Abstract
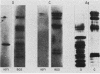
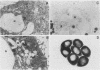
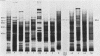
Two monoclonal antibodies have been produced against the human 85,000-molecular-weight heat shock protein (hsp85). One of these, 16F1, cross-reacts with the murine homolog and is shown by peptide map immunoblots to be directed against an epitope different from that recognized by the other monoclonal antibody, 9D2. Both monoclonal antibodies recognize only a single Mr-85,000 species in two-dimensional immunoblots. Immunoprecipitation did not reveal an association of this heat shock protein with any other protein in HeLa cells. Immunoperoxidase staining showed a purely cytosolic distribution at both light and electron microscopic levels and no association with membranes, mitochondria, or other organelles. The 9D2 monoclonal and a polyclonal antimurine hsp85 antibody were used to identify the antigens and to quantitate their levels in a variety of normal tissues by immunoautoradiography. Relative abundance in the various tissues as determined by Coomassie blue staining correlates reasonably well with the immunoreactivity. Testis and brain, for example, have high hsp85 levels, whereas heart and skeletal muscle have little or none. The Mr-85,000 sodium dodecyl sulfate-polyacrylamide gel band in testis and brain lysates was further confirmed to be hsp85 by one-dimensional partial proteolytic peptide mapping. Based on these data and our previous observations showing that synthesis and levels of the protein are altered by depriving cultured cells of glucose, we speculate that intracellular hsp85 levels depend on differences in the intermediary metabolism of glucose in the various tissues. Furthermore, it appears that high basal levels of this heat shock protein may not necessarily protect cells against heat shock, since testis is one of the most heat-sensitive tissues and has the highest hsp85 level.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Atkinson B. G. Synthesis of heat-shock proteins by cells undergoing myogenesis. J Cell Biol. 1981 Jun;89(3):666–673. doi: 10.1083/jcb.89.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. W., Lanks K. W. Use of immobilized lactoperoxidase to label L cell proteins involved in adhesion to polystyrene. J Cell Biol. 1980 May;85(2):402–413. doi: 10.1083/jcb.85.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cotton P. C., Brugge J. S. Neural tissues express high levels of the cellular src gene product pp60c-src. Mol Cell Biol. 1983 Jun;3(6):1157–1162. doi: 10.1128/mcb.3.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie R. W., White F. P. Trauma-induced protein in rat tissues: a physiological role for a "heat shock" protein? Science. 1981 Oct 2;214(4516):72–73. doi: 10.1126/science.7280681. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. L., Lai Y. K., Markert C. L. Diverse forms of stress lead to new patterns of gene expression through a common and essential metabolic pathway. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3485–3488. doi: 10.1073/pnas.79.11.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins R. C., Dahmus M. E. Rapid visualization of protein bands in preparative SDS-polyacrylamide gels. Anal Biochem. 1979 Mar;93(2):257–260. doi: 10.1016/s0003-2697(79)80148-7. [DOI] [PubMed] [Google Scholar]
- Hightower L. E., White F. P. Cellular responses to stress: comparison of a family of 71--73-kilodalton proteins rapidly synthesized in rat tissue slices and canavanine-treated cells in culture. J Cell Physiol. 1981 Aug;108(2):261–275. doi: 10.1002/jcp.1041080216. [DOI] [PubMed] [Google Scholar]
- Hughes E. N., August J. T. Coprecipitation of heat shock proteins with a cell surface glycoprotein. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2305–2309. doi: 10.1073/pnas.79.7.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasambalides E. J., Lanks K. W. Dexamethasone can modulate glucose-regulated and heat shock protein synthesis. J Cell Physiol. 1983 Jan;114(1):93–98. doi: 10.1002/jcp.1041140116. [DOI] [PubMed] [Google Scholar]
- Kasambalides E. J., Lanks K. W. Effects of low molecular weight nutrients on the pattern of proteins synthesized by non-proliferating murine L cells. Exp Cell Res. 1981 Mar;132(1):31–39. doi: 10.1016/0014-4827(81)90079-3. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landry J., Bernier D., Chrétien P., Nicole L. M., Tanguay R. M., Marceau N. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 1982 Jun;42(6):2457–2461. [PubMed] [Google Scholar]
- Lanks K. W., Kasambalides E. J., Chinkers M., Brugge J. S. A major cytoplasmic glucose-regulated protein is associated with the Rous sarcoma virus pp60src protein. J Biol Chem. 1982 Aug 10;257(15):8604–8607. [PubMed] [Google Scholar]
- Lanks K. W., Kasambalides E. J. Purification and characterization of a major component from the cytoplasmic matrix of cultured murine L cells. Biochim Biophys Acta. 1979 May 23;578(1):1–12. doi: 10.1016/0005-2795(79)90106-5. [DOI] [PubMed] [Google Scholar]
- Lanks K. W. Metabolite regulation of heat shock protein levels. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5325–5329. doi: 10.1073/pnas.80.17.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinger L., Varshavsky A. Heat-shock proteins of Drosophila are associated with nuclease-resistant, high-salt-resistant nuclear structures. J Cell Biol. 1981 Sep;90(3):793–796. doi: 10.1083/jcb.90.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. C., Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982 May;79(10):3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. G., Moran L. A. Proteins related to the mouse L-cell major heat shock protein are synthesized in the absence of heat shock gene expression. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2317–2321. doi: 10.1073/pnas.81.8.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran L. A., Chauvin M., Kennedy M. E., Korri M., Lowe D. G., Nicholson R. C., Perry M. D. The major heat-shock protein (hsp70) gene family: related sequences in mouse, Drosophila, and yeast. Can J Biochem Cell Biol. 1983 Jun;61(6):488–499. doi: 10.1139/o83-065. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Omar R. A., Lanks K. W. Heat shock protein synthesis and cell survival in clones of normal and simian virus 40-transformed mouse embryo cells. Cancer Res. 1984 Sep;44(9):3976–3982. [PubMed] [Google Scholar]
- Rosenthal J. D., Hayashi K., Notkins A. L. Comparison of direct and indirect solid-phase microradioimmunoassays for the detection of viral antigens and antiviral antibody. Appl Microbiol. 1973 Apr;25(4):567–573. doi: 10.1128/am.25.4.567-573.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinibaldi R. M., Morris P. W. Putative function of Drosophila melanogaster heat shock proteins in the nucleoskeleton. J Biol Chem. 1981 Nov 10;256(21):10735–10738. [PubMed] [Google Scholar]
- Subjeck J. R., Sciandra J. J., Johnson R. J. Heat shock proteins and thermotolerance; a comparison of induction kinetics. Br J Radiol. 1982 Aug;55(656):579–584. doi: 10.1259/0007-1285-55-656-579. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y., Whitman M., Cantley L. C., Erikson R. L. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2117–2121. doi: 10.1073/pnas.81.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldik L. J., Watterson D. M. Reproducible production of antiserum against vertebrate calmodulin and determination of the immunoreactive site. J Biol Chem. 1981 May 10;256(9):4205–4210. [PubMed] [Google Scholar]
- Velazquez J. M., Lindquist S. hsp70: nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell. 1984 Mar;36(3):655–662. doi: 10.1016/0092-8674(84)90345-3. [DOI] [PubMed] [Google Scholar]
- Vincent M., Tanguay R. M. Different intracellular distributions of heat-shock and arsenite-induced proteins in Drosophila Kc cells. Possible relation with the phosphorylation and translocation of a major cytoskeletal protein. J Mol Biol. 1982 Dec 5;162(2):365–378. doi: 10.1016/0022-2836(82)90532-0. [DOI] [PubMed] [Google Scholar]
- Voellmy R., Bromley P. A. Massive heat-shock polypeptide synthesis in late chicken embryos: convenient system for study of protein synthesis in highly differentiated organisms. Mol Cell Biol. 1982 May;2(5):479–483. doi: 10.1128/mcb.2.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Purification of the major mammalian heat shock proteins. J Biol Chem. 1982 Dec 25;257(24):14949–14959. [PubMed] [Google Scholar]