Abstract
There are conflicting reports as to whether malignant peripheral nerve sheath tumor (MPNST) patients with neurofibromatosis type 1 (NF1) have worse prognosis than non-NF1 MPNST patients. Large clinical studies to address this problem are lacking due to the rareness of MPNST. We have performed meta-analyses testing the effect of NF1 status on MPNST survival based on publications from the last 50 years, including only nonoverlapping patients reported from each institution. In addition, we analyzed survival characteristics for 179 MPNST patients from 3 European sarcoma centers. The meta-analyses including data from a total of 48 studies and >1800 patients revealed a significantly higher odds ratio for overall survival (OROS) and disease-specific survival (ORDSS) in the non-NF1 group (OROS = 1.75, 95% confidence interval [CI] = 1.28–2.39, and ORDSS = 1.68, 95% CI = 1.18–2.40). However, in studies published in the last decade, survival in the 2 patient groups has been converging, as especially the NF1 group has shown improved prognosis. For our own MPNST patients, NF1 status had no effect on overall or disease-specific survival. The compiled literature from 1963 to the present indicates a significantly worse outcome of MPNST in patients with NF1 syndrome compared with non-NF1 patients. However, survival for the NF1 patients has improved in the last decade, and the survival difference is diminishing. These observations support the hypothesis that MPNSTs arising in NF1 and non-NF1 patients are not different per se. Consequently, we suggest that the choice of treatment for MPNST should be independent of NF1 status.
Keywords: MPNST, neurofibromatosis, NF1, meta-analysis
Twenty-seven years after the seminal publication on neurofibromatosis by Friedrich von Recklinghausen in 1882,1 the first 2 cases of malignant tumors of the peripheral nerves associated with neurofibromatosis type 1 (NF1) in Norway were described in a publication by Francis Harbitz (1867–1950), a professor of pathology and pathological anatomy at the Royal Frederick University in Christiania, since 1939 known as the University of Oslo.2,3 In his report, Harbitz describes 2 women aged 32 and 44 with >20-year histories of neurofibromatosis and multiple operations, both of whom died shortly after malignant transformation of preexisting plexiform neuromas. In the hundred years that have passed since then, our knowledge about neurofibromatosis and cancer biology, as well as surgical and therapeutic techniques, has developed tremendously. Still, even today, the medical histories of the 2 women are more typical than exceptional for patients showing malignant transformation of tumors in the peripheral nervous system.
Malignant peripheral nerve sheath tumor (MPNST) is currently the recommended term for all malignancies that arise from the peripheral nervous system or that show nerve sheath differentiation and includes tumors previously also known as malignant neuroma, malignant neurilemmoma, neurogenic sarcoma, neurofibrosarcoma, and malignant schwannoma.4,5 MPNST is a rare disease, with an incidence of 1 in 100 000 in the general population,6 and the prognosis is poor, with only 20%–50% of patients surviving 5 years from diagnosis. The correct primary diagnosis of MPNST remains a challenge due to morphological complexity as described in the soft tissue sarcoma reference textbooks,7,8 and typically, expert pathologists at reference institutions for sarcomas are responsible for conducting the diagnostic procedures.9 Treatment of MPNST also represents a great challenge, as there is currently no standardized treatment other than radical surgery. Chemotherapy is used for some patients with unresectable tumors or metastatic disease, and radiotherapy is occasionally used, but due to the rareness of the disease, it is not possible in a realistic time frame to conduct randomized controlled trials for MPNST patients only, and the documentation for the efficacy of any adjuvant treatment is limited.10–13
Roughly half of all MPNSTs are sporadic; they are found in patients who do not carry any known genetic predisposition for cancer. The remaining tumors are found in patients who are diagnosed with the genetic disorder NF1, an autosomal dominant disease with characteristic clinical manifestations such as multiple benign neurofibromas, Lisch nodules, and café-au-lait spots. The lifetime risk for developing malignant tumors in NF1 patients has been estimated to be up to 10%.14 NF1 patients have a shorter life expectancy compared with the general population, and in addition to their increased incidence of MPNST, they have a higher mortality rate from brain tumors and respiratory diseases.15,16 The NF1 syndrome is caused by alterations in the NF1 gene, mapping to the long arm of chromosome 17, which encodes the tumor suppressor protein neurofibromin. The complete function of this large protein is only partly understood, but a central GTPase domain is known to inhibit cell proliferation by inactivation of Ras proteins.17
So far, no decisive molecular differences have been identified in the tumors from NF1 and non-NF1 MPNST patients. Biallelic mutations of the NF1 gene are found in a significant portion of all MPNSTs, and among patients with NF1, one of the alleles is altered in the germline.18–20 Multiple chromosome alterations are typical for MPNSTs from both NF1 and non-NF1 patients and include frequent losses of chromosome arm 9p and gains of the whole or part of 17q.21–24
The reported survival rates of NF1 patients with MPNST compared with those of patients with sporadic MPNST are conflicting. In several reports, NF1 patients have a lower survival rate than non-NF1 patients,6,25–27 while other reports suggest that there is no difference.28–31 Here, we present updated and new survival data for a total of 179 MPNST patients from 2 Scandinavian and 1 Italian sarcoma center, as well as a comprehensive review of the literature and a meta-analysis summarizing the mortality risk in NF1 versus non-NF1 patients.
Materials and Methods
Patients
This study included 98 Norwegian, 26 Swedish, and 55 Italian patients with localized MPNST who were initially diagnosed between 1970 and 2011 and treated at the Norwegian Radium Hospital, Oslo, Norway; at Skåne University Hospital, Lund, Sweden; and at the Istituto Ortopedico Rizzoli, Bologna, Italy, respectively. The updated clinical data for each country are summarized in Supplementary Tables 1–3. Parts of the patient data have been presented in previous publications.24,32–34 The biobanks and projects were approved according to national legislation.
Statistical Analyses
Five-year overall survival (OS), disease-specific survival (DSS), and disease-free survival (DFS), as well as P values for other clinical associations, were calculated using SPSS software version 18.0 (see Supplementary material for details).
Literature Review and Meta-analyses
A MedLine (PubMed) search was performed using the search string “(“peripheral nervous system neoplasms” [MeSH] OR “nerve sheath neoplasms” [MeSH] AND malignant AND humans [MeSH] AND English [lang] AND (prognosis OR mortality OR survival OR clinicopathologic)”. The abstracts from all the hits were browsed to identify relevant citations, and the full manuscripts of these were read to identify all studies describing survival data for 10 or more MPNST patients. In addition to the citations identified through the MedLine search, we included relevant studies cited within the selected studies. The procedures for extraction of data and calculation of odds ratios (ORs) and hazard ratios (HRs) are described in the Supplementary material. Only studies with at least 5 NF1 and 5 non-NF1 patients were included for OR calculations in order to avoid ORs of zero or infinity. For HRs, all studies that included both patient groups were included.
The meta-analyses and assessment of heterogeneity and publication bias were performed using the software MIX Meta-analysis in Excel version 2.0,35 as described in the Supplementary material.
Results
Clinical Associations in 3 European Patient Groups
In the combined patient series from the 3 European sarcoma centers, 35% of the MPNST patients were diagnosed with NF1 (Table 1)—33% in the Norwegian series, 38% in the Swedish series, and 36% in the Italian series (Supplementary Tables 1–3). Generally, the NF1 patients were significantly younger than the non-NF1 patients at the time of the initial MPNST diagnosis, and they more often received adjuvant radio- and chemotherapy (Table 1). However, these associations were not seen for the Italian patients, where NF1 and non-NF1 patients were of equal age and the fractions of patients within each patient group receiving adjuvant treatment were similar (Supplementary Table 3). Tumors in the head, neck, or trunk were rarely seen among the Italian patients or among the Swedish non-NF1 patients. At the Norwegian hospital, the majority of tumors were found in the head, neck, or trunk, and no difference was seen between NF1 and non-NF1 patients in terms of tumor site. For all 179 patients from the 3 countries combined, the primary malignant tumors in NF1 patients were found to be slightly larger than tumors in non-NF1 patients (Table 1), but this observation was not significant for any of the national centers alone (Supplementary Tables 1–3). For the other clinical observables—gender, tumor grade, initial metastases, and remission status—we did not find any differences between the patients with sporadic and NF1-associated MPNST.
Table 1.
Clinical parameters and association to 5-year DSS
| All Patients |
Non-NF1 Patients |
NF1 Patients |
Distribution in NF1 vs Non-NF1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | DSS, % (SE) | Pa | No. | DSS, % (SE) | Pa | No. | DSS, % (SE) | Pa | Pb | |
| All patients | 179 | 46 (4) | 117 | 47 (5) | 62 | 45 (7) | ||||
| History of NF1 | .41 | |||||||||
| No | 117 | 47 (5) | ||||||||
| Yes | 62 | 45 (7) | ||||||||
| Country | .1 | .2 | .28 | .82 | ||||||
| Italy | 55 | 48 (7) | 35 | 53 (9) | 20 | 38 (11) | ||||
| Norway | 98 | 43 (5) | 66 | 42 (7) | 32 | 43 (9) | ||||
| Sweden | 26 | 59 (10) | 16 | 54 (13) | 10 | 67 (16) | ||||
| Age quartiles, yc | .21 | .08 | .9 | 1 × 10–7 | ||||||
| 11–25 | 46 | 50 (8) | 20 | 48 (12) | 26 | 53 (11) | ||||
| 26–42 | 44 | 52 (8) | 25 | 62 (10) | 18 | 39 (12) | ||||
| 43–59 | 44 | 42 (8) | 32 | 45 (9) | 12 | 31 (14) | ||||
| 60–86 | 45 | 42 (8) | 40 | 40 (8) | 5 | 60 (22) | ||||
| Gender | .57 | .91 | .44 | .53 | ||||||
| Female | 87 | 44 (6) | 59 | 47 (7) | 28 | 39 (10) | ||||
| Male | 92 | 48 (6) | 58 | 47 (7) | 34 | 50 (9) | ||||
| Grade | .002 | .02 | .05 | .33 | ||||||
| Low | 20 | 82 (9) | 15 | 76 (12) | 5 | 100 | ||||
| High | 151 | 41 (4) | 95 | 43 (5) | 56 | 39 (7) | ||||
| Missing | 8 | 7 | 1 | |||||||
| Tumor size quartiles, cmc | 7 × 10–7 | .00002 | .02 | .03 | ||||||
| 1–5 | 44 | 74 (7) | 37 | 75 (8) | 7 | 67 (19) | ||||
| 6–8 | 39 | 49 (8) | 24 | 46 (11) | 15 | 53 (13) | ||||
| 9–13 | 42 | 41 (8) | 20 | 43 (12) | 22 | 40 (11) | ||||
| 14–40 | 37 | 31 (8) | 22 | 27 (10) | 15 | 37 (13) | ||||
| Missing | 17 | 13 | 3 | |||||||
| Complete remission | .0005 | .0004 | .24 | 1.0 | ||||||
| No | 49 | 31 (7) | 31 | 28 (9) | 18 | 35 (12) | ||||
| Yes | 106 | 57 (5) | 67 | 59 (6) | 39 | 54 (8) | ||||
| Missing | 24 | 19 | 5 | |||||||
| Metastasis at time of diagnosis | 4 × 10–13 | .00003 | 4 × 10–10 | .33 | ||||||
| No | 154 | 52 (4) | 102 | 53 (5) | 52 | 52 (7) | ||||
| Yes | 20 | 5 (5) | 11 | 9 (9) | 9 | 0 | ||||
| Missing | 5 | 4 | 1 | |||||||
| Location | .07 | .06 | .66 | .87 | ||||||
| Non-extremities | 75 | 38 (6) | 48 | 38 (8) | 27 | 38 (10) | ||||
| Extremities | 102 | 52 (5) | 67 | 53 (6) | 35 | 50 (9) | ||||
| Missing | 2 | 2 | ||||||||
| Radiotherapy | .91 | .41 | .31 | .04 | ||||||
| No | 101 | 48 (5) | 73 | 47 (6) | 28 | 52 (10) | ||||
| Yes | 78 | 44 (6) | 44 | 47 (8) | 34 | 39 (9) | ||||
| Chemotherapy | .02 | .05 | .31 | .0003 | ||||||
| No | 116 | 52 (5) | 87 | 52 (6) | 29 | 52(10) | ||||
| Yes | 63 | 36 (6) | 30 | 32 (9) | 33 | 40 (9) | ||||
aSignificance from Breslow test for binary variables and Wald test for continuous variables (age and tumor size).
bTwo-sided Fisher exact test for categorical data. Two-sided t-test for continuous data: age, assuming nonequal variance; tumor size, assuming equal variance.
cSurvival percentages are shown for each quartile. P values were calculated using uncategorized continuous data.
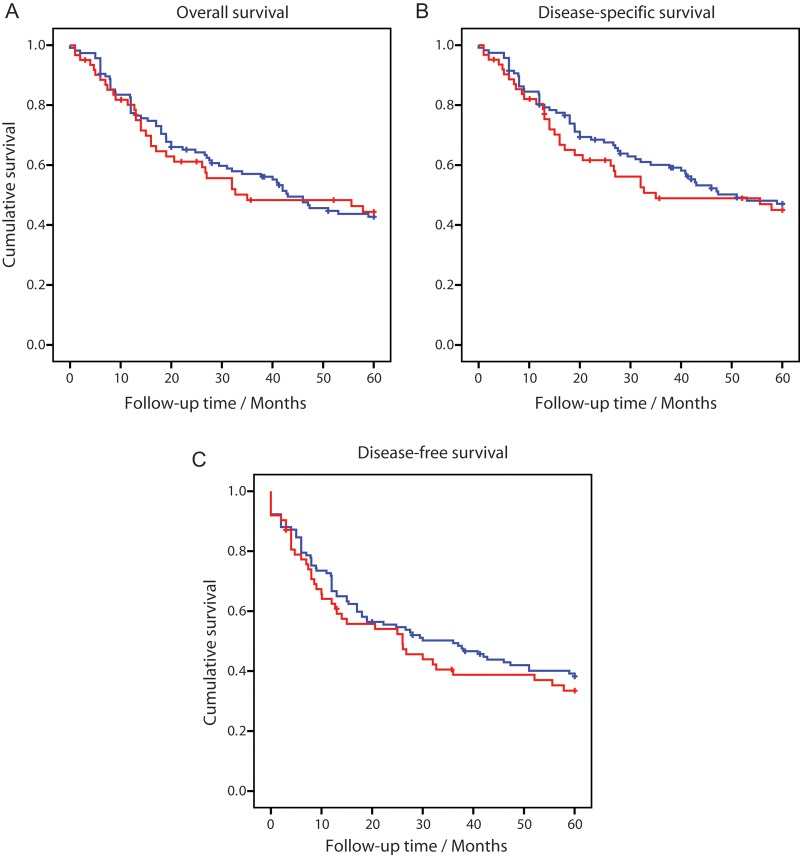
Patient Survival
OS, DSS, and DFS curves for NF1 and non-NF1-associated MPNST are shown in Fig. 1. Among the 179 patients presented here, 90 were recorded to have died of MPNST, while 6 died of other causes within 5 years of MPNST diagnosis. Neither OS (P = .70) nor DSS (P = .41) showed any difference between NF1 and non-NF1 patients (Fig. 1A and B; Table 1 and Supplementary Table 4). For DFS, a slightly lower survival percentage was observed among NF1 patients, although not significantly different (P = .42) (Fig. 1C; Supplementary Table 5). An overview of the relationship between DSS and clinical factors is given in Table 1, and correspondingly for OS and DFS in Supplementary Tables 4 and 5, respectively. Tumor grade, tumor size, surgical remission status, and metastatic disease at time of initial diagnosis were all significantly associated with survival (Table 1). Patients who were selected for chemotherapy also seem to have a worse prognosis, while patients receiving radiotherapy had no significant difference in survival compared with those not receiving such treatment. When patients were stratified by NF1 association, several of these prognostic factors had a more pronounced effect for the non-NF1 patients than for the NF1 patients, which may be partly explained by a lower number of observations in the NF1 group. Most strikingly, remission status failed to be a significant predictor of survival in the NF1 group, although the actual survival percentages in the 2 patient groups were similar (Table 1). The effect of age was highly significant for OS, and to a lesser extent for DFS, among non-NF1 patients, while no such effect could be seen for the NF1 patients, who were generally younger (Supplementary Tables 4 and 5).
Fig. 1.
Kaplan–Meier plots for 5-year OS (A), DSS (B), and DFS (C) from MPNST patients with NF1 (n = 62, red lines) and without NF1 (n = 117, blue lines).
Literature Search
The MedLine search for citations reporting survival data for patients with nerve sheath neoplasms resulted in a total of 747 hits from 1965 to February 2012 (Fig. 2). After browsing all the abstracts to identify studies that reported patient survival in NF1 and non-NF1-associated malignant nerve sheath neoplasms, 197 citations were found relevant. All of these 197 publications were screened in depth to identify studies that included at least 10 MPNSTs and that specified clinical data for the individual patients or separate survival percentages for NF1 and non-NF1 patients. Fifty-nine citations fulfilled these criteria. In addition, 14 studies that did not appear on the initial MedLine search were included based on citations within the selected studies; 2 recently published studies were also included that were still not indexed for MedLine.36,37 The extracted data from each of the 73 studies are listed in Supplementary Table 6. Finally, the patient origins and inclusion time frames in each study were compared, and studies with overlapping patient material were excluded, leaving only the largest study from each institution. Studies reporting only one patient group, NF1 or non-NF1, were also excluded. Exceptions were made for the 2 papers published by D'Agostino et al.38,39 that covered the 2 patient groups separately; these 2 papers were merged into a single study. Also for the 2 papers by Schmidt et al.,23,40 overlapping patients could be identified and excluded, which allowed us to combine the remaining patients into a single larger study. A total of 48 studies was thus selected, covering more than 1850 unique MPNST patients.
Fig. 2.
Flowchart of literature review and study selection for meta-analyses.
Meta-analysis Synthesis
Two meta-analyses were performed comparing ORs for mortality in NF1-associated MPNST versus non-NF1-associated MPNST; one included studies reporting OS23,25–28,31,38–60 (Fig. 3A), and the other included studies reporting DSS29,30,36,38,39,41,42,46,48,50,51,53,61–65 (Fig. 3B) and (Fig. 3C). Both showed a significantly worse prognosis for NF1-associated MPNST versus non-NF1-associated MPNST. Similar results were obtained for HRs as effect measure (Supplementary Fig. 1A and B for OS23,27,31,37–41,44,46,48,50,51,53,54,57–59,61,63,66–74 and DSS,29,30,36–39,41,46,48,50,51,53,61,63,66,69,70,72,74 respectively). A summary of the meta-analyses with quality assessment parameters for heterogeneity and publication bias can be found in Table 2.
Fig. 3.
Meta-analyses of OR for mortality from MPNST in NF1 patients compared with non-NF1 patients using OS (A and C) and DSS (B and D) as clinical endpoints, and 1963 to present (A and B) and 2001 to present (C and D) as publication time frames. The OR for each study is represented by a square; horizontal lines represent 95% CIs. The size of the square represents the weight (inverse variance). The diamonds represent the pooled ORs using a random effects model.
Table 2.
Results and quality assessment of the 4 meta-analyses measuring the effect of NF1 status on MPNST mortality
| Meta-analysis Identifiers |
Synthesis |
Quality Assessment |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Heterogeneity |
Publication Bias |
||||||||
| Effect measurea | Survival endpoint | n included studies | n patients | Pooled effectb (95% CI) | P | Cochran's Q-test | P | I2 | Pc |
| OR | OS | 28 | 1652 | 1.75 (1.28–2.39) | .0004 | 44 | .02 | 39% | .84 |
| OR | DSS | 17 | 1041 | 1.68 (1.18–2.40) | .004 | 22 | .15 | 27% | .56 |
| HR | OS | 28 | 969 | 1.38 (1.10–1.72) | .004 | 33 | .17 | 20% | .17 |
| HR | DSS | 19 | 975 | 1.40 (1.13–1.75) | .002 | 14 | .71 | 0% | .06d |
aThe effect measures, OR and HR, indicate the risk for death in NF1-associated MPNST vs non-NF1 MPNST.
bRandom effect model.
cEgger's regression test for zero intercept.
dThe Egger test indicates that there might be publication bias. Trim-and-fill correction gives HRDSS = 1.33 (1.08–1.65).
When only studies published after 2000 were included in the meta-analyses, significance is greatly reduced (Table 3). For OS, neither OR26–28,31,54–60 (Fig. 3B) nor HR27,31,37,54,57–59,72–74 (Supplementary Fig. 1C) showed a statistically significant difference between the 2 MPNST patient groups (P = .12 and 0.30, respectively). For DSS, the OR29,30,36,65 (Fig. 3D) and HR29,30,36,37,72,74 (Supplementary Fig. 1D) were still borderline significant (P = .02 and .05, respectively).
Table 3.
Results and quality assessment of the 4 meta-analyses measuring the effect of NF1 status on MPNST mortality for studies published after year 2000 only
| Meta-analysis Identifiers |
Synthesis |
Quality Assessment |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Heterogeneity |
Publication Bias |
||||||||
| Effect measurea | Survival endpoint | n included studies | n patients | Pooled effectb (95% CI) | P | Cochran's Q-test | P | I2 | Pc |
| OR | OS | 12 | 975 | 1.47 (0.91–2.39) | .12 | 25 | .007 | 57% | .79 |
| OR | DSS | 5 | 720 | 1.47 (1.06–2.04) | .02 | 4 | .42 | 0% | .73 |
| HR | OS | 11 | 572 | 1.19 (0.85–1.66) | .30 | 16 | .10 | 37% | .87 |
| HR | DSS | 7 | 701 | 1.32 (1.00–1.74) | .05 | 3 | .83 | 0% | .19 |
aThe effect measures, OR and HR, indicate the risk for death in NF1-associated MPNST versus non-NF1 MPNST.
bRandom effect model.
cEgger's regression test for zero intercept.
Correlations between MPNST Survival and Time of Report
To further analyze dependency of the survival data with date of publication, individual patient data were extracted. For studies from the same institution, duplicate patients were identified and excluded if 2 patients had matching age, gender, and NF1 status, as well as identical follow-up information or extended follow-up information in the most recent report. In summary, follow-up data for 910 unique MPNST patients were extracted: 398 NF1 and 512 non-NF1.9,23,31,37–41,44,46,50,51,53,54,57,58,61,63,66–81
A univariate Cox regression analysis using publication year before or within the last decade (ie, published 1963–2000 vs 2001–2012) as a binary explanatory variable for OS showed that recent publication was significantly correlated with improved OS for NF1 patients with MPNST (HR, 0.71; 95% CI, 0.56–0.90; P = .004), and Kaplan–Meier analysis showed that OS improved from 26% before 2001 to 39% after 2001 for this patient group (Fig. 4). For non-NF1 patients an opposite tendency was observed, with 43% OS before 2001 and 36% after 2001, although this finding was less significant in the Cox regression analysis (HR, 1.24; 95% CI, 0.98–1.55; P = .07).
Fig. 4.

Kaplan–Meier plots comparing time dependency of 5-year OS for MPNST patients with NF1 (red lines) and without NF1 (blue lines). Thick lines include studies published after 2000 (n = 207 NF1 and n = 246 non-NF1) and thin lines include studies published between 1963 and 2000 (n = 191 NF1 and n = 266 non-NF1).
Discussion
The present report summarizes the survival data for 179 MPNST patients from 3 European sarcoma centers. We found a 5-year DSS of 46%, an OS of 44%, and a DFS of 37%, and there was no statistically significant difference in survival between patients with and without NF1. A similar conclusion has been reported in other large studies28–31; however, there are also several studies that report a significantly worse outcome for MPNST in NF1 patients.6,25–27
To address this problem, we present 4 meta-analyses that compare the risk for death from MPNST in NF1 patients versus non-NF1 patients: 2 meta-analyses comparing OS and 2 comparing DSS, using both OR and HR as effect measures. The combined literature on MPNST from 1963 to the present suggests that the NF1 patient group has worse prognosis than the non-NF1 group irrespective of the effect measure and survival endpoint analyzed. However, there is a trend toward larger patient series and a smaller difference between NF1 and non-NF1 patients in more recent studies. When only studies published in the last decade were included in the meta-analyses (2001–2012), OS was no longer significantly different between NF1 and non-NF1 MPNST patients (Table 3). For DSS, the difference between non-NF1 and NF1 in studies published after 2001 was also reduced but still borderline significant.
The Kaplan–Meier plots based on data from more than 900 individual patients that could be extracted from 38 studies listed in Supplementary Table 6 further illustrate the trend that NF1 patients approach the survival levels of non-NF1 patients (Fig. 4). The observation that survival from MPNST for NF1 patients has improved in recent years has also been described in an independent study from the United Kingdom in which the authors report improved 5-year survival in the range of ∼25% (for NF1 patients diagnosed in 1980–1996) to ∼55% (for NF1 patients diagnosed in 1997–2010).82
There might be at least 4 explanations for why NF1 patients have been reported to have poorer outcomes after an MPNST diagnosis than non-NF1 patients: (1) MPNSTs in NF1 patients are biologically different and inherently more aggressive, (2) the natural tumor defense systems in NF1 patients are less fit to combat cancer, thus allowing for more rapid growth of the malignant tumor, (3) the MPNST diagnosis is delayed in NF1 patients, resulting in more advanced tumors, and (4) treatment of MPNST in NF1 patients differs from that in non-NF1 patients. While the first 2 alternatives describe biological differences, the last 2 depend on when and how patients are received in the clinic.
If there are biological differences between MPNSTs in NF1 patients compared with non-NF1 patients, one would expect to find molecular differences in the DNA, RNA, or protein level in these tumors. DNA copy number variation and large chromosomal rearrangements have long been known to occur in MPNSTs, while in neurofibromas, which may be regarded as benign counterparts to MPNSTs, far fewer or no genomic aberrations are found.9,83–86 However, while several recurrent changes have been reported for MPNSTs, no data on consistent differences between NF1 and non-NF1 tumors have been extracted from these studies. A handful of studies have analyzed mRNA expression profiles in MPNST,57,87–93 and gene profiles that distinguish MPNSTs from neurofibromas have been suggested. However, none of these studies could find a reliable distinction between patients with NF1 and non-NF1 MPNST. Watson et al.57 did note a higher average expression of EGFR in NF1-associated MPNSTs, but this was not related to survival and has so far not been verified in other studies. TP53 mutations are frequent in many cancer types, and some small studies report mutations in up to 70% of NF1-associated MPNSTs94,95; others claim that TP53 mutations are mainly found in non-NF1 MPNSTs,96 while we and others have reported that TP53 mutations are rare in MPNST.97–99 Several immunohistochemical markers have been suggested to have prognostic information for MPNST, but none has consistently distinguished between NF1 and non-NF1 MPNSTs.30–32,87,98,100–103 In conclusion, the current literature provides very little evidence to support a biological difference between NF1 and non-NF1-associated MPNST; however, as more advanced technologies are continuously being implemented in research and clinical use, we will not rule out that such differences may be found in the future.
Clinical parameters may also provide hints to any differences between MPNST patients with and without the NF1 syndrome. A review of the literature listed in Supplementary Table 6 showed that NF1 patients were generally significantly younger than patients without NF1 in practically all MPNST reports. This finding is as expected because NF1 carries a germline mutation of the NF1 gene, which is believed to be an initiating factor for development of both neurofibromas and MPNSTs.104,105 However, since the same NF1 mutations are found in sporadic tumors, this cannot alone explain any difference in outcome. Population studies on NF1 patients have shown that the mortality from causes other than MPNST in this group is higher than in the general population,15,16,106–108 suggesting that there will be a bias toward lower OS in the NF1 group. On the other hand, age may be a contributing factor for lower OS in the non-NF1 group, as MPNST patients without NF1 were on average 20 years older than MPNST patients with NF1. In our data covering 179 patients, 6/96 deaths within 5 years after diagnosis were attributed to causes other than MPNST, and coincidentally, none of these 6 patients had NF1.
Associations between NF1 status and other clinical parameters are less clear and not confirmed by independent studies. In a recent study of MPNST patients with NF1,82 the authors report significantly improved survival for female NF1 patients, but this is in contrast to our data in which female NF1 patients had a worse prognosis, although not significantly so (see Table 1). Some studies report that a higher proportion of malignant tumors in NF1 patients are located in the trunk than in the extremities and that NF1-associated MPNSTs have a larger average size than sporadic tumors.6,25,27 In our data, we did not see any difference in tumor localization. A slightly larger tumor size in NF1 could be detected when the patients from all 3 hospitals were merged (Table 1), but not on the level of each hospital (Supplementary Tables 1–3). Similar results have been reported by others.26,29 Larger tumor size in NF1 patients might well be a sign of a more aggressive tumor, but without any molecular data to support this, it is just as likely that the tumors are detected at a later stage.
The observation that survival for NF1 patients has improved in recent years suggests that exogenous factors have changed during this period. It seems reasonable that detection of a novel malignant tumor in an NF1 patient who carries the burden of multiple benign neurofibromas can be more challenging than detection of a single tumor in a non-NF1 patient, and as a consequence, some NF1 patients may have presented more advanced tumors than the average non-NF1 patient at the time of diagnosis. A delayed MPNST diagnosis may also be rationalized by social stigmatization experienced by NF1 patients, which may prevent them from seeking medical assistance at an early stage of cancer development.109 This bias would lead to higher ORs and HRs for deaths of NF1 patients versus non-NF1 patients. Therefore, one might speculate that awareness among NF1 patients and the monitoring of routines have improved over the last few years, allowing for earlier detection of potentially malignant tumors in this group of patients, thus explaining the smaller difference compared with non-NF1 MPNST patients. A puzzling observation is that we found the opposite tendency for non-NF1 patients: survival has decreased slightly for these patients in the last few years. A possible explanation for this observation is that better consensus agreements for soft tissue sarcoma diagnostics in the last decade have reduced the number of less aggressive tumors being misclassified as MPNST, especially for non-NF1 patients.
In summary, the molecular characteristics of NF1 and non-NF1 MPNSTs known today do not provide any obvious explanation for more aggressive behavior by NF1-associated MPNST, nor can they identify any subgroups of poor disease outcome within either of the cohorts. Based on the presented survival analysis and meta-analyses, MPNST patients with and without NF1 have similar DSS prognoses, although NF1 patients appear to have an increased overall mortality, which may result from increased mortality from causes other than MPNST.
Supplementary Material
Funding
This work was financed by grants to R. A. L. from the Faculty of Medicine, University of Oslo (supporting M. H. as a PhD student), from the Norwegian Cancer Society (supporting T. H. Å. as a PhD student), and from the Comprehensive Cancer Centre–the Norwegian Radium Hospital. F. M. received support from the Swedish Childhood Cancer Foundation.
Conflict of interest statement: None declared.
Supplementary Material
References
- 1.von Recklinghausen F. Über die multiplen Fibrome der Haut und ihre Beziehung zu den multiplen Neuromen. Festschrift für Rudolf Virchow. Berlin, Germany: Hirschwald; 1882. p. 138. [Google Scholar]
- 2.Harbitz F. Multiple neurofibromatosis (von Recklinghausen's disease) Arch Int Med. 1909;3:32–65. [Google Scholar]
- 3.Hosoi K. Multiple neurofibromatosis (von Recklinghausen's disease) Arch Surg. 1931;22:258–281. [Google Scholar]
- 4.Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002;62:1573–1577. [PubMed] [Google Scholar]
- 5.Perrin RG, Guha A. Malignant peripheral nerve sheath tumors. Neurosurg Clin N Am. 2004;15:203–216. doi: 10.1016/j.nec.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006–2021. doi: 10.1002/1097-0142(19860515)57:10<2006::aid-cncr2820571022>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002. [Google Scholar]
- 8.Weiss SW, Goldblum JR. Enzinger and Weiss's Soft Tissue Tumors. 5th edn. St. Louis, MO: Mosby Elsevier; 2008. [Google Scholar]
- 9.Mertens F, Dal Cin P, De Wever I, et al. Cytogenetic characterization of peripheral nerve sheath tumours: a report of the CHAMP study group. J Pathol. 2000;190:31–38. doi: 10.1002/(SICI)1096-9896(200001)190:1<31::AID-PATH505>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Grobmyer SR, Reith JD, Shahlaee A, Bush CH, Hochwald SN. Malignant peripheral nerve sheath tumor: molecular pathogenesis and current management considerations. J Surg Oncol. 2008;97:340–349. doi: 10.1002/jso.20971. [DOI] [PubMed] [Google Scholar]
- 11.Gupta G, Mammis A, Maniker A. Malignant peripheral nerve sheath tumors. Neurosurg Clin N Am. 2008;19:533–543. doi: 10.1016/j.nec.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Widemann BC. Current status of sporadic and neurofibromatosis type 1–associated malignant peripheral nerve sheath tumors. Curr Oncol Rep. 2009;11:322–328. doi: 10.1007/s11912-009-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari A, Miceli R, Rey A, et al. Non-metastatic unresected paediatric non-rhabdomyosarcoma soft tissue sarcomas: results of a pooled analysis from United States and European groups. Eur J Cancer. 2011;47:724–731. doi: 10.1016/j.ejca.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCaughan JA, Holloway SM, Davidson R, Lam WW. Further evidence of the increased risk for malignant peripheral nerve sheath tumour from a Scottish cohort of patients with neurofibromatosis type 1. J Med Genet. 2007;44:463–466. doi: 10.1136/jmg.2006.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans DG, O'Hara C, Wilding A, et al. Mortality in neurofibromatosis 1: in North West England: an assessment of actuarial survival in a region of the UK since 1989. Eur J Hum Genet. 2011;19:1187–1191. doi: 10.1038/ejhg.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masocco M, Kodra Y, Vichi M, et al. Mortality associated with neurofibromatosis type 1: a study based on Italian death certificates (1995–2006) Orphanet J Rare Dis. 2011;6:11. doi: 10.1186/1750-1172-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker D, Wright E, Nguyen K, et al. Gene for von Recklinghausen neurofibromatosis is in the pericentromeric region of chromosome-17. Science. 1987;236:1100–1102. doi: 10.1126/science.3107130. [DOI] [PubMed] [Google Scholar]
- 18.Messiaen LM, Callens T, Mortier G, et al. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum Mutat. 2000;15:541–555. doi: 10.1002/1098-1004(200006)15:6<541::AID-HUMU6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 19.Upadhyaya M, Kluwe L, Spurlock G, et al. Germline and somatic NF1 gene mutation spectrum in NF1-associated malignant peripheral nerve sheath tumors (MPNSTs) Hum Mutat. 2008;29:74–82. doi: 10.1002/humu.20601. [DOI] [PubMed] [Google Scholar]
- 20.Bottillo I, Ahlquist T, Brekke H, et al. Germline and somatic NF1 mutations in sporadic and NF1-associated malignant peripheral nerve sheath tumours. J Pathol. 2009;217:693–701. doi: 10.1002/path.2494. [DOI] [PubMed] [Google Scholar]
- 21.Lothe RA, Karhu R, Mandahl N, et al. Gain of 17q24-qter detected by comparative genomic hybridization in malignant tumors from patients with von Recklinghausen's neurofibromatosis. Cancer Res. 1996;56:4778–4781. [PubMed] [Google Scholar]
- 22.Berner JM, Sorlie T, Mertens F, et al. Chromosome band 9p21 is frequently altered in malignant peripheral nerve sheath tumors: studies of CDKN2A and other genes of the pRB pathway. Genes Chromosomes Cancer. 1999;26:151–160. [PubMed] [Google Scholar]
- 23.Schmidt H, Wurl P, Taubert H, et al. Genomic imbalances of 7p and 17q in malignant peripheral nerve sheath tumors are clinically relevant. Genes Chromosomes Cancer. 1999;25:205–211. [PubMed] [Google Scholar]
- 24.Brekke HR, Ribeiro FR, Kolberg M, et al. Genomic changes in chromosomes 10, 16, and X in malignant peripheral nerve sheath tumors identify a high-risk patient group. J Clin Oncol. 2010;28:1573–1582. doi: 10.1200/JCO.2009.24.8989. [DOI] [PubMed] [Google Scholar]
- 25.Sordillo PP, Helson L, Hajdu SI, et al. Malignant schwannoma—clinical characteristics, survival, and response to therapy. Cancer. 1981;47:2503–2509. doi: 10.1002/1097-0142(19810515)47:10<2503::aid-cncr2820471033>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Carli M, Ferrari A, Mattke A, et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol. 2005;23:8422–8430. doi: 10.1200/JCO.2005.01.4886. [DOI] [PubMed] [Google Scholar]
- 27.Porter DE, Prasad V, Foster L, Dall GF, Birch R, Grimer RJ. Survival in malignant peripheral nerve sheath tumours: a comparison between sporadic and neurofibromatosis type 1–associated tumours. Sarcoma. 2009;2009:756395. doi: 10.1155/2009/756395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okada K, Hasegawa T, Tajino T, et al. Clinical relevance of pathological grades of malignant peripheral nerve sheath tumor: a multi-institution TMTS study of 56 cases in northern Japan. Ann Surg Oncol. 2007;14:597–604. doi: 10.1245/s10434-006-9053-5. [DOI] [PubMed] [Google Scholar]
- 29.Anghileri M, Miceli R, Fiore M, et al. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2006;107:1065–1074. doi: 10.1002/cncr.22098. [DOI] [PubMed] [Google Scholar]
- 30.Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249:1014–1022. doi: 10.1097/SLA.0b013e3181a77e9a. [DOI] [PubMed] [Google Scholar]
- 31.Yu J, Deshmukh H, Payton JE, et al. Array-based comparative genomic hybridization identifies CDK4 and FOXM1 alterations as independent predictors of survival in malignant peripheral nerve sheath tumor. Clin Cancer Res. 2011;17:1924–1934. doi: 10.1158/1078-0432.CCR-10-1551. [DOI] [PubMed] [Google Scholar]
- 32.Brekke HR, Kolberg M, Skotheim RI, et al. Identification of p53 as a strong predictor of survival for patients with malignant peripheral nerve sheath tumors. Neuro Oncol. 2009;11:514–528. doi: 10.1215/15228517-2008-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Storlazzi CT, Brekke HR, Mandahl N, et al. Identification of a novel amplicon at distal 17q containing the BIRC5/SURVIVIN gene in malignant peripheral nerve sheath tumours. J Pathol. 2006;209:492–500. doi: 10.1002/path.1998. [DOI] [PubMed] [Google Scholar]
- 34.Longhi A, Errani C, Magagnoli G, et al. High grade malignant peripheral nerve sheath tumors: outcome of 62 patients with localized disease and review of the literature. J Chemother. 2010;22:413–418. doi: 10.1179/joc.2010.22.6.413. [DOI] [PubMed] [Google Scholar]
- 35.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KG. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stucky CCH, Johnson KN, Gray BA, et al. Malignant peripheral nerve sheath tumors (MPNST): the Mayo Clinic experience. Ann Surg Oncol. 2012;19:878–885. doi: 10.1245/s10434-011-1978-7. [DOI] [PubMed] [Google Scholar]
- 37.Demir HA, Varan A, Yalcn B, Akyuz C, Kutluk T, Buyukpamukcu M. Malignant peripheral nerve sheath tumors in childhood: 13 cases from a single center. J Pediatr Hematol Oncol. 2012;34:204–207. doi: 10.1097/MPH.0b013e31822d4cef. [DOI] [PubMed] [Google Scholar]
- 38.D'Agostino AN, Soule EH, Miller RH. Primary malignant neoplasms of nerves (malignant neurilemomas) in patients without manifestations of multiple neurofibromatosis (von Recklinghausen's disease) Cancer. 1963;16:1003–1014. doi: 10.1002/1097-0142(196308)16:8<1003::aid-cncr2820160807>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 39.D'Agostino AN, Soule EH, Miller RH. Sarcomas of the peripheral nerves and somatic soft tissues associated with multiple neurofibromatosis (von Recklinghausen's disease) Cancer. 1963;16:1015–1027. doi: 10.1002/1097-0142(196308)16:8<1015::aid-cncr2820160808>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt H, Taubert H, Meye A, et al. Gains in chromosomes 7, 8q, 15q and 17q are characteristic changes in malignant but not in benign peripheral nerve sheath tumors from patients with Recklinghausen's disease. Cancer Lett. 2000;155:181–190. doi: 10.1016/s0304-3835(00)00426-2. [DOI] [PubMed] [Google Scholar]
- 41.White HR. Survival in malignant schwannoma. An 18-year study. Cancer. 1971;27:720–729. doi: 10.1002/1097-0142(197103)27:3<720::aid-cncr2820270331>3.0.co;2-d. Jr. [DOI] [PubMed] [Google Scholar]
- 42.Storm FK, Eilber FR, Mirra J, Morton DL. Neurofibrosarcoma. Cancer. 1980;45:126–129. doi: 10.1002/1097-0142(19800101)45:1<126::aid-cncr2820450122>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 43.Ariel IM. Tumors of the peripheral nervous system. CA Cancer J Clin. 1983;33:282–299. doi: 10.3322/canjclin.33.5.282. [DOI] [PubMed] [Google Scholar]
- 44.Bojsen-Møller M, Myhre-Jensen O A consecutive series of 30 malignant schwannomas. Survival in relation to clinico-pathological parameters and treatment. Acta Pathol Microbiol Immunol Scand A. 1984;92:147–155. [PubMed] [Google Scholar]
- 45.Nambisan RN, Rao U, Moore R, Karakousis CP. Malignant soft tissue tumors of nerve sheath origin. J Surg Oncol. 1984;25:268–272. doi: 10.1002/jso.2930250410. [DOI] [PubMed] [Google Scholar]
- 46.Raney B, Schnaufer L, Ziegler M, Chatten J, Littman P, Jarrett P. Treatment of children with neurogenic sarcoma. Experience at the Children's Hospital of Philadelphia, 1958–1984. Cancer. 1987;59:1–5. doi: 10.1002/1097-0142(19870101)59:1<1::aid-cncr2820590105>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 47.Greager JA, Reichard KW, Campana JP, DasGupta TK. Malignant schwannoma of the head and neck. Am J Surg. 1992;163:440–442. doi: 10.1016/0002-9610(92)90050-2. [DOI] [PubMed] [Google Scholar]
- 48.Chang SM, Ho WL. Malignant peripheral nerve sheath tumor: a study of 21 cases. Zhonghua Yi Xue Za Zhi (Taipei) 1994;54:122–130. [PubMed] [Google Scholar]
- 49.Doorn PF, Molenaar WM, Buter J, Hoekstra HJ. Malignant peripheral nerve sheath tumors in patients with and without neurofibromatosis. Eur J Surg Oncol. 1995;21:78–82. doi: 10.1016/s0748-7983(05)80073-3. [DOI] [PubMed] [Google Scholar]
- 50.DeCou JM, Rao BN, Parham DM, et al. Malignant peripheral nerve sheath tumors: the St. Jude Children's Research Hospital experience. Ann Surg Oncol. 1995;2:524–529. doi: 10.1007/BF02307086. [DOI] [PubMed] [Google Scholar]
- 51.Kunisada T, Kawai A, Ozaki T, Sugihara S, Taguchi K, Inoue H. A clinical analysis of malignant schwannoma. Acta Med Okayama. 1997;51:87–92. doi: 10.18926/AMO/30784. [DOI] [PubMed] [Google Scholar]
- 52.Wong WW, Hirose T, Scheithauer BW, Schild SE, Gunderson LL. Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Biol Phys. 1998;42:351–360. doi: 10.1016/s0360-3016(98)00223-5. [DOI] [PubMed] [Google Scholar]
- 53.Angelov L, Davis A, O'Sullivan B, Bell R, Guha A. Neurogenic sarcomas: experience at the University of Toronto. Neurosurgery. 1998;43:56–64. doi: 10.1097/00006123-199807000-00035. [DOI] [PubMed] [Google Scholar]
- 54.Ganju A, Roosen N, Kline DG, Tiel RL. Outcomes in a consecutive series of 111 surgically treated plexal tumors: a review of the experience at the Louisiana State University Health Sciences Center. J Neurosurg. 2001;95:51–60. doi: 10.3171/jns.2001.95.1.0051. [DOI] [PubMed] [Google Scholar]
- 55.Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39:311–314. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cashen DV, Parisien RC, Raskin K, Hornicek FJ, Gebhardt MC, Mankin HJ. Survival data for patients with malignant schwannoma. Clin Orthop Relat Res. 2004;426:69–73. doi: 10.1097/01.blo.0000131256.82455.c5. [DOI] [PubMed] [Google Scholar]
- 57.Watson MA, Perry A, Tihan T, et al. Gene expression profiling reveals unique molecular subtypes of neurofibromatosis type I–associated and sporadic malignant peripheral nerve sheath tumors. Brain Pathol. 2004;14:297–303. doi: 10.1111/j.1750-3639.2004.tb00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holtkamp N, Atallah I, Okuducu AF, et al. MMP-13 and p53 in the progression of malignant peripheral nerve sheath tumors. Neoplasia. 2007;9:671–677. doi: 10.1593/neo.07304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tabone-Eglinger S, Bahleda R, Cote JF, et al. Frequent EGFR positivity and overexpression in high-grade areas of human MPNSTs. Sarcoma. 2008;2008:849156. doi: 10.1155/2008/849156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Endo M, Kobayashi C, Setsu N, et al. Prognostic significance of p14ARF, p15INK4b and p16INK4a inactivation in malignant peripheral nerve sheath tumors. Clin Cancer Res. 2011;17:3771–3782. doi: 10.1158/1078-0432.CCR-10-2393. [DOI] [PubMed] [Google Scholar]
- 61.Hruban RH, Shiu MH, Senie RT, Woodruff JM. Malignant peripheral nerve sheath tumors of the buttock and lower extremity. A study of 43 cases. Cancer. 1990;66:1253–1265. doi: 10.1002/1097-0142(19900915)66:6<1253::aid-cncr2820660627>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 62.Meis JM, Enzinger FM, Martz KL, Neal JA. Malignant peripheral nerve sheath tumors (malignant schwannomas) in children. Am J Surg Pathol. 1992;16:694–707. doi: 10.1097/00000478-199207000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Kourea HP, Bilsky MH, Leung DH, Lewis JJ, Woodruff JM. Subdiaphragmatic and intrathoracic paraspinal malignant peripheral nerve sheath tumors: a clinicopathologic study of 25 patients and 26 tumors. Cancer. 1998;82:2191–2203. doi: 10.1002/(sici)1097-0142(19980601)82:11<2191::aid-cncr14>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 64.Loree TR, North JH, Jr., Werness BA, Nangia R, Mullins AP, Hicks WL., Jr. Malignant peripheral nerve sheath tumors of the head and neck: analysis of prognostic factors. Otolaryngol Head Neck Surg. 2000;122:667–672. doi: 10.1016/S0194-5998(00)70193-8. [DOI] [PubMed] [Google Scholar]
- 65.Hagel C, Zils U, Peiper M, et al. Histopathology and clinical outcome of NF1-associated vs. sporadic malignant peripheral nerve sheath tumors. J Neurooncol. 2007;82:187–192. doi: 10.1007/s11060-006-9266-2. [DOI] [PubMed] [Google Scholar]
- 66.Trojanowski JQ, Kleinman GM, Proppe KH. Malignant tumors of nerve sheath origin. Cancer. 1980;46:1202–1212. doi: 10.1002/1097-0142(19800901)46:5<1202::aid-cncr2820460521>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 67.Arpornchayanon O, Hirota T, Itabashi M, et al. Malignant peripheral nerve tumors: a clinicopathological and electron microscopic study. Jpn J Clin Oncol. 1984;14:57–74. [PubMed] [Google Scholar]
- 68.Daimaru Y, Hashimoto H, Enjoji M. Malignant peripheral nerve-sheath tumors (malignant schwannomas). An immunohistochemical study of 29 cases. Am J Surg Pathol. 1985;9:434–444. doi: 10.1097/00000478-198506000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Lodding P, Kindblom LG, Angervall L. Epithelioid malignant schwannoma. A study of 14 cases. Virchows Arch A Pathol Anat Histopathol. 1986;409:433–451. doi: 10.1007/BF00705415. [DOI] [PubMed] [Google Scholar]
- 70.McCarron KF, Goldblum JR. Plexiform neurofibroma with and without associated malignant peripheral nerve sheath tumor: a clinicopathologic and immunohistochemical analysis of 54 cases. Mod Pathol. 1998;11:612–617. [PubMed] [Google Scholar]
- 71.Casanova M, Ferrari A, Spreafico F, et al. Malignant peripheral nerve sheath tumors in children: a single-institution twenty-year experience. J Pediatr Hematol Oncol. 1999;21:509–513. [PubMed] [Google Scholar]
- 72.Minovi A, Basten O, Hunter B, Draf W, Bockmuhl U. Malignant peripheral nerve sheath tumors of the head and neck: management of 10 cases and literature review. Head Neck. 2007;29:439–445. doi: 10.1002/hed.20537. [DOI] [PubMed] [Google Scholar]
- 73.Scheithauer BW, Erdogan S, Rodriguez FJ, et al. Malignant peripheral nerve sheath tumors of cranial nerves and intracranial contents: a clinicopathologic study of 17 cases. Am J Surg Pathol. 2009;33:325–338. doi: 10.1097/PAS.0b013e31818d6470. [DOI] [PubMed] [Google Scholar]
- 74.Moretti VM, Crawford EA, Staddon AP, Lackman RD, Ogilvie CM. Early outcomes for malignant peripheral nerve sheath tumor treated with chemotherapy. Am J Clin Oncol–Cancer Clin Trials. 2011;34:417–421. doi: 10.1097/COC.0b013e3181e9c08a. [DOI] [PubMed] [Google Scholar]
- 75.Ducatman BS, Scheithauer BW. Malignant peripheral nerve sheath tumors with divergent differentiation. Cancer. 1984;54:1049–1057. doi: 10.1002/1097-0142(19840915)54:6<1049::aid-cncr2820540620>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 76.Bailet JW, Abemayor E, Andrews JC, Rowland JP, Fu YS, Dawson DE. Malignant nerve sheath tumors of the head and neck: a combined experience from two university hospitals. Laryngoscope. 1991;101:1044–1049. doi: 10.1288/00005537-199110000-00003. [DOI] [PubMed] [Google Scholar]
- 77.Shearer P, Parham D, Kovnar E, et al. Neurofibromatosis type I and malignancy: review of 32 pediatric cases treated at a single institution. Med Pediatr Oncol. 1994;22:78–83. doi: 10.1002/mpo.2950220203. [DOI] [PubMed] [Google Scholar]
- 78.Halling KC, Scheithauer BW, Halling AC, et al. p53 expression in neurofibroma and malignant peripheral nerve sheath tumor: an immunohistochemical study of sporadic and NF1-associated tumors. Am J Clin Pathol. 1996;106:282–288. doi: 10.1093/ajcp/106.3.282. [DOI] [PubMed] [Google Scholar]
- 79.Kluwe L, Friedrich RE, Peiper M, Friedman J, Mautner VF. Constitutional NF1 mutations in neurofibromatosis 1 patients with malignant peripheral nerve sheath tumors. Hum Mutat. 2003;22:420. doi: 10.1002/humu.9193. [DOI] [PubMed] [Google Scholar]
- 80.Tucker T, Wolkenstein P, Revuz J, Zeller J, Friedman JM. Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology. 2005;65:205–211. doi: 10.1212/01.wnl.0000168830.79997.13. [DOI] [PubMed] [Google Scholar]
- 81.Brenner W, Friedrich RE, Gawad KA, et al. Prognostic relevance of FDG PET in patients with neurofibromatosis type-1 and malignant peripheral nerve sheath tumours. Eur J Nucl Med Mol Imaging. 2006;33:428–432. doi: 10.1007/s00259-005-0030-1. [DOI] [PubMed] [Google Scholar]
- 82.Ingham S, Huson SM, Moran A, Wylie J, Leahy M, Evans DG. Malignant peripheral nerve sheath tumours in NF1: improved survival in women and in recent years. Eur J Cancer. 2011;47:2723–2728. doi: 10.1016/j.ejca.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 83.Lothe RA, Slettan A, Saeter G, Brogger A, Borresen AL, Nesland JM. Alterations at chromosome 17 loci in peripheral nerve sheath tumors. J Neuropathol Exp Neurol. 1995;54:65–73. doi: 10.1097/00005072-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 84.Mechtersheimer G, Otano-Joos M, Ohl S, et al. Analysis of chromosomal imbalances in sporadic and NF1-associated peripheral nerve sheath tumors by comparative genomic hybridization. Genes Chromosomes Cancer. 1999;25:362–369. [PubMed] [Google Scholar]
- 85.Plaat BE, Molenaar WM, Mastik MF, Hoekstra HJ, te Meerman GJ, van den Berg E. Computer-assisted cytogenetic analysis of 51 malignant peripheral-nerve-sheath tumors: sporadic vs. neurofibromatosis-type-1–associated malignant schwannomas. Int J Cancer. 1999;83:171–178. doi: 10.1002/(sici)1097-0215(19991008)83:2<171::aid-ijc5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 86.Koga T, Iwasaki H, Ishiguro M, Matsuzaki A, Kikuchi M. Frequent genomic imbalances in chromosomes 17, 19, and 22q in peripheral nerve sheath tumours detected by comparative genomic hybridization analysis. J Pathol. 2002;197:98–107. doi: 10.1002/path.1101. [DOI] [PubMed] [Google Scholar]
- 87.Skotheim RI, Kallioniemi A, Bjerkhagen B, et al. Topoisomerase-II alpha is upregulated in malignant peripheral nerve sheath tumors and associated with clinical outcome. J Clin Oncol. 2003;21:4586–4591. doi: 10.1200/JCO.2003.07.067. [DOI] [PubMed] [Google Scholar]
- 88.Holtkamp N, Reuss DE, Atallah I, et al. Subclassification of nerve sheath tumors by gene expression profiling. Brain Pathol. 2004;14:258–264. doi: 10.1111/j.1750-3639.2004.tb00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Henderson SR, Guiliano D, Presneau N, et al. A molecular map of mesenchymal tumors. Genome Biol. 2005;6:R76. doi: 10.1186/gb-2005-6-9-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karube K, Nabeshima K, Ishiguro M, Harada M, Iwasaki H. cDNA microarray analysis of cancer associated gene expression profiles in malignant peripheral nerve sheath tumours. J Clin Pathol. 2006;59:160–165. doi: 10.1136/jcp.2004.023598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller SJ, Rangwala F, Williams J, et al. Large-scale molecular comparison of human schwann cells to malignant peripheral nerve sheath tumor cell lines and tissues. Cancer Res. 2006;66:2584–2591. doi: 10.1158/0008-5472.CAN-05-3330. [DOI] [PubMed] [Google Scholar]
- 92.Francis P, Namløs HM, Muller C, et al. Diagnostic and prognostic gene expression signatures in 177 soft tissue sarcomas: hypoxia-induced transcription profile signifies metastatic potential. BMC Genomics. 2007;8:73. doi: 10.1186/1471-2164-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lévy P, Ripoche H, Laurendeau I, et al. Microarray-based identification of tenascin C and tenascin XB, genes possibly involved in tumorigenesis associated with neurofibromatosis type 1. Clin Cancer Res. 2007;13:398–407. doi: 10.1158/1078-0432.CCR-06-0182. [DOI] [PubMed] [Google Scholar]
- 94.Legius E, Dierick H, Wu R, et al. TP53 mutations are frequent in malignant NF1 tumors. Genes Chromosomes Cancer. 1994;10:250–255. doi: 10.1002/gcc.2870100405. [DOI] [PubMed] [Google Scholar]
- 95.Leroy K, Dumas V, Martin-Garcia N, et al. Malignant peripheral nerve sheath tumors associated with neurofibromatosis type 1: a clinicopathologic and molecular study of 17 patients. Arch Dermatol. 2001;137:908–913. [PubMed] [Google Scholar]
- 96.Birindelli S, Perrone F, Oggionni M, et al. Rb and TP53 pathway alterations in sporadic and NF1-related malignant peripheral nerve sheath tumors. Lab Invest. 2001;81:833–844. doi: 10.1038/labinvest.3780293. [DOI] [PubMed] [Google Scholar]
- 97.Taubert H, Wurl P, Bache M, et al. The p53 gene in soft tissue sarcomas: prognostic value of DNA sequencing versus immunohistochemistry. Anticancer Res. 1998;18:183–187. [PubMed] [Google Scholar]
- 98.Watanabe T, Oda Y, Tamiya S, Kinukawa N, Masuda K, Tsuneyoshi M. Malignant peripheral nerve sheath tumours: high Ki67 labelling index is the significant prognostic indicator. Histopathology. 2001;39:187–197. doi: 10.1046/j.1365-2559.2001.01176.x. [DOI] [PubMed] [Google Scholar]
- 99.Lothe RA, Smith-Sorensen B, Hektoen M, et al. Biallelic inactivation of TP53 rarely contributes to the development of malignant peripheral nerve sheath tumors. Genes Chromosomes Cancer. 2001;30:202–206. [PubMed] [Google Scholar]
- 100.Ågesen TH, Flørenes VA, Molenaar WM, et al. Expression patterns of cell cycle components in sporadic and neurofibromatosis type 1–related malignant peripheral nerve sheath tumors. J Neuropathol Exp Neurol. 2005;64:74–81. doi: 10.1093/jnen/64.1.74. [DOI] [PubMed] [Google Scholar]
- 101.Keizman D, Issakov J, Meller I, et al. Expression and significance of EGFR in malignant peripheral nerve sheath tumor. J Neurooncol. 2009;94:383–388. doi: 10.1007/s11060-009-9862-z. [DOI] [PubMed] [Google Scholar]
- 102.Kobayashi C, Oda Y, Takahira T, et al. Aberrant expression of CHFR in malignant peripheral nerve sheath tumors. Mod Pathol. 2006;19:524–532. doi: 10.1038/modpathol.3800548. [DOI] [PubMed] [Google Scholar]
- 103.Kourea HP, Cordon-Cardo C, Dudas M, Leung D, Woodruff JM. Expression of p27(kip) and other cell cycle regulators in malignant peripheral nerve sheath tumors and neurofibromas: the emerging role of p27(kip) in malignant transformation of neurofibromas. Am J Pathol. 1999;155:1885–1891. doi: 10.1016/S0002-9440(10)65508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cichowski K, Shih TS, Schmitt E, et al. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286:2172–2176. doi: 10.1126/science.286.5447.2172. [DOI] [PubMed] [Google Scholar]
- 105.Vogel KS, Klesse LJ, Velasco-Miguel S, Meyers K, Rushing EJ, Parada LF. Mouse tumor model for neurofibromatosis type 1. Science. 1999;286:2176–2179. doi: 10.1126/science.286.5447.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sørensen SA, Mulvihill JJ, Nielsen A. Long-term follow-up of von Recklinghausen neurofibromatosis. Survival and malignant neoplasms. N Engl J Med. 1986;314:1010–1015. doi: 10.1056/NEJM198604173141603. [DOI] [PubMed] [Google Scholar]
- 107.Zöller M, Rembeck B, Akesson HO, Angervall L. Life expectancy, mortality and prognostic factors in neurofibromatosis type 1. A twelve-year follow-up of an epidemiological study in Goteborg, Sweden. Acta Derm Venereol. 1995;75:136–140. doi: 10.2340/0001555575136140. [DOI] [PubMed] [Google Scholar]
- 108.Rasmussen SA, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am J Hum Genet. 2001;68:1110–1118. doi: 10.1086/320121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ablon J. Living with Genetic Disorder: The Impact of Neurofibromatosis 1. Westport, CT: Auburn House; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.