Figure 1. Relative binding of PHT-427 analogs with different carbon chain lengths to the expressed PH domains of Akt and PDK.
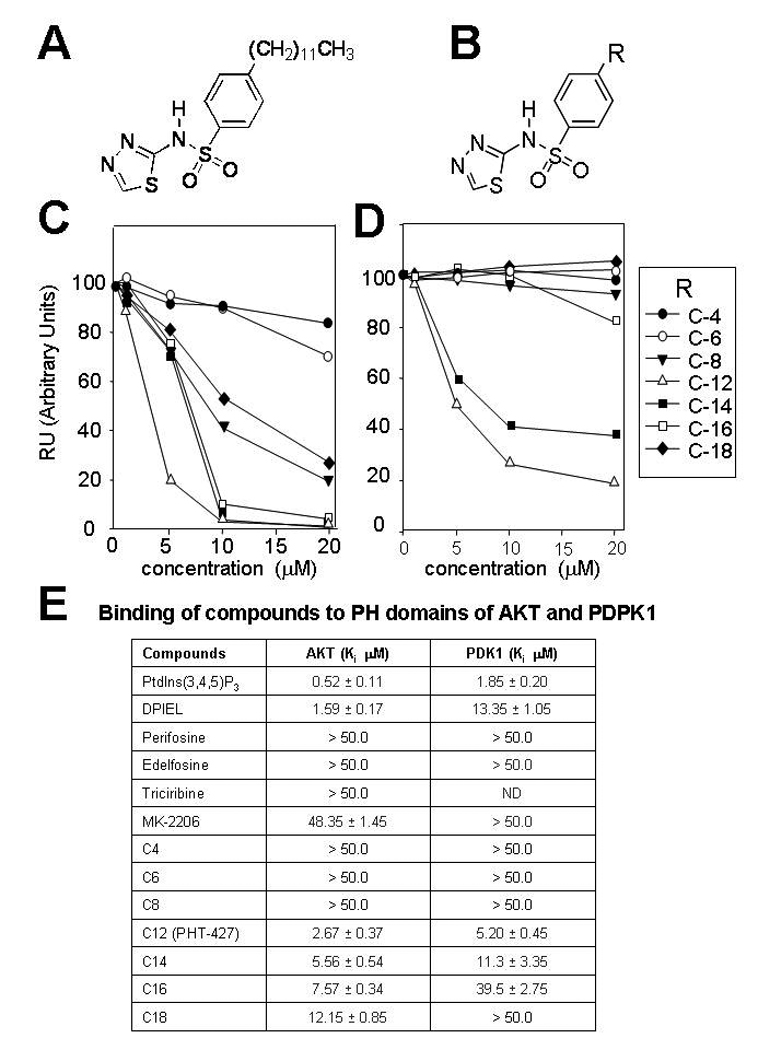
A, structure of PHT-427; B Structure of analogs where R is a C-4 to C-14 carbon chain. C, Surface plasmon resonance spectroscopy (SPR) was used to measure the binding affinity (Ki) for the expressed PH domain of Akt2 and of D, PDPK1 by competitive binding of the compounds for the natural ligand phosphatidylinositol-(3,4,5)-P3. E, Kis for reported Akt PH domain inhibitors, and PHT-427 and its C-4- C-14 carbon chain analogs. Ki, were measured as the concentration of compound that displace 50% of the PH domain bound to lipid vesicles enriched in PdtIns(3,4,5)P3 in the absence of drug. Values are the mean of 4 determinations and bars are SE. ND is not determined