.Gharbi SI, Zvelebil MJ, Shuttleworth SJ, et al. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007;404:15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wipf P, Minion DJ, Halter RJ, et al. Synthesis and biological evaluation of synthetic viridins derived from C(20)-heteroalkylation of the steroidal PI-3-kinase inhibitor wortmannin. Org Biomol Chem. 2004;2:1911–20. doi: 10.1039/b405431h. [DOI] [PubMed] [Google Scholar]
- 17.Ihle NT, Paine-Murrieta G, Berggren MI, et al. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts. Molecular cancer therapeutics. 2005;4:1349–57. doi: 10.1158/1535-7163.MCT-05-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ihle NT, Williams R, Chow S, et al. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Molecular cancer therapeutics. 2004;3:763–72. [PubMed] [Google Scholar]
- 19.Mehrian-Shai R, Chen CD, Shi T, et al. Insulin growth factor-binding protein 2 is a candidate biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer. Proc Natl Acad Sci U S A. 2007;104:5563–8. doi: 10.1073/pnas.0609139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saal LH, Johansson P, Holm K, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7564–9. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akashi T, Nishimura Y, Wakatabe R, Shiwa M, Yamori T. Proteomics-based identification of biomarkers for predicting sensitivity to a PI3-kinase inhibitor in cancer. Biochem Biophys Res Commun. 2007;352:514–21. doi: 10.1016/j.bbrc.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 22.Shirasawa S, Furuse M, Yokoyama N, Sasazuki T. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science (New York, NY. 1993;260:85–8. doi: 10.1126/science.8465203. [DOI] [PubMed] [Google Scholar]
- 23.White MA, Nicolette C, Minden A, et al. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–41. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 24.Khosravi-Far R, White MA, Westwick JK, et al. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Molecular and cellular biology. 1996;16:3923–33. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamad NM, Elconin JH, Karnoub AE, et al. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes & development. 2002;16:2045–57. doi: 10.1101/gad.993902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim KH, Counter CM. Reduction in the requirement of oncogenic Ras signaling to activation of PI3K/AKT pathway during tumor maintenance. Cancer cell. 2005;8:381–92. doi: 10.1016/j.ccr.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Nishizuka S, Charboneau L, Young L, et al. Proteomic profiling of the NCI-60 cancer cell lines using new high-density reverse-phase lysate microarrays. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14229–34. doi: 10.1073/pnas.2331323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu J, He X, Baggerly KA, Coombes KR, Hennessy BT, Mills GB. Non-parametric quantification of protein lysate arrays. Bioinformatics. 2007;23:1986–94. doi: 10.1093/bioinformatics/btm283. [DOI] [PubMed] [Google Scholar]
- 29.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer cell. 2004;6:91–9. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Santana C, Ortega E, Garcia-Carranca A. Oncogenic H-ras induces cyclin B1 expression in a p53-independent manner. Mutat Res. 2002;508:49–58. doi: 10.1016/s0027-5107(02)00172-0. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd AC, Paterson HF, Morris JD, Hall A, Marshall CJ. p21H-ras-induced morphological transformation and increases in c-myc expression are independent of functional protein kinase C. EMBO J. 1989;8:1099–104. doi: 10.1002/j.1460-2075.1989.tb03479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sears R, Leone G, DeGregori J, Nevins JR. Ras enhances Myc protein stability. Mol Cell. 1999;3:169–79. doi: 10.1016/s1097-2765(00)80308-1. [DOI] [PubMed] [Google Scholar]
- 33.Martin AP, Miller A, Emdad L, et al. Lapatinib resistance in HCT116 cells is mediated by elevated MCL-1 expression, decreased BAK activation, and not by ERBB receptor mutation. Mol Pharmacol. 2008 doi: 10.1124/mol.108.047365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hao Y, Wong R, Feig LA. RalGDS couples growth factor signaling to Akt activation. Molecular and cellular biology. 2008;28:2851–9. doi: 10.1128/MCB.01917-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dietel M. Predictive medicine: incipient reality or fata morgana? J Pathol. 2007;212:353–5. doi: 10.1002/path.2191. [DOI] [PubMed] [Google Scholar]
- 36.Chow JY, Dong H, Quach KT, Van Nguyen PN, Chen K, Carethers JM. TGF-beta mediates PTEN suppression and cell motility through calcium-dependent PKC-alpha activation in pancreatic cancer cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G899–905. doi: 10.1152/ajpgi.00411.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caron RW, Yacoub A, Li M, et al. Activated forms of H-RAS and K-RAS differentially regulate membrane association of PI3K, PDK-1, and AKT and the effect of therapeutic kinase inhibitors on cell survival. Molecular cancer therapeutics. 2005;4:257–70. [PubMed] [Google Scholar]
- 38.Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer cell. 2005;7:561–73. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Massarelli E, Varella-Garcia M, Tang X, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res. 2007;13:2890–6. doi: 10.1158/1078-0432.CCR-06-3043. [DOI] [PubMed] [Google Scholar]
- 40.Khambata-Ford S, Garrett CR, Meropol NJ, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–7. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 41.Qin B, Ariyama H, Baba E, et al. Activated Src and Ras induce gefitinib resistance by activation of signaling pathways downstream of epidermal growth factor receptor in human gallbladder adenocarcinoma cells. Cancer chemotherapy and pharmacology. 2006;58:577–84. doi: 10.1007/s00280-006-0219-4. [DOI] [PubMed] [Google Scholar]
- 42.Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–62. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armengol G, Rojo F, Castellvi J, et al. 4E-binding protein 1: a key molecular “funnel factor” in human cancer with clinical implications. Cancer research. 2007;67:7551–5. doi: 10.1158/0008-5472.CAN-07-0881. [DOI] [PubMed] [Google Scholar]
- 44.She QB, Solit DB, Ye Q, O’Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer cell. 2005;8:287–97. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas RK, Baker AC, Debiasi RM, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007;39:347–51. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 46.Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer research. 2005;65:10669–73. doi: 10.1158/0008-5472.CAN-05-2620. [DOI] [PubMed] [Google Scholar]