Abstract
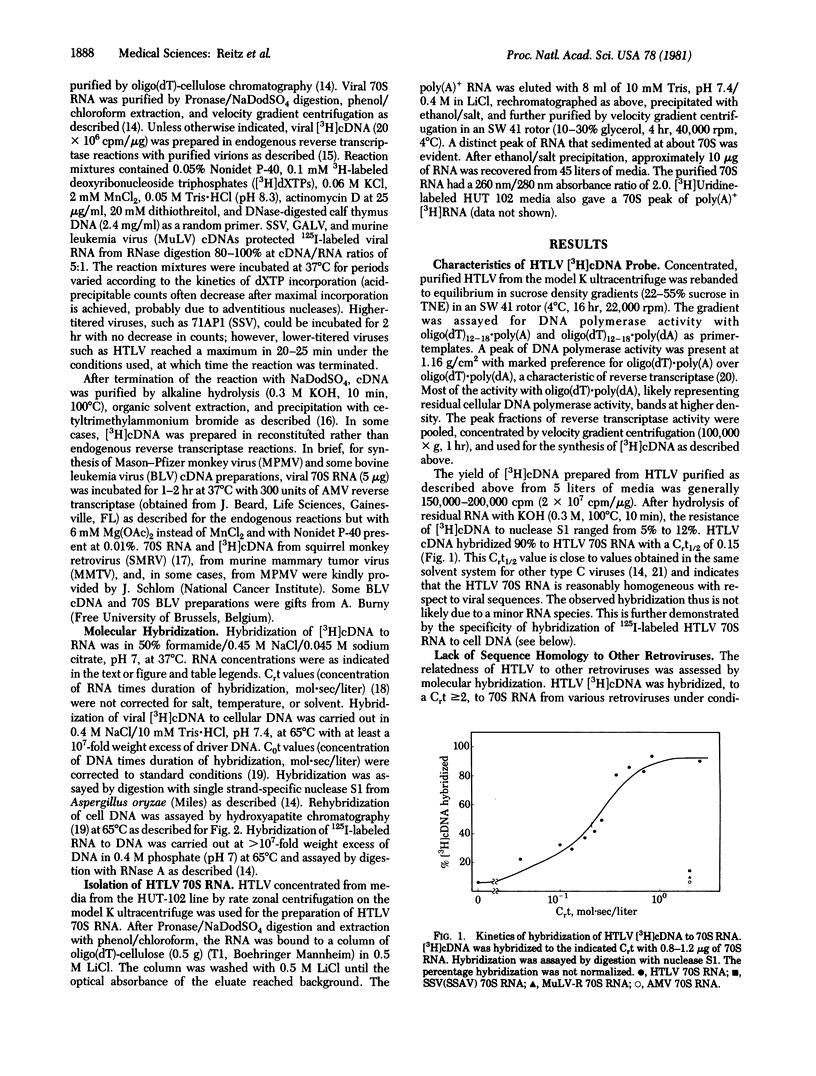
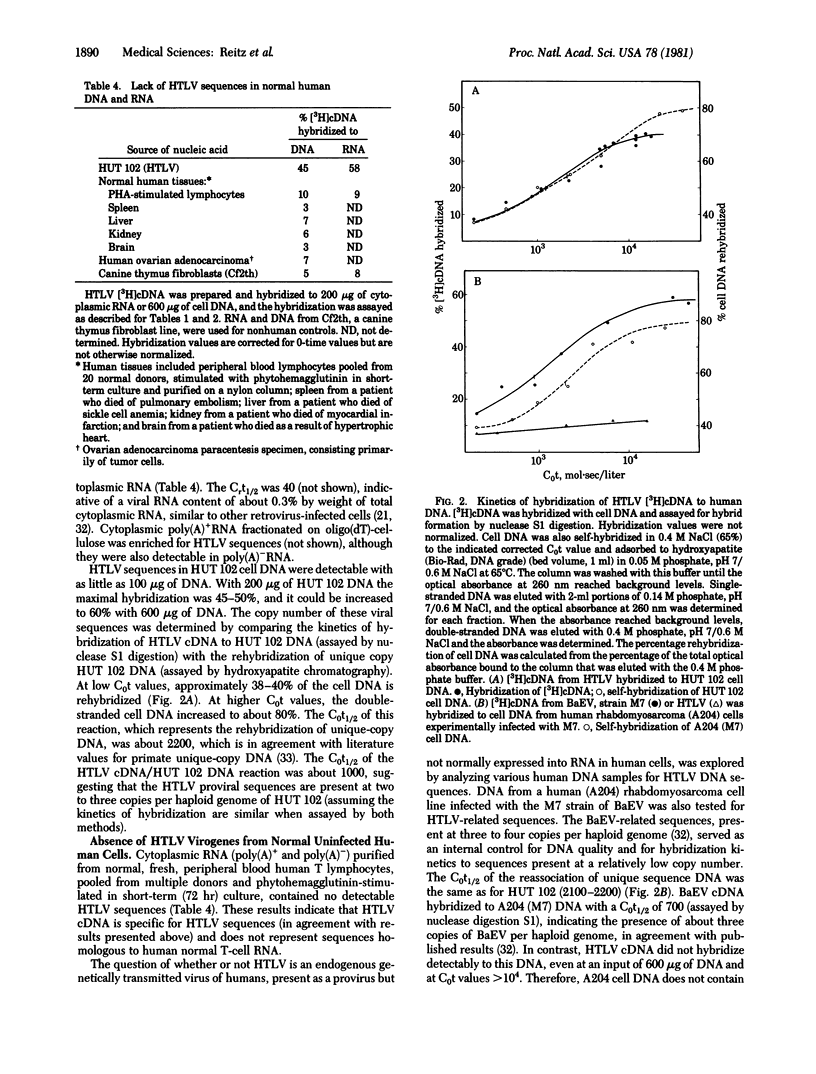
A type C retrovirus (designated HTLV) recently isolated from a cell line derived from a lymph node and later from peripheral blood of a person with cutaneous T-cell lymphoma (mycosis fungoides) was characterized by nucleic acid hybridization experiments. HTLV [3H]cDNA hybridized 90% to its own 70S RNA with kinetics consistent with the genetic complexity of other retroviruses, but it did not hybridize substantially to RNA or proviral DNA from any animal retroviruses (types B, C, and D), including those from nonhuman primates. Conversely, [3H]cDNA from other retroviruses did not hybridize to RNA or DNA of the human T-cell line producing HTLV. HTLV proviral sequences were present (two to three copies per haploid genome) in DNA of these cells, and homologous sequences were present in the cell cytoplasmic RNA (0.3% viral sequences by weight). HTLV-related nucleic acid sequences were not found in DNA from various other human tissues. The results indicate that HTLV is a new class of type C virus that is not an endogenous (genetically transmitted) retrovirus in man.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Endogenous type-C RNA viruses of mammalian cells. Biochim Biophys Acta. 1976 Dec 23;458(4):323–354. doi: 10.1016/0304-419x(76)90006-8. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Tronick S. R., Aaronson S. A. Isolation and characterization of an endogenous type C RNA virus of mink (Mv1Lu) cells. J Virol. 1978 Jan;25(1):129–137. doi: 10.1128/jvi.25.1.129-137.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of primate oncornaviruses: An endogenous virus from langurs (Presbytis spp.) with related virogene sequences in other Old World monkeys. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4557–4561. doi: 10.1073/pnas.74.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Sells B. H., Purdom I. F. Kinetic complexity of RNA molecules. J Mol Biol. 1972 Jan 14;63(1):21–39. doi: 10.1016/0022-2836(72)90519-0. [DOI] [PubMed] [Google Scholar]
- Bobrow S. N., Smith R. G., Reitz M. S., Gallo R. C. Stimulated normal human lymphocytes contain a ribonuclease-sensitive DNA polymerase distinct from viral RNA-directed DNA polymerase. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3228–3232. doi: 10.1073/pnas.69.11.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Kelloff G. J., Vernon M. L., Lane W. T., Capps W. I., Bumgarner S. D., Turner H. C., Huebner R. J. Prevalence of endogenous type-C virus in normal hamster tissues and hamster tumors induced by chemical carcinogens, simian virus 40, and polyoma virus. J Natl Cancer Inst. 1974 May;52(5):1469–1476. doi: 10.1093/jnci/52.5.1469. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Gallagher R. E., Wong-Staal F., Aoki T., Markham P. D., Schetters H., Ruscetti F., Valerio M., Walling M. J., O'Keeffe R. T. Isolation and tissue distribution of type-C virus and viral components from a gibbon ape (Hylobates lar) with lymphocytic leukemia. Virology. 1978 Feb;84(2):359–373. doi: 10.1016/0042-6822(78)90255-6. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Reitz M. S., Jr Molecular probes for tumor viruses in human cancer. Int Rev Exp Pathol. 1976;16:1–58. [PubMed] [Google Scholar]
- Goodenow R. S., Kaplan H. S. Characterization of the reverse transcriptase of a type C RNA virus produced by a human lymphoma cell line. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4971–4975. doi: 10.1073/pnas.76.10.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberling R. L., Barker S. T., Kalter S. S., Smith G. C., Helmke R. J. Oncornavirus: isolation from a squirrel monkey (Saimiri sciureus) lung culture. Science. 1977 Jan 21;195(4275):289–292. doi: 10.1126/science.63993. [DOI] [PubMed] [Google Scholar]
- Hino S., Tronick S. R., Heberling R. L., Kalter S. S., Hellman A., Aaronson S. A. Endogenous New World primate retrovirus: interspecies antigenic determinants shared with the major structural protein of type-D RNA viruses of Old World monkeys. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5734–5738. doi: 10.1073/pnas.74.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung G. D. Activation of guinea pig C-type virus in cultured spleen cells by 5-bromo-2'-deoxyuridine. J Natl Cancer Inst. 1972 Aug;49(2):567–570. [PubMed] [Google Scholar]
- Kaplan H. S. Leukemia and lymphoma in experimental and domestic animals. Ser Haematol. 1974;7(2):94–163. [PubMed] [Google Scholar]
- Kawakami T. G., Huff S. D., Buckley P. M., Dungworth D. L., Synder S. P., Gilden R. V. C-type virus associated with gibbon lymphosarcoma. Nat New Biol. 1972 Feb 9;235(58):170–171. doi: 10.1038/newbio235170a0. [DOI] [PubMed] [Google Scholar]
- Kurnick J. T., Grönvik K. O., Kimura A. K., Lindblom J. B., Skoog V. T., Sjöberg O., Wigzell H. Long term growth in vitro of human T cell blasts with maintenance of specificity and function. J Immunol. 1979 Apr;122(4):1255–1260. [PubMed] [Google Scholar]
- Mier J. W., Gallo R. C. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6134–6138. doi: 10.1073/pnas.77.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. M., Miller L. D., Olson C., Gillette K. G. Virus-like particles in phytohemagglutinin-stimulated lymphocyte cultures with reference to bovine lymphosarcoma. J Natl Cancer Inst. 1969 Dec;43(6):1297–1305. [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Mier J. W., Woods A. M., Gallo R. C. T-cell lines established from human T-lymphocytic neoplasias by direct response to T-cell growth factor. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6815–6819. doi: 10.1073/pnas.77.11.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Abrell J. W., Trainor C. D., Gallo R. C. Precipitation of nucleic acids with cetyltrimethylammonium bromide: a method for preparing viral and cellular DNA polymerase products for cesium sulfate density gradient analysis. Biochem Biophys Res Commun. 1972 Oct 6;49(1):30–38. doi: 10.1016/0006-291x(72)90005-8. [DOI] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Luczak J. C., Gallo R. C. Mapping of related and nonrelated sequences of RNA from wooly monkey virus and gibbon ape leukemia virus. Virology. 1979 Feb;93(1):48–56. doi: 10.1016/0042-6822(79)90274-5. [DOI] [PubMed] [Google Scholar]
- Robert M. S., Smith R. G., Gallo R. C., Sarin P. S., Abrell J. W. Viral and cellular DNA polymerase: comparison of activities with synthetic and natural RNA templates. Science. 1972 May 19;176(4036):798–800. doi: 10.1126/science.176.4036.798. [DOI] [PubMed] [Google Scholar]
- Ruscetti F. W., Morgan D. A., Gallo R. C. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol. 1977 Jul;119(1):131–138. [PubMed] [Google Scholar]
- Scolnick E. M., Maryak J. M., Parks W. P. Levels of rat cellular RNA homologous to either Kirsten sarcoma virus or rat type-C virus in cell lines derived from Osborne-Mendel rats. J Virol. 1974 Dec;14(6):1435–1444. doi: 10.1128/jvi.14.6.1435-1444.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock N. D., Ferrer J. F. Replicating C-type virus in phytohemagglutinin-treated buffy-coat cultures of bovine origin. J Natl Cancer Inst. 1972 Apr;48(4):985–996. [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Lieber M. M., Sherr C. J. Characterization of a type C virus released from the porcine cell line PK(15). Virology. 1974 Mar;58(1):65–74. doi: 10.1016/0042-6822(74)90141-x. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Sen A., King N., Daniel M. D., Fleckenstein B. Endogenous New World primate type C viruses isolated from owl monkey (Aotus trivirgatus) kidney cell line. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1004–1008. doi: 10.1073/pnas.75.2.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Maaten M. J., Boothe A. D., Seger C. L. Isolation of a virus from cattle with persistent lymphocytosis. J Natl Cancer Inst. 1972 Dec;49(6):1649–1657. doi: 10.1093/jnci/49.6.1649. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Reitz M. S., Paran M., Gallo R. C. Mechanism of stimulation of murine type-C RNA tumor virus production by glucocorticoids: post-transcriptional effects. J Virol. 1974 Oct;14(4):802–812. doi: 10.1128/jvi.14.4.802-812.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
