Abstract
Background
Previous research has shown that rats reared in an enriched condition (EC) are more sensitive to the acute effects of amphetamine than rats reared in an isolated condition (IC); yet, EC rats self-administer less amphetamine than IC rats. The present study used cocaine to further explore this environmental enrichment behavioral phenotype, as well as the underlying molecular mechanisms involved.
Methods
Enriched condition and IC rats were studied in a broad battery of behavioral tests, including cocaine conditioned place preference (CPP) and self-administration and several measures of anxiety- and depression-related behavior. The involvement of the transcription factor, cyclic adenosine monophosphate (cAMP) response element binding protein (CREB), in mediating EC versus IC differences was investigated.
Results
Enriched condition rats exhibited less cocaine self-administration, despite showing enhanced cocaine CPP. Enriched condition rats also displayed less depression-like behavior but higher levels of anxiety-like behavior. This behavioral phenotype is consistent with low CREB activity in the nucleus accumbens, a key brain reward region. Indeed, EC rats have less phospho-CREB (the transcriptionally active form of the protein) in the nucleus accumbens than IC rats, and a selective knockdown of CREB in this brain region of normally reared rats, by use of a novel viral vector expressing a short hairpin RNA (shRNA) directed against CREB, reproduced the EC behavioral phenotype.
Conclusions
These studies identify a potential molecular mechanism for how rearing environment—a nonpharmacological, nonsurgical manipulation— can modify a wide range of complex emotional behaviors.
Keywords: Addiction, anxiety, craving, depression, differential rearing, drug abuse, drug addiction, forced swim test, incentive sensitization, relapse, salience
A majority of people who experiment with drugs of abuse do not become addicted, despite feeling the full euphorigenic effects of the drug, while others can become addicted upon initial drug exposures. Thus, understanding the neurobiology of resistance to addiction can be crucial in preventing addiction and treating those already addicted.
Environmental factors contribute to addiction susceptibility in humans. The environmental enrichment paradigm has shown great potential as a rodent model of environment-based resistance to addiction. In this model, rats are divided into differing environmental conditions at 21 days of age. Rats in the isolated condition (IC) are single-housed with no exposure to social contact or novelty, while rats in the enriched condition (EC) are housed 12 per cage in large home cages containing novel objects that are rearranged and replaced daily. Prior research has shown that this differential rearing for 30 days produces reproducible and robust behavioral changes in rats. Enriched condition rats exhibit a protective addiction phenotype: they are less prone to self-administer amphetamine compared with IC rats (1,2). This effect is paradoxical because EC rats are more sensitive to the locomotor-activating, dopamine-releasing, and rewarding effects (as measured by conditioned place preference [CPP]) of amphetamine (3–5). Thus, environmental enrichment appears to decrease addiction liability without decreasing drug sensitivity. These results add to the growing literature that drug self-administration and CPP measure different aspects of addiction-related behavior (6).
A separate line of research has found that blocking activity of the transcription factor, cyclic adenosine monophosphate (cAMP) response element binding protein (CREB), in nucleus accumbens (NAc), a critical brain reward region, exerts robust effects in several behavioral models related to anxiety, depression, and addiction. Blocking CREB in rat NAc produces an antidepressant and anxiogenic phenotype and increases sensitivity to cocaine reward as measured by CPP (7–13). Interestingly, blocking CREB in NAc decreases self-administration of cocaine (14), again revealing a discordance between CPP and self-administration identical to that induced by environmental enrichment.
The central hypothesis of this report was that the enrichment phenotype is mediated, at least partly, by decreased CREB activity in NAc. The results of the present study further define the EC behavioral phenotype: we show that EC rats, compared with IC rats, exhibit reduced cocaine self-administration but higher cocaine CPP, decreased depression-related behavior but increased anxiety-related behavior, and decreased CREB activity in NAc, which is associated with reduced expression of brain-derived neurotrophic factor (BDNF)—an established target gene for CREB—in this brain region. Moreover, we employ a novel viral vector to decrease endogenous CREB activity in NAc and show that this manipulation replicates the EC phenotype in nonenriched rats. Because any one behavioral paradigm is open to alternate interpretations and caveats, we employ multiple behavioral paradigms for measuring aspects of addiction, depression, and anxiety.
Methods and Materials
Environmental Conditions
Male Sprague-Dawley rats were assigned randomly to either EC or IC home-cage conditions from 21 days to 51 days of age. Enriched condition rats were housed in large cages with 12 rats per cage, containing novel hard plastic objects (14 per cage). Rats were removed briefly each day and seven of the objects were replaced; the remaining objects were rearranged into novel configurations. Isolated condition rats were housed individually in standard-sized cages and were not handled except to change bedding as needed. Selected experiments were performed on IC versus social condition (SC) rats, which were pair-housed in standard cages.
Cocaine Reward and Reinforcement
Conditioned place preference provides an indirect measure of drug reward (6). Rats were conditioned with 10 mg/kg cocaine hydrochloride (HCl) or saline intraperitoneal (IP) for 30 min per session using an 8-day unbiased conditioning procedure. Enriched condition and IC rats were also compared in the acquisition, maintenance, extinction, and reinstatement of cocaine taking or seeking behavior by use of an intravenous self-administration paradigm (see Supplement 1 for specific details of the experimental paradigms used in this study).
Anxiety-Related Behavior
Three tests of anxiety-like behavior were used: defecation in response to a mild stressor (new cage and cold conditions), neophobia to a novel taste (sucrose), and exploratory behavior in response to an anxiety-producing novel environment (elevated plus maze [EPM]). These tests are considered measures of anxiety-like behavior because they are each sensitive to anxiolytic benzodiazepine drugs (15,16).
Depression-Related Behavior
We employed three tests to model distinct aspects of depression-like behavior: sucrose preference to model anhedonia, social interaction to model social withdrawal, and forced swim test (FST) to model behavioral despair. Spontaneous locomotor activity was measured in EC and IC rats for 120 min in circular corridor activity chambers, since locomotor activity can confound several of the behavioral assays used in this study.
Construction and Packaging of Adeno-Associated Virus Short Hairpin RNA to CREB
To directly decrease CREB activity in NAc, a novel adeno-associated virus (AAV)-based viral vector system was developed to express a short hairpin RNA (shRNA) directed at CREB using the target sequence 5′-GAGCAATACAGCTGGCTAACA-3′, which is conserved across rat, mouse, and human CREB. The vector was injected bilaterally into rat NAc shell. A scrambled (scr) AAV-shRNA that does not correspond to any known rodent messenger RNA (mRNA) sequence was used as a control condition.
Results
Cocaine Reward and Reinforcement
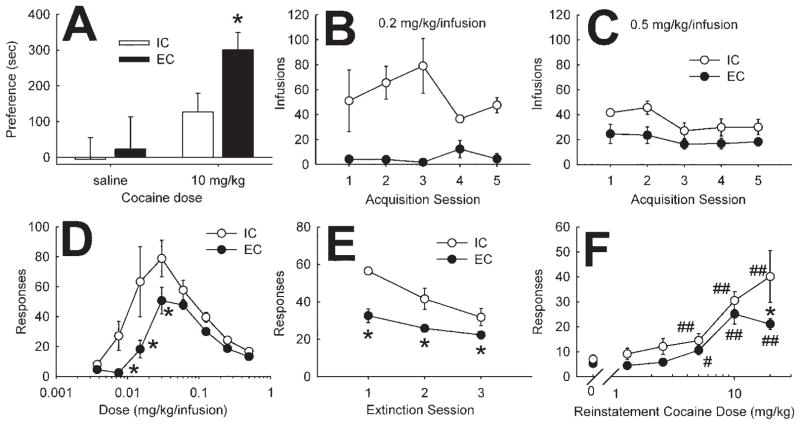
As a first measure of the sensitivity of EC and IC rats to the initial rewarding effects of cocaine, animals were studied using CPP. The results showed that EC rats are more sensitive than IC rats to the rewarding effects of cocaine at a 10 mg/kg dose [F (1,25) = 4.6, p < .05; Figure 1A]. This is similar to previous findings with amphetamine (4).
Figure 1.
Effect of environmental enrichment on reward- and reinforcement-related behaviors. (A) Effect of enrichment on cocaine-induced CPP. Data show mean (± SEM) second preference for saline- or cocaine-paired side. *Significant difference from IC rats (p < .05). (B and C) Effect of enrichment on acquisition of cocaine self-administration. Points represent mean (± SEM) number of infusions of cocaine in EC and IC rats across 60 min. Separate groups of rats self-administered .2 mg/kg/infusion (B) or .5 mg/kg/infusion (C) for five consecutive sessions. (D) Effect of enrichment on cocaine self-administration dose-response function. Points represent mean (± SEM) number of responses by EC (n = 11) and IC (n = 9) rats. *Significant difference in responding from IC rats at the same unit dose (p < .05). (E) Effect of enrichment on extinction of cocaine self-administration. Points represent mean (± SEM) number of responses by EC and IC rats during a 3-hour extinction period after a 1-hour period of self-administration (n = 13 and 9, respectively). *Significant difference in responding from IC group (p < .05). (F) Effect of enrichment on reinstatement of cocaine responding. Points represent mean (± SEM) number of responses by EC (n = 11) and IC (n = 8) rats during reinstatement of cocaine seeking. *Significant difference in reinstatement responding between EC and IC rats (p < .05). #Significant difference (p < .05) from respective saline control. ##Significance at p < .01. CPP, conditioned place preference; EC, enriched condition; IC, isolated condition.
We next studied intravenous self-administration of cocaine. However, before doing so, it was important to know whether EC and IC rats had a similar ability to perform operant responses necessary for self-administration. Accordingly, EC and IC rats were compared for operant responding for sucrose pellets. We found that EC rats responded less for sucrose pellets when not motivated by hunger but responded more than IC rats when motivated by hunger (at 85% free feed body weight), as analyzed by a two-factor analysis of variance (ANOVA) [F(1,20) = 9.3, p < .01; Figure 1A in Supplement 1]. Because response rates for sucrose pellets are far higher than that seen with cocaine self-administration, these results demonstrate that both groups of rats are fully capable of responding at high rates.
For acquisition of cocaine self-administration, EC and IC rats were allowed five sessions (60 min each) to acquire self-administration of .2 mg/kg/infusion (n = 4–7) or .5 mg/kg/infusion (n = 5–8). The results demonstrated that EC rats responded less than IC rats [F(1,19) = 46.1, p < .001; Figure 1B,C] overall and that EC and IC rats responded differently to dose. Specifically, EC rats responded more at the higher dose of cocaine compared with EC rats at the lower dose, while IC rats responded less for the high dose than the low dose [F(1,19) = 13.7, p < .005]. In fact, EC rats did not acquire stable responding at the low unit dose of cocaine.
A within-session dose-response procedure was next used to study stable maintenance responding (17). All rats were allowed to acquire stable responding of .5 mg/kg/infusion before the dose-response procedure was instituted. Rats were then allowed 30 min to self-administer .5 mg/kg/infusion before the dose was halved. The rats were allowed to respond another 30 min, at which time the dose was halved again. The session continued as such for a total of eight doses. This procedure yields a consistent inverse-U shaped dose-response function. Results revealed that EC rats responded significantly fewer times than IC rats for low unit doses of cocaine [F(7,112) = 2.3, p < .05; Figure 1D]. Enriched condition rats also displayed significantly less total drug intake at higher doses [F(7,112) = 3.17, p < .005; Figure 1B in Supplement 1].
Responding on the cocaine-paired lever during extinction of self-administration is used as a measure of craving. Accordingly, EC and IC rats were measured for within-session extinction, where self-administration of cocaine (.5 mg/kg/infusion) was allowed for 60 min followed by 180 min of extinction, where a response elicited the normal 60-sec signalled time-out but no drug was delivered. Using this procedure, EC rats responded fewer times during extinction compared with IC rats [F(1,18) = 15.7, p < .001; Figure 1E].
Reinstatement, where extinguished responding can be reinstated with a noncontingent injection (IP in this case) of the previously self-administered drug, is used as a model of relapse. Using a validated method (18), rats were allowed to self-administer .5 mg/kg/infusion cocaine for 60 min followed by a 180-min extinction period. Once responding extinguished, one of five cocaine doses or saline was injected IP and the rats were placed back into the operant chamber. Nonreinforced responding was recorded for an additional 180 min. Overall, EC rats responded less than IC rats across the dose range [main effect of environment F(1,15) = 5.1, p < .05; Figure 1F], but the interaction term was not significant. Planned comparisons revealed a significant decrease in reinstatement responding at the 20 mg/kg cocaine dose in EC rats.
Anxiety-Related Behavior
The effect of environmental enrichment on anxiety-like behavior was measured in three assays. First, EC and IC rats were placed in novel polycarbonate cages on ice for 20 min, and the number of fecal boli was recorded. Enriched condition rats defecated more than IC rats [F(1,7) = 294.0, p < .001; Figure 2A].
Figure 2.
Effect of environmental enrichment on behaviors relating to activity and anxiety. (A) Effect of enrichment on anxiety-induced defecation in response to cold stress. Points represent mean (± SEM) number of fecal boli across 20-min session. (B) Effect of enrichment on anxiety-induced taste neophobia. Points represent mean (± SEM) milliliters of 1% sucrose solution consumed during 30 min (n = 12). (C) Effect of environmental enrichment on spontaneous locomotor activity. Points represent mean (± SEM) photocell beam breaks across 10-min time bins (n = 12). *Statistically significant difference from IC rats. IC, isolated condition; EC, enriched condition.
Sucrose neophobia is unique among anxiety models, since rats are not placed in a novel environment; rather, the novel stimulus (a 1% wt/vol sucrose solution) is introduced directly into the home cage. This eliminates possible confounds related to exposure to novel environments, where EC rats are clearly more experienced than IC rats. Water was removed from the home cage at 5:00 PM. At 7:00 PM, the sucrose solution was introduced to the home cage in the normal water bottle with sipper tube. Rats were allowed to drink for 30 min. Enriched condition rats exhibited more neophobia to an unfamiliar sucrose taste than IC rats [F(1,23) = 9.5, p < .005; Figure 2B].
The EPM measures exploratory behavior upon placement in a novel and potentially threatening environment. Time on open arms was measured for 5 min, and behavior was scored using an automated video-tracking system. An ANOVA of these data revealed no difference in open-arm time between EC and IC rats [F (1,17) = .186, ns; Figure 1C in Supplement 1].
Locomotor activity in a novel environment also represents a stress-related measure and, in addition, is an important control for several behavioral assays. Therefore, locomotor activity was measured in EC and IC rats. Enriched condition rats exhibited lower rates of spontaneous locomotor activity compared with IC rats across the entire session [F(1,22) = 49.1, p < .001; Figure 2C].
Depression-Related Behavior
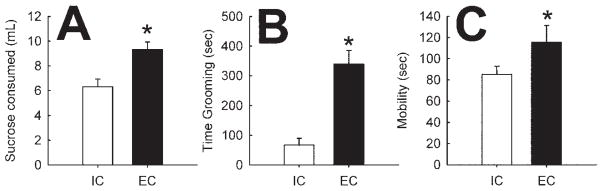
Enriched condition and IC rats were studied in three tests of depression-like behavior, which assess distinct behavioral domains. The first was sucrose preference. Although sucrose neophobia (described above) is an index of anxiety-like behavior, preference for a familiar sucrose solution is used as a measure of natural reward. Rats are preexposed to a 1% (wt/vol) sucrose solution for 3 days, and 2 days later, they are tested for their preference for sucrose versus water. Enriched condition rats consumed more sucrose than IC rats [F(1,23) = 11.9, p < .005; Figure 3A], behavior similar to that induced by antidepressant drugs.
Figure 3.
Effect of environmental enrichment on behaviors modeling depression. (A) Effect of environmental enrichment on sucrose preference. Points represent mean (± SEM) milliliters of 1% sucrose consumed during the 10-min test session (n = 12). (B) Effect of enrichment on social behavior. Points represent mean (± SEM) seconds grooming across the 30-min session (n = 6). (C) Effect of enrichment on mobility in the FST. Points represent mean (± SEM) seconds of mobility time during a 5-min test session (n = 12). *Statistically significant difference from IC rats. FST, forced swim test; IC, isolated condition; EC, enrichedcondition.
Withdrawal from social contact is a hallmark symptom in many cases of depression. To investigate social interaction, all rats were isolated for 24 hours before testing, after which time interactions between pairs of familiar EC or IC rats were quantified. We found that EC rats engaged in more social grooming and other types of social interaction than IC rats [F(1,11) = 28.42, p < .001; Figure 3B].
The FST is a widely used assay of depression-like behavior, where an antidepressive-like phenotype is marked by increased mobility (time struggling or swimming). As shown in Figure 3C, EC rats exhibited increased mobility time [F(1,22) = 3.0, p < .05; one-tailed test based on results from (19)].
Comparison of IC Rats to Pair-Housed Rats
To identify the specific effects of social isolation in IC rats, a separate set of IC rats was compared with rats housed in pairs but without further enrichment, referred to as SC rats. The rats were then subjected to behavioral tests modeling cocaine-conditioned reward and anxiety- and depression-related behaviors. The moderate amount of enrichment from pair-housing rats was not sufficient to produce CPP to 10 mg/kg cocaine [F(1,22) = .5, ns; Figure 4A]. However, the anxiety measures were sensitive enough to resolve differences between IC and SC rats in the stress-induced defecation [F(1,21) = 4.98, p < .05; Figure 4B] and the sucrose neophobia [F(1,20) = 4.47, p < .05; Figure 4C] paradigms. Furthermore, the FST was able to resolve a small but significant difference in mobility between IC and SC rats [F(1,22) = 8.6, p < .01; Figure 4D]. In contrast, the intermediate level of enrichment from social contact was unable to produce significant results in the sucrose preference test [F(1,22) = .01, ns; Figure 4E]. An ANOVA did, however, reveal a significant difference between IC and SC rats with respect to social grooming [F(1,11) = 48.05, p < .001; Figure 4F].
Figure 4.
Effect of moderate enrichment (pair-housing) on behaviors relating to cocaine conditioned reward, anxiety, and depression. (A) Effect of pair-housing (SC) on cocaine-induced CPP. Points represent mean (± SEM) second preference for cocaine-paired side. (B) Effect of pair-housing on anxiety-induced defecation (cold stress). Points represent mean (± SEM) number of fecal boli across 20-min session. (C) Effect of pair-housing on anxiety-induced taste neophobia. Points represent mean (± SEM) milliliters of 1% sucrose solution consumed during 30 min (n = 11). (D) Effect of pair-housing on mobility in the FST. Points represent mean (± SEM) seconds of mobility time during a 5-min test session (n = 12). (E) Effect of pair-housing on sucrose preference. Points represent mean (± SEM) milliliters of 1% sucrose consumed during the 10-min test session (n = 12). (F) Effect of pair-housing on social behavior. Points represent mean (± SEM) seconds grooming across the 30-min session (n = 6). *Statistically significant difference from IC rats. CPP, conditioned place preference; FST, forced swim test; IC, isolated condition; SC, social condition.
Reduced CREB Activity in NAc Mediates the EC Phenotype
Prior research has shown that blocking CREB activity in NAc produces a behavioral phenotype that in many respects resembles the changes in drug reward, anxiety, and depression measures seen in EC versus IC rats. This led us to hypothesize that the EC behavioral phenotype may be partly mediated by decreased CREB activity in NAc.
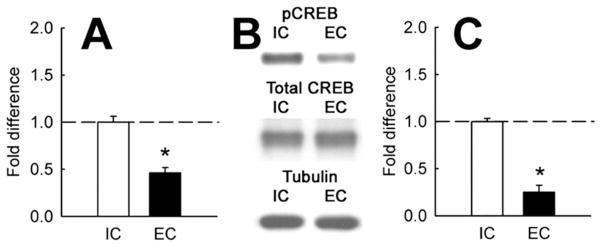
To test whether EC rats have lower CREB activity in NAc than IC rats, we measured levels of phospho-CREB (pCREB), the active form of the protein, in this brain region by Western blotting. Results showed that EC rats had significantly lower basal levels of pCREB in this brain region compared with IC rats [F(1,7) = 41.3, p < .001; Figure 5A]. In contrast, there was no difference in total CREB levels [F(1,7) = 1.0, ns].
Figure 5.
Effect of environmental enrichment on CREB activity in the nucleus accumbens. (A) Effect of environmental enrichment on pCREB levels in the NAc. Points represent mean (± SEM) fold difference in optical density of pCREB immunoreactivity, normalized to levels of total CREB, as measured by a Western blot (n = 4). (B) Lanes of representative blots of pCREB and total CREB in EC and IC animals. (C) Effect of environmental enrichment on BDNF mRNA levels in NAc in EC and IC rats under basal conditions. Points represent mean (± SEM) fold difference in relative BDNF signal as measured by real-time qPCR (n = 4). *Statistically significant difference from IC rats. BDNF, brain-derived neurotrophic factor; CREB, cyclic adenosine monophosphate (cAMP) response element binding protein; EC, enriched condition; IC, isolated condition; mRNA, messenger RNA; NAc, nucleus accumbens; pCREB, phospho-CREB; qPCR, quantitative polymerase chain reaction.
Brain-derived neurotrophic factor is a well-studied CREB target gene with relevance to addiction- and depression-related behavior in NAc. If decreased CREB activity in this brain region has functional consequences, one would expect concomitant decreases in expression of CREB target genes. Accordingly, quantitative polymerase chain reaction (PCR) was used to measure BDNF mRNA expression in NAc. Brain-derived neurotrophic factor was decreased in this brain region of EC rats compared with that of IC rats [F(1,7) = 12.3, p < .05; Figure 5C]. Subsequent Western blotting confirmed a decrease in BDNF protein levels as well (data not shown).
We next tested directly whether decreased CREB activity in NAc mimics the EC behavioral phenotype independent of environment. To accomplish this, we developed a novel shRNA-based method to knock down levels of endogenous CREB in NAc selectively. We first validated that the shRNA sequence directed against CREB mRNA was capable of knocking down CREB expression in human embryonic kidney cells compared with a green fluorescent protein (GFP) shRNA control plasmid (Figure 6A,B). We then incorporated the CREB shRNA into an AAV vector, which was stereotactically injected into rat NAc shell for in vivo validation. The AAV vector also expressed a GFP reporter. This vector mediated robust transgene expression as demonstrated by the high levels of GFP in the injected area (Figure 6C). Moreover, we found that the vector consistently reduced levels of CREB expression in NAc (Figure 6D). This effect was even more striking at higher magnification (Figure 6E–H). Further, analysis of pixel intensity of individual cells confirms an average ~ 75% reduction in CREB immunoreactivity in shRNA-transduced cells (data not shown). This effect was not seen with a control vector expressing a scrambled shRNA sequence, which showed CREB levels indistinguishable from uninjected control conditions (data not shown).
Figure 6.
Validation of AAV-CREB RNAi. (A and B) shRNA to CREB or GFP was transfected into HEK cells and 3 to 5 days later CREB levels were measured by Western blot (n = 2 in duplicate). (C) Representative transduction of AAV-CREB RNAi in NAc as seen from GFP expression and (D) CREB immunoreactivity in the same section. (E) Closeup of cells expressing GFP had lower CREB immunoreactivity (F) compared with comparable nontransduced cells (G and H). *Statistically significant difference from GFP control. AAV, adeno-associated virus; ac, anterior commissure; CREB, cyclic adenosine monophosphate (cAMP) response element binding protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; HEK, human embryonic kidney; NAc, nucleus accumbens; RNAi, RNA interface; shRNA, short hairpin RNA.
Having validated this shRNA approach, we injected AAVs (expressing CREB shRNA or scrambled shRNA) bilaterally into NAc shell of pair-housed rats. We found that the shRNA-mediated knockdown of CREB in NAc, compared with animals receiving scrambled shRNA, produced a behavioral phenotype in pair-housed rats generally similar to the phenotype from environmental enrichment. The one exception was an inability to decrease spontaneous locomotor activity (Figure 7A). Local CREB knockdown in NAc did, however, produce increased anxiety-like behavior in the stress-induced defecation [F(1,23) = 9.5, p < .01; Figure 7B] and sucrose neophobia [F(1,12) = 4.4, p < .05; one-tailed test based on results from Green et al. [12]; Figure 7C) tests. The CREB knockdown had no effect in the EPM [F (1,20) = .01, ns; Figure 7D), but it should be noted that environmental enrichment did not produce an effect in this model either. Moreover, local CREB knockdown produced an antidepressant effect similar to environmental enrichment in the sucrose preference [F(1,12) = 3.7, p < .05; one-tailed test per Green et al. [12]; Figure 7E] and social grooming [F(1,12) = 5.66, p < .05; Figure 7F] tests.
Figure 7.
Effect of AAV-CREB RNAi in nucleus accumbens on behaviors relating to activity, anxiety, and depression in pair-housed rats. Rats received bilateral injections with CREB RNAi AAV or with a control AAV expressing a scrambled shRNA. (A) Effect of CREB RNAi on spontaneous locomotor activity. Points represent mean (± SEM) photocell beam breaks across 10-min time bins (n = 12). (B) Effect of CREB RNAi on anxiety-induced defecation (cold stress). Points represent mean (± SEM) number of fecal boli across 20-min session (n = 12). (C) Effect of CREB RNAi on anxiety-induced taste neophobia. Points represent mean (± SEM) milliliters of 1% sucrose solution consumed during 30 min (n = 6). (D) Effect of CREB RNAi on behavior in the EPM. Points represent mean (±SEM) seconds on the open arm. (E) Effect of CREB RNAi on sucrose preference. Points represent mean (±SEM) milliliters of 1% sucrose consumed during the 10-min test session (n = 6). (F) Effect of CREB RNAi on social behavior. Points represent mean (±SEM) seconds grooming across the 30-min session (n = 6). *Statistically significant difference from GFP control. AAV, adeno-associated virus; CREB, cyclic adenosine monophosphate (cAMP) response element binding protein; EPM, elevated plus maze; RNAi, RNA interface; shRNA, short hairpin RNA.
Discussion
A major question in the drug addiction field is why most people experimenting with drugs do not become addicted despite feeling the euphoria associated with drug exposure (20). While there are no doubt many factors that contribute to this phenomenon, one way to model this in animals is to define experimental conditions where volitional self-administration of a drug is reduced without a decrease in its acute rewarding effects. The environmental enrichment paradigm is one of the few known experimental manipulations that achieve this goal: it increases the rewarding cues of stimulants as measured by CPP, while at the same time decreases the abuse liability of these drugs as measured by drug self-administration (present study [1–5]). Such opposite effects may seem paradoxical, but considerable research has shown that place conditioning and self-administration measure distinct aspects of drug action: CPP measures reward-related associative processes in a drug-free state, whereas self-administration measures reinforcement-related drug-taking behavior (6). This emphasizes the importance of analyzing the actions of drugs of abuse in a range of behavioral models as done in the present study. We hypothesize that the repeated activation of reward pathways by novelty, exercise, and social contact leaves EC rats less vulnerable to compulsive drug self-administration. While speculative, this hypothesis is consistent with human data of what makes individuals vulnerable or resistant to addiction.
We then delineate a behavioral phenotype in EC versus IC rats that reaches far beyond addiction-related behavior. Enriched condition rats show an antidepressant-like phenotype compared with IC rats based on results in the FST, social grooming behavior, and sucrose preference paradigms. Also, EC rats show increased anxiety-like behavior in two of three tests performed. The entire behavioral phenotype of EC versus IC rats is consistent with the behavioral phenotype produced by low CREB activity in NAc achieved by overexpression of a dominant negative CREB inhibitor. This includes dissociation of drug reward sensitivity, which is increased in low CREB animals, and drug self-administration, which is decreased (7,14). This led us to examine levels of active CREB in EC versus IC rats. Consistent with the behavioral data, EC rats exhibit reduced levels of active CREB in NAc. To causally link the EC-IC behavioral phenotype to reduced CREB activity, we downregulated levels of endogenous CREB in NAc by use of a novel RNA interface (RNAi) vector. Our findings, along with the aforementioned published studies of a dominant negative CREB mutant, show that reduced CREB activity in this brain region induces an EC-like behavioral phenotype in normally housed animals.
The decreased operant responding for cocaine by EC rats is not a function of decreased ability to press a lever or general malaise, because EC rats consistently outperform IC rats in operant tests for sucrose pellets when motivated by hunger. Enriched condition rats perform more poorly than IC rats in operant sucrose tests when not motivated by hunger. Much of this decreased responding occurs at low unit doses or during extinction and reinstatement when the magnitude of the reinforcer is low (2). We hypothesize that reinforcer magnitude or saliency is important for determining operant responding of differentially reared rats.
Environmental enrichment also decreased levels of BDNF, a CREB target, in NAc. Such low BDNF levels in this brain region of EC rats would be expected to decrease depressive-like behavior, based on studies of rodent models and postmortem tissue of depressed humans (21). The present results offer additional evidence for the influence of CREB and BDNF in NAc in modulating depression-like behavior (22).
The EC phenotype is similar to the low CREB phenotype in numerous tests used here. The one inconsistency was seen in the EPM. Prior research has shown that blockade of CREB activity in this brain region using a dominant-negative CREB mutant increases anxiety-like behavior, reflected by decreased time spent on the open arm of the EPM (10,12). Here, we found no significant difference between EC and IC rats in anxiety on the EPM, and other laboratories have even reported decreased anxiety in EC mice on the EPM (23). However, EC rats are habituated to novel environments from daily reorganization of novel objects in their home cage and avidly explore novel environments. It is very likely that the novelty of the EPM did not represent an anxiety-provoking stimulus as for the IC rats, thereby confounding the results. Enriched condition rats did, however, show increased anxiety-like behavior in the sucrose neophobia and stress-induced defecation tests, both independent of exploratory activity.
The novel CREB RNAi AAV vector produced EC-like effects in pair-housed rats in four of six behaviors examined. The inability of CREB RNAi to decrease spontaneous locomotor activity and increase anxiety-like behavior on the EPM suggests that other CREB family genes may partially compensate for the loss of CREB (24), because overexpression of dominant-negative CREB inhibitors, which inhibit all cAMP response element activity, produces clear results in these tests (10,12). Alternatively, a decrease in total CREB protein using RNAi might produce different effects than decreased pCREB with no change in total CREB, as seen in EC rats. As well, other brain regions may be involved in the EC phenotype (see below). Regardless, these experiments show that decreasing endogenous CREB protein in NAc mimics most aspects of environmental enrichment.
Depending upon the brain region involved, CREB produces vastly different behavioral effects (8). For example, CREB activity in NAc is prodepressant (see above), while similar activity in the hippocampus is antidepressant (25). It is interesting that EC rats exhibit less CREB activity in NAc (present experiments) but display increased CREB activity in the hippocampus (26,27). This suggests a coordinated regulation of behavior where decreased CREB activity in NAc in concert with increased CREB activity in hippocampus both contribute to the antidepressant-like phenotype observed with environmental enrichment.
While anxiety- and depression-related behavior are oppositely modulated in EC versus IC rats, humans often display comorbidity of depression and anxiety. By contrast, depression and anxiety symptoms occur independently in many humans and the two show distinct responses to many psychotropic medications. The answer is not entirely obvious, but the opposite modulation of these behaviors is a clear hallmark of CREB regulation in NAc (8,12,24).
Environment is an important factor for most psychiatric conditions including anxiety, depression, and addiction, and the interaction between environment and genes is paramount for emergence of these disorders. The environmental enrichment paradigm is of great utility because it is a nonsurgical, noninvasive manipulation that decreases drug-taking behavior without decreasing general drug sensitivity or natural reward. Gene transcription, the interface between genes and environment, provides important insight into the etiology of these conditions and may identify potential targets for their future treatment. The experiments reported here extend earlier research on the unique effects of environmental enrichment on complex behaviors and identify decreased CREB activity specifically in NAc as a central mechanism underlying the protective effects of environmental enrichment. Therapeutic interventions aimed at decreasing CREB function, or decreasing its effects on key target genes such as BDNF, in NAc are likely to provide beneficial effects on addiction- and depression-related behaviors.
Supplementary Material
Acknowledgments
These experiments were funded by several grants from the National Institute on Drug Abuse and National Institute of Mental Health, including R21DA18333-02 and DA12964.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- 2.Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: Dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl) 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- 3.Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–893. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- 4.Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol Biochem Behav. 1994;48:459–464. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 5.Bardo MT, Valone JM, Robinet PM, Shaw WB, Dwoskin LP. Environmental enrichment enhances the stimulant effect of intravenous amphetamine: Search for a cellular mechanism in the nucleus accumbens. Psychobiology. 1999;27:292–299. [Google Scholar]
- 6.Bardo MT, Bevins RA. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 7.Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 8.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrot M, Wallace-Black D, Bolaños CA, Graham D, Perrotti LI, Neve RL, et al. Regulation of anxiety and initiation of sexual anxiety by CREB in the nucleus accumbens. Proc Natl Acad Sci U S A. 2005;102:8357–8362. doi: 10.1073/pnas.0500587102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green TA, Alibhai IN, Hommel JD, DiLeone RJ, Kumar A, Theobald DE, et al. Induction of inducible cAMP early repressor (ICER) in nucleus accumbens by stress or amphetamine increases behavioral responses to emotional stimuli. J Neurosci. 2006;26:8235–8242. doi: 10.1523/JNEUROSCI.0880-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi KH, Whisler K, Graham DL, Self DW. Antisense-induced reduction in nucleus accumbens cyclic AMP response element binding protein attenuates cocaine reinforcement. Neuroscience. 2006;137:373–383. doi: 10.1016/j.neuroscience.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 15.Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: A review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 16.Marti J, Armario A. Effects of diazepam and desipramine in the forced swimming test: Influence of previous experience with the situation. Eur J Pharmacol. 1993;236:295–299. doi: 10.1016/0014-2999(93)90601-d. [DOI] [PubMed] [Google Scholar]
- 17.Schenk S, Partridge B. Influence of a conditioned light stimulus on cocaine self-administration in rats. Psychopharmacology (Berl) 2001;154:390–396. doi: 10.1007/s002130000608. [DOI] [PubMed] [Google Scholar]
- 18.Green TA, Schenk S. Dopaminergic mechanism for caffeine-produced cocaine seeking in rats. Neuropsychopharmacology. 2002;26:422–430. doi: 10.1016/S0893-133X(01)00343-8. [DOI] [PubMed] [Google Scholar]
- 19.Magalhaes A, Summavielle T, Tavares MA, de Sousa L. Effects of postnatal cocaine exposure and environmental enrichment on rat behavior in a forced swim test. Ann N Y Acad Sci. 2004;1025:619–629. doi: 10.1196/annals.1316.077. [DOI] [PubMed] [Google Scholar]
- 20.Wagner FA, Anthony JC. From first drug use to drug dependence. Developmental periods of risk for dependence upon marijuana, cocaine and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit and depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Friske JE, Gammie SC. Environmental enrichment alters plus maze, but not maternal defense performance in mice. Physiol Behav. 2005;85:187–194. doi: 10.1016/j.physbeh.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Green TA, Alibhai IN, Unterberg S, Ghose S, Tamminga CA, Neve RL, Nestler EJ. Induction of activating transcription factor 2 (ATF2), ATF3 and ATF4 in the nucleus accumbens and effects on behavior related to addiction, anxiety and depression. J Neurosci. 2008;28:2025–2032. doi: 10.1523/JNEUROSCI.5273-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry. 2001;49:753–762. doi: 10.1016/s0006-3223(00)01114-8. [DOI] [PubMed] [Google Scholar]
- 26.Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- 27.Huang FL, Huang KP, Wu J, Boucheron C. Environmental enrichment enhances neurogranin expression and hippocampal learning and memory but fails to rescue impairments of neurogranin null mutant mice. J Neurosci. 2006;26:6230–6237. doi: 10.1523/JNEUROSCI.1182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.