Abstract
Physical activity decreases cocaine self-administration in laboratory animals and is associated with positive outcomes in substance abuse treatment programs; however, less is known about its efficacy in preventing the establishment of regular patterns of substance use in drug-naive individuals. The purpose of the present study was to examine the effects of access to a running wheel on the acquisition of cocaine self-administration in experimentally naive rats. Male, Long-Evans rats were obtained at weaning and assigned to sedentary (no wheel) or exercising (access to wheel) conditions immediately upon arrival. After six weeks, rats were surgically implanted with intravenous catheters and placed in operant conditioning chambers for 2 hr/day for 15 consecutive days. Each session began with a noncontingent priming infusion of cocaine, followed by a free-operant period in which each response on the active lever produced an infusion of cocaine on a fixed ratio (FR1) schedule of reinforcement. For days 1–5, responding was reinforced with 0.25 mg/kg/infusion cocaine; for days 6–15, responding was reinforced with 0.75 mg/kg/infusion cocaine. In addition, all rats were calorically restricted during days 11–15 to 85% to 95% of their free-feeding body weight. Compared to sedentary rats, exercising rats acquired cocaine self-administration at a significantly slower rate and emitted significantly fewer active lever presses during the 15 days of behavioral testing. These data indicate that access to a running wheel inhibits the acquisition of cocaine self-administration, and that physical activity may be an effective intervention in substance abuse prevention programs.
Keywords: Acquisition, Cocaine, Exercise, Rat, Sedentary, Self-Administration
1. Introduction
A rapid transition from initial drug exposure to regular patterns of drug use is considered an important prognosticator of whether an individual will later develop problems with substance abuse and dependence (U.S. Congress, Office of Technology Assessment, 1994). Consequently, one of the goals of substance abuse prevention programs is to discourage the development of regular patterns of drug use in at-risk populations. The acquisition of regular patterns of drug intake after initial drug exposure can be modeled in the laboratory by exposing an animal to noncontingent drug infusions and then permitting the animal to self-administer that drug in free-operant test sessions. Factors that influence the acquisition of drug self-administration in the laboratory are often identical to the factors that influence the likelihood an individual will develop problems with substance abuse and dependence. For instance, social isolation (Kosten et al., 2000), stress (Tidey and Miczek, 1997), and previous drug exposure (Fletcher et al., 2001) increase the rate of acquisition in laboratory animals and are considered risk factors for developing problems with substance use in humans. Moreover, interventions that decrease the rate of acquisition in laboratory animals, such as access to alternative nondrug reinforcers, decrease the probability that human populations will develop problems with substance use (see review by Campbell and Carroll, 2000). For instance, providing laboratory rats with concurrent access to a palatable drinking solution reduces the acquisition of intravenous cocaine self-administration (Carroll and Lac, 1993), whereas providing adolescents with educational programs and social activities reduces the initiation of alcohol drinking (Perry et al., 1996; 2002).
Physical activity is one intervention that may reduce the likelihood that an individual will transition into regular patterns of drug use. Epidemiological studies report that adolescents who engage in regular physical activity are less likely to use drugs and alcohol (Field et al., 2001; Kirkcaldy et al., 2002; Ströhle et al., 2007; Iannotti et al., 2009) and have fewer risk factors that are associated with the development of substance use disorders (Collingwood et al., 1991; 2000) than their peers who do not engage in regular physical activity. Preclinical studies reveal that physical activity reduces drug self-administration in procedures that model different transitional stages in the development of substance use disorders. For instance, access to a running wheel decreases the maintenance of cocaine-reinforced behavior in animals with well-established self-administration histories (Cosgrove et al., 2002; Smith et al., 2008), and reduces drug-primed (Zlebnik et al., 2010) and cue-induced (Lynch et al., 2010) reinstatement of cocaine-seeking behavior in animals after a period of forced abstinence. The ability of wheel running to influence the acquisition of cocaine self-administration (i.e., the establishment of regular patterns of cocaine self-administration) is not known.
The purpose of the present study was to examine the effects of access to a running wheel on the acquisition of cocaine self-administration. To this end, sedentary (no wheel) and exercising (access to wheel) rats were implanted with intravenous catheters and allowed to self-administer cocaine in free-operant test sessions. Each session began with a priming infusion of cocaine, followed by a 2-hr period in which cocaine was available on a fixed ratio (FR1) schedule of reinforcement. Previous studies report that the rate of acquisition is influenced by both the dose of cocaine used to reinforce behavior (Carrol and Lac, 1997) and the level of caloric restriction at the time of testing (Specker et al., 1994; Campbell and Carroll, 2001). In the present study, acquisition testing advanced through three distinct stages designed to systematically increase the probability that self-administration would be acquired. In phase 1 (days 1–5), responding was reinforced with 0.25 mg/kg/infusion cocaine; in phase 2 (days 6–10), responding was reinforced with 0.75 mg/kg/infusion cocaine; and in phase 3 (days 11–15) responding was reinforced with 0.75 mg/kg/infusion cocaine and animals were calorically restricted to 85% to 95% of their free-feeding body weight. We hypothesized that rates of active lever pressing would increase over the 15-day period, and that access to a running wheel would significantly decrease the rate of acquisition.
2. Material and methods
2.1. Animals and apparatus
Male Long-Evans rats were obtained at 21 days of age from Charles River Laboratories (Raleigh, NC, USA) and assigned randomly to sedentary and exercising conditions immediately upon arrival. Sedentary rats were housed in polycarbonate cages (interior dimensions: 50 × 28 × 20 cm) that permitted no physical activity beyond normal cage ambulation. Exercising rats were housed in identical cages but with a running wheel (interior diameter: 35 cm) from Harvard Apparatus (Holliston, MA, USA) affixed to the interior of the cage. Mechanical switches on each wheel recorded the number of revolutions. Cages with locked or inactive wheels were not used for the sedentary control group because rodents climb in locked running wheels (Koteja et al., 1999), potentially comprising the primary experimental manipulation of the study (i.e., physical activity). All rats remained in their respective sedentary and exercising conditions for the duration of the study. During the period of behavioral testing, exercising rats had full access to their running wheels before and after each session. Drinking water was continuously available in the home cages. Food was freely available in the home cages except for the final few days of the study during which the rats were lightly food restricted (see below). Rats were maintained on a 12-hr light/dark cycle (lights on: 7:00 a.m.) in a temperature- and humidity controlled vivarium. All subjects were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animals Resources, 1996) and the Institutional Animal Care and Use Committee of Davidson College. A total of 46 rats were assigned to the sedentary and exercising conditions (n = 23 sedentary; n = 23 exercising). Rats that lost catheter patency at any point during testing were removed from the study and their data were not included in the statistical analysis. A total of 38 rats completed the study (n = 19 sedentary; n = 19 exercising).
All experiments were conducted in polycarbonate and aluminum operant conditioning chambers (interior dimensions: 31 × 24 × 21 cm) from Med Associates, Inc. (St Albans, VT, USA). Each chamber was equipped with two response levers on one wall, a white stimulus light located above each lever, and a houselight located on the opposite wall. Drug infusions were delivered from an infusion pump mounted outside the chamber via Tygon tubing protected by a stainless steel spring and attached to a counter-balanced swivel suspended above the chamber. All experimental events were programmed and data were collected with software and interfacing from Med Associates, Inc.
2.2. Procedures
Approximately six weeks after arrival, rats were anesthetized with a combination of ketamine (100 mg/kg, ip) and xylazine (8 mg/kg, ip) and surgically implanted with intravenous catheters into the right jugular vein. Butorphanol (1.0 mg/kg, sc) was given after surgery and the following morning as an analgesic. A solution of heparinized saline and ticarcillin (20 mg/kg, iv) was infused through the catheter daily to prevent infection and maintain catheter patency. After 7 days, ticarcillin was discontinued and only heparinized saline was used to maintain patency. All rats were allowed to recover for 3–4 days prior to the beginning of self-administration testing.
During self-administration tests, rats were removed from their home cages, placed in the operant conditioning chambers, and connected to the infusion pumps via Tygon tubing. Each session began with illumination of the house light, illumination of the white stimulus light above the active lever, and a noncontingent infusion of cocaine. During all sessions, lever presses were reinforced on a fixed ratio (FR1) schedule of reinforcement. On this schedule, responses on the active lever produced an infusion of cocaine, with infusion duration varying between 2.5 and 3.5 seconds depending on body weight. Coincident with the start of each infusion, the stimulus light above the active lever turned off for 20 seconds to signal a timeout during which cocaine was not available and responses had no programmed consequences. For all sessions, responses on the inactive lever were recorded but had no programmed consequences. Sessions lasted 2 hr or until 50 infusions were delivered, whichever occurred first. One session was conducted each day for 15 consecutive days.
Behavioral testing advanced through three consecutive five-day phases. During the first phase (days 1–5), each infusion, including the priming infusion, delivered 0.25 mg/kg cocaine. During the second phase (days 6–10), each infusion, including the priming infusion, delivered 0.75 mg/kg cocaine. During the third phase (days 11–15), each infusion delivered 0.75 mg/kg cocaine (the same as the second phase). Also during the third phase, rats were calorically restricted to 85% to 95% of their free-feeding body weight. Food restriction began immediately after the 10th session, and continued until the end of testing. Food restriction was individualized for each rat, and body weight was not allowed to drop greater than 5% in any rat during any 24-hr period. The three phases were designed to systematically increase the probability that rats would acquire cocaine self-administration. Progression through the three stages was the same for all rats, regardless of when they acquired cocaine self-administration.
Acquisition was operationally defined as obtaining 12 infusions on each of two consecutive days, with the first of those days marking the date of acquisition. Thus, it was possible for a rat to meet the acquisition criterion on the first day, provided that at least 12 infusions were obtained on both the first and second day of testing. Any rat that obtained 12 infusions for the first time on the 15th day received one additional test day. If that rat obtained at least 12 infusions on the 16th day, then it was considered to have met the acquisition criterion on the 15th day. During the 16th session, all conditions were identical to those present during the 15th session. No rat ever obtained at least 12 infusions on one day of testing without obtaining at least 12 infusions on all subsequent days of testing, the lowest number for which this was true. Consequently, the 12-infusion criterion was able to discriminate between those rats that had acquired cocaine self-administration and those that had not.
2.3. Data analysis
Wheel running (rev/day) and body weight (g) during the first six weeks of the study were analyzed via repeated-measures ANOVA using week as the factor. Wheel running and body weights during the 15 days of behavioral testing were analyzed via repeated-measures ANOVA using day as the factor.
Time to acquisition, total active lever presses, and total inactive lever presses were compared between groups (sedentary vs. exercise) via independent-samples t-tests. Active and inactive lever presses were also compared between groups and across sessions via mixed-factor ANOVA using group as the between-subjects factor and session as the repeated-measure.
Four rats (2 sedentary rats and 2 exercising rats) developed a stereotypy directed toward the inactive lever immediately after reaching the acquisition criterion, resulting in hundreds or thousands of responses on the inactive lever during the 2-hr test session. This effect was transient and only observed during the first one or two sessions following acquisition. Consequently, inactive lever data were not included in the statistical analysis if the number of inactive lever presses on a given day was greater than 10 standard deviations from the mean for that group. Using this criterion, inactive lever data were eliminated for two days from Rat 1418 (sedentary), one day from Rat 1466 (sedentary), two days from Rat 1299 (exercising) and one day from Rat 1428 (exercising). The elimination of these data did not impact any group effect (or lack thereof) between sedentary and exercising rats.
Pearson product-moment correlations were used to examine the relationship between wheel running (before surgery, after surgery, and throughout the entire study) and cocaine acquisition (days to criterion and total active lever presses). Pearson product-moment correlations were also used to examine the relationship between wheel running and active lever presses during each of the three phases of testing.
3. Results
Wheel running, measured in revolutions per day, increased significantly during the six weeks prior to catheter implantation [Figure 1, main effect of week: F (5, 90) = 32.038, p < .001]. Wheel running reached a plateau immediately after surgery and no further increases were observed. Although small day-to-day variations in wheel running were apparent over the 15 days of testing, this effect failed to reach statistical significance (p =. 094). Body weights increased significantly during the first six weeks of the study [main effect of week: F (5, 180) = 113.464, p < .001], but no differences were observed between the two groups. Body weights also varied significantly over the 15 days of behavioral testing [main effect of day: F (14, 504) = 123.184, p < .001], but no group differences were observed.
Figure 1.
Wheel running (upper panel) and body weight (lower panel) over the course of the study. Wheel running is expressed in revolutions per day (rev/day); body weight is expressed in grams (g). Horizontal axes depict time expressed in weeks (weeks 1–6) and days (days 1–15). Vertical reference lines represent transitions between different experimental events: home cage and running wheel acclimation (weeks 1–6); self-administration testing with 0.25 mg/kg/infusion cocaine (days 1–5); self-administration testing with 0.75 mg/kg/infusion cocaine (days 6–10); self-administration testing with 0.75 mg/kg/infusion cocaine during food restriction (days 11–15). Vertical lines surrounding data points represent the SEM; where not indicated, the SEM fell within the data point. Data are shown from 19 sedentary rats and 19 exercising rats.
The percentage of rats acquiring cocaine self-administration was greater in sedentary rats than exercising rats on all days of testing after the first day (Figure 2A). Sedentary rats acquired cocaine self-administration significantly more rapidly than exercising rats [Figure 2B, effect of group: t (36) = 2.502, p = .017], reaching the acquisition criterion, on average, three days sooner. The number of active lever presses increased significantly over the 15 days of testing [Figure 2C, main effect of session: F (14, 504) = 16.289, p < .001], and sedentary rats emitted a significantly greater number of active lever presses than exercising rats [main effect of group: F (1, 36) = 6.698, p = .014]. Over the 15 days of testing, the total number of active lever presses was approximately twice as great in sedentary rats than in exercising rats, an effect that was statistically significant [Figure 2D, effect of group: t (36) = 2.588, p = .014]. In contrast, the number of inactive lever presses did not differ between the two groups during the 15 days of testing (Figure 2E). Although some day-to-day variation was observed, this effect failed to reach statistical significance (p = .072). Unlike that seen with active lever presses, the total number of inactive lever presses over the 15 days of testing did not differ between sedentary and exercising rats (Figure 2F).
Figure 2.
A. Percent of rats reaching the acquisition criterion over 15 days of testing. B. Number of days to reach the acquisition criterion. C. Number of active lever presses per session over 15 days of testing. D. Total number of active lever presses over 15 days of testing. E. Number of inactive lever presses per session over 15 days of testing. F. Total number of inactive lever presses over 15 days of testing. Asterisks (*) indicate significant difference as determined by independent-samples t-test. For panels A, C, and E, vertical reference lines represent transitions between different experimental events: self-administration testing with 0.25 mg/kg/infusion cocaine (days 1–5); self-administration testing with 0.75 mg/kg/infusion cocaine (days 6–10); self-administration testing with 0.75 mg/kg/infusion cocaine during food restriction (days 11–15). For all panels, vertical lines surrounding data points represent the SEM; where not indicated, the SEM fell within the data point. Data are shown from 19 sedentary rats and 19 exercising rats.
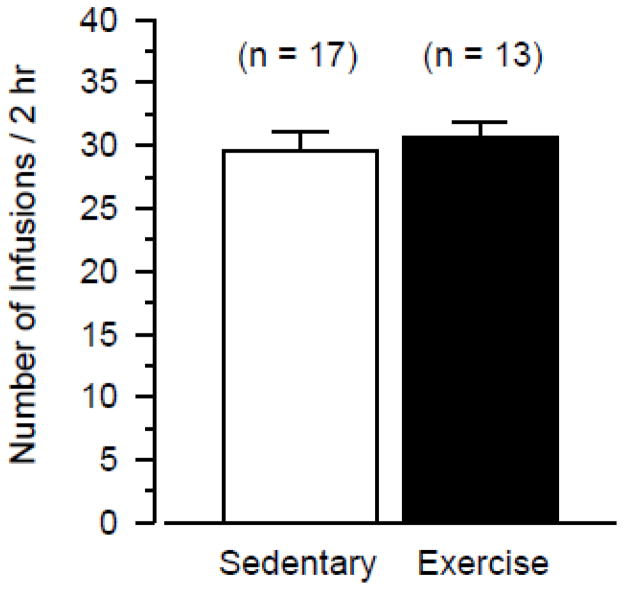
To examine the maintenance of cocaine-reinforced responding in the two groups, cocaine self-administration was compared on day 15 using only data from rats that met the acquisition criterion (Figure 3). In the 17 sedentary rats and 13 exercising rats meeting the acquisition criterion, cocaine self-administration did not differ between the two groups. Analysis of individual event records revealed stable response rates with regular post-reinforcement pauses in both sedentary and exercising rats (data not shown).
Figure 3.
Cocaine self-administration during the 15th session in only those rats meeting the acquisition criterion. Vertical axis indicates number of infusions during 2-hr test session. Data are shown from 17 sedentary rats and 13 exercising rats meeting the acquisition criterion.
Pearson product-moment correlations did not reveal any significant relationship between wheel running (measured before surgery, after surgery, and throughout the entire study) and cocaine acquisition (measured as days to criterion and total active lever presses). Similarly, wheel running was not predictive of active lever pressing during any of the three phases of behavioral testing. Correlations between wheel running and active lever responding ranged from −.227 to .204 for all comparisons (data not shown).
4. Discussion
The main finding of this study is that access to a running wheel significantly decreases the rate of acquisition of cocaine self-administration in male rats responding in a free operant procedure. On average, rats with access to a running wheel acquired cocaine self-administration 3 days later than sedentary rats, and emitted fewer active lever presses during the 15 days of behavioral testing. Differences between the two groups were observed as early as the second day of testing, and remained evident until the conclusion of the experiment. The differential rate of acquisition could not be attributed to differences in nonspecific responding between the two groups. Rates of inactive lever pressing did not differ between the two groups, suggesting that the increased rate of acquisition observed in sedentary rats was not due to higher rates of general operant responding. Additionally, the differential rate of acquisition could not be attributed to differences in sensitivity to cocaine. When only rats meeting the acquisition criterion were compared, cocaine self-administration did not differ between the two groups. This latter finding is consistent with our previous work showing that wheel running does not alter sensitivity to cocaine when unit price (i.e., ratio value) and income (i.e., session length) is low (Smith et al., 2011). Only when ratio values are high, such as on a progressive ratio schedule of reinforcement, or when session lengths are extended to 6 or 23 hours do we see significant differences between sedentary and exercising rats (Smith et al., 2008; Smith et al., 2011).
It is important to note that access to a running wheel is a form of environmental enrichment, and the present data may be due to an enrichment-related effect. Environmental enrichment has been shown to influence the positive reinforcing effects of cocaine (Smith et al., 2009), and its protective effects on measures of drug-seeking behavior have been well documented (Bardo et al., 2001; Stairs et al., 2006; Thiel et al., 2010; 2011). Although traditional models of environmental enrichment typically include multiple enriching stimuli (e.g., access to running or climbing devices, access to novel objects or toys, access to same-sex cagemates), the present data suggest that access to a running wheel in the absence of other enriching stimuli is sufficient for decreasing drug-seeking behavior in acquisition procedures.
Although there is not a standard procedure for measuring the acquisition of drug self-administration in laboratory animals, most studies using intravenous self-administration methods begin by priming the animal with noncontingent infusions of the drug, and then allowing the animal to respond for contingent infusions under a schedule of positive reinforcement. The noncontingent infusions may be provided as a single priming infusion at the beginning of each session (as in the current study) or as part of an auto-shaping procedure in which each infusion is paired with the insertion/retraction of a response lever. Both methods are believed to model human patterns of acquisition in which drugs are initially provided freely (perhaps by friends or dealers) and then the monetary or behavioral costs of obtaining the drug increase as the individual shifts to regular patterns of use (for review and discussion, see Carroll and Meisch, 2011). Regardless of the method employed, studies have consistently shown that increasing the dose of the drug and increasing the level of caloric restriction increases the likelihood that drug self-administration will be acquired (Specker et al., 1994; Carrol and Lac, 1997; Campbell and Carroll, 2001).
In the present study, testing advanced through three distinct phases in which the dose and level of caloric restriction were systematically varied. These phases were not designed to measure the impact of dose and caloric restriction on cocaine self-administration; rather, these phases were designed to progressively increase the likelihood that self-administration would be acquired. Consistent with this aim, acquisition increased progressively in both groups over the 15 days of testing. Sedentary rats acquired cocaine self-administration at a faster rate than exercising rats, especially during the first two phases of testing (days 1–10). The majority of exercising rats that acquired did so during the third phase of testing, during which responding was maintained by the higher dose of cocaine and all rats were calorically restricted to 85% – 95% of their free-feeding body weight. It is important to note that body weights did not differ between sedentary and exercising rats at any point during the study. Previous studies report that rats with access to running wheels increase their energy intake to compensate for their increase in energy expenditure (Holloszy, 1993), and we have found that exercising rats consume approximately 35% more food than sedentary rats under free-feed conditions (unpublished observations). In the present study, exercising rats consumed 33% more food than sedentary rats during the 5 days of caloric restriction when food consumption was specifically being monitored. The finding that body weights did not differ between sedentary and exercising rats suggest that both the dose manipulation (expressed as mg/kg of body weight) and the caloric restriction manipulation (expressed as a percentage of free-feeding body weight) was equivalent between the two groups.
Consistent with previous studies (Smith and Yancey, 2003; Smith and Lyle, 2006), wheel running increased over the first several weeks of the study. Wheel running leveled off immediately after surgery, and remained relatively stable across the 15 days of testing. Previous studies have reported that both contingent (Cosgrove et al., 2002; Smith et al., 2008) and noncontingent (Iijima et al., 1995; Santucci et al., 2008) cocaine suppresses wheel running in laboratory animals. One possibility that may account for the lack of suppression in the present study is the low levels of cocaine intake when averaged across 19 rats over 15 days of testing. Because very low levels of cocaine intake were observed during the first phase of testing (few rats had acquired), and because some rats never acquired cocaine self-administration by the end of the third phase, average levels of cocaine intake were less in the present study than in earlier studies. It should be noted that our electronic counters record only the number of wheel revolutions and do not permit a temporal analysis of wheel running. It is likely that circadian patterns of wheel running were disrupted during the period of behavioral testing, but future studies using a more detailed analysis of running patterns will be needed to confirm this possibility. We previously reported that wheel running prior to surgery was later predictive of cocaine-maintained breakpoints on a progressive ratio schedule of reinforcement (Smith et al., 2008), and we predicted that wheel running would be predictive of acquisition in the present study. Contrary to our prediction, wheel running was not correlated with any measure of cocaine self-administration during any phase of testing.
Physical activity induces many neurobiological changes that could account for its ability to protect against the establishment of regular patterns of drug self-administration. For instance, running increases central dopamine concentrations (Meeusen and De Meirleir, 1995) and regular bouts of running increase the density of dopamine D2 receptors (Gilliam et al., 1984; MacRae et al., 1987). The latter finding is particularly relevant to the present study because a higher density of D2 receptors is associated with decreased cocaine self-administration in laboratory animals (Morgan et al., 2002). Wheel running also modulates the intracellular signaling molecule ERK, which is important for drug-induced synaptic plasticity. ERK is particularly important for drug craving (Thomas et al. 2008), and levels of ERK in central reward pathways are correlated with cocaine-seeking behavior (Koya et al., 2009; Lynch et al., 2010; Lu et al., 2006; Edwards et al., 2011). Importantly, access to a running wheel during a period of forced abstinence from cocaine self-administration reduces phosphorylated levels of ERK and decreases cocaine-seeking behavior (Lynch et al., 2010).
On a neuroanatomical level, voluntary (van Praag et al., 1999; Rhodes et al., 2003; Bednarczyk et al., 2009) and forced (Uda et al., 2006; Lou et al., 2008; Kim et al., 2010) running procedures reliably increase neurogenesis in the hippocampal formation. Hippocampal neurogenesis has protective effects in models of drug-seeking behavior, and reductions in hippocampal neurogenesis have been implicated in an increased propensity to self-administer cocaine (Noonan et al., 2010). The relationship between cocaine exposure and hippocampal neurogenesis is reciprocal, in that contingent (Sudai et al., 2011) and noncontingent (Yamaguchi et al., 2004) cocaine administration reliably decrease neurogenesis. Physical activity may thus be producing it protective effects, in part, by enhancing the ability of the hippocampus to mitigate the establishment of regular patterns of drug intake. Wheel running also increases gliogenesis in the prefrontal cortex (Mandyam et al., 2007) and enhances prefrontal-mediated executive functioning (Small et al., 2006; Yanagisawa et al., 2010). A deficit in prefrontal functioning is believed to play a role in the development of substance use disorders (Goldstein and Volkow, 2002), and physical activity may protect against the establishment of regular and compulsive patterns of drug intake via a prefrontal mechanism.
Epidemiological studies consistently reveal that adolescents who engage in regular physical activity are less likely to use drugs and have fewer risk factors associated with the development of substance use disorders (Collingwood et al., 1991; 2000; Field et al., 2001; Kirkcaldy et al., 2002; Ströhle et al., 2007; Iannotti et al., 2009). This inverse relationship between physical activity and substance use may be due to three possibilities: (1) physical activity may produce a causal decrease in drug use, either by serving as an alternative nondrug reinforcer or by promoting neurobiological changes that protect the individual from developing regular patterns of drug intake; (2) drug use may produce a causal decrease in physical activity, either by decreasing aerobic capacity or by reducing discretionary time that would otherwise be spent on nondrug-related activities; or (3) a third factor (e.g., parents, peers, and role models) may have a causal influence on both activities. Although these three possibilities are not mutually exclusive of one another, an accumulating body of evidence from preclinical studies suggests that physical activity plays a causal role in the reduction of drug-seeking behavior. This point is particularly relevant from a translational perspective, because it supports the expanded use of activity-based interventions in drug abuse prevention programs.
Research Highlights.
Male rats were assigned to sedentary (no wheel) and exercising (access to wheel) conditions at weaning and later allowed to self-administer cocaine in free-operant test sessions.
Exercising rats acquired cocaine self-administration at a slower rate than sedentary rats.
Relative to sedentary rats, exercising rats emitted fewer active lever presses over 15 days of testing.
The number of inactive lever presses did not differ between exercising and sedentary rats.
These data indicate that access to a running wheel inhibits the acquisition of cocaine self-administration.
Acknowledgments
This study was supported by the National Institutes of Health (NIDA Grant DA027485 to MAS). The authors thank the National Institute on Drug Abuse for supplying the study drug, Amy Becton for providing animal care, and Kimberly Lang for providing expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology. 2001;155:278–84. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Bednarczyk MR, Aumont A, Décary S, Bergeron R, Fernandes KJ. Prolonged voluntary wheel-running stimulates neural precursors in the hippocampus and forebrain of adult CD1 mice. Hippocampus. 2009;19:913–27. doi: 10.1002/hipo.20621. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Acquisition of drug self-administration: Environmental and pharmacological interventions. Exp Clin Psychopharm. 2000;8:312–25. doi: 10.1037//1064-1297.8.3.312. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Effects of ketoconazole on the acquisition of intravenous cocaine self-administration under different feeding conditions in rats. Psychopharmacology. 2001;154:311–8. doi: 10.1007/s002130000627. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Autoshaping i.v. cocaine self-administration in rats: effects of nondrug alternative reinforcers on acquisition. Psychopharmacology. 1993;110:5–12. doi: 10.1007/BF02246944. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Acquisition of i.v. amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacology. 1997;129:206–14. doi: 10.1007/s002130050182. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Acquisition of Drug Self-Administration. Animal Models of Drug Addiction. In: Olmstead MCC, editor. Neuromethods. Part 2. Vol. 53. New York: Humana Press; 2011. pp. 237–65. [Google Scholar]
- Collingwood TR, Reynolds R, Kohl HW, Smith W, Sloan S. Physical fitness effects on substance abuse risk factors and use patterns. J Drug Educ. 1991;21:73–84. doi: 10.2190/HV5J-4EYN-GPP7-Y3QG. [DOI] [PubMed] [Google Scholar]
- Collingwood TR, Sunderlin J, Reynolds R, Kohl HW., 3rd Physical training as a substance abuse prevention intervention for youth. J Drug Educ. 2000;30:435–51. doi: 10.2190/RVUE-9XW7-TYRQ-EJR8. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav. 2002;73:663–71. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Edwards S, Bachtell RK, Guzman D, Whisler KN, Self DW. Emergence of context-associated GluR(1) and ERK phosphorylation in the nucleus accumbens core during withdrawal from cocaine self-administration. Addict Biol. 2011;16:450–7. doi: 10.1111/j.1369-1600.2010.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Sanders CE. Exercise is positively related to adolescents’ relationships and academics. Adolescence. 2001;36:105–10. [PubMed] [Google Scholar]
- Fletcher PJ, Robinson SR, Slippoy DL. Pre-exposure to (+/−)3,4-methylenedioxy-methamphetamine (MDMA) facilitates acquisition of intravenous cocaine self-administration in rats. Neuropsychopharmacology. 2001;25:195–203. doi: 10.1016/S0893-133X(01)00222-6. [DOI] [PubMed] [Google Scholar]
- Gilliam PE, Spirduso WW, Martin TP, Walters TJ, Wilcox RE, Farrar RP. The effects of exercise training on [3H]-spiperone binding in rat striatum. Pharmacol Biochem Behav. 1984;20:863–7. doi: 10.1016/0091-3057(84)90008-x. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO. Exercise increases average longevity of female rats despite increased food intake and no growth retardation. Gerontol. 1993;48:B97–100. doi: 10.1093/geronj/48.3.b97. [DOI] [PubMed] [Google Scholar]
- Iannotti RJ, Kogan MD, Janssen I, Boyce WF. Patterns of adolescent physical activity, screen-based media use, and positive and negative health indicators in the U.S. and Canada. J Adolesc Health. 2009;44:493–9. doi: 10.1016/j.jadohealth.2008.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima Y, Shinoda M, Kuribara H, Asami T, Uchihashi Y. Evaluation of acute and sub-acute effects of cocaine by means of circadian variation in wheel-running and drinking in mice. Nihon Shinkei Seishin Yakurigaku Zasshi. 1995;15:315–21. [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010;45:357–65. doi: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Kirkcaldy BD, Shephard RJ, Siefen RG. The relationship between physical activity and self-image and problem behaviour among adolescents. Soc Psychiatry Psychiatr Epidemiol. 2002;37:544–50. doi: 10.1007/s00127-002-0554-7. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875:44–0. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Koteja P, Garland T, Jr, Sax JK, Swallow JG, Carter PA. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Anim Behav. 1999;58:1307–1318. doi: 10.1006/anbe.1999.1270. [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56 (Suppl 1):177–85. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou SJ, Liu JY, Chang H, Chen PJ. Hippocampal neurogenesis and gene expression depend on exercise intensity in juvenile rats. Brain Res. 2008;1210:48–55. doi: 10.1016/j.brainres.2008.02.080. [DOI] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biol Psychiatry. 2010;68:774–7. doi: 10.1016/j.biopsych.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae PG, Spirduso WW, Walters TJ, Farrar RP, Wilcox RE. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolites in presenescent older rats. Psychopharmacology. 1987;92:236–40. doi: 10.1007/BF00177922. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci. 2007;27:11442–50. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen R, De Meirleir K. Exercise and brain neurotransmission. Sports Med. 1995;20:160–88. doi: 10.2165/00007256-199520030-00004. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–74. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–15. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CL, Williams CL, Komro KA, Veblen-Mortenson S, Stigler MH, Munson KA, Farbakhsh K, Jones RM, Forster JL. Project Northland: long-term outcomes of community action to reduce adolescent alcohol use. Health Educ Res. 2002;17:117–32. doi: 10.1093/her/17.1.117. [DOI] [PubMed] [Google Scholar]
- Perry CL, Williams CL, Veblen-Mortenson S, Toomey TL, Komro KA, Anstine PS, McGovern PG, Finnegan JR, Forster JL, Wagenaar AC, Wolfson M. Project Northland: outcomes of a communitywide alcohol use prevention program during early adolescence. Am J Public Health. 1996;86:956–65. doi: 10.2105/ajph.86.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr, et al. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003;117:1006–16. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- Santucci AC, Hernandez L, Caba J. Wheel-running behavior is altered following withdrawal from repeated cocaine in adult rats. Behav Neurosci. 2008;122:466–70. doi: 10.1037/0735-7044.122.2.466. [DOI] [PubMed] [Google Scholar]
- Small GW, Silverman DH, Siddarth P, Ercoli LM, Miller KJ, Lavretsky H, et al. Effects of a 14-day healthy longevity lifestyle program on cognition and brain function. Am J Geriatr Psychiatry. 2006;14:538–45. doi: 10.1097/01.JGP.0000219279.72210.ca. [DOI] [PubMed] [Google Scholar]
- Smith MA, Iordanou JC, Cohen MB, Cole KT, Gergans SR, Lyle MA, Schmidt KT. Effects of environmental enrichment on sensitivity to cocaine in female rats: importance of control rates of behavior. Behav Pharmacol. 2009;20:312–21. doi: 10.1097/FBP.0b013e32832ec568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Lyle MA. Chronic exercise decreases sensitivity to mu opioids in female rats: correlation with exercise output. Pharmacol Biochem Behav. 2006;85:12–22. doi: 10.1016/j.pbb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98:129–35. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Walker KL, Cole KT, Lang KC. The effects of aerobic exercise on cocaine self-administration in male and female rats. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2321-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Yancey DL. Sensitivity to the effects of opioids in rats with free access to exercise wheels: mu opioid tolerance and physical dependence. Psychopharmacology. 2003;167:426–34. doi: 10.1007/s00213-003-1471-5. [DOI] [PubMed] [Google Scholar]
- Specker SM, Lac ST, Carroll ME. Food deprivation history and cocaine self-administration: an animal model of binge eating. Pharmacol Biochem Behav. 1994;48:1025–9. doi: 10.1016/0091-3057(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Klein ED, Bardo MT. Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behav Pharmacol. 2006;17:597–604. doi: 10.1097/01.fbp.0000236271.72300.0e. [DOI] [PubMed] [Google Scholar]
- Ströhle A, Höfler M, Pfister H, Müller AG, Hoyer J, Wittchen HU, et al. Physical activity and prevalence and incidence of mental disorders in adolescents and young adults. Psychol Med. 2007;37:1657–66. doi: 10.1017/S003329170700089X. [DOI] [PubMed] [Google Scholar]
- Sudai E, Croitoru O, Shaldubina A, Abraham L, Gispan I, Flaumenhaft Y, Roth-Deri I, Kinor N, Aharoni S, Ben-Tzion M, Yadid G. High cocaine dosage decreases neurogenesis in the hippocampus and impairs working memory. Addict Biol. 2011;16:251–60. doi: 10.1111/j.1369-1600.2010.00241.x. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Engelhardt B, Hood LE, Peartree NA, Neisewander JL. The interactive effects of environmental enrichment and extinction interventions in attenuating cue-elicited cocaine-seeking behavior in rats. Pharmacol Biochem Behav. 2011;97:595–602. doi: 10.1016/j.pbb.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Pentkowski NS, Peartree NA, Painter MR, Neisewander JL. Environmental living conditions introduced during forced abstinence alter cocaine-seeking behavior and Fos protein expression. Neuroscience. 2010;171:1187–96. doi: 10.1016/j.neuroscience.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–42. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology. 1997;130:203–12. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- U.S. Congress, Office of Technology Assessment. Technologies for Understanding and Preventing Substance Abuse and Addiction, OTA-EHR-597. Washington DC: U.S. Government Printing Office; 1994. [Google Scholar]
- Uda M, Ishido M, Kami K, Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Res. 2006;1104:64–72. doi: 10.1016/j.brainres.2006.05.066. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Juan R, Arai H, Hori T, Asada T. Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Ann N Y Acad Sci. 2004;1025:351–62. doi: 10.1196/annals.1316.043. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Dan I, Tsuzuki D, Kato M, Okamoto M, Kyutoku Y, et al. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage. 2010;50:1702–10. doi: 10.1016/j.neuroimage.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME. Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology. 2010;209:113–25. doi: 10.1007/s00213-010-1776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]