Abstract
Sensation-seeking is a personality characteristic that has been associated with drug abuse. Some evidence suggests that sensation-seekers might experience increased rewarding effects from drugs of abuse, possibly contributing to the association between sensation-seeking and risk for drug abuse. The present study examined the effects of three doses of alcohol (0.0 g/kg, 0.45 g/kg, and 0.65 g/kg) on inhibitory control, information processing, and subjective ratings in a group of high sensation-seekers and a group of low sensation-seekers (N = 20). Inhibitory control was measured by a cued go/no-go task and speed of information processing was assessed by the Rapid Information Processing (RIP) task. Alcohol impaired inhibitory control and information processing. Group differences were also observed. Compared with their low sensation-seeking counterparts, high sensation-seekers demonstrated increased sensitivity to the subjective rewarding effects of alcohol and a poorer degree of inhibitory control that was further impaired by alcohol. The findings highlight reward- and cognitive-based mechanisms by which sensation-seeking could operate to increase risk for alcohol abuse.
Keywords: Alcohol, Inhibitory Control, Sensation-Seeking, Information Processing
1. Introduction
Alcohol is well-known for its acute “disinhibiting” effects on behavior and its chronic use is associated with sustained states of under-controlled behavior often described as impulsivity (e.g., Bates et al., 2002; Fillmore, 2003, 2007; Jentsch and Taylor, 1999; Lyvers, 2000). Alcohol abuse disorders are considered by many investigators to represent a disinhibitory psychopathology (Cloninger, 1987; Finn et al., 1994, Sher and Trull, 1994; Widiger and Smith, 1994). A large body of research reports associations between problem drinking and impulsivity as expressed through personality attributes, externalizing disorders, and patterns of maladaptive behaviors (Flory et al., 2003; Iacono et al., 1999; Kirisci et al., 2004). Moreover, there is growing evidence that impulsivity might play an important causal role in problem drinking. Prospective studies have shown that impulsive characteristics often precede the onset of problem alcohol use. Longitudinal studies of children and adolescents have shown that impulsivity predicts early-onset drinking age and development of heavy drinking and alcohol-dependence in young adults (August et al., 2006; Ernst et al., 2006). There also is considerable evidence that externalizing disorders, such as ADHD, increase risk for alcohol and other drug use (Barkley, 2006; Flory et al., 2003; Molina et al., 1999).
Such evidence raises the possibility that certain personality characteristics could confer increased risk for the display of disinhibited behavior under alcohol, and possibly explain some of the association between traits and problem drinking behaviors. One such personality trait is sensation-seeking. Sensation-seeking bares close similarity with impulsivity (Zuckerman et al., 1993; Aluja et al., 2002) and has been of considerable interest to researchers in recent years because of its association with drug abuse (Ball, 2004; Hawkins et al., 1992). Sensation-seeking is considered to be a neurobiologically-based tendency to seek novel, complex, intense sensations and a proclivity to take physical, social, legal, and financial risks in order to achieve such experiences (Zuckerman, 1994). The construct is multifaceted with four major characteristics: disinhibition, thrill and adventure seeking, experience seeking, and boredom susceptibility (Zuckerman, 1994). A variety of self-report instruments have been developed to assess the facets of sensation-seeking, such as the Sensation-Seeking Scale Form V (SSS-V), the Tridimensional Personality Questionnaire, and the Multidimensional Personality Questionnaire-Brief Form (Cloninger, 1987; Patrick et al., 2002; Zuckerman et al., 1978). These self-report instruments are commonly used to assess sensation-seeking in relation to risk for substance abuse (e.g., Hawkins et al., 1992; White et al., 2005).
With respect to alcohol abuse, there is a large body of evidence that points to sensation-seeking as a risk factor for alcohol-related problems. For example, recent research shows that early-onset alcoholics tend to report greater sensation-seeking than late-onset alcoholics, with levels of sensation-seeking predicting overall severity of the alcohol abuse disorder (Dom et al., 2006). Moreover, a recent meta-analysis of 61 studies that examined relations between sensation-seeking and alcohol use concluded that sensation-seeking was associated with increased alcohol use and alcohol-related problems (Hittner and Swickert, 2006).
One explanation for greater substance abuse risk among sensation-seekers could be that these individuals are more responsive to the rewarding effects of many abused drugs, including alcohol. Several studies have examined this hypothesis with respect to the effects of psychostimulant drugs, such as nicotine and d-amphetamine (e.g., Hutchison et al., 1999; Perkins et al., 2000; Stoops et al., 2007). In these studies, individual differences in response to the administration of these drugs were examined in relation to individual differences in sensation-seeking as assessed by self-report instruments. In general, these studies showed that high sensation-seekers report more rewarding effects from psychostimulants compared with low sensation-seekers. A recent study by our group also found that high sensation-seekers reported more subjective rewarding effects in response to the administration of d-amphetamine (Kelly et al., 2006). In that study, subjects with no stimulant or other drug abuse history completed the Impulsive Sensation-Seeking Scale (Impulsive SSS) of the Zuckerman-Kuhlman Personality Questionnaire (ZKPQ) (Zuckerman et al., 1993). Those scoring in the upper and bottom norm-based quartiles were classified as high and low sensation-seekers, respectively. Performance measures and visual analogue measures of subjective rewarding effects (e.g., “feel the drug”, “like the drug”, “feel high”) were then assessed following three oral doses of d-amphetamine: 0 mg, 7.5 mg, and 15 mg/per 70 kg body weight. Results showed that, compared with low sensation-seekers, high sensation-seekers reported greater subjective rewarding effects in response to the active doses. Finally, some preclinical studies parallel the findings with humans. For example, research shows that novelty-seeking in laboratory animals (e.g., rats) is associated with increased sensitivity to stimulant drugs (e.g., Bardo et al., 1996)
Taken together, these studies provide some intriguing evidence to suggest that sensation-seekers might experience increased rewarding effects from drugs of abuse, possibly contributing to the association between sensation-seeking and risk for drug abuse. In contrast to studies of psychostimulant drugs, less research has examined the role of sensation-seeking in response to alcohol. However, there is some evidence that high sensation-seekers also might display increased reactions to alcohol. Ray et al. (2006) reported that, compared with low sensation-seekers, high sensation-seekers reported more subjective “vigor” and less sedation in response to an acute dose of alcohol. In general, subjective stimulant-like effects of alcohol are considered to reflect the rewarding properties of the drug (e.g., Corbin et al., 2008; Fillmore, 2001), and are typically more pronounced during the ascending limb of the blood alcohol curve (Martin et al., 1993). As such, the findings by Ray et al. (2006) could indicate some heightened sensitivity to the rewarding effects of alcohol among high sensation-seekers. However, given the lack of research in this area it is difficult to draw any definitive conclusions about sensation-seeking and alcohol sensitivity.
In addition to questions about subjective effects, little is known about potential differences between high and low sensation-seekers in their actual behavioral response to alcohol. This is somewhat surprising given that alcohol is well known for its disinhibiting effects on behavior which is a characteristic of sensation-seeking and impulsivity. During the past decade, laboratory studies have provided considerable support for the notion that alcohol can actually promote impulsive actions by impairing basic inhibitory mechanisms which normally serve to suppress inappropriate behavior. Stop-signal and cued go/no-go tasks are reaction time tasks used to model behavioral control as the ability to quickly activate a response to a go-signal and suddenly inhibit a response when a stop-signal occurs (Logan, 1994; Logan and Cowan, 1984; Miller et al., 1991). Studies using these tasks have found that alcohol impairs the ability to inhibit behavior (e.g., de Wit et al., 2000; Fillmore and Vogel-Sprott, 2000; Marczinski and Fillmore, 2003; Mulvihill et al., 1997). The frequency of failures to inhibit responses to stop signals increases in drinkers following moderate doses of alcohol (e.g., 0.65 g/kg). The findings are important because they identify a basic inhibitory mechanism that is impaired by alcohol which could contribute to the display of impulsive, aggressive, and other socially inappropriate behaviors under the drug (Fillmore, 2003, 2007; Jentsch and Taylor, 1999).
Given the link between sensation-seeking, impulsivity, and alcohol abuse, it is important to test the possibility that sensation-seekers might be more sensitive to the acute disinhibiting effects of alcohol. The present study used the cued go/no-go task to examine the degree to which three doses of alcohol (0.0 g/kg, 0.45 g/kg, and 0.65 g/kg) impaired inhibitory control of behavior in a group of young adult social drinkers classified as high sensation-seekers and a demographically-similar group classified as low sensation-seekers. The study also included self-report ratings of rewarding effects from alcohol in order to test the possibility that high sensation-seekers experience greater subjective reward in response to the drug. The impairing effect of alcohol was also examined on a more general indicator of information processing (i.e., speed of information processing). Speed of information processing was measured using the Rapid Information Processing (RIP) task. The task is widely used and is a reliable model of information processing speed (Battig and Buzzi, 1986; Baldinger et al., 1995; Hasenfratz and Battig, 1994) that is highly sensitive to the impairing (i.e., slowing) effects of alcohol (Fillmore et al., 1998; Fillmore and Vogel-Sprott, 1997).
Finally, the study also compared the intensity of alcohol effects during each limb of the blood alcohol curve (ascending vs. descending). During the ascending limb of the blood alcohol curve the effects of alcohol tend to be more intense and are characterized by greater stimulant-like effects (Fillmore et al., 2005; Martin et al., 1993). Accordingly, it is possible that any heightened sensitivity to alcohol effects among high sensation-seekers might be especially evident during the ascending limb of the curve.
2. Method
2.1 Participants
Volunteers were recruited via notices posted on community bulletin boards and by word of mouth. All potential volunteers completed a brief telephone interview or an internet-based questionnaire addressing general medical and legal status. Volunteers had to have: a minimum of grade 8 education, demonstrated reading ability and no uncorrected vision or auditory problems. All respondents had to be in good health and report occasional alcohol use (e.g., consumed alcohol on at least one occasion per week, and consumed at least 3 drinks on one occasion within the past 30 days). Potential volunteers with histories of serious physical disease, current physical disease, impaired cardiovascular functioning, chronic obstructive pulmonary disease, seizure, head trauma, CNS tumors, or past histories of psychiatric disorder, (i.e., Axis I, DSM IV) were excluded from participation.
Potential volunteers also completed the Impulsive Sensation-Seeking scale of the Zuckerman-Kuhlman Personality Questionnaire (ZKPQ). They were selected for participation if their Impulsive Sensation-Seeking scale score classified them as either high sensation-seekers (HS) or low sensation-seekers (LS). Classifications were determined by comparing potential participants’ scores to a norm-based sample of scale scores from 2969 college students (Zuckerman, personal correspondence). To be classified as high sensation-seeking, potential volunteers had to have a scale score in the upper quartile of the distribution (score > 14 for males, and > 13 for females). To be classified as low sensation-seeking, they had to have a scale score in the lower quartile of the distribution (score < 7 for males, and < 6 for females). Our group has used these norms to classify high and low sensation-seekers in previous research (Kelly et al., 2006) and we have demonstrated criterion-related validity for this approach based on other sensation-seeking scales, such as the Zuckerman Sensation-Seeking Scale Form V (SSS-V) (Zuckerman et al., 1978).
For the present study, this classification scheme was used to identify ten high sensation-seeking (HS) subjects and ten low sensation-seeking (LS) subjects, who were invited for testing. Each sensation-seeking group was comprised of five men and five women. The age range of the sample was between 21 and 31 years (mean = 23.2, SD = 3.1). In terms of racial make-up, one volunteer identified himself as Asian and the rest identified themselves as Caucasian. All volunteers reported using alcohol and 18 volunteers reported using caffeine. Two volunteers reported tobacco use (one HS and one LS subject). These subjects reported smoking less than four cigarettes per day. Four subjects reported some marijuana use. All four subjects were in the HS group. The modal frequency of marijuana use was less than once per month. Finally, one subject also reported using codeine within one month of the initial screening. No other substance use was reported within one month prior to initial screening. The education level of the sample ranged from 12 to 16 years (mean = 15.2, SD = 1.0). The study was reviewed and approved by the University of Kentucky Medical Institutional Review Board, and all subjects provided written informed consent. Subjects earned $420 for participating in the study.
2.2 Apparatus and Materials
2.2.1 Cued Go/No-Go Task
Inhibitory control was measured by a cued go/no-go reaction time task used in other research to measure the disinhibiting effects of alcohol (e.g., Fillmore et al., 2005a; Marczinski and Fillmore, 2003). Cues provide preliminary information regarding the type of imperative target stimulus (i.e., go or no-go) that is likely to follow. The cues have a high probability of signaling the correct target. Inhibitory and activational tendencies show rapid development of cue-dependence as the cues come to elicit preparatory processes for the inhibition or execution of behavior (e.g., Posner, 1980; Miller et al., 1991). The go cue conditions are of particular interest. Go cues generate response prepotency which speeds response time to go targets. However, subjects must overcome this response prepotency in order to inhibit the response if a no-go target is subsequently displayed. Failures to inhibit responses to no-go targets are more frequent following go cues compared with no-go cues, indicating that it is more difficult to inhibit prepotent responses (Miller et al., 1991). Moreover, inhibitory control in this prepotent, go cue condition appears to be highly sensitive to the effects of alcohol and other psychoactive drugs (for a review see Fillmore, 2003).
The task was operated using E-Prime software (Schneider et al., 2002) and was performed on a PC. A trial involved the following sequence of events: a fixation point (+) for 800 ms; a blank, white screen for 500 ms; a cue (a white rectangle) displayed for one of five stimulus onset asynchronies (SOAs: 100, 200, 300, 400, and 500 ms); a go or no-go target (green or blue rectangle) that remained visible until the participant made a response or 1000 ms had elapsed; and an inter-trial interval of 700 ms.
The cue was presented either horizontally or vertically. Participants were instructed to press the forward slash (/) key on the keyboard if the cue turned green (i.e., go target) and to suppress the response if the cue turned blue (i.e., no-go target). Participants were encouraged to make fast responses and the computer displayed how quickly they responded to each go target by presenting their reaction time in milliseconds.
The orientation of the cue (horizontal or vertical) signaled the probability that a go or no-go target would be displayed. Cues presented horizontally preceded the go target on 80% of the trials and preceded the no-go target on 20% of the trials. Cues presented vertically preceded the no-go target on 80% of the trials and preceded the go target on 20% of the trials. Previous research has demonstrated that this level of cue validity produces prepotent responding (Abroms et al., 2003; Marczinski and Fillmore, 2003, 2005). The different SOAs between cues and targets encouraged participants in both conditions to pay attention to the cues, and the variability and randomness of the SOAs prevented the participants from anticipating the exact onset of the targets.
A test consisted of 250 trials that presented the four possible cue-target combinations. An equal number of vertical (125) and horizontal (125) cues were presented before an equal number of go (125) and no-go (125) target stimuli. Each cue-target combination was presented at each of the five SOAs, and an equal number of SOAs separated each cue-target combination. The presentation of cue-target combinations and SOAs was random. A test required approximately 15 minutes to complete.
2.2.2 Rapid Information Processing (RIP) Task
A self-paced, interactive task measured subjects’ information processing speed. The task was performed on a PC and operated by Micro Experimental Laboratory software (Schneider, 1995). A fixed pseudo random sequence of 250 digits consisting of the digits one to eight was presented on the display. Each digit was presented for 67 ms with an initial inter-stimulus-interval (ISI) of 600 ms. Subjects were required to press a key whenever they saw a digit that represented the third digit of a 3-digit sequence (a triad) that was comprised of even digits (e.g., 6,2,4) or of odd digits (e.g., 5,1,7). Thus the task required subjects to constantly and quickly update information in working memory in order to detect triads. The entire 250 digit sequence contained eleven even-digit triads and ten odd-digit triads. The initial presentation rate of a test was 90 digits per minute. Each correct response to a triad speeded the digit presentation rate by decreasing the ISI by 33 ms. A failure to respond to a triad or a response to a non-triad slowed the presentation rate by increasing the ISI by 33 ms. Thus the task measured rate of information processing by adjusting the presentation rate according to the subject’s ability to constantly encode and update information in working memory in order to detect triads. A test lasted five minutes during which the 250 digit sequence was presented in a repeated loop. The speed of the subject’s information processing during a test was measured by the average number of digits presented per minute on the test, with fewer digits per minute indicating slower information processing capacity.
2.2.3 Subjective Effects
A 4-item visual analogue scale (VAS) was used to measure subjective effects of alcohol. The items ask participants to rate the subjective effects of alcohol in terms of how much they: “feel the alcohol” (feel), “like the effects” (like), “feel high” (high), and “feel stimulated” (stimulated). Participants indicate their response to each item by placing a vertical mark through a 100 mm line, with the left-side (0 mm) indicating “not at all”, and the right-side (100 mm) indicating “very much”. The scale has been used in other research to demonstrate the subjective rewarding effects of alcohol (e.g., Chutuape et al., 1994; Fillmore, 2001).
2.2.4 Sensation-Seeking
To provide additional validation for the classification of high and low sensation-seekers according to the norms from ZKPQ, participants also completed the Zuckerman Sensation-Seeking Scale Form V (SSS-V) (Zuckerman et al., 1993; Zuckerman et al., 1978). The SSS-V consists of four subscales: Thrill and Adventure Seeking (sample item: “I would like to go scuba diving.”), Experience Seeking (sample item: “I like some of the earthy body smells.”), Disinhibition (sample item: “I feel best after taking a couple of drinks.”) and Boredom Susceptibility (sample item: “I can’t stand watching a movie that I’ve seen before.”). Each subscale consists of ten items. Each item contains two choices (statement A or statement B), one of which describes sensation-seeking behavior and one its opposite. Participants are told to choose the statement that best describes themselves.
2.3 Procedure
2.3.1 Intake Screen and Assessment
The study was conducted at the Residential Research Facility at the University of Kentucky. All volunteers were tested individually and were informed that the study examined the effects of alcohol on mood and behavior. During an initial screening/assessment session all potential volunteers provided informed consent and completed a comprehensive medical history questionnaire, as well as questionnaires about drug-use and demographics. Participants also completed the Zuckerman SSS Form V (Zuckerman et al., 1978). Vital signs were assessed and routine, clinical laboratory blood chemistry tests were conducted on all potential volunteers. All medical information was reviewed by a physician who determined eligibility for study participation. Volunteers were familiarized and practiced on the cued go/no-go and RIP tasks to ensure asymptotic and stable performance levels. Volunteers participated in one “practice” session to acclimate them to the entire testing procedure of the dose administration sessions. This session was identical to the dose administration sessions, however, no alcohol was given during the practice session.
2.3.2 Dose Administration Sessions
Performance was tested in response to three alcohol doses: 0 g/kg, 0.45 g/kg, and 0.65 g/kg. Doses were administered in random order, under double-blind conditions. Each dose was administered twice, on two separate sessions. The replication of each dose administration tested the reproducibility of the dose-response effects observed. Each of the six dose administration sessions required 6 hours to complete and was conducted on a different day, with a minimum inter-session interval of 48 hours and a maximum interval of one week. Subjects were at the laboratory only for scheduled sessions and returned home after each session was completed. On average, subjects completed approximately two sessions per week.
2.3.2.1 Pre-Session Preliminary Checks
Volunteers were required to fast and abstain from caffeinated beverages for four hours prior to each session. At the beginning of each session, volunteers completed a pre-session questionnaire that collected information about tobacco and alcohol consumption, hours slept during the past 24 hours, recent medication use, and illness since the previous session. Subjects performed a standard field sobriety test of motor coordination and provided a breath sample using a breath-analyzer to verify a zero blood alcohol concentration (BAC) (Alco-Sensor III, Intoximeters, Inc., St Louis, MO). Carbon-monoxide level from a breath sample (piCO Smokerlyzer Breath CO Monitor, Bedfont Scientific Ltd, UK) was also measured to verify smoker status. A urine sample was obtained to test for the presence of cocaine/benzoylecgonine, benzodiazepines, barbiturates, tetrahydrocannabinol (THC), d-amphetamine, and opiates (On Trak TesTstiks, Roche Diagnostics Corporation, Indianapolis, IN). Two subjects who tested positive were informed of the result and had their session rescheduled. No other volunteers tested positive for any drug during this study. No smoking was allowed during the sessions. Females were also tested for pregnancy via urine analysis.
2.3.2.2 Baseline Testing and Dose Administration
Testing began in the late morning with a baseline (i.e., pre-administration) test battery that included tests on the cued go/no-go task, the RIP task and completion of the VAS. The test battery required approximately 30 minutes to complete. Immediately after baseline testing, participants received one of the three alcohol doses: 0 g/kg, 0.45 g/kg, or 0.65 g/kg. Doses were calculated based on body weight and were administered as absolute alcohol divided equally into two drinks, each containing one part alcohol and three parts carbonated mix. Placebo doses consisted of four parts carbonated mix with 3 ml of alcohol floated on the surface. Participants had 6 minutes to consume the drinks. The 0.65 g/kg dose produces an average peak BAC of 80 mg/100 ml at approximately 60 minutes following the beverage administration and begins to decline at about 70 minutes post-administration. The 0.45 g/kg dose yields an average peak BAC of 50 mg/100 ml approximately 40 minutes post-administration and begins to decline at about 50 minutes post-administration.
2.3.2.3 Post-administration testing
After dose administration, performance on the tasks and VAS measures was assessed twice. The first test was timed to coincide with the ascending limb of the BAC curve and occurred 30 minutes post-administration. The timing of the second test coincided with the descending phase of the curve. This test occurred 75 minutes post-administration for the 0.45 g/kg dose and 90 minutes post-administration for the 0.65 g/kg dose. The testing schedule in the placebo condition was identical to the 0.45 g/kg dose condition. BAC was measured just prior to and following the completion of each test battery. This occurred at 30, 60, 75, and 105 minutes post-administration for the 0.45 g/kg dose and 30, 60, 90, and 120 minutes post-administration for the 0.65 g/kg dose. Subjects relaxed and read magazines or watched television during the periods between testing. No volunteers experienced any adverse reaction to the drug or any procedure.
2.4 Criterion Measures and Data Analyses
2.4.1 Cued go/no-go Performance
The cued go/no-go task measured failures to inhibit responses to no-go targets (failures of inhibition) and speed of responding to go targets (response activation). Failure of response inhibition was measured as the proportion (p) of no-go targets in the pre-potent, go cue condition which a subject failed to inhibit a response during a test. Higher p-inhibition failure scores indicated poorer inhibitory control (i.e., increased disinhibition). Response activation was measured by the reaction time (RT) to go targets in the go cue condition. A mean RT score for a subject was calculated for a test. Individual trial RTs that were less than 100 ms or greater than 1000 ms were excluded. Longer mean RTs indicate slower response activation on a test. Omission errors were also recorded. These errors occurred when subjects failed to respond to go targets. These errors were infrequent, occurring on less than 2% of trials and precluded statistical analyses.
2.4.2 RIP Task Performance
Information processing speed was measured by the participant’s mean digit processing rate during a test. The measure was expressed as the average number of digits processed per minute. Slower mean processing rates indicate less efficient information processing.
2.4.3 Data Analyses
Group differences in sample characteristics were examined by t tests and by multivariate analysis of variance (MANOVA). The treatment effects on performance and VAS measures were each initially analyzed by a 2 Group (HS vs. LS) × 3 Dose (0.0 g/kg, 0.45 g/kg, 0.65 g/kg) × 3 Test (baseline, first post-administration test, second post-administration test) × 2 Replication (first vs. second dose administration) mixed-design analysis of variance (ANOVA). With the exception of the VAS rating of “like”, there were no main effects or interactions involving the Replication factor for any other measure. In the absence of any main effects or interactions involving the Replication factor, a subject’s score on a measure for each time period under a dose was averaged across the two replications. The Results Section reports 2 (Group) × 3 (Dose) × 3 (Test) ANOVAs of the averaged scores and all tables and figures report these averaged scores. Simple effects comparisons of dose effects were examined by Bonferroni-adjusted t tests that maintained a family-wise error rate of .05.
3. Results
3.1 Sample Characteristics
No significant difference between HS and LS groups was obtained with respect to age or education level (ps > .295). HS subjects reported significantly higher levels of weekly alcohol use compared with LS subjects, t(18) = 2.2, p = .043. The average typical weekly consumption in terms of standard alcoholic drinks for HS and LS groups was 11.9 (SD = 6.8) and 5.8 (SD = 5.6), respectively.
To check the validity of the group classification based on the ZKPQ norms, group differences in the four subscales of the SSS-V were examined by MANOVA. There was a significant multivariate difference between the groups, Hotelling’s T2 (4,15) = 7.1, p = .002, and univariate F tests showed that the HS group had significantly higher scale scores for the Thrill and Adventure Seeking scale, F(1,18) = 27.1, p < .001, and the Experience Seeking scale, F(1,18) = 10.0, p = .005. The HS group also reported a significantly higher composite (i.e., total) score compared with the LS group, t(18) = 4.5, p < .001. Group means for the four subscales and total scale are presented in Table 1. Although group differences for the Disinhibition and Boredom Susceptibility scales did not attain statistical significance, the table shows that the HS group had higher scores on both of these scales as well.
Table 1.
SSS-V Scores for High and Low Sensation-Seekers
| Group |
Contrasts |
||||
|---|---|---|---|---|---|
| HS |
LS |
||||
| M | SD | M | SD | ||
| Thrill and Adventure Seeking | 9.4 | 0.84 | 4.4 | 2.9 | Sig** |
| Experience Seeking | 6.9 | 2.6 | 3.5 | 2.2 | Sig** |
| Boredom Susceptibility | 3.3 | 2.3 | 2.3 | 1.6 | ns. |
| Disinhibition | 5.4 | 2.5 | 3.6 | 2.6 | ns. |
| Sensation-Seeking Composite Score | 25.0 | 5.3 | 13.8 | 5.7 | Sig** |
Note. The numerical values presented are the means and standard deviations of SSS-V scores for high and low sensation-seekers. All group contrasts were tested by univariate F tests.
Sig indicates a significance value of p < .05,
Sig indicates a significance value of p < .01, and ns. indicates no significant difference.
3.2 Blood Alcohol Concentrations
There was no detectable BAC in the placebo condition. BAC in the active dose conditions was analyzed by a 2 Group (HS vs. LS) × 2 Dose (0.45 g/kg, 0.65 g/kg) × 4 Time mixed-design analysis of variance (ANOVA). The ANOVA revealed a significant main effect of Dose, F(1, 18) = 252.7, p < .01, and Time, F(3, 54) = 43.0, p < .01. BACs were similar in each group and no significant main effects or interactions involving group were obtained (ps > .12). The mean BACs based on the entire sample showed that subjects achieved higher BACs following the 0.65 g/kg dose and BACs were lower during the second of the two tests under each dose (i.e., during the declining limb of the curve). The mean BACs following 0.45 g/kg alcohol during the first test (i.e., at 30 and 60 minutes) were 52.3 mg/100 ml (SD = 10.6) and 47.0 mg/100 ml (SD = 9.0), respectively. During the second test, at 75 and 105 minutes, mean BACs were 41.7 mg/100 ml (SD = 7.2) and 33.4 mg/100 ml (SD = 6.7), respectively. The mean BACs under 0.65 g/kg alcohol during the first test at 30 and 60 minutes were 72.9 mg/100 ml (SD = 16.9) and 73.4 mg/100 ml (SD = 13.3), respectively. During the second test, at 90 and 120 minutes, mean BACs were 63.5 mg/100 ml (SD = 10.4) and 55.0 mg/100 ml (SD = 10.0), respectively.
3.3 Cued go/no-go Performance
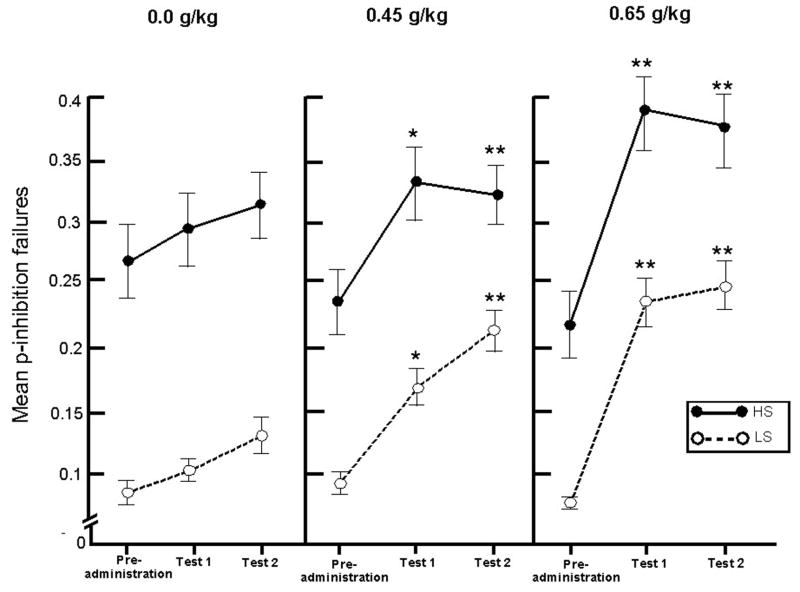
A 2 Group × 3 Dose × 3 Test (pre-administration, test 1, test 2) ANOVA of subjects’ p-inhibition failures revealed a significant main effect of Group, F(1, 18) = 5.4, p = .033, and a significant Dose × Test interaction, F(4, 72) = 10.0, p < .001. Subjects’ p-inhibition failures are presented for each group in Figure 1. The figure shows that HS subjects displayed more inhibition failures (i.e., more disinhibition) compared with LS subjects across all dose and test conditions. The figure also shows that inhibitory failures increased from pre-administration levels in response to both active doses and these dose effects appeared similar in both groups. Follow-up simple effects tests compared p-inhibition failures at pre-administration to test 1 and to test 2 in each dose condition and significant differences are shown in Figure 1. Both HS and LS groups displayed significant increases in inhibition failures in response to both 0.45 g/kg and 0.65 g/kg alcohol. The lack of a 3-way interaction involving Group indicates that the groups did not differ in terms of their sensitivity to the disinhibiting effect of alcohol. No significant changes were observed in response to placebo.
Figure 1.
Mean proportion of inhibition failures on the cued go/no-go task for both groups in response to three doses of alcohol (0.0 g/kg, 0.45 g/kg, 0.65 g/kg). Means are presented at baseline (i.e., pre-administration) and two times following dose administration (i.e., test 1 and test 2). Means represent the average of two separate dose replications. Capped vertical lines show the standard error of the mean. Asterisks indicate significant differences from baseline. * p < .05, ** p < .01.
Subjects’ RTs to the go target were examined by a 2 Group × 3 Dose × 3 Test ANOVA that obtained a significant Dose × Test interaction, F(4, 72) = 4.9, p = .002. No main effect of Group was observed (p = .478). Table 2 reports the mean RT scores of the entire sample for each time period under each dose. Although there appears to be some slowing effects on RT following administration of active doses, simple effects tests revealed no significant slowing of RT compared with pre-administration in response to either active dose (ps > .05)
Table 2.
Average Reaction Time and Processing Rate for High and Low Sensation-Seekers
| Dose |
||||||
|---|---|---|---|---|---|---|
| 0.0 g/kg | 0.45 g/kg | 0.65 g/kg | ||||
| M | SEM | M | SEM | M | SEM | |
| Reaction Time (Cued Go/No-Go Task) | ||||||
| Baseline | 275.9 | 6.2 | 274.2 | 5.3 | 276.5 | 5.3 |
| Test 1 | 273.9 | 6.3 | 278.7 | 7.6 | 274.0 | 6.4 |
| Test 2 | 267.6 | 5.1 | 279.8 | 6.9 | 282.3 | 6.4 |
| Processing Rate (RIP Task) | ||||||
| Baseline | 106.0 | 4.6 | 105.8 | 3.8 | 105.7 | 4.3 |
| Test 1 | 107.7 | 4.7 | 99.4 | 4.4 | 97.1 | 4.7 |
| Test 2 | 103.0 | 4.5 | 101.1 | 5.3 | 98.2 | 4.5 |
Note. The numerical values presented are the means and standard error of means for reaction time on the cued go/no-go task and rate of information processing on the RIP task for high and low sensation-seekers.
3.4 RIP Task Performance
A 2 Group × 3 Dose × 3 Test ANOVA of subjects’ processing rates revealed a significant Dose × Test interaction, F(4, 72) = 4.4, p = .003. No main effect of Group was observed (p = .859). Table 2 reports the mean processing rate of the entire sample for each time period under each dose. The table shows that processing rates were slowed (i.e., impaired) in response to the active doses. Simple effects analyses showed that 0.65 g/kg alcohol significantly slowed processing rates at test 1 compared with pre-administration (p < .05).
3.5 Subjective Effects
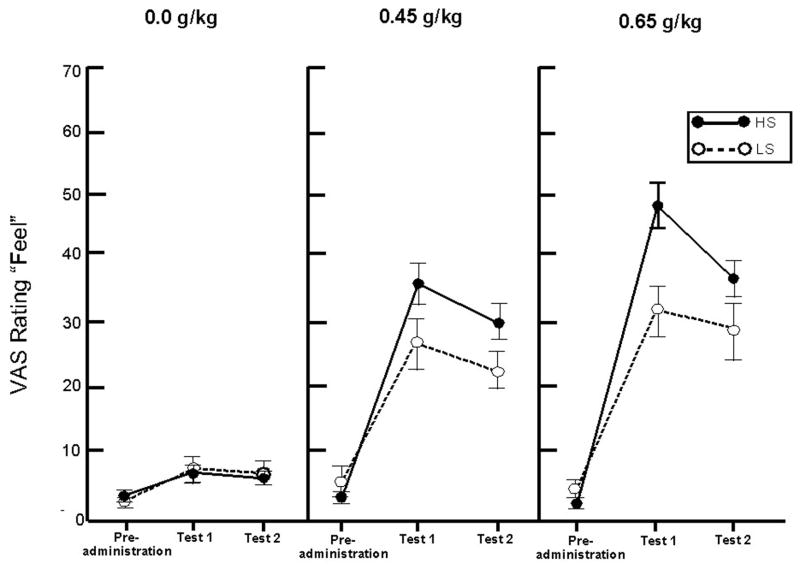
Ratings of the four VAS measures were analyzed by separate ANOVAs. Ratings of feel revealed a significant Group × Dose × Test interaction, F(4,72) = 2.4, p = .05. Figure 2 shows that ratings generally increased as a function of dose and were maximal during test 1 (i.e., during the ascending limb). The figure also shows that the 3-way interaction is due to group differences in response to alcohol. Ratings were comparable following placebo, but differed in response to the active doses whereby the HS group reported greater subjective effects during each post-administration test. These observations were confirmed by simple effect group comparisons of the increase in feel rating from the first test under placebo to the first test under each active dose. The HS reported a significantly greater increase compared with LS subjects following 0.65 g/kg alcohol (p = .038). No significant group difference was observed following 0.45 g/kg alcohol (p = .150).
Figure 2.
Mean VAS rating of “feel” for both groups in response to three doses of alcohol (0.0 g/kg, 0.45 g/kg, 0.65 g/kg). Means are presented at baseline (i.e., pre-administration) and two times following dose administration (i.e., test 1 and test 2). Means represent the average of two separate dose replications. Capped vertical lines show the standard error of the mean.
For the rating of like, the initial 4-way ANOVA showed interactions of the Replication factor with Test and Dose factors (ps < .015). Figure 3 plots the groups’ ratings under each dose separately by the Replication factor. Ratings of like generally increased as a function of dose and also were maximal at 30 minutes post-administration (i.e., during the ascending limb). Similar to feel ratings, ratings of like also showed group differences in the response to alcohol. Ratings were comparable following placebo, but differed in response to the active doses whereby the HS group reported greater subjective effects during each post-administration test. This observation was confirmed by a significant Group × Dose × Test interaction, F(4,72) = 3.2, p = .018. Simple effect group comparisons of the increase in like rating from the first test under placebo to the first test under each active dose showed a significantly greater increase for the HS group compared with LS subjects following both 0.65 g/kg (p = .029) and 0.45 g/kg alcohol (p = .035). With regard to the Replication factor, the figure shows that responses to the active doses were consistent across the Replication factor and that the interactions involving Replication appear due to differences in ratings between the first and second administration of the placebo.
Figure 3.
Mean VAS rating of “like” for both groups in response to three doses of alcohol (0.0 g/kg, 0.45 g/kg, 0.65 g/kg). Means are presented at baseline (i.e., pre-administration) and two times following dose administration (i.e., test 1 and test 2). The left panel illustrates the first administration of each dose and the right panel illustrates the second administration of each dose. Capped vertical lines show the standard error of the mean.
Finally, ratings of stimulated and high both showed significant Dose × Test interactions (ps < .001), but no main effects or interactions involving the Group factor were observed (ps > .321). Ratings were similar for both groups; generally increasing as a function of dose and peaking during test 1.
4. Discussion
This study examined the acute behavioral and subjective rewarding effects of alcohol in groups of adults who were classified as high and low sensation-seekers. In terms of behavioral effects, the study showed that alcohol impaired inhibitory control as evident by increased inhibition failures in the cued go/no-go task following the two active doses. Although the two groups did not differ in the intensity of alcohol-induced impairment, high sensation-seekers demonstrated considerably poorer levels inhibitory control and this difference was evident in all test and dose conditions, including the pre-administration tests and the placebo condition (i.e., drug-free). In terms of subjective effects, VAS ratings increased in response to the active doses. Moreover, group differences were evident in these dose effects, with high sensation-seekers reporting more pronounced increases in ratings of feeling and liking the effects. Alcohol also slowed subjects’ information processing as measured by the RIP task. Subjects’ processing rates slowed in response to the higher of the two active doses (0.65 g/kg). However, unlike inhibitory control, processing rate showed no difference between high and low sensation-seekers. In sum, compared with their low sensation-seeking counterparts, high sensation-seekers demonstrated increased sensitivity to the subjective rewarding effects of alcohol and a poorer degree of inhibitory control that was further impaired by alcohol. These findings are based on well-established assessments that provided highly reliable and stable measures over time. The evidence is further strengthened by the reproducibility of the effects. Each dose was administered twice in the experiment and no differences in dose response functions were observed between administrations for any measure.
Evidence that the high sensation-seekers experienced a greater subjective rewarding effect from alcohol is important because it could help explain their increased risk for alcohol abuse. There is considerable empirical evidence from human and animal studies to support the notion that rewarding effects of drugs play a major role in drug abuse. Preclinical studies show that a priming dose of alcohol can reinstate alcohol consumption in recently detoxified laboratory animals (Katz and Higgins, 2003; Shaham et al., 2003). In humans, priming doses increase self-reports of the euphorigenic effects, such as “liking” and “wanting to drink more”, as well as behavioral measures of drug reinforcement, such as increased self-administration of alcohol and work output to acquire subsequent doses (Fillmore, 2001; Fillmore and Rush, 2001; de Wit, 1996; Ludwig et al., 1974). As such, a heightened sensitivity to the rewarding properties of alcohol in high sensation-seekers could operate to reinforce excessive alcohol intake, leading to a pattern of abusive drinking.
However, it is important to note that these subjective effect ratings, themselves, are not direct assessments of the reinforcing properties of a drug. More direct evidence would require actual behavioral assessments of the reinforcing effects of alcohol, such as measuring the drinkers’ level of operant responding for the drug following a preload, priming dose. In addition to providing only indirect assessment of rewarding effects, there are other limitations of subjective ratings of drug effects as well. For instance, group differences in subjective ratings could simply reflect a response style among sensation-seekers whereby they are willing to rate effects as being more intense regardless of their own actual experience.
Evidence that alcohol impairs the ability to inhibit prepotent responses on the cued go/no-go task is consistent with findings from other studies that have used this task in our laboratory (Fillmore et al., 2005; Marczinski and Fillmore, 2003). Participants’ reaction times are unlikely to account for the increased inhibition failures under alcohol. Faster reaction times allow less time to inhibit a response and thus could increase inhibition failures. However, a speed-accuracy trade-off cannot explain the increased p-inhibition failures in response to alcohol because reaction times were unaffected by the drug. Similarly, faster reaction times cannot explain the greater inhibitory failures associated with high sensation-seeking as both groups displayed similar reaction times on the go/no-task.
Evidence for alcohol-induced impairment of inhibitory control is important because poor inhibitory control is considered to contribute to the display of impulsivity. Moreover, the heightened level of disinhibition under alcohol in high sensation-seekers might also help explain the link between sensation-seeking and problem drinking at a more fundamental level of behavioral control. Although there is little dispute that reward mechanisms play an important role in abuse potential, neurocognitive mechanisms have become another focus in recent years (Fillmore, 2003, 2007; Lyvers, 2000). Terminating a drinking episode requires inhibition of ongoing alcohol-administration behaviors and the reallocation of attention away from alcohol-related stimuli. Any impairment of normal inhibitory mechanisms resulting from an initial dose of alcohol could compromise the ability to stop additional alcohol administrations in a drinking situation, leading to excessive, binge drinking. Indeed, a recent laboratory study by our group shows that drinkers who are more disinhibited in response to an initial dose of alcohol also display greater consumption of the drug when given ad lib access (Weafer and Fillmore, in press). Thus acute alcohol-induced impairment of inhibitory processes could represent an important cognitive mechanism by which an initial alcohol dose promotes subsequent self-administration. As such, the heightened level of disinhibition that is characteristic of high sensation-seekers might represent an under-controlled behavioral state that could compromise their ability to stop additional alcohol administrations in a drinking situation, leading to binge drinking. Although speculative at this point, the account highlights the importance of understanding how cognitive processes, such as inhibitory control, are acutely affected by the administration of alcohol.
It is also interesting to note that the group differences observed in response to alcohol run counter to what would be predicted based on basic principles of pharmacological tolerance to alcohol. High sensation-seekers reported higher levels of weekly alcohol consumption than did low sensation-seekers. However, despite the greater alcohol use among high sensation-seekers, these individuals were not more tolerant to the behaviorally-impairing effects of alcohol than were low sensation-seekers, and actually displayed more sensitivity to the subjective effects of the drug. In a supplemental set of analyses, we examined the possibility that group differences in weekly alcohol consumption might have mediated the alcohol effects on performance and VAS measures. All ANOVA models were re-analyzed with weekly alcohol use as a covariate and the results of these ANCOVAs were consistent with the ANOVA models for all measures. Thus, weekly alcohol use did not appear to influence the responses to the drug.
The study also showed no evidence of “acute tolerance”. Reduced impairment at a given BAC on the descending limb is commonly referred to as acute tolerance, suggesting that the reduction might be due to some adaptive process occurring during physiological exposure to the drug over time. Impairment of subjects’ inhibitory control was evident on both the ascending limb (test 1) and the descending limb (test 2). In fact, no appreciable recovery of impaired inhibitory control was evident during test 2 in either group, despite lower average BACs during the declining limb. With respect to information processing speed, subjects’ processing rates also showed little recovery when tested during the declining limb of the BAC curve. However, in contrast to the behavioral effects, the intensity of the subjective effects of alcohol abated somewhat in both groups during the declining limb. Although some evidence shows that acute tolerance develops to alcohol-induced impairment of motor functions, a recent study found that alcohol-induced impairment of inhibitory control showed no evidence of acute tolerance during the descending limb of the BAC curve (Fillmore et al., 2005). Thus, the argument that impairment at a given BAC is diminished during the descending versus ascending limb curve might be an over-generalization given that acute tolerance might not develop uniformly across all aspects of behavioral functioning (Schweizer and Vogel-Sprott, 2008).
In conclusion, the present research highlights reward- and cognitive-based mechanisms by which sensation-seeking could operate to increase risk for alcohol abuse. The dearth of information concerning the link between alcohol effects on basic mechanisms of self-control and the drug’s potential for abuse represents a serious gap in our understanding of the transition from social drinking to abusive drinking. Given its relationship to binge drinking and its implication as a contributor to impulsivity, drinkers’ lack of inhibitory control under alcohol may prove to be an important dose challenge indicator of current or future alcohol-related problems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abroms BD, Fillmore MT, Marczinski CA. Alcohol-induced impairment of behavioral control: Effects on the alternation and suppression of prepotent responses. J Stud Alcohol. 2003;64:687–695. doi: 10.15288/jsa.2003.64.687. [DOI] [PubMed] [Google Scholar]
- Aluja A, Garcia O, Garcia LF. A comparative study of Zuckerman’s three structural models for personality through the NEO-PI-R, ZKPQ-III-R, EPQ-RS and Goldberg’s 50-bipolar adjectives. Pers Individ Dif. 2002;33:713–725. [Google Scholar]
- August GJ, Winters KC, Realmuto GM, Fahnhorst T, Botzet A, Lee S. Prospective study of adolescent drug use among community samples of ADHD and non-ADHD participants. J Am Acad Child and Adolesc Psychiatry. 2006;45:824–832. doi: 10.1097/01.chi.0000219831.16226.f8. [DOI] [PubMed] [Google Scholar]
- Baldinger B, Hasenfratz M, Battig K. Comparison of the effects of nicotine on a fixed rate and a subject-paced version of the rapid information processing task. Psychopharmacology. 1995;121:396–400. doi: 10.1007/BF02246080. [DOI] [PubMed] [Google Scholar]
- Ball SA. Personality traits, disorders, and substance abuse. In: Stelmack RM, editor. On the Psychobiology of Personality: Essays in Honor of Marvin Zuckerman. Elsevier; San Diego, CA: 2004. pp. 203–222. [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Barkley R. A handbook for diagnosis and treatment. 3. Guilford Press; New York: 2006. Attention-deficit hyperactivity disorder. [Google Scholar]
- Bates ME, Bowden SC, Barry D. Neurocognitive impairment associated with alcohol use disorders: Implications for treatment. Exp Clin Psychopharmacol. 2002;10:193–212. doi: 10.1037//1064-1297.10.3.193. [DOI] [PubMed] [Google Scholar]
- Battig K, Buzzi R. Effect of coffee on the speed of subject-paced information processing. Neuropsychobiology. 1986;16:126–130. doi: 10.1159/000118312. [DOI] [PubMed] [Google Scholar]
- Chutuape MD, Mitchell SH, de Wit H. Ethanol preloads increase preference under concurrent random-ratio schedules in social drinkers. Exp Clin Psychopharmacol. 1994;2:310–318. [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Corbin WR, Gearhardt A, Fromme K. Stimulant alcohol effects prime within session drinking behavior. Psychopharmacology. 2008;197:327–337. doi: 10.1007/s00213-007-1039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Exp Clin Psychopharmacol. 1996;4:5–10. [Google Scholar]
- de Wit H, Crean J, Richards JB. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neurosci. 2000;114:830–837. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- Dom G, Hulstijn W, Sabbe B. Differences in impulsivity and sensation seeking between early- and late-onset alcoholics. Addict Behav. 2006;31:298–308. doi: 10.1016/j.addbeh.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Ernst M, Luckenbach DA, Moolchan ET, Leff MK, Allen R, Eshel N, London ED, Kimes A. Behavioral predictors of substance-use initiation in adolescence with and without attention-deficit/hyperactivity disorder. Pediatrics. 2006;117:2030–2039. doi: 10.1542/peds.2005-0704. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Acute alcohol induced impairment of cognitive functions: Past and present findings. Int J Disabil Hum Dev. 2007;6:115–125. [Google Scholar]
- Fillmore MT. Drug abuse as a problem of impaired control: current approaches and findings. Behav Cogn Neurosci Rev. 2003;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Cognitive preoccupation with alcohol and binge drinking in college students: Alcohol-induced priming of the motivation to drink. Psychol Addict Behav. 2001;15:325–332. [PubMed] [Google Scholar]
- Fillmore MT, Carscadden J, Vogel-Sprott M. Alcohol, cognitive impairment, and expectancies. J Stud Alcohol. 1998;59:174–179. doi: 10.15288/jsa.1998.59.174. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. J Stud Alcohol. 2005;66:663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Alcohol effects on inhibitory and activational response strategies in the acquisition of alcohol and other reinforcers: Priming the motivation to drink. J Stud Alcohol. 2001;62:646–656. doi: 10.15288/jsa.2001.62.646. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Response inhibition under alcohol: Effects of cognitive and motivational conflict. J Stud Alcohol. 2000;61:239–246. doi: 10.15288/jsa.2000.61.239. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Resistance to cognitive impairment under alcohol: The role of environmental consequences of cognitive performance. Exp Clin Psychopharmacol. 1997;5:251–255. doi: 10.1037//1064-1297.5.3.251. [DOI] [PubMed] [Google Scholar]
- Finn PR, Kessler DN, Hussong AM. Risk for alcoholism and classical conditioning to signals for punishment: Evidence for a weak behavioral inhibition system? J Abnorm Psychol. 1994;103:293–301. doi: 10.1037//0021-843x.103.2.293. [DOI] [PubMed] [Google Scholar]
- Flory K, Milich R, Lynam DR, Leukefeld C, Clayton R. Relation between childhood disruptive behavior and substance use and dependence symptoms in young adulthood: Individuals with symptoms of attention-deficit/hyperactivity disorder and conduct disorder are uniquely at risk. Psychol Addict Behav. 2003;17:151–158. doi: 10.1037/0893-164x.17.2.151. [DOI] [PubMed] [Google Scholar]
- Hasenfratz M, Battig K. Acute dose-effect relationships of caffeine and mental performance, EEG, cardiovascular and subjective parameters. Psychopharmacology. 1994;114:281–287. doi: 10.1007/BF02244850. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: Implications for substance abuse prevention. Psychol Bull. 1992;112:64–105. doi: 10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- Hittner JB, Swickert R. Sensation seeking and alcohol use: A meta-analytic review. Addict Behav. 2006;31:1383–1401. doi: 10.1016/j.addbeh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Wood MD, Swift R. Personality factors moderate subjective and psychophysiological responses to d-amphetamine in humans. Exp Clin Psychopharmacol. 1999;7:493–501. doi: 10.1037//1064-1297.7.4.493. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: Findings from the Minnesota Twin Family Study. Dev Psychopathol. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implication for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Robbins G, Martin CA, Fillmore MT, Lane SD, Harrington NG, Rush CR. Individual differences in drug abuse vulnerability: d-Amphetamine and sensation-seeking status. Psychopharmacology. 2006;189:17–25. doi: 10.1007/s00213-006-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisci L, Tarter RE, Vanyukov M, Reynolds M, Habeych M. Relation between cognitive distortion and neurobehavior disinhibition on the development of substance use during adolescence and substance use disorder by young adulthood: A prospective study. Drug Alcohol Depend. 2004;76:125–133. doi: 10.1016/j.drugalcdep.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: A user’s guide to the stop-signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory Processes in Attention, Memory, and Language. Academic Press; San Diego, CA: 1994. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Ludwig AM, Wikler A, Stark LH. The first drink: Psychobiological aspects of craving. Arch Gen Psychiatry. 1974;30:539–547. doi: 10.1001/archpsyc.1974.01760100093015. [DOI] [PubMed] [Google Scholar]
- Lyvers M. “Loss of control” in alcoholism and drug addiction: A neuroscientific interpretation. Exp Clin Psychopharmacol. 2000;8:225–249. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Alcohol increases reliance on cues that signal acts of control. Exp Clin Psychopharmacol. 2005;13:15–24. doi: 10.1037/1064-1297.13.1.15. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Preresponse cues reduce the impairing effects of alcohol on the execution and suppression of responses. Exp Clin Psychopharmacol. 2003;11:110–117. doi: 10.1037//1064-1297.11.1.110. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res. 1993;22:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Miller J, Schaffer R, Hackley SA. Effects of preliminary information in a go versus no-go task. Acta Psychologica. 1991;76:241–292. doi: 10.1016/0001-6918(91)90022-r. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Smith BH, Pelham WE. Interactive effects of attention deficit hyperactivity disorder and conduct disorder on early adolescent substance use. Psychol Addict Behav. 1999;13:348–358. [Google Scholar]
- Mulvihill LE, Skilling TA, Vogel-Sprott M. Alcohol and the ability to inhibit behavior in men and women. J Stud Alcohol. 1997;58:600–605. doi: 10.15288/jsa.1997.58.600. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychol Assess. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Grobe JE. Greater sensitivity to subjective effects of nicotine in nonsmokers high in sensation seeking. Exp Clin Psychopharmacol. 2000;8:462–471. doi: 10.1037//1064-1297.8.4.462. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Ray LA, McGeary J, Marshall E, Hutchison KE. Risk factors for alcohol misuse: Examining heart rate reactivity to alcohol, alcohol sensitivity, and personality constructs. Addict Behav. 2006;31:1959–1973. doi: 10.1016/j.addbeh.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime User’s Guide. Pittsburgh, PA: Psychology Software Tools; 2002. [Google Scholar]
- Schneider W. Volume 1: Perception. In: Bridgeman B, Prinz W, editors. Handbook of perception and action. Academic Press; San Diego, CA: 1995. pp. 103–141. [Google Scholar]
- Schweizer TA, Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: a review of acute tolerance and recovery of cognitive performance. Exp Clin Psychopharmacol. 2008;16:240–250. doi: 10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology. 2003;168(1–2):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: Alcoholism and antisocial personality disorder. J Abnorm Psychol. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Robbins CG, Martin CA, Rush CR, Kelly TH. The reinforcing, subject-rated, performance, and cardiovascular effects of d-amphetamine: Influence of sensation-seeking status. Addict Behav. 2007;32:1177–1188. doi: 10.1016/j.addbeh.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Individual differences in acute alcohol impairment of inhibitory control predict ad libitum alcohol consumption. Psychopharmacology. doi: 10.1007/s00213-008-1284-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Labouvie EW, Papadaratsakis V. Changes in substance use during the transition to adulthood: A comparison of college students and their noncollege age peers. J Drug Issues. 2005;35:281–306. [Google Scholar]
- Widiger TA, Smith GT. Substance use disorder: Abuse, dependence and dyscontrol. Addiction. 1994;89:267–282. doi: 10.1111/j.1360-0443.1994.tb00889.x. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. Cambridge University Press; New York: 1994. [Google Scholar]
- Zuckerman M, Eysenck S, Eysenck HJ. Sensation seeking in England and America: Cross-cultural, age, and sex comparisons. J Consult Clin Psychol. 1978;46:139–149. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM, Joireman J, Teta P, Kraft M. A comparison of three structural models for personality: The big three, the big five, and the alternative five. J Pers Soc Psychol. 1993;65:757–768. [Google Scholar]