Abstract
Drug addicts have deficits in frontocortical function and cognition even long after the discontinuation of drug use. It is not clear, however, whether the cognitive deficits are a consequence of drug use, or are present prior to drug use, and thus are a potential predisposing factor for addiction. To determine if self-administration of cocaine is capable of producing long-lasting alterations in cognition rats were allowed access to cocaine for either 1 hr/day (short access; ShA) or 6 hrs/day (long access; LgA) for 3 weeks. Between 1 and 30 days after the last self-administration session we examined performance on a cognitively demanding test of sustained attention that requires an intact medial prefrontal cortex. The expression of dopamine D1 and D2 receptor mRNA and D2 protein levels in the prefrontal cortex were also examined. Early after the discontinuation of drug use LgA (but not ShA) animals were markedly impaired on the sustained attention task. Although the LgA animals improved over time, they continued to show a persistent pattern of performance deficits indicative of a disruption of cognitive flexibility up to 30 days after the discontinuation of drug use. This was accompanied by a significant decrease in DA D2 (but not D1) mRNA in the medial and orbital prefrontal cortex, and D2 receptor protein in the medial prefrontal cortex of LgA (but not ShA) animals. These findings establish that repeated cocaine use is capable of producing persistent alterations in the prefrontal cortex and in cognitive function, and illustrate the usefulness of extended access self-administration procedures for studying the neurobiology of addiction.
Keywords: self-administration, attention, addiction, dopamine receptor, medial prefrontal cortex, orbital frontal cortex, D2
Introduction
Cocaine addicts have deficits in cognitive function, including deficits on tests of short-term memory, attentional control, and decision-making, and these are evident even long after the discontinuation of drug use (Miller, 1985; Hoff, et al, 1996; Rosselli and Ardila, 1996; Bolla, et al, 1999). Many of these cognitive functions are known to require the prefrontal cortex, and structural and functional imaging studies suggest that indeed, prefrontal cortical function is impaired in addicts (Volkow, et al, 1988; Volkow, et al, 1991; Goldman-Rakic, 1995; Robbins, 1996; Bolla, et al, 1998; Liu, et al, 1998; Ernst, et al, 2002; Bolla, et al, 2003; Matochik, et al, 2003; Bolla, et al, 2004; Kosten, et al, 2006). It is not clear, however, whether these behavioral and neurobiological deficits precede drug use, and thus potentially contribute to the propensity for addiction, or are a consequence of drug use. It is difficult to address this issue in humans, but studies with non-human animals allow one to determine whether exposure to a drug of abuse is capable of producing changes in brain and behavior comparable to those seen in addicts.
Studies using non-human animals have shown that when psychostimulant drugs are administered by an experimenter they can produce both acute and persistent deficits in cognitive function (Crider et al., 1982; Jentsch et al., 2002; Kondrad and Burk, 2004; Schoenbaum et al., 2004; Burke et al., 2006). For example, Schoenbaum et al. (2004) reported that experimenter-administered cocaine produces persistent reversal deficits on an odor discrimination task mediated by the orbital frontal cortex in rats, and similar deficits have been seen in non-human primates (Jentsch et al., 2002). However, with one exception (Calu et al., 2007), experiments in which animals were allowed to self-administer cocaine have not resulted in persistent cognitive deficits. The self-administration of psychostimulant drugs is reported to produce cognitive deficits during the period of acute withdrawal, for a few days after the discontinuation of drug treatment, but these do not persist unless animals are given an additional drug challenge (Crider, et al, 1982; Kondrad and Burk, 2004; Dalley, et al, 2005a; Dalley, et al, 2005b; Kantak, et al, 2005). The absence of persistent deficits following cocaine self-administration in some studies may be due to the cognitive tasks utilized, or alternatively, the self-administration procedures used may not accurately model the addictive state.
Preclinical studies involving self-administration have largely used limited access procedures (Dalley, et al, 2005a; Dalley, et al, 2005b; Kantak, et al, 2005), but these may not produce some of the key changes in brain and behavior that characterize addiction. Recent reports suggest that extended access procedures are required to produce many symptoms characteristic of addiction, including escalation of drug-intake (Ahmed and Koob, 1998; Deroche-Gamonet, et al, 2004; Vanderschuren and Everitt, 2004; Ferrario, et al, 2005). We hypothesized, therefore, that extended (but not limited) access to self-administered cocaine may produce persistent deficits on a demanding task that probes attentional processes, and associated changes in prefrontal cortical dopamine function.
Our focus on the long-term consequences of self-administered cocaine specifically on attentional functions is based on evidence indicating marked impairments in attentional processes in psychostimulant abusers (e.g., Omstein, et al, 2000; Goldstein, et al, 2007), and on the view that persistent impairments in attentional processes and capacities fundamentally contribute to, or even underlie, deficits in executive functions (e.g., Posner and Rothbart, 2007; Sarter and Lustig, 2007) - deficits that are thought to contribute to addiction (Rogers, et al, 1999). Furthermore, repeated (experimenter-administered) amphetamine treatment has been shown to disrupt attentional performance on a task very similar to that employed here (Martinez, et al, 2005; Kozak, et al, 2007), indicating that the task should be adequately sensitive.
Materials and Methods
Subjects
One hundred and thirty-eight male Wistar rats (Harlan, Indianapolis, IL) weighing 200–225g at the start of the experiment were individually housed in square plastic hanging cages (8 × 9 × 8 cm). The animals were housed in a temperature and humidity controlled room with a 14:10 light/dark cycle, with water available ad libitum. Animals were food restricted throughout the experiment to maintain at least 90% of their free feeding body weight. All procedures were approved by the University of Michigan Committee on the Use and Care of Animals (UCUCA).
Apparatus
Behavioral training, testing, and drug administration took place in 16 operant chambers measuring 22 × 18 × 13 cm (Med Associates, St. Albans, VT, USA) located inside larger sound-attenuating chambers. For the sustained attention task, each operant chamber was equipped with a panel consisting of one light (2.8 W), two retractable levers, and a food pellet dispenser, all located on the front wall. A house light (2.8 W) was located close to the ceiling of the back wall. The floor of the chamber consisted of 19 stainless steel rods (4 mm in diameter) spaced 1.5 cm apart (center-to-center). For the drug self-administration procedure, each operant chamber had two nose poke holes equipped with cue lights. A tone (2900 Hz) was also available inside of the chamber. The floor of the chamber consisted of a solid piece of black plastic placed over the stainless steel rods. Med-PC for Windows software (v. 1.1, Med Associates) controlled all signal presentation, lever operation, pellet delivery, drug delivery, tone presentation and data collection via a Pentium PC.
Experiment I: Within-Subjects Study
Sustained Attention Task Training
For a detailed description of sustained attention procedures see McGaughy & Sarter (1995). The task required four phases of training. Phase I. All animals were shaped to lever press for food (45-mg banana flavored pellet, Bioserv, Frenchtown, NJ) utilizing a fixed ratio 1 (FR1) schedule such that one lever press resulted in the delivery of one food pellet. During this stage both of the levers were extended into the box at the beginning of each session and remained extended for the duration of the session. Training continued until the animals had at least three days of 120 reinforced lever presses. Phase II. After reaching this criterion, rats were then shaped to discriminate between signals (illumination of the central signal light for a period of 1 s) and non-signals (no illumination). For this training phase the start of each individual trial was indicated by the extension of both levers into the box. Response on one lever was reinforced if it followed a signal, while response on the other lever was reinforced if it followed a non-signal. The assignment of levers was counter-balanced across subjects to minimize side-bias. To increase the salience of the signal light and facilitate acquisition of the appropriate response, the house light was not illuminated during this training step. Signal and non-signal trials were presented pseudo-randomly with a mean intertrial interval (ITI) of 9±3 s. Four responses were possible during this second phase of training: hit, defined as the correct response to a signal; miss, the incorrect response to a signal; correct rejection, the correct response to a non-signal; and false alarm, the erroneous response to a non-signal (i.e., a claim for a signal following a non-signal; see Figure 1 for schematic). If the animal responded incorrectly, the previous trial was repeated up to three times. If a rat continued to respond incorrectly, it received a forced-choice trial in which only the correct lever was extended into the box while the signal was presented. Forced-choice trials also served to impede the generation of a side bias. Trials in which the rats failed to emit a response within 4 s of lever insertions were counted as omissions. Animals reached criterion for this portion of training when they responded correctly to 65% of the signal and non-signal trials for five consecutive days. Phase III. For the third stage of training the 1.0 s signal was replaced with three different signal lengths of 500, 50, and 25 ms, equally distributed across the session. Following correct responding to 65% of the 500 ms and non-signal trials, the final stage of training began. Phase IV. During the final stage of training, the house light was illuminated; this is a critical step in the acquisition of the sustained attention task as it forces animals to limit competitive behaviors and to closely monitor the intelligence panel in order to detect signal events. The final task consisted of three blocks of 54 trials, with nine trials of each signal length and 27 non-signal trials. The signal and non-signal trials were pseudo-randomized. Animals reached criterion when their performance became asymptotic at a level of at least 70% hits on the longest (500 ms) signal length trials, 70% correct rejections on the non-signal trials, and less than 30% omissions.
Figure 1.
Schematic illustration of the of the sustained attention task. Correct responses on signal trials (hits) and non-signal trials (correct rejections) were rewarded (arrows) and incorrect responses (misses and false alarms, respectively) were not.
Surgical Procedures
Individual animals reached performance criterion on the sustained attention task at different rates, and therefore cocaine self-administration and subsequent attention testing occurred at different times for each animal. After reaching criterion on the attention task, animals were anaesthetized with ketamine and xylazine anesthesia (77:1.5 mg/ml, intraperitoneal [IP], at 0.1 mL/100g of body weight) and a silicone catheter (Plastics One, Roanoke, VA) was inserted into the right jugular vein and passed subcutaneously to exit from the animals’ back (see Caine, et al, 1993). Animals were allowed to recover from surgery for a minimum of 3 days prior to drug administration. Catheters were flushed daily with 0.1 mL of gentamicin (50 mg/kg, in 0.9% sterile bacteriostatic saline).
Cocaine Self-Administration
Rats were transported from their home cage to an operant chamber 6 days a week for 4 weeks, where they were allowed to nose-poke for cocaine (0.4 mg/kg/infusion in 50 µL of saline administered over 1.6 s) on a continuous reinforcement schedule (FR1) with a time-out of 20 s. A training session commenced with the illumination of the “active” nose-poke hole stimulus light. Responding in this hole resulted in drug delivery. Responding in the other nose-poke hole, designated inactive, had no consequences. Rats were initially trained during daily one hour sessions for a total of six days. Animals that did not acquire stable self-administration behavior (at least 5 infusions each day for 3 consecutive days) were removed from the study. After the initial 6-day training period animals were divided into two groups: a long access group (LgA) and a short access group (ShA). These groups were balanced according to both the amount of drug they administered during the first week of training and their performance during attention training. Animals in the ShA group continued to receive one-hour test sessions, whereas animals in the LgA group received six-hour test sessions for an additional 16–17 sessions. A third group of rats (no-drug control group) received sham surgery and were transported each day to a novel test room where they were placed into Plexiglas chambers, similar to the operant chambers.
Sustained Attention Testing
One day following four weeks of drug self-administration (or transport in the no-drug control groups), animals were retested on the sustained attention task (n=12–16). Animals were tested again two weeks after the last self-administration session, followed by daily retraining for 1 week following the test on day 14.
Experiment II: Between-Subjects Study
Experiment II utilized a between-subjects design to control for potentially confounding effects of repeated testing on the performance on the attention task in Experiment I. This study also examined performance on the sustained attention task one month after the last self-administration session to determine the persistence of any drug-induced cognitive deficits. Along with the behavioral analysis, Experiment II was also designed to examine changes in the expression of dopamine D1 and D2 receptor mRNA in the prefrontal cortex.
The methods for sustained attention task training and drug self-administration were identical to those in Experiment I.
Sustained Attention Testing
Following the completion of self-administration, animals in the No Drug, ShA, and LgA groups were divided into two more groups. One group (n=5–6) was tested on the attention task 1 day following the last self-administration session and another independent group (n=6–8) was tested 30 days following the last self-administration session.
Tissue Collection
Three days following their respective sustained attention testing session (at days 4 & 33 following the last self-administration session) all animals were decapitated and their brains removed and frozen in isopentane (−40 to −50°C) on dry ice. The brains were then stored at −80°C. Coronal brain sections (10 µm) were cut on a cryostat at 200 µm intervals, thaw mounted on Superfrost/Plus slides (4 sections per slide, Fisher Scientific, Pittsburgh, PA) and stored at −80°C until further processing.
In Situ Hybridization
For detailed methodology of the in situ hybridization techniques used see Kabbaj et al. (2000). Following fixation, sections were hybridized with 35S-labeled cRNA probes produced using standard in vitro transcription methodology. Prefrontal cortex sections were processed for in situ hybridization of dopamine D1 and D2 receptor mRNA (480-mer). The probes were diluted in hybridization buffer and slides were coverslipped and incubated overnight at 55°C. Following rinses and dehydration, slides were exposed to x-ray film (Kodak Biomax-MR, Eastman Kodak, Rochester, NY) for 2 weeks. The specificity of the hybridization signal was confirmed with sense probe controls (data not shown).
Quantification
Autoradiographs were digitized using a Scanmaker 1000XL scanner (Microtek, Carson, CA) and the magnitude of the signal of the radioactive probes was determined using Scion Image (Scion Corporation, Frederick, MD). A macro was used to automatically determine signal above background in each brain region (written by Dr. S. Campeau, University of Colorado, Boulder, CO). A background value was obtained from a white matter region such as the corpus callosum in each section and only values exceeding the background by 3.5 times the standard deviation were included in the analysis. Results are expressed as the mean integrated density of signal pixels divided by the total number of pixels in the selected region. The person quantifying was blind to group assignments. Anatomical regions were identified and subdivided for densitometric analysis according to the stereotaxic atlas of Paxinos and Watson (Paxinos and Watson, 1998). More specifically, the medial prefrontal cortex included the cingulate, prelimbic, and infralimbic subregions of the frontal cortex at 2.7mm anterior from bregma, as defined by Paxinos and Watson (1998). The orbital frontal cortex included the medial, lateral, and ventral subregions, at 4.2mm anterior to bregma, as defined by Paxinos and Watson (1999).
Experiment III. D2 Receptor Protein
Experiment III was designed to examine the effects of limited vs. extended access to self-administered cocaine on levels of DA D1 and D2 receptor protein in the prefrontal cortex. The methods for drug self-administration were identical to those in Experiment I.
Tissue Collection and Sample Preparation
Three or thirty days following their final self-administration session animals were decapitated and their brains removed. The entire prefrontal cortex was dissected, fast frozen on dry ice and kept at −80°C until further processing. Frozen brain tissue was homogenized (1:10, wt/vol) in cold 50 mM Tris-HCl buffer, pH 6.8, containing 2% SDS, 1 mM EDTA, and various protease inhibitors as previously reported (Garcia-Fuster et al., 2007). Aliquots of total homogenate were mixed with equal volumes of electrophoresis loading buffer, denatured and stored at −20°C until use. Protein concentrations were determined by the BCA Protein Assay Reagent (Pierce Biotechnology, Rockford, IL, USA).
Western Blot Analysis
For western blot analysis, 33 µg of each protein sample (determined to be within the linear range for immunobloting of D2DR) was separated by electrophoresis on 10% acrylamide/bisacrylamide minigels and transferred electrophoretically to nitrocellulose membranes. The membranes were subsequently blocked for 1 hour at room temperature in phosphate buffered saline (PBS, in mM: 137 NaCl, 2.68 KCl, 10.14 Na2HPO4, 1.76 KH2PO4) containing 0.2% Tween-20, 0.5% bovine serum albumin (BSA), and 5% non-fat dry milk. Incubation with the primary antibodies (mouse anti-dopamine D2 receptor, 1:400 dilution, Santa Cruz Biotechnology, Inc., Santa Cruz, CA; mouse anti-dopamine D1 receptor, 1:1000 dilution, Santa Cruz Biotechnology Inc, Santa Cruz; mouse anti-β-actin, 1:10,000 dilution, Sigma-Adrich, St. Louis, MO) was performed overnight at 4°C. After extensive washing in PBS the membranes were incubated with the secondary antibody, horseradish peroxidase-linked anti-mouse IgG, (1:5,000 dilution) in blocking solution at room temperature for 1 h. Immunoreactivity of target proteins was detected with the ECL Western Blot Detection system (Amersham International, Buckinghamshire, UK) and visualized by exposure to Hyperfilm ECL film (Amersham) for 60 s to 60 min. The autoradiograms were then quantified by densitometric scanning (IOD) using Image J software. Each protein sample was run on 3–4 gels and an average value for each animal was calculated. The amount of target protein in brain samples of treated rats was compared to an internal control sample in the same gel composed of prefrontal cortices from 3 control rats that had not received any treatment. This internal control was run 3 times on each gel and an average was calculated for comparison with other samples on that gel. Finally, percent changes in immunoreactivity with respect to internal controls (100%) were calculated for each sample in each of the various gels. In all the experiments the content of b-actin, a cytoskeletal protein not altered in brain by cocaine treatments (Imam, et al, 2005), was quantified as a loading control.
Statistical Analyses
Cocaine Self-Administration
The number of infusions for the first hour of each session and for the entirety of the session was determined for each day of self-administration. Two-way repeated measures ANOVAs were performed with escalation session (1–16) as the repeated variable, drug group (ShA, LgA) as the independent variable and first hour or total infusions as the dependent variable. For all analyses, alpha was set at 0.05.
Sustained Attention
For each sustained attention session, the number of hits, misses, correct rejections, false alarms, and omissions was calculated. To examine each animal’s accuracy on the task, the relative number of hits [hits/(hits + misses)] for each signal length, and the relative number of correct rejections [correct rejections/(correct rejections + false alarms)] were calculated. In addition the vigilance index (VI), an overall measure of sustained attention performance, was calculated using the relative number of hits (h) and false alarms (fa) with the following formula: VI= (h-fa) / [2(h+fa)−(h+fa)2]. This index is similar to the sensitivity index (Frey and Colliver, 1973) except that omitted trials are excluded from the calculation. The VI provides a sensitive measure of overall performance, taking into account both trial types; VI varies between 1 and −1, with 1 indicating that all responses were hits and correct rejections, 0 that responses were randomly distributed over the four response types (hits, misses, false alarms, correct rejections), and −1 that all responses were misses and false alarms. Performance on each of the trial types (signal vs. non-signal) was also examined to reveal the specific responses contributing to the overall deficits. Two-way ANOVAs were performed with withdrawal day (1 or 30) and drug group (ND, ShA, LgA) as the independent variables and VI or the relative number of hits or correct rejections as the dependent variables. When significant main effects or interactions were revealed, individual group comparisons were made using either additional ANOVAs, or Fisher’s post-hoc tests.
Gene Expression
The levels of mRNA in the experimental groups were expressed as a percent of the mean value for the control group. Group differences in gene expression were assessed using two-way ANOVAs with withdrawal time (4 or 33 days) and drug group (ND, ShA, LgA) as the independent variables and percent of control mRNA levels as the dependent variable. Fisher’s post-hoc comparisons were conducted when main effects or interactions were present.
Western Blot Analysis
The levels of D1 and D2 receptor protein were expressed as a percent of internal control values for each individual gel (see above). Group differences in protein expression were assessed using a two-way ANOVA with withdrawal time (3 or 30 days) and drug group (ND, ShA, LgA) as the independent variables and percent of control protein levels as the dependent variable.
Results
Experiment I: Within-Subjects Study
Cocaine Self-administration
Following the initial 6-day training period ShA animals continued with 1-hour self-administration sessions and LgA animals were given 6 hr daily sessions. When examining the number of infusions taken during the first hour of each of these sessions (Figure 2A), both the ShA [One-way ANOVA, day, F(15,240)=1.86, p=.03] and LgA groups [day, F(15,224)=4.89, p<.0001] animals increased their cocaine intake over time. However, the effect was much greater in the LgA group than the ShA group, as indicated by a significant group by time interaction [main effect of group, F(1,15)=16.49, p=0.0003, main effect of day, F(15,435)=12.87, p<0.0001, group by day interaction, F(15,435)=2.90, p=0.0002]. In addition, the LgA animals exhibit a significant increase in the number of infusions over the entire session over the course of self-administration (Figure 2B).
Figure 2.
A. Mean (+/− SEM) number of cocaine infusions during the first hour of each self-administration session in Experiment I. Both the short (ShA, 1 hr sessions) and the long (LgA, 6 hr sessions) access groups increased their intake over time, but this effect was significantly greater in the LgA group than the ShA group. B. The mean (+/− SEM) number of cocaine infusions over the entire session for LgA and ShA groups.
Sustained Attention Performance
Animals were trained on the sustained attention task for approximately 8 weeks prior to drug self-administration and animals assigned to the LgA, ShA and no-drug groups were matched based on training performance (Figure 3 a & d). Twenty-four hours following the final self-administration session animals in the LgA group were markedly impaired on the sustained attention task, as illustrated by a significant decrease in VI relative to no-drug controls. In contrast, the ShA group did not differ from control [Overall ANOVA, main effect of group, comparing all groups, F(2,80)= 11.49, p=0.0001, group by signal length interaction, F(4,80)= 5.68, p=.0004. Individual group comparisons: LgA vs. ND, group, F(1,50)= 17.13, p=0.0003, interaction, F(2,50)=11.59, p<.0001; LgA vs. ShA, group, F(1,58)=12.56, p=0.0014, interaction, F(2,58)=4.36, p=0.0172; Figure 3b]. At this point in time the performance of animals in the LgA group was impaired with respect to their ability to detect signals as well as to respond correctly to non-signal events [Signal Trials Overall ANOVA, interaction, F(4,90)= 6.92, p<.0001. Individual group comparisons: LgA vs. ND, F(2,50)=13.57, p<.0001; LgA vs. ShA, F(2,58)=5.37, p=0.007. Non-signal Trials ANOVA, F(2,40)=4.28, p=0.02; *, differs from ShA and No Drug groups as determined by Fisher’s Tests; Figure 3e]. Fourteen days following the last self-administration session the LgA animals were still impaired relative to the control group, as indicated by a significant decrease in VI [Overall ANOVA, group, F(2,80)= 4.78, p=.01, interaction, F(4,80)= 4.28, p=0.004. Individual group comparisons: LgA vs. ND, group, F(1,50)=5.48, p=.028; LgA vs. ShA, group, F(1,58)=5.69, p=0.016; Figure 3c]. However, by 14 days of withdrawal the deficit was due primarily to an increase in the number of false alarms (“claims” for signals on non-signal trials), as there was no longer a significant effect of prior self-administration on signal trial performance.
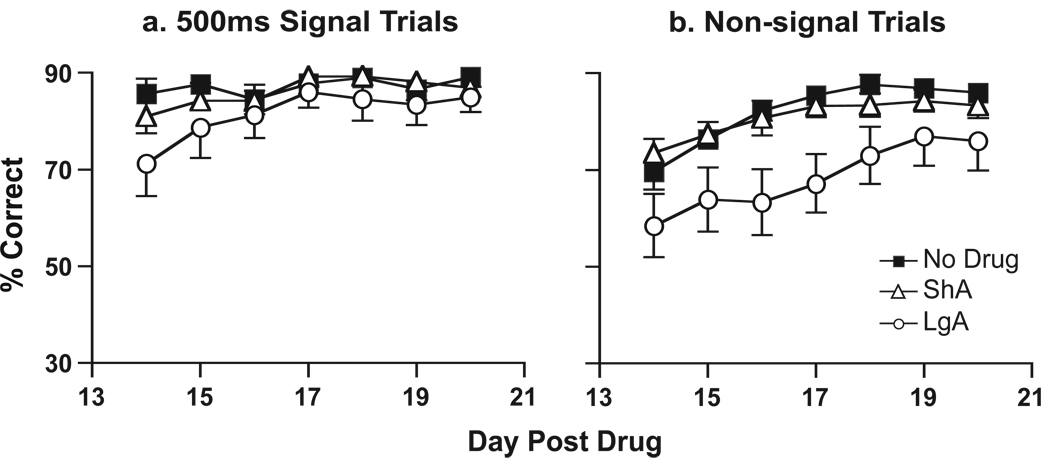
Figure 3.
The effect of extended vs. limited access to self-administered cocaine on performance on the sustained attention task as a function of time following the last self-administration session. The top three panels show the data expressed as a vigilance index (VI), which provides an overall index of performance incorporating both signal and non-signal trials (see Methods). The bottom three panels show the percent correct responses for signal trials (as a function of signal duration; line graph) and non-signal trials (bar graph) separately. The panels on the left (a, d) show performance on the last day of training, prior to any cocaine self-administration experience, in the animals that will eventually be given limited (ShA) or extended (LgA) access to cocaine, or no treatment (No Drug). The groups were balanced to equate performance at the end of training, so there are no group differences on any measure at this point in time. The panels in the middle (b, e) show performance one day following the final self-administration session. Panel b shows that at this time there was a significant decrease in VI in the LgA group relative to both the No Drug and ShA groups, which did not differ from one another. Panel e shows that the LgA group performed more poorly than either the ShA or No Drug groups on both signal and non-signal trials, and the ShA and No Drug groups did not differ from one another on either trial type (* differs from ShA and No Drug groups as determined by Fisher’s Tests). The panels on the right (c, f) show performance when the same animals were retested 14 days following the final self-administration session. Panel c shows that at this time there was still a significant decrease in VI in the LgA group, relative to both the No Drug and ShA groups. However, by this time the deficit was primarily a function of impaired performance on non-signal trials (see Panel f, * differs from ShA and No Drug groups as determined by Fisher’s Tests).
Following the test on day 14, animals were tested daily for an additional 7 days. Throughout this retraining period, there were no group differences on signal trials, i.e., in the relative number of hits (Figure 4a). However, the LgA group continued to show a significant deficit on non-signal trials relative to the control and ShA groups throughout this period [Overall ANOVA, group, F(2,240)= 3.80, p=0.03. Individual group comparisons: LgA vs. ND, group, F(1,150)=4.15, p=0.05, LgA vs. ShA, group, F(1,174)=4.1, p=0.05; Figure 4b]. It is important to note that there were no group differences in either response latencies or the number of omissions at any point in time, indicating that all animals were equally motivated to perform the task (Figure 5).
Figure 4.
The effect of one week of “retraining” on the sustained attention task beginning 14 days after the last self-administration session. Data are shown as percent correct responses (a) on signal trials in which the signal duration was 500 msec, and (b) on non-signal trials. There were no group differences on signal trials over this period of time. However, on non-signal trials the LgA group showed significantly fewer correct responses than either of the other two groups.
Figure 5.
The effect of extended vs. limited access to self-administered cocaine on response latency and omission rate (Mean ± SEM) as a function of time following the last self-administration session in Experiment I. There were no group differences on either measure on either day.
Experiment II. Between-subjects study
Cocaine Self-administration
As in Experiment I, ShA and LgA groups were balanced to match drug intake during the initial 6-day self-administration training period and ShA animals continued with 1-hour self-administration sessions and LgA animals were given 6 hr daily sessions for 3 weeks. When examining the number of infusions taken during the first hour of each of these sessions (Figure 6a), LgA animals showed a progressive increase in intake over time [main effect of drug condition, F(1,768)=19.92, p<0.0001, main effect of day, F(16,768)=6.05, p<0.0001, interaction, F(16,768)=3.11, p<0.0001, pair-wise comparisons, p<0.05 (LgA vs. ShA, days 8–17)]. In addition, the LgA animals exhibit a significant increase in the number of infusions over the entire session over the course of self-administration (Figure 6B). In this experiment there was no significant change in drug intake in the ShA group over days of testing (One-way ANOVA, day, F(16,340)=.64, p=.85; Figure 6).
Figure 6.
A. Mean (+/− SEM) number of cocaine infusions during the first hour of each self-administration session in Experiment II. The LgA group significantly increased their intake over time, but there were no significant changes in cocaine intake over time in the ShA group. B. The mean (+/− SEM) number of cocaine infusions over the entire session for LgA and ShA groups.
Sustained Attention Performance
All groups were balanced based on their attention training performance (Figure 7a & d). As in Experiment 1, when tested 24 hours after the final self-administration session animals in the LgA group were markedly impaired on the sustained attention task, as indicated by a significant decrease in VI [Overall ANOVA, group, F(2,26)=6.06, p=0.01. Individual group comparisons: LgA vs. ND, group, F(1,16)=32.49, p=0.0005; LgA vs. ShA, group, F(1,18)=4.1, p=0.05; Figure 7b]. This was due to a decrease in performance on both signal and non-signal trials, although only the effect on signal trials was statistically significant [Overall ANOVA, group, F(2,39)= 5.6, p=0.007. Individual group comparisons: LgA vs. ND, group, F(1,24)=7.7, p=0.01; LgA vs. ShA, group, F(1,27)=5.4, p=0.028; Figure 7e]. The absence of a significant effect on non-signal trials, as seen in Experiment I, was probably due to a lack of power because a relatively small number of animals were tested at this time point. A larger independent group of LgA animals was tested 30 days after the last self-administration session, and these animals were significantly impaired on non-signal, but not signal trials [Overall ANOVA, F(2,29)= 3.37, p =0.04; Figure 7f]. There were no differences between the ShA and control groups at either point in time. Also, as in Exp. I, the LgA group did not differ from controls in either response latencies or rate of omissions at either time point examined (data not shown).
Figure 7.
The effect of extended vs. limited access to self-administered cocaine on performance on the sustained attention task as a function of time following the last self-administration session in Experiment 2. The data are presented as described for Figure 3. As in Experiment I, there was a significant decrease in VI in the LgA group relative to both the No Drug and ShA groups when animals were tested one day after the last self-administration session, and the latter two groups did not differ from one another (Panel b). Panel e shows that the LgA group made significantly fewer correct responses on signal trials, but the effect on non-signal trials was not statistically significant, presumably because a relatively small number of animals were tested on this day. The panels on the right (c, f) show the performance of an independent group of animals tested for the first time 30 days following the final self-administration session. At this time there were no significant group differences on VI. Panel f shows that, as in Experiment I, when tested long after the last self-administration session the LgA group was not impaired in their performance on signal trials but was impaired on non-signal trials (* differs from ShA and No Drug groups as determined by Fisher’s Tests).
Influence of Instrumental Experience
In Experiment I and II the LgA group spent 6 hours per day actively engaged in an operant task during the 4 week self-administration period, whereas the ShA group only spent 1 hour per day and the ND animals none. It is possible, therefore, that group differences in time spent performing this instrumental response could in some way have contributed to group differences in performance when the animals were retested on the sustained attention task. To address this issue we conducted a separate control experiment in which rats were given extended experience (3 weeks) making a similar instrumental response (nose-poking for a food reward), and then 24 hrs later retested on the sustained attention task. There was no effect of 3 weeks of training on a food-rewarded instrumental task (relative to controls that did not work for food) when animals were re-exposed to the sustained attention task 24 hrs following the last session of instrumental training (data not shown). We conclude, therefore, that group differences in the time spent simply making an instrumental response during the self-administration phase of Experiments I and II were not responsible for the subsequent group differences in performance on the sustained attention task.
Dopamine Receptor mRNA Expression in the Prefrontal Cortex
Brains were obtained for in situ hybridization histochemistry 3 days after the final test on the sustained attention task, which for one group was 4 days and for the other group 33 days following the last self-administration session. We quantified DA receptor mRNA expression in the two main sub-regions of the prefrontal cortex, the medial prefrontal cortex (cingulate, prelimbic, and infralimbic cortices) and the orbitofrontal cortex (medial, ventral, and lateral orbital cortices), as defined by Paxinos and Watson (1999). Medial Prefrontal Cortex. There were no differences between the control and ShA groups in D2 mRNA expression in the medial prefrontal cortex at either time point (Figure 8a). In contrast, relative to the control group, the LgA animals showed a significant decrease in D2 mRNA expression in the medial prefrontal cortex, which was evident at both 4 and 33 days after the last self-administration session [Overall ANOVA, group, F(2,24)=3.87, p=0.03; Individual group comparisons: LgA vs. ND, group, F(1,15)=6.94, p=0.02, LgA vs. ShA, group, F(1,16)=7.99, p=0.01; Figure 8a]. There were no group differences in levels of D1 receptor mRNA in medial prefrontal cortex (Figure 8b). Orbitofrontal Cortex. As in the medial prefrontal cortex, the LgA group (but not the ShA group) exhibited a decrease in D2 receptor mRNA in the orbitofrontal cortex at both withdrawal times [D2 Overall ANOVA, group, F(2,22)=3.48, p=0.05; Individual group comparisons: LgA vs. ND, group, F(1,14)=5.66, p=0.03, LgA vs. ShA, group, F(1,15)=5.97, p=0.03; Figure 9a] and no alterations in D1 receptor mRNA expression (Figure 9).
Figure 8.
Dopamine D2 receptor (a) and D1 receptor (b) mRNA expression in the medial prefrontal cortex of rats allowed extended (LgA) or limited (SgA) access to self-administered cocaine, and then examined either 4 or 33 days after the last self-administration session. The data represent the mean (+ SEM) levels of mRNA in the LgA and SgA groups expressed as a percent of the average amount of mRNA in the No Drug control group (represented by the horizontal dashed lines). The LgA group showed a significant decrease in D2 receptor mRNA expression, relative to both the No Drug control group and the ShA group, which did not differ from one another (*, differs from ShA and No Drug groups). There were no group differences in D1 mRNA expression in the medial prefrontal cortex.
Figure 9.
Dopamine D2 receptor (a) and D1 receptor (b) mRNA expression in the orbital prefrontal cortex of rats allowed extended (LgA) or limited (ShA) access to self-administered cocaine, and then examined either 4 or 33 days after the last self-administration session. The data represent the mean (+ SEM) levels of mRNA in the LgA and ShA groups expressed as a percent of the average amount of mRNA in the no drug control group (see horizontal dashed lines). The LgA group showed a significant decrease in D2 receptor mRNA expression in the orbitofrontal cortex relative to both the ShA and control animals at both withdrawal time points. There were no significant group differences in D1 receptor mRNA expression.
Brain Behavior Correlations
Correlational analyses were used to examine the relationship between sustained attention performance and dopamine receptor mRNA levels. The VI score was used to take into account both trial types in the attention task. There was significant positive correlation between performance and D2 receptor mRNA levels in the prefrontal cortex (r = 0.36, p = 0.05; data not shown). There were no significant correlations between D1 mRNA expression and performance.
Experiment III. Dopamine Receptor Protein in Medial Prefrontal Cortex
Cocaine Self-administration
As in Experiment I & II, ShA and LgA groups were balanced to match drug intake during the initial 6-day self-administration training period. Animals allowed extended access to cocaine showed a progressive increase in both their total intake and their intake during the first hour of each session [main effect of drug condition, F(1,476)= 64.24, p<0.0001, main effect of day, F(17,476)=6.61, p<0.0001, interaction, F(17,476)= 6.97, p<0.0001, pair-wise comparisons, p<0.05, LgA vs. ShA, escalation days 7–18)]. As in Exp. II, the ShA group did not significantly change drug intake over time [One-way ANOVA, day, F(17,270)=1.57, p=.07]. These data are similar to those described in Experiment I (Figure 2) and Experiment II (Figure 6) and are therefore not shown.
Western Blot Analysis
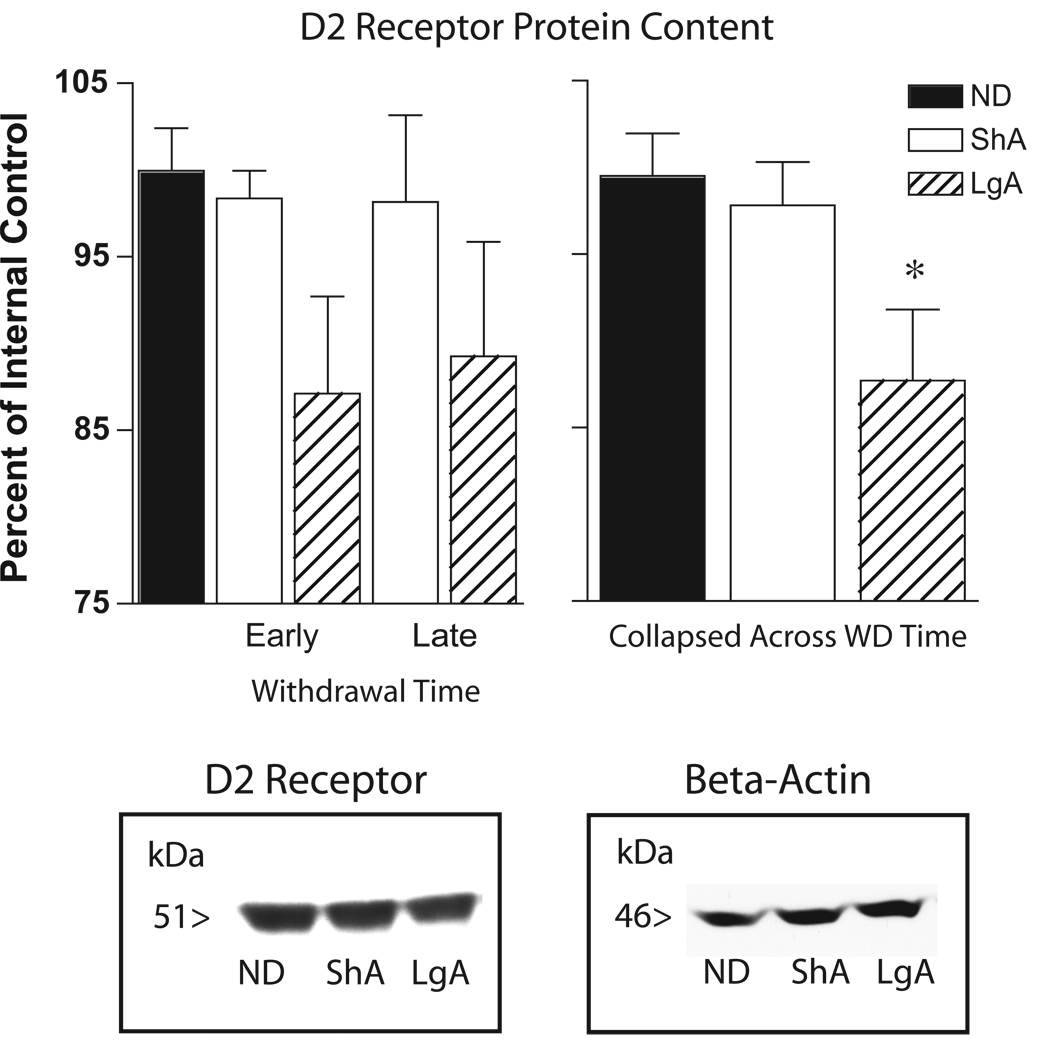
Analysis of the amount of D2 receptor protein in the prefrontal cortex resulted in a significant main effect of group but no effect of time or a group by time interaction [Overall ANOVA, group, F(2,42)=4.08, p=0.02, withdrawal time, NS, interaction, NS; see Figure 10). Further analysis indicated that this was because the LgA group showed a small but statistically significant decrease in D2 receptor protein relative to the Control and ShA groups, which did not differ from one another [Individual group comparisons: LgA vs. ND, group, F(1,28)=6.71, p=0.02, LgA vs. ShA, group, F(1,25)=4.34, p=0.05]. There were no group differences in the amount of D1 receptor protein in the prefrontal cortex (data not shown).
Figure 10.
Dopamine D2 receptor protein levels in the medial prefrontal cortex of rats allowed extended (LgA), limited (ShA) or no (ND) access to self-administered cocaine examined either 3 or 30 days after the last self-administration session. The data represent the mean (+SEM) of D2 receptor protein levels relative to an internal control. The LgA group showed a significant decrease in D2 receptor protein levels, relative to both the No Drug control group and the ShA group, which did not differ from one another. As there was a significant effect of group, but no effect of withdrawal time and no group by time interaction, the data were collapsed across the two time points to demonstrate that the group effect is due to a decrease in LgA group (panel b; * differs from ShA and No Drug groups). No group differences were seen in a beta-actin control.
Discussion
Effects of past experience with self-administered cocaine on cognitive function
The day after the discontinuation of cocaine self-administration, during acute withdrawal, animals that had extended access to self-administered cocaine (LgA) had a profound deficit on the sustained attention task, as indicated by a large decrease in the vigilance index (VI), and by impairments on both signal and non-signal trials. In contrast, animals that had limited access to cocaine (SgA) showed no deficits. With time (within 14 days) performance on signal trials recovered in the LgA group. However, the deficit on non-signal trials persisted for at least 30 days. Experimenter-administered cocaine has been reported to produce persistent cognitive deficits in both rats (Schoenbaum, et al, 2004; Burke, et al, 2006) and monkeys (Jentsch, et al, 2002), but other than the present study, and a recent study by Calu and colleagues (2007) showing persistent effects of self-administered cocaine on reversal learning, most studies utilizing cocaine self-administration procedures have not found evidence of persistent cognitive deficits.
It is not clear why evidence of persistent deficits in cognitive function following cocaine self-administration have been elusive. However, most previous studies involved limited access procedures and it is possible that these do not produce some of the changes in brain and behavior characteristic of addiction. Indeed, accumulating evidence suggests that extended access to cocaine has a greater likelihood of producing a number of symptoms of addiction, relative to limited access, one of which is the tendency to escalate drug intake (Ahmed and Koob, 1998; Paterson and Markou, 2003; Deroche-Gamonet, et al, 2004; Vanderschuren and Everitt, 2004; Ferrario, et al, 2005). However, in one previous study rats were allowed to self-administer a maximum of 150 injections of cocaine over 8 hours and these animals achieved a level of intake comparable to the LgA group here, but did not show persistent deficits on a 5-choice serial reaction time task (2005a). There are at least two possible reasons for this. One, the self-administration procedure used by Dalley et al. (2005a) may not have resulted in an escalation of drug intake or other changes in brain and behavior characteristic of addiction. However, a more likely reason is that in the 5-choice serial reaction time task all trials are signal trials, in that animals are required to report the location of a brief visual stimulus. We also saw only a transient deficit on signal trials, and in this sense the results reported here are consistent with those reported by Dalley and his colleagues (2005a; 2005b).
In the present study there was a persistent deficit only on non-signal trials, and therefore, it is important to consider how the cognitive demands of the task used here differ as a function of trial type, and what this might tell us about the nature of the deficit. First, it is important to emphasize that there were no group differences in omissions or response latencies in any of the experiments, suggesting that the animals were equally motivated to perform and did not suffer from obvious sensorimotor deficits. In contrast, in studies using the 5-choice serial reaction time task drug-treated animals did show a significant increase in omissions and response latencies, complicating interpretation of the nature of their deficit (2005a; 2005b). In addition, after the initial withdrawal period LgA animals’ signal trial performance was normal suggesting that the deficits are not attributable to a failure to retrieve the rules governing the responses in the two types of trials. Nevertheless, on non-signal trials the LgA animals generated an abnormally high number of false alarms, reporting a signal had occurred when it had not. Although more work is required to determine the exact nature of this deficit, the behavioral and cognitive operations underlying performance during non-signal trials certainly differ from those governing signal trials, and/or tasks that involve exclusively signal trials. On signal trials the correct response is prompted by the presence of a visual stimulus and animals typically select the correct lever side and await lever insertion to respond. On non-signal trials, however, animals must first probe their memory as to whether or not a signal occurred before initiating an action. This may not only be more demanding in terms of taxing memory, but it also represents a more ambiguous situation, as the decision that a signal did not occur would be expected to be associated with high levels of uncertainty and therefore a reduced response criterion. Finally, performance on this task also taxes cognitive flexibility, which is required to shift constantly between the processing of the conflicting response rules governing the unpredictable occurrence of trials with or without signal events. Therefore, we hypothesize that prior cocaine use may have produced a persistent impairment in cognitive flexibility, which is required to respond correctly in trials involving high levels of uncertainty about signal status (see also Yu & Dayan 2005), while leaving the ability to respond correctly to a signal intact.
Effects of past experience with self-administered cocaine on brain dopamine
Perhaps the most striking finding in the present study was that extended (but not limited) access to self-administered cocaine decreased dopamine D2 (but not D1) receptor mRNA expression in both the medial and orbital prefrontal cortices and this effect persisted for at least 34 days after the last self-administration session. Furthermore, there was a small but statistically significant decrease in levels of D2 receptor protein in the prefrontal cortex of LgA but not ShA animals at both points in time after the last exposure to cocaine. There has been relatively little research on the long-term effects of cocaine on D2 receptors in the frontal cortex. However, Nogueira et al. (2006) recently reported that experimenter-administered cocaine produces a persistent (2–4 weeks) decrease in, “D2-mediated regulation of cortical excitability” (p. 12308), which may be related to the alterations in D2 receptor mRNA and protein described here, and to the well-documented “hypofrontality” seen in cocaine addicts (Bolla, et al, 2003; Bolla, et al, 2004) and in animals treated repeatedly with cocaine (Sun and Rebec, 2005; Beveridge, et al, 2006; Sun and Rebec, 2006). The decreases in D2 receptor mRNA in the orbitofrontal cortex are particularly relevant in light of data demonstrating persistent deficits in reversal learning following cocaine administration, a task thought to be dependent on the orbitofrontal cortex (Schoenbaum, et al, 2004; Calu, et al, 2007).
These data suggest, therefore, that self-administered cocaine is capable of producing a marked and persistent dysregulation of dopamine function in the prefrontal cortex, which may represent another manifestation of marked frontal cortical dysfunction associated with cocaine addiction. Given that this effect was only seen in animals that received extended access to cocaine it is possible that dysregulation of dopamine D2 receptor signaling contributes to the cognitive deficit, which was also only seen in the LgA group. The integrity of D2 receptor function is closely associated with the ability to manipulate acutely relevant information in prefrontal regions (Wang et al. 2004) and inhibition of D2 receptor activation in both humans and animals, either by antagonist administration or genetic manipulation, results in a decrease in the acquisition of working memory task rules (Glickstein, et al, 2005), attentional set-shifting (Tost, et al, 2006), and spatial working memory (Von Huben, et al, 2006; Rinaldi, et al, 2007). This is consistent with the idea that cocaine impaired cognitive flexibility primarily by disrupting prefrontal D2 receptor function. Finally, we found no effect of cocaine self-administration experience on D1 mRNA expression in the prefrontal cortex, consistent with the recent report that experimenter-administered cocaine has no effect on D1 receptor sensitivity in this region (Nogueira, et al, 2006).
Although the decrease in D2 mRNA in medial prefrontal cortex was large (~70%), the decrease in protein was much more modest (~10%). Discrepancies between mRNA and protein levels are not uncommon (Mansour, et al, 1990; Mansour, et al, 1991) and may be due to a number of different factors. For example, D2 receptor mRNA is localized primarily in the cell bodies of prefrontal neurons but D2 receptor protein is present in both presynaptic and postsynaptic elements, and therefore the population of neurons sampled by the two measures may be quite different. More research is needed to determine if the decreases seen in the current study are primarily due to changes in a specific cell type. It is also possible that rather than reflecting a cell type specificity, the dissociation between mRNA and protein is due to alterations in the translation of mRNA into protein. Nevertheless, despite the difference in the magnitude of the effect of cocaine on mRNA versus protein, both were decreased, indicating a potential persistent dysregulation of D2 function in the prefrontal cortex of animals that had extended access to self-administered cocaine.
Conclusions
Extended, but not limited, access to self-administered cocaine produced a persistent deficit on a sustained attention task indicative of reduced cognitive flexibility, and this was accompanied by a persistent decrease in both dopamine D2 receptor mRNA expression and D2 protein levels in the medial prefrontal cortex, and of D2 mRNA in the orbitofrontal cortex. These findings are consistent with the hypothesis that similar deficits in brain and cognitive function seen in addicts may be a consequence of drug use, rather than just reflecting a premorbid condition. Furthermore, the fact that these changes in brain and behavior were only evident in animals allowed extended assess to self-administered cocaine suggests that this procedure may be especially valuable in studying the neurobiology of addiction.
Acknowledgements
We thank Sharon Burke, Jeffrey P. Gross, James M. Dell’Orco and Tracy Simmons for technical assistance.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci. 2006;23:3109–3118. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. Journal of Neuropsychiatry & Clinical Neurosciences. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Cadet JL, London ED. The neuropsychiatry of chronic cocaine abuse. J Neuropsychiatry Clin Neurosci. 1998;10:280–289. doi: 10.1176/jnp.10.3.280. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. JNeuropsychiatry ClinNeurosci. 1999;11:361–369. doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke KA, Franz TM, Gugsa N, Schoenbaum G. Prior cocaine exposure disrupts extinction of fear conditioning. Learn Mem. 2006;13:416–421. doi: 10.1101/lm.216206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Lintz R, Koob GF. Intravenous drug self-administration techniques in animals. In: Sahgal A, editor. Behavioral Neuroscience, a practical approach. New York: Oxford University Press; 1993. pp. 117–143. [Google Scholar]
- Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem. 2007;14:325–328. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider A, Solomon PR, McMahon MA. Disruption of selective attention in the rat following chronic d-amphetamine administration: relationship to schizophrenic attention disorder. BiolPsychiatry. 1982;17:351–361. [PubMed] [Google Scholar]
- Dalley JW, Laane K, Pena Y, Theobald DE, Everitt BJ, Robbins TW. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology (Berl) 2005a;182:579–587. doi: 10.1007/s00213-005-0107-3. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Berry D, Milstein JA, Laane K, Everitt BJ, Robbins TW. Cognitive sequelae of intravenous amphetamine self-administration in rats: evidence for selective effects on attentional performance. Neuropsychopharmacology. 2005b;30:525–537. doi: 10.1038/sj.npp.1300590. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat.[see comment] Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Frey PW, Colliver JA. Senstivity and responsivitiy meausres for discrimination learning. Learning & Motivation. 1973;4:327–342. [Google Scholar]
- Glickstein SB, Desteno DA, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors exhibit differential activation of prefrontal cortical neurons during tasks requiring attention. Cereb Cortex. 2005;15:1016–1024. doi: 10.1093/cercor/bhh202. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Ann N Y Acad Sci. 1995;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Zhang L, Telang F, Volkow ND. The effect of practice on a sustained attention task in cocaine abusers. Neuroimage. 2007;35:194–206. doi: 10.1016/j.neuroimage.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff AL, Riordan H, Morris L, Cestaro V, Wieneke M, Alpert R, Wang GJ, Volkow N. Effects of crack cocaine on neurocognitive function. Psychiatry Res. 1996;60:167–176. doi: 10.1016/0165-1781(96)02758-8. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Duhart HM, Skinner JT, Ali SF. Cocaine induces a differential dose-dependent alteration in the expression profile of immediate early genes, transcription factors, and caspases in PC12 cells: a possible mechanism of neurotoxic damage in cocaine addiction. Ann N Y Acad Sci. 2005;1053:482–490. doi: 10.1111/j.1749-6632.2005.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Udo T, Ugalde F, Luzzo C, Di Pietro N, Eichenbaum HB. Influence of cocaine self-administration on learning related to prefrontal cortex or hippocampus functioning in rats. Psychopharmacology (Berl) 2005;181:227–236. doi: 10.1007/s00213-005-2243-1. [DOI] [PubMed] [Google Scholar]
- Kondrad RL, Burk JA. Transient disruption of attentional performance following escalating amphetamine administration in rats. Psychopharmacology (Berl) 2004;175:436–442. doi: 10.1007/s00213-004-1857-z. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Kozak R, Martinez V, Young D, Brown H, Bruno JP, Sarter M. Toward a neuro-cognitive animal model of the cognitive symptoms of schizophrenia: disruption of cortical cholinergic neurotransmission following repeated amphetamine exposure in attentional task-performing, but not non-performing, rats. Neuropsychopharmacology. 2007;32:2074–2086. doi: 10.1038/sj.npp.1301352. [DOI] [PubMed] [Google Scholar]
- Liu X, Matochik JA, Cadet JL, London ED. Smaller volume of prefrontal lobe in polysubstance abusers: a magnetic resonance imaging study. Neuropsychopharmacology. 1998;18:243–252. doi: 10.1016/S0893-133X(97)00143-7. [DOI] [PubMed] [Google Scholar]
- Mansour A, Meador-Woodruff JH, Bunzow JR, Civelli O, Akil H, Watson SJ. Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. J Neurosci. 1990;10:2587–2600. doi: 10.1523/JNEUROSCI.10-08-02587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Meador-Woodruff JH, Zhou QY, Civelli O, Akil H, Watson SJ. A comparison of D1 receptor binding and mRNA in rat brain using receptor autoradiographic and in situ hybridization techniques. Neuroscience. 1991;45:359–371. doi: 10.1016/0306-4522(91)90233-e. [DOI] [PubMed] [Google Scholar]
- Martinez V, Parikh V, Sarter M. Sensitized attentional performance and Fos-immunoreactive cholinergic neurons in the basal forebrain of amphetamine-pretreated rats. Biol Psychiatry. 2005;57:1138–1146. doi: 10.1016/j.biopsych.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Miller L. Neuropsychological assessment of substance abusers: review and recommendations. J Subst Abuse Treat. 1985;2:5–17. doi: 10.1016/0740-5472(85)90017-0. [DOI] [PubMed] [Google Scholar]
- Nogueira L, Kalivas PW, Lavin A. Long-term neuroadaptations produced by withdrawal from repeated cocaine treatment: role of dopaminergic receptors in modulating cortical excitability. J Neurosci. 2006;26:12308–12313. doi: 10.1523/JNEUROSCI.3206-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Academic: San Diego; 1998. The Rat Brain in Stereotaxic Coordinates. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Rinaldi A, Mandillo S, Oliverio A, Mele A. D1 and D2 receptor antagonist injections in the prefrontal cortex selectively impair spatial learning in mice. Neuropsychopharmacology. 2007;32:309–319. doi: 10.1038/sj.npp.1301176. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Dissociating executive functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1463–1470. doi: 10.1098/rstb.1996.0131. discussion 1470-1461. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rosselli M, Ardila A. Cognitive effects of cocaine and polydrug abuse. JClinExpNeuropsychol. 1996;18:122–135. doi: 10.1080/01688639608408268. [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C. Attentional functions in learning and memory. In: Squire LR, Albright T, Bloom FE, Gage FH, Spitzer N, editors. New Encyclopedia of Neuroscience. Elsevier: Oxford; in press. [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;177:315–323. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26:8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Meyer-Lindenberg A, Klein S, Schmitt A, Hohn F, Tenckhoff A, Ruf M, Ende G, Rietschel M, Henn FA, Braus DF. D2 antidopaminergic modulation of frontal lobe function in healthy human subjects. Biol Psychiatry. 2006;60:1196–1205. doi: 10.1016/j.biopsych.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. British Journal of Psychiatry. 1988;152:641–648. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Von Huben SN, Davis SA, Lay CC, Katner SN, Crean RD, Taffe MA. Differential contributions of dopaminergic D1- and D2-like receptors to cognitive function in rhesus monkeys. Psychopharmacology (Berl) 2006;188:586–596. doi: 10.1007/s00213-006-0347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]