Abstract
In this study, we investigated behavioral factors underlying conditioned place preference (CPP) induced by social interaction in adolescent rats. We found that the magnitude of socially-induced CPP depended on the social motivation of the animals and on the amount of training. After extinction, socially-induced CPP could be reinstated by a single reconditioning session. Treatment with methylphenidate, which disrupts social play behavior in adolescent rats, but not social exploratory behavior, prevented the development of socially-induced CPP. Interestingly, methylphenidate by itself induced CPP. These data demonstrate that: 1. social interaction is rewarding in adolescent rats; 2. appetitive and mnemonic factors influence the development of socially-induced CPP; 3. comparable to drug-induced CPP, socially-induced CPP can be extinguished and reinstated; 4. social play is likely to be the most rewarding aspect of social interaction in adolescent rats; 5. social context influences the subjective effects of methylphenidate.
INTRODUCTION
Natural rewards are linked with behaviors that are essential for survival of the individual, group, or species. Feeding, drinking, and sexual behavior are such pleasurable activities that can function as reinforcers. Alongside these generally known natural reinforcers, social interactions are also known to be pleasurable, and it is quite clear that social interactions serve to acquire and maintain important intra-species relationships. In most mammals, interactions with peers are particularly abundant during adolescence, and it is thought that peer-peer interactions during adolescence are of major importance for social and cognitive development (Fagen, 1981; Vanderschuren et al., 1997; Nelson et al., 2005). Early laboratory experiments have shown that social interactions can function as reinforcers in young primates (Falk, 1958; Mason et al., 1963), and later work demonstrated that the opportunity to engage in social play was a reinforcer for maze learning in adolescent rats (Humphreys and Einon, 1981; Normansell and Panksepp, 1990). Place conditioning, where an animal comes to approach previously neutral environmental cues after these have been repeatedly paired with a pleasurable drug or event, can be used to investigate the neural and psychological basis of reward (Schechter and Calcagnetti, 1993; Bardo and Bevins, 2000; Tzschentke, 1998; -2007). Indeed, social play behavior in adolescent rats has been shown to induce conditioned place preference (CPP; Calcagnetti and Schechter, 1992; Crowder and Hutto, 1992; Van den Berg et al., 1999; Douglas et al., 2004; Thiel et al., 2008; -2009).
Although social play behavior is known to induce CPP, the exact underlying behavioral mechanisms remain unclear. Therefore, the present study aimed to identify appetitive and mnemonic factors underlying socially-induced CPP in adolescent rats. In addition, we aimed to investigate whether it was social play behavior specifically, or social interaction in general, that caused socially-induced CPP in adolescent rats. Thus, the first three experiments investigated how social motivation influences the development of socially-induced CPP, by comparing groups of rats isolated for different periods before place conditioning, as it is known that the amount of social play behavior displayed by adolescent rats is a function of the duration of the preceding social isolation period (Niesink and Van Ree, 1989; Vanderschuren et al., 1995a; -2008). These experiments provide important information about how changes in social motivation influence socially-induced CPP. Also, by varying the number and duration of conditioning sessions, we aimed to understand whether the amount of training influences the development of CPP. Third, we tested whether socially-induced CPP was subject to extinction. In general, CPP extinguishes with repeated exposure to the conditioning environment without the primary pleasurable stimulus, but several studies have shown that CPP can be remarkably persistent (e.g. Mueller et al., 2002; Mueller and Stewart, 2000), perhaps demonstrating that the reward-associated cues had become attractive stimuli by themselves (see Di Ciano and Everitt, 2004). In recent years, extinction/reinstatement procedures have been successfully applied to place conditioning experiments, showing that expression of drug-induced CPP can be reinstated by a single drug re-exposure or by stress (for review see Tzschentke, 2007). Therefore, we tested whether a reconditioning session could reinstate extinguished socially-induced CPP.
In the second part of the study, we addressed the notion (Humphreys and Einon, 1981; Pellis and McKenna, 1995) that play is the most positive, rewarding aspect of the social repertoire of adolescent rats. To that aim, we treated the animals with methylphenidate to interfere with the expression of social play. We have recently shown that methylphenidate suppresses social play behavior in adolescent rats. Methylphenidate suppressed both play solicitation and responsiveness to play solicitation, without affecting social exploratory behavior. However, vehicle-treated animals still solicited play from methylphenidate-treated test partners (Vanderschuren et al., 2008). Thus, by treating either the test animal, the stimulus animal, or both with methylphenidate, we investigated the extent to which play solicitation, being solicited, social interaction with a non-playful partner and full expression of social play behavior contribute to the development of socially-induced CPP. Since social behaviors related and unrelated to play have different ontogenetic profiles and are also differently affected by drug treatments (Vanderschuren et al., 1997), understanding their relative contribution to the positive subjective properties of social interaction will help to understand the brain mechanisms involved in the regulation of social reward.
METHODS
Animals
Male Wistar rats (Charles River, Sulzfeld, Germany) arrived in our animal facility at 21 days of age and were housed in groups of three in 40 × 26 × 20 (l × w × h) Macrolon cages under controlled conditions (i.e. temperature 20–21 °C, 60–65% relative humidity and 12/12 h light cycle with lights on at 7.00 a.m.). Upon arrival, the animals were allowed at least five days of acclimatization to our facility and they were handled for 3 days before the start of the experiments. Food and water were available ad libitum. All animals were experimentally naive and were used only once, with the following exception: in experiment 2, 12-week-old Wistar rats that had previously been used undrugged in a behavioral experiment were used as housing partners for the experimental animals. All experiments were approved by the Animal Ethics Committee of Utrecht University and were conducted in agreement with Dutch laws (Wet op de Dierproeven, 1996) and European regulations (Guideline 86/609/EEC).
Apparatus
The place conditioning setup (TSE Systems, Bad Homburg, Germany) comprised 8 boxes, each consisting of three compartments with removable Plexiglas lids: two equally sized large conditioning compartments (30 × 25 × 30 cm; l × w × h) separated by a smaller, neutral compartment (10 × 25 × 30 cm; l × w × h). The two conditioning compartments had different visual and tactile cues: one had black-and-white striped walls and a floor with wide metal mesh, the other had black walls and a floor with fine metal mesh. The compartment with black walls had a white light (2 watt) mounted on the Plexiglas lid, to achieve a comparable light intensity in both conditioning compartments. The middle compartment had white walls, a smooth floor and a white light (2 watt) on the lid. Pilot studies performed in our laboratory have shown that the rats had no unconditioned preference for one of the compartments.
During conditioning, closed dividers between the compartments were used to confine the animals to the conditioning compartment. During habituation and testing, the closed dividers were replaced by dividers that contained an arched gateway (9 × 11.5 cm; w × h), allowing the rats access to all compartments. During habituation and testing, the position of the animal in the apparatus was monitored by an array of infrared photobeam sensors located 2.5 cm above the floor. A computer recorded the time (in sec) that the animals spent in each compartment. All experiments were performed in a sound attenuated and dimly lit room, where the eight place conditioning boxes were located. This test room was connected to an outer pre-test room, where the animals were housed throughout the experiment.
Drugs
Methylphenidate-HCl (BUFA, Castricum, The Netherlands) was dissolved in saline and administered s.c. in a dose of 1.0 mg/kg, 30 min before the conditioning session. Injection volume was 2 ml/kg.
EXPERIMENT 1. ROLE OF SOCIAL MOTIVATION AND AMOUNT OF TRAINING IN SOCIALLY-INDUCED CPP I
The aim of experiment 1 was twofold: 1. investigate whether preference for the social-paired compartment was influenced by changes in social motivation induced by different degrees of social isolation before conditioning; 2. investigate whether preference for the social-paired compartment was influenced by the amount of training by varying the number of conditioning sessions per day.
Procedure
At 24 days of age, rats were transported to the outer pre-test room, where they remained until the end of the experiment. The following day (day 1), each rat was placed into the CPP apparatus and allowed to move freely around the three compartments of the apparatus for 15 min. The number of seconds spent in each compartment was recorded to determine baseline side preference for each subject.
We used a counterbalanced place conditioning design (Tzschentke, 2007). Thus, on the basis of their baseline preference scores (i.e., time spent in each of the two conditioning compartments), the rats were assigned to a compartment in which they would be allowed social interaction during conditioning, so that the baseline preference in each test group for the (to be) social-paired and (to be) non-social paired compartments approximated 50%. Thus, some rats, based on the baseline preference, would be conditioned in their preferred compartment, but others would be conditioned on their nonpreferred compartment. This procedure rules out the possibility that preference shifts are the result of decreased avoidance of the non-preferred compartment.
After the baseline preference session, the rats were allocated to one of three different housing conditions during conditioning: 1. individually housed rats: these animals were placed in individual cages after the baseline preference session, and remained individually housed throughout the experiment; 2. short-term isolated rats: these rats were housed in groups of three and were isolated for 3.5 h before each conditioning and test session; 3. socially housed rats: these rats were housed in groups of three throughout the experiment.
Place conditioning began on day 2. To determine whether the development of socially-induced CPP varied with the number of conditioning sessions per day, rats in each housing group were divided into two sub-groups that received either one (Once/day group) or two (Twice/day group) conditioning sessions per day. The Once/day group underwent eight consecutive days of conditioning: on days 2, 4, 6 and 8 of the experiment, rats were placed for 15 min in one compartment with an initially unfamiliar partner that did not differ more than 15 g in body weight (social session). On days 3, 5, 7 and 9 they were placed alone in the other compartment for 15 min (non social session). The Twice/day group underwent eight consecutive days of conditioning, with two conditioning sessions per day: on days 2, 4, 6 and 8 of the experiment, rats were placed for 15 min in one compartment with an initially unfamiliar partner (social session) in the morning, and were placed alone in the other compartment (non social session) during the afternoon. On days 3, 5, 7 and 9, the order of sessions was reversed, i.e. rats were placed alone in one side of the CPP apparatus during the morning session, and were placed in the other compartment with the social partner in the afternoon session. Morning and afternoon sessions were separated by at least 2 h. This procedure prevented the rats to associate a certain form of conditioning session with a particular time of day. The partners used in the social sessions were housed in the same way as the respective experimental rats. On day 10, at 34 days of age, the rats were placed in the middle compartment and allowed to freely move throughout the apparatus for 15 min. Time spent in each compartment was recorded.
Statistical analysis
Data were analyzed with a mixed ANOVA, taking the interdependence of the time spent in each compartment into account, and using housing condition (three levels: individually housed rats, short-term isolated rats, socially housed rats) as between-subjects factor, and compartment as within-subjects factor (two levels: social compartment and non social compartment). Mixed ANOVA was followed by Tukey’s post hoc test performed on significant housing condition × compartment interaction.
Results
All groups failed to develop socially-induced CPP, regardless of whether they received one ([F(housing condition)2,29=2.65, n.s.; F(compartment)2,29=1.58, n.s.; F(housing condition × compartment)2,29=0.37, n.s.], figure 1A) or two conditioning sessions per day ([F(housing condition)2,29=1.13, n.s.; F(compartment)2,29=2.60, n.s.; F(housing condition × compartment)2,29=0.17, n.s.], figure 1B). In rats subjected to one conditioning session per day (figure 1A), the mean differences between the time spent in the social- versus the non social-paired compartment were 59.5 ± 26.2 sec (individually housed rats), 31.2 ± 41.2 sec (short-term isolated rats) and 4.6 ± 58.1 sec (socially housed rats). In rats subjected to two conditioning sessions per day (figure 1B), the mean differences between the time spent in the social- vs non social-paired compartment were 80.9 ± 47.3 sec (individually housed rats), 40.7 ± 58.9 sec (short-term isolated rats) and 37.3 ± 60.9 sec (socially housed rats). Thus, although these differences did not reach statistical significance, socially isolated rats seemed to spend more time in the environment paired with a social partner, compared to both the short-term isolated and socially housed rats. Moreover, long-term isolated rats conditioned twice per day showed the highest preference for the social-paired compartment.
Figure 1.
Effect of number of conditioning sessions/day on socially-induced CPP in individually housed, short-term isolated and socially housed rats. Rats received either one (Panel A) or two (Panel B) 15-min conditioning sessions/day, for eight days. Data represent mean ± SEM time spent in the social paired- (social, white bar) and non social-paired (non social, black bar) compartments on the test day. n=10–12 per group. The data in panels A and B are from two separate experiments.
EXPERIMENT 2. ROLE OF SOCIAL MOTIVATION AND AMOUNT OF TRAINING IN SOCIALLY-INDUCED CPP II
Experiment 2 aimed to determine whether using longer conditioning sessions would facilitate the development of socially-induced CPP in rats housed under different conditions.
Procedure
At 25 days of age, rats received a baseline preference test as described above (experiment 1, day 1). On the basis of their baseline preference scores (as in experiment 1), the rats were allocated to different housing conditions: 1. individually housed rats: these animals were placed in individual cages after the baseline preference session, and remained individually housed throughout the experiment; 2. short-term isolated rats: these rats were housed in groups of three and were isolated for 3.5 h before each conditioning and test session; 3. rats housed with an adult: these rats were housed with a 12-week-old partner, that we reasoned would show relatively low levels of play, given the ontogenetic profile of social play (Baenninger, 1967; Panksepp, 1981). The groups were conditioned similar to the Twice/day group from experiment 1, i.e. eight conditioning days, with two conditioning sessions (social and non social session) per day, but each conditioning session lasted 30 min instead of 15 min. Testing took place on day 10, when rats were 34 days old, as in experiment 1.
Statistical analysis
Data were analyzed with a mixed ANOVA, taking the interdependence of the time spent in each compartment into account, and using housing condition (three levels: individually housed rats, short-term isolated rats, rats housed with an adult) as between-subjects factor, and compartment as within-subjects factor (two levels: social compartment and non social compartment). Mixed ANOVA was followed by Tukey’s post hoc test performed on significant housing condition × compartment interaction.
Results
Mixed ANOVA for the time spent in the social/non social compartment on the test day gave the following results: [F(housing condition)2,33=1.09, n.s.; F(compartment)2,33=9.61, p<0.01; F(housing condition × compartment)2,33=3.99, p<0.01]. Post hoc analysis performed on the housing condition × compartment interaction showed that socially isolated rats displayed socially-induced CPP, since they spent significantly more time in the social-paired compartment on the test day. Briefly isolated rats tended to spend more in the social-paired compartment, while rats housed with a 12-week-old rat did not show preference or aversion for the social-paired compartment (figure 2).
Figure 2.
Socially-induced CPP in individually housed rats, short-term isolated rats and rats housed with a 12-week-old partner. Rats received two 30-min conditioning sessions/day, for eight days. Data represent mean ± SEM time spent in the social paired- (social, white bar) and non social-paired (non social, black bar) compartments on the test day. **p<0.01 for difference in time spent in the social- and non social-paired compartments on the test day (Tukey’s post hoc test, n=12 per group).
EXPERIMENT 3. EXTINCTION AND REINSTATEMENT OF SOCIALLY-INDUCED CPP
The aim of experiment 3 was threefold: 1. investigate the persistence of socially-induced CPP; 2. investigate whether extinguished socially-induced CPP could be reinstated by single reconditioning session; 3. determine the persistence of CPP after reinstatement.
Procedure
At 25 days of age, rats were socially isolated following the baseline preference session, conditioned during the following eight days, and tested for their preference on day 10, when they were 34 days old, as in experiment 2 (i.e. two 30 min conditioning sessions/day). On day 11, the rats were placed into the CPP apparatus with free access to all compartments for 15 min, and time spent in each compartment was recorded. This test was repeated daily until four sessions had passed in which the difference between the time spent in the social-paired and non-social-paired compartments was not statistically significant; this took seven sessions (including the first test for CPP on day 10, which is essentially an extinction session). Twenty-four hours after the last extinction session, the rats received one day of reconditioning. They were placed in the social-paired compartment with a social partner for 30 min (social session); two hours later, they were placed in the non social-paired compartment alone for 30 min (non social session). The day after, rats were placed in the apparatus with free access to all compartments, to evaluate whether extinguished socially-induced CPP had been reinstated. Testing was repeated daily until three sessions had passed in which the difference between the time spent in the social-paired and non-social-paired compartments was not statistically significant.
Unlike the usual procedure employed in extinction-reinstatement setups (Shaham et al., 2003; Tzschentke, 2007), we conducted a reconditioning session to reinstate CPP, rather than a non-contingent exposure to the unconditioned stimulus outside of the testing environment immediately prior to testing for reinstatement. Recent evidence on drug-induced reinstatement of drug seeking suggests that not only motivational effects of the reinstating stimulus, but also its interoceptive properties, play a major role in the expression of reinstatement (Keiflin et al., 2008). However, unlike drug stimuli, whose interoceptive effects are subject to the pharmacokinetic properties of the drug, it is not known whether the interoceptive effects of a social interaction will outlast the interaction itself. We therefore reasoned that subjecting the animals to social interaction outside of the CPP-apparatus immediately before a reinstatement test would actually enhance the expression of extinction, because this would comprise explicit unpairing of the CPP-apparatus (including its social-paired compartment) and social interaction.
Statistical analysis
The difference in time spent in the social- versus the non social-paired compartment was calculated within the same experimental group and the data were analyzed using Student’s t test.
Results
Figure 3 shows the mean (± SEM) time spent in the social- and non social-paired compartment during the first test day, during extinction and reinstatement of CPP. On the first test session, the rats showed socially-induced CPP, since they spent significantly more time in the social-paired compartment (t= 4.25, p<0.001). The following two sessions, rats still spent more time in the compartment previously associated with the social partner (extinction session 1: t= 4.17, p<0.001; extinction session 2: t= 2.86, p<0.01). From the third extinction session on, CPP was no longer apparent, with the difference in time spent in the social- versus the non social-paired compartment not being significant anymore (extinction session 3: t= 0.89, n.s.; extinction session 4: t= 1.27, n.s.; extinction session 5: t= 1.47, n.s.; extinction session 6: t= 0.82, n.s.). A single reconditioning day reinstated CPP (reinstatement session 1: t= 2.53, p<0.01). However, the duration of socially-induced CPP after reinstatement was short, since it was no longer present during the following sessions (reinstatement session 2: t= 0.07, n.s.; reinstatement session 3: t= 0.53, n.s.; reinstatement session 4: t= 0.1, n.s.; figure 3).
Figure 3.
Extinction and reinstatement of socially-induced CPP in individually housed rats. Data represent mean ± SEM time spent in the social paired- (social, white bar) and non social-paired (non social, black bar) compartments on the test day (TEST), during extinction (EX) and reinstatement (REIN) of the conditioned response. ***p<0.001, **p<0.01 for difference in time spent in the social- and non social-paired compartments (Student’s t test, n=12 per group).
EXPERIMENT 4. EFFECT OF METHYLPHENIDATE ON SOCIALLY-INDUCED CPP I
Experiment 4 was designed to test the hypothesis that conditioning with a play-unresponsive partner would prevent the development of socially-induced CPP. To this aim, adolescent rats were conditioned with partners treated either with vehicle or with a dose of mehylphenidate that selectively suppresses social play behavior in adolescent rats, without affecting social exploration (Vanderschuren et al., 2008).
Procedure
Socially isolated rats were divided in two groups. Both groups were tested for baseline preference at 25 days of age, and conditioned as in experiment 2, i.e. eight conditioning days, with two 30 min conditioning sessions (social and non social session) per day. During the social session, the control group was conditioned with a vehicle-treated social partner, whereas the methylphenidate group was conditioned with a partner treated with methylphenidate (1 mg/kg, s.c., 30 min before conditioning). The animals did not receive any treatment before the non social sessions. Rats from both groups were tested on day 10, at 34 days of age, as described above.
Statistical analysis
Data were analyzed with a mixed ANOVA, taking the interdependence of the time spent in each compartment into account, and using treatment of the partner (two levels: methylphenidate or vehicle) as between-subjects factor, and compartment as within-subjects factor (two levels: social compartment and non social compartment). Mixed ANOVA was followed by Tukey’s post hoc test performed on significant group × compartment interaction.
Results
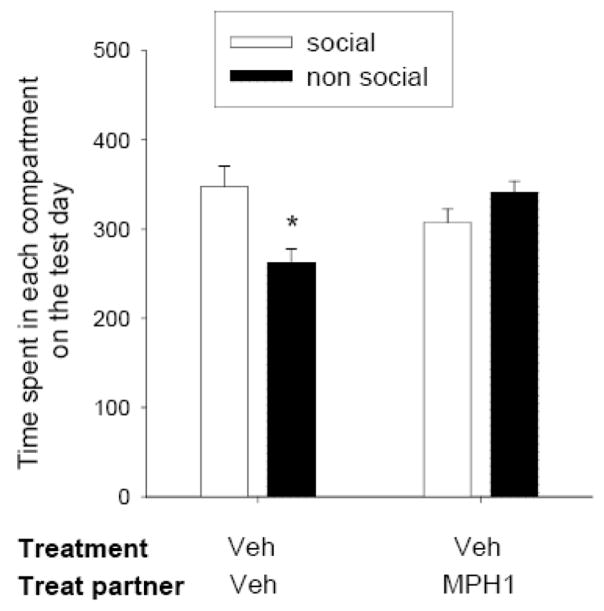
Mixed ANOVA for the time spent in the social/non social compartment on the test day gave the following results: [F(partner treatment)1,22=4.43, p<0.05; F(compartment)1,22=1.32, n.s.; F(partner treatment × compartment)1,22=6.92, p<0.05]. Post hoc analysis performed on the partner treatment × compartment interaction showed that, in line with results from experiment 2, socially isolated rats conditioned with vehicle-treated partners showed socially-induced CPP (figure 4). However, the rats conditioned with a methylphenidate-treated partner did not display preference for the social-paired compartment (figure 4). The dose of methylphenidate used in this experiment has been shown to suppress both the initiation to play and the responsivity to play initiation, leaving social exploration and locomotor activity during social encounters unaffected (Vanderschuren et al., 2008). These data therefore suggest that social interaction with a play-unresponsive partner does not induce CPP in adolescent rats.
Figure 4.
Effect of methylphenidate on the development of socially-induced CPP. Experimental rats were conditioned either vehicle- or methylphenidate- (1 mg/kg, s.c.) treated partners. “Treatment” indicates the treatment (vehicle, Veh) of experimental rats, whereas “Treat partner” indicates the treatment (either vehicle (Veh) or methylphenidate (MPH1)) of the social partner. Data represent mean ± SEM time spent in the social paired- (social, white bar) and non social-paired (non social, black bar) compartments on the test day. *p<0.05 for difference in time spent in the social- and non social-paired compartments (Tukey’s post hoc test, n=12 per group).
EXPERIMENT 5. EFFECT OF METHYLPHENIDATE ON SOCIALLY-INDUCED CPP II
Experiment 4 showed that rats treated with methylphenidate were unrewarding social partners, because their vehicle-treated experimental counterparts did not display CPP. In our previous work, we found that methylphenidate suppressed both the initiation to play and the responsivity to play initiation. However, vehicle-treated animals still solicited play from methylphenidate-treated test partners (Vanderschuren et al., 2008). In experiment 5, we evaluated the contribution of play solicitation in the development of socially-induced CPP. By treating either the experimental animal with methylphenidate (that will not solicit play and will not respond to solicitation, but will be solicited by a vehicle-treated partner), or its test partner (that will not solicit play, or respond to solicitation, but will allow the experimental animal to solicit), or both with methylphenidate (completely eliminating social play from the interaction), we investigated the extent to which play solicitation or being solicited, social interaction with a non-playful partner and the entire interaction of social play contribute to the development of socially-induced CPP.
Procedure
Socially isolated rats were tested for baseline preference at 25 days of age, and conditioned as in experiment 2, i.e. eight conditioning days, with two 30 min conditioning sessions (social and non social session) per day. After testing for baseline preference, rats were randomly allocated to one of the following treatment groups: Veh-Veh (rats injected with vehicle 30 min before the beginning of the social session and conditioned with a vehicle-treated social partner); Veh-MPH (rats injected with vehicle 30 min before the beginning of the social session and conditioned with a methylphenidate (1 mg/kg, s.c.)-treated social partner); MPH-Veh (rats injected with methylphenidate (1 mg/kg, s.c.) 30 min before the social session and conditioned with a vehicle-treated social partner); MPH-MPH (rats injected with methylphenidate (1 mg/kg, s.c.) 30 min before the social session and conditioned with a methylphenidate (1 mg/kg, s.c.)-treated social partner). The animals did not receive any treatment before the non social conditioning sessions. Rats from all groups were tested on day 10, at 34 days of age, as described above.
Statistical analysis
Data were analyzed with a mixed ANOVA, taking the interdependence of the time spent in each compartment into account, and using treatment of the experimental rat (two levels: methylphenidate or vehicle) and treatment of the partner (two levels: methylphenidate or vehicle) as between-subjects factors, and compartment as within-subjects factor (two levels: social compartment and non social compartment). Mixed ANOVA was followed by Tukey’s post hoc test performed on significant treatment/partner treatment × compartment interaction.
Results
Statistical analysis for the time spent in the social/non social compartment on the test day gave the following results: [F(treatment)1,32=0.10, n.s.; F(partner treatment)1,32=0.98, n.s.; F(treatment × partner treatment)1,32=2.06, n.s.; F(compartment)1,32=2.98, n.s.; F(compartment × treatment)1,32=0.6, n.s.; F(compartment × partner treatment)1,32=1.73, n.s.; F(compartment × treatment × partner treatment)1,20=5.98, p<0.05;]. Post hoc analysis performed on the compartment × treatment × partner treatment interaction showed that only vehicle-treated rats conditioned with vehicle-treated partners displayed socially-induced CPP, whereas vehicle-treated rats conditioned with methylphenidate-treated partners did not (figure 5). Consistent with experiment 4, this result suggests that being conditioned with a play-responsive partner is necessary for the development of socially-induced CPP. Treatment of the experimental animal with methylphenidate blocked the development of CPP (figure 5). This effect was independent of the treatment received by the social partner, since methylphenidate-treated rats conditioned with either vehicle- or methylphenidate-treated partners spent a comparable amount of time in the social- and non social-paired compartments (figure 5).
Figure 5.
Effect of methylphenidate (1 mg/kg, s.c., 30 min before the social session) on socially-induced CPP. “Treatment” indicates the treatment (either vehicle (Veh) or methylphenidate (MPH1)) of the experimental rats, whereas “Treat partner” indicates the treatment (either vehicle (Veh) or methylphenidate (MPH1)) of the social partner. Data represent mean ± SEM time spent in the social paired- (social, white bar) and non social-paired (non social, black bar) compartments on the test day. **p<0.01 for difference in time spent in the social- and non social-paired compartments (Tukey’s post hoc test, n=8–12 per group).
EXPERIMENT 6. EFFECT OF METHYLPHENIDATE ON PLACE CONDITIONING
The aim of experiment 6 was to exclude the possibility that methylphenidate blocked the development of socially-induced CPP because of aversive properties of methylphenidate itself, thus opposing the positive subjective properties of social behavior.
Procedure
The procedure was comparable to experiment 5, except that the rats were not allowed to socially interact during conditioning. On day 1, at 25 days of age, all rats were tested for baseline preference in the CPP apparatus and then socially isolated. Starting from day 2, the rats were conditioned for eight consecutive days, with two conditioning sessions per day. Thirty min before the drug session, half of the rats were injected with methylphenidate (1 mg/kg, s.c.), whereas the other half were injected with vehicle. The conditioning session consisted of placing the rats individually in one side of the CPP apparatus (drug compartment) for 30 min. On the same day, 30 min before the vehicle session, rats from both groups were injected with vehicle, and then placed in the other side of the apparatus (vehicle compartment) for 30 min. The order of drug and vehicle sessions was counterbalanced over days: on days 2, 4, 6 and 8, rats were conditioned with methylphenidate in the morning session, and with vehicle in the afternoon session; on days 3, 5, 7 and 9, the order of sessions was reversed, i.e. rats were conditioned with vehicle in the morning session, and with methylphenidate in the afternoon session. Rats were tested on day 10, at 34 days of age, as described above.
Statistical analysis
Data were analyzed with a mixed ANOVA, taking the interdependence of the time spent in each compartment into account, and using treatment of the experimental rat (two levels: methylphenidate or vehicle) as between-subjects factor, and compartment as within-subjects factor (two levels: drug compartment and vehicle compartment). Mixed ANOVA was followed by Tukey’s post hoc test performed on significant treatment × compartment interaction.
Results
Mixed ANOVA for the time spent in the drug/vehicle compartment on the test day gave the following results: [F(treatment)1,10=0.036, n.s.; F(compartment)1,10=7.74, p<0.05; F(treatment × compartment)1,10=5.74, p<0.05]. Post hoc analysis performed on the treatment × compartment interaction showed that vehicle-treated rats, as expected, spent an equal amount of time in both compartments on the test day. In contrast, methylphenidate-treated rats spent more time in the drug than in the vehicle compartment (figure 6). This shows that 1 mg/kg methylphenidate, administered 30 min before conditioning, has positive subjective properties in adolescent rats.
Figure 6.
Effect of methylphenidate (1 mg/kg, s.c., 30 min before the drug session) on place conditioning. Data represent mean ± SEM time spent in the drug paired- (drug, white bar) and vehicle-paired (vehicle, black bar) compartments on the test day. *p<0.05 for difference in time spent in the drug- and vehicle-paired compartments (Tukey’s post hoc test, n=6 per group).
DISCUSSION
The present study had the following aims: 1. to understand how motivational aspects of social interaction in adolescent rats contribute to the development of socially-induced CPP. 2. to understand how the amount of training influences socially-induced CPP and whether socially-induced CPP is susceptible to extinction and reinstatement, like drug-induced CPP. 3. to test the hypothesis that conditioning with play-unresponsive partners would prevent the development of socially-induced CPP, and to investigate the relative contribution of play solicitation to socially-induced CPP.
By and large, previous studies of socially-induced CPP in adolescent rats used only socially isolated animals as experimental subjects (Calcagnetti and Schechter, 1992; Crowder and Hutto, 1992; Van den Berg et al., 1999; Thiel et al., 2008; -2009, but see Douglas et al., 2004). These studies have not systematically investigated whether changes in the incentive value of social interaction modify the acquisition of CPP. To address this issue, we compared the magnitude of socially-induced CPP in groups of rats subjected to different housing conditions. Furthermore, given the paucity of data about how the amount of training influences CPP, we investigated how different conditioning parameters, such as number and length of conditioning sessions per day, influenced the development of socially-induced CPP. Our analysis showed that when a protocol consisting of two daily 30-min conditioning sessions for eight days was used, socially isolated rats displayed robust and reproducible socially-induced CPP. Rats that were briefly isolated (i.e. 3.5 h) before conditioning, which yields an about half-maximal induction of social play behavior (Niesink and Van Ree, 1989; Vanderschuren et al., 1995a; -2008) showed a trend towards CPP, whereas adolescent rats housed with a 12-week-old social partner did not show CPP. These data demonstrate that motivational factors make a major contribution to socially-induced CPP in adolescent rats. In fact, in keeping with previous studies (Douglas et al., 2004), graded levels of social isolation during conditioning lead to graded levels of CPP (see Fig. 2), with animals most motivated for social interaction displaying the most pronounced CPP. The same holds true for other natural rewards: for example, a food-deprived rat will prefer contextual cues previously associated with food, while the same rat, when water deprived, will approach cues associated with fluid (Perks and Clifton, 1997). Like feeding and drinking, social play can be seen as a homeostatically controlled behavior, which is increased by social deprivation and reduced by social satiation. Indeed, social isolation strongly increases social play in adolescent rats, with the amount of social play displayed being a function of the duration of social isolation preceding the test (Panksepp and Beatty, 1980; Niesink and Van Ree, 1989; Vanderschuren et al., 1995a; -2008). In our experiments, individually housed rats had the opportunity to engage in social interaction and social play only once per day, i.e. during conditioning with a social partner. Thus, maximal levels of social play experienced during conditioning and high motivation for social interaction as a result of social isolation might account for the strong CPP displayed by socially isolated rats. In support of the notion that prolonged social isolation increases the appetitive motivation for social interactions, it has been shown that: 1. individually housed rats tested in a T-maze chose social rewards over food rewards more often than socially housed animals (Ikemoto and Panksepp, 1992); 2. social housing reduced the 50-kHz ultrasonic vocalizations (USVs) emitted by rats in response to rewarding manual tickling by an experimenter, and increased the time to approach the experimenter’s hand (Burgdorf and Panksepp, 2001); 3. compared to socially housed rats, individually housed animals showed faster acquisition of instrumental tasks for tickling (Burgdorf and Panksepp, 2001) and rapid acquisition of 50-kHz USVs to a conditioned stimulus that predicted tickle reward (Panksepp and Burgdorf, 2000).
Remarkably, we observed no CPP in adolescent rats housed with a 12-week old rat. We reasoned that the low levels of social play displayed by rats of this age (Baenninger, 1967; Panksepp, 1981) would substantially enhance social play in the adolescents during conditioning. The lack of CPP in these animals suggests that the high intrinsic motivation for play in adolescent animals caused the adults to engage in play as well (Pellis and Pellis, 1992; Pellis and Pellis, 1991), so that the levels of play performed by the adolescents during conditioning were not sufficient to evoke CPP. However, since we did not measure home cage social interactions between adolescents and 12-week old cagemates, this interpretation remains speculative.
From a methodological point of view, two more issues need to be addressed. First, our experiments used a counterbalanced place conditioning procedure (Tzschentke, 2007). This means that the animals were allocated to test groups and conditioning compartments on the basis of their baseline side preference. Thus, in each group, approximately half of the rats was conditioned in their preferred compartment and half in their non-preferred one, so that the baseline preference in each test group for the (to be) social-paired and (to be) non-social paired compartments approximated 50%. Therefore, preference shifts after conditioning reflect socially-induced CPP, rather than decreased avoidance for the initially non-preferred compartment. Second, in our experimental setting, 30-min conditioning sessions induced robust CPP; shorter conditioning sessions, conducted either once or twice/day, caused a trend towards CPP that was not consistent across experiments. However, a recent study found no difference in the magnitude of CPP in adolescent rats conditioned with a social partner for either 10 or 30 min (Thiel et al., 2008). This discrepancy might be due to differences in the experimental procedures (biased vs unbiased design) and apparatus used (two- vs three-compartment CPP boxes). In adolescent rats, social behavior, and particularly social play, peaks in the first 5–10 min of the social encounter, depending on the familiarity with the environment, and then wanes (Panksepp et al., 1984; Vanderschuren et al., 1995b). Thus, a possible explanation of our findings is that, at the beginning of the conditioning session, animals paid more attention to the social than to the environmental stimuli. Over time, the animals would become more aware of the contextual stimuli, allowing for the association between the positive social experience and the environmental stimuli to take place. It is also possible that in the socially isolated rats, the need for social interaction was so high that allowing them social interaction for only 15 min/day was not enough to fulfill their social needs, so that frustration about the briefness of the interaction, and the interruption of the interaction after 15 min diluted the positive value of the social experience in the conditioning environment.
In experiment 3, CPP was extinguished by daily exposure to the CPP apparatus (including, but not restricted to the social-paired compartment, as the rats were free to move throughout the apparatus during testing) in the absence of the social partner. The initial CPP lasted for three daily test sessions. However, after seven extinction sessions, CPP could be reinstated by a single reconditioning session. These data demonstrate that the acquired positive value of neutral environmental stimuli by association with a positive social experience is quite persistent, since a single re-exposure to the social partner in the social-paired compartment after extinction reinstated CPP. Interestingly, the duration of reinstated CPP was shorter than the original CPP. This could be the result of two factors. First, the reconditioning took place at an age (approximately 42 days) when levels of social play might already be waning (Baenninger, 1967; Panksepp, 1981). The social repertoire of adolescent rats changes during development. Social behaviors related to play peak between 30 and 40 days of age, and then gradually decline, being replaced by more adult-like sexual and agonistic behaviors. In contrast, social behaviors unrelated to play occur during the entire lifespan of rats (Panksepp, 1981; Thor and Holloway, 1984; Vanderschuren et al., 1997). Thus, since our results (see below) suggest that social play rather than social exploratory behavior underlies the development of socially-induced CPP (Humphreys and Einon, 1981; Pellis and McKenna, 1995), it is possible that lower levels of play experienced by older animals during the reconditioning session might account for a weaker CPP. Second, the animals’ training history might explain the short duration of the reinstated CPP. During the eight initial training sessions, an unambiguous association was made between the social-paired compartment and social interaction. The CPP evoked by this association was then extinguished over seven sessions, followed by a single reconditioning session. Thus, the fact that animals had experienced the positive association in nine sessions, but extinction in seven sessions, might have caused the social-paired compartment to become an ambiguous environment, paired with social interaction on somewhat more than half of the occasions, thus reducing the strength of CPP.
In experiment 4, we compared the magnitude of CPP in adolescent rats conditioned with either vehicle- or methylphenidate-treated partners. Interestingly, when adolescent rats were conditioned with methylphenidate-treated partners, place preference was completely abolished. We have previously shown that the dose of methylphenidate used in this experiment reduced both play solicitation and responsiveness to play solicitation, without affecting social exploratory behavior or locomotor activity during social interaction (Vanderschuren et al., 2008). Furthermore, rats treated with this dose of methylphenidate indirectly reduced social play in vehicle-treated rats (Vanderschuren et al., 2008). This is consistent with findings showing that social play behavior is influenced by the level of social activity of the partner (Pellis and McKenna, 1992; -1995; Trezza and Vanderschuren, 2008). In the present study, rats conditioned with methylphenidate-treated partners spent equal amounts of time in the social- and non social-paired compartment. Thus, in keeping with previous studies (Calcagnetti and Schechter, 1992), conditioning with play-unresponsive partners prevented the development of CPP. A likely explanation of the results of experiment 4 is that social interaction, without the opportunity to engage in reciprocal rough-and-tumble play, is not rewarding for adolescent rats. These findings are consistent with the observations that adolescent rats tested in a T-maze preferred a play-responsive over a play-unresponsive partner (Humphreys and Einon, 1981). Interestingly, recent studies, using suboptimal conditioning parameters, have shown that cocaine and nicotine can enhance socially-induced CPP, even though these drugs reduced social play behavior during conditioning (Thiel et al., 2008; -2009), suggesting that drugs and social interaction can have additive rewarding effects in adolescent rats under certain circumstances. Our analysis of the effects of methylphenidate on social play behavior showed that vehicle-treated rats did solicit play from their methylphenidate-treated partners, even if they were not reciprocated (Vanderschuren et al., 2008). We therefore performed a next experiment, in which the experimental animal, the stimulus animal, or both were treated with the drug, to test whether play solicitation itself, or being solicited would be rewarding. The data from experiment 4 suggested that unreciprocated play solicitation was not rewarding, and this was confirmed in experiment 5, because vehicle-treated animals conditioned with a methylphenidate-treated partner did not show CPP. In addition, the results of experiment 5 suggest that being solicited but not responding is not rewarding either, since methylphenidate-treated rats conditioned with a vehicle-treated partner did not show CPP either. As might then be expected, methylphenidate-treated animals conditioned with a methylphenidate-treated partner, in which social play would be completely eliminated (Vanderschuren et al., 2008), also displayed no CPP. Thus, the lack of socially-induced CPP in methylphenidate-treated rats is likely the result of the suppression of social play, that occurred when one or both animals were treated with the drug (Vanderschuren et al., 2008). A limitation of our study is that we did not measure social play behavior during conditioning. Therefore, although our data suggest that social play is the most pleasurable aspect of the social repertoire of adolescent rats, further research is needed to support this interpretation. Methylphenidate is widely prescribed and very effective for the treatment of ADHD. In view of the importance of peer relationships during development, the finding that methylphenidate markedly disrupts social reward in adolescent rats highlights the need for a better understanding of the effects of psychostimulant drugs on the developing brain.
The effects of methylphenidate on socially-induced CPP were not the result of methylphenidate-induced place aversion counteracting the positive properties of social play. In fact, this dose of methylphenidate itself induced CPP (experiment 6). This result confirms the influence of social context on the subjective effects of drugs of abuse. Interestingly, whereas the subjective effects of several other drugs of abuse are enhanced in a social context (Newman et al., 2007; Thiel et al., 2008; -2009; Tomie et al., 2004), the play-unresponsive state induced by methylphenidate apparently blunted its own positive properties. Another remarkable detail to experiment 6 is the fact that methylphenidate induced CPP, despite the fact that it was administered 30 min before conditioning (to be consistent with experiments 4 and 5, in which the drug was administered 30 min before conditioning with a social partner, since this pretreatment interval has been shown to suppress social play; Vanderschuren et al., 2008). Previous research has indicated that imposing an interval between drug treatment and place conditioning can lead to place aversion, because when positive subjective properties of the drug have subsided, opponent negative effects may prevail (Ettenberg et al., 1999). Apparently, the slower kinetics of methylphenidate compared to cocaine (Volkow and Swanson, 2003) permit for positive properties of this drug to be seen even after a considerable interval between drug treatment and conditioning.
In conclusion, the present study provides more insights into how social rewards can be used in a place conditioning setup in adolescent rats. In particular, we showed that: 1. the magnitude of socially-induced CPP depends upon the number and length of conditioning sessions, demonstrating that the amount of training contributes to the development of CPP; 2. similar to other natural rewards, socially-induced CPP is directly influenced by changes in the motivational state of the subject, because socially-induced CPP was most reliably found in animals socially isolated during conditioning; 3. socially-induced CPP is persistent and, comparable to drug-induced CPP, can be reinstated after extinction; 4. social play behavior is likely the most pleasurable aspect of the social repertoire of adolescent rats; in fact, it is essential that both social partners engage in a playful interaction for social behavior to be rewarding. Together, these findings help to understand how learning on the basis of social information, i.e. the association of neutral environmental stimuli with positive social experiences, leads to long-lasting changes in behavior.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baenninger LP. Comparison of behavioural development in socially isolated and grouped rats. Anim Behav. 1967;15:312–323. doi: 10.1016/0003-3472(67)90018-8. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. Tickling induces reward in adolescent rats. Physiol Behav. 2001;72:167–73. doi: 10.1016/s0031-9384(00)00411-x. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav. 1992;51:667–72. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- Crowder WF, Hutto CW., Jr Operant place conditioning measures examined using two nondrug reinforcers. Pharmacol Biochem Behav. 1992;41:817–24. doi: 10.1016/0091-3057(92)90233-6. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacol. 2004;47:202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45:153–62. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav. 1999;64:507–12. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Fagen R. Animal Play Behavior. Oxford University Press; 1981. [Google Scholar]
- Falk JL. The grooming behavior of the chimpanzee as a reinforcer. J Exp Anal Behav. 1958;1:83–5. doi: 10.1901/jeab.1958.1-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys AP, Einon DF. Play as a reinforcer for maze-learning in juvenile rats. Anim Behav. 1981;29:259–270. [Google Scholar]
- Ikemoto S, Panksepp J. The effects of early social isolation on the motivation for social play in juvenile rats. Dev Psychobiol. 1992;25:261–74. doi: 10.1002/dev.420250404. [DOI] [PubMed] [Google Scholar]
- Keiflin R, Isingrini E, Cador M. Cocaine-induced reinstatement in rats: evidence for a critical role of cocaine stimulus properties. Psychopharmacology. 2008;197:649–60. doi: 10.1007/s00213-008-1083-1. [DOI] [PubMed] [Google Scholar]
- Mason WA, Saxon SV, Sharpe LG. Preferential responses of young chimpanzees to food and social rewards. Psychol Rec. 1963:341–345. [Google Scholar]
- Mueller D, Perdikaris D, Stewart J. Persistence and drug-induced reinstatement of a morphine-induced conditioned place preference. Behav Brain Res. 2002;136:389–397. doi: 10.1016/s0166-4328(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, Mcclure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Newman JL, Perry JL, Carroll ME. Social stimuli enhance phencyclidine (PCP) self-administration in rhesus monkeys. Pharmacol Biochem Behav. 2007;87:280–8. doi: 10.1016/j.pbb.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesink RJM, Van Ree JM. Involvement of opioid and dopaminergic systems in isolation-induced pinning and social grooming of young rats. Neuropharmacology. 1989;28:411–8. doi: 10.1016/0028-3908(89)90038-5. [DOI] [PubMed] [Google Scholar]
- Normansell L, Panksepp J. Effects of morphine and naloxone on play-rewarded spatial discrimination in juvenile rats. Dev Psychobiol. 1990;23:75–83. doi: 10.1002/dev.420230108. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14:327–32. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Beatty W. Social deprivation and play in rats. Behavioral and Neural Biology. 1980:197–206. doi: 10.1016/s0163-1047(80)91077-8. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Burgdorf J. 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats: effects of social housing and genetic variables. Behav Brain Res. 2000;115:25–38. doi: 10.1016/s0166-4328(00)00238-2. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8:465–92. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Pellis SM, McKenna MM. Intrinsic and extrinsic influences on play fighting in rats: effects of dominance, partner’s playfulness, temperament and neonatal exposure to testosterone propionate. Behav Brain Res. 1992;50:135–45. doi: 10.1016/s0166-4328(05)80295-5. [DOI] [PubMed] [Google Scholar]
- Pellis SM, McKenna M. What do rats find rewarding in play fighting?--an analysis using drug-induced non-playful partners. Behav Brain Res. 1995;68:65–73. doi: 10.1016/0166-4328(94)00161-8. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Juvenilized play fighting in subordinate male rats. Aggressive Behavior. 1992;18:449–457. [Google Scholar]
- Pellis SM, Pellis VC. Role of reversal changes during the ontogeny of play fighting in male rats: attack versus defence. Aggressive Behavior. 1991;17:179–189. [Google Scholar]
- Perks SM, Clifton PG. Reinforcer revaluation and conditioned place preference. Physiol Behav. 1997;61:1–5. doi: 10.1016/s0031-9384(96)00243-0. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Calcagnetti DJ. Trends in place preference conditioning with a cross-indexed bibliography: 1957–1991. Neurosci Biobehav Rev. 1993;17:21–41. doi: 10.1016/s0149-7634(05)80228-3. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: A model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–12. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology. 2009 doi: 10.1007/s00213-009-1470–2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor DH, Holloway WR., Jr Social play in juvenile rats: a decade of methodological and experimental research. Neurosci Biobehav Rev. 1984;8:455–64. doi: 10.1016/0149-7634(84)90004-6. [DOI] [PubMed] [Google Scholar]
- Tomie A, Uveges JM, Burger KM, Patterson-Buckendahl P, Pohorecky LA. Effects of ethanol sipper and social opportunity on ethanol drinking in rats. Alcohol Alcohol. 2004;39:197–202. doi: 10.1093/alcalc/agh055. [DOI] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJMJ. Cannabinoid and opioid modulation of social play behavior in adolescent rats: Differential behavioral mechanisms. Eur Neuropsychopharmacol. 2008;18:519–30. doi: 10.1016/j.euroneuro.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Van den Berg CL, Pijlman FT, Koning HA, Diergaarde L, Van Ree JM, Spruijt BM. Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats. Behav Brain Res. 1999;106:133–42. doi: 10.1016/s0166-4328(99)00099-6. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Spruijt BM, Van Ree JM. Effects of morphine on different aspects of social play in juvenile rats. Psychopharmacology. 1995a;117:225–31. doi: 10.1007/BF02245191. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Spruijt BM, Van Ree JM. Influence of environmental factors on social play behavior of juvenile rats. Physiol Behav. 1995b;58:119–123. doi: 10.1016/0031-9384(94)00385-i. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–26. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Trezza V, Griffioen-Roose S, Schiepers OJG, Van Leeuwen N, De Vries TJ, Schoffelmeer ANM. Methylphenidate disrupts social play Behavior in adolescent rats. Neuropsychopharmacology. 2008;33:2946–2956. doi: 10.1038/npp.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160:1909–18. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]