Abstract
The origins and evolution of higher cognitive functions including complex forms of learning, attention and executive functions are unknown. A potential mechanism driving the evolution of vertebrate cognition early in the vertebrate lineage (550 My ago) was genome duplication and subsequent diversification of postsynaptic genes. Here we report the first genetic analysis of a vertebrate gene family in cognitive functions measured using computerized touchscreens. Comparison of mice carrying mutations in all four Dlg paralogs show simple associative learning required Dlg4, while Dlg2 and Dlg3 diversified to play opposing roles in complex cognitive processes. Exploiting the translational utility of touchscreens in humans and mice, testing Dlg2 mutations in both species showed Dlg2’s role in complex learning, cognitive flexibility and attention has been highly conserved over 100 My. Dlg family mutations underlie psychiatric disorders suggesting genome evolution expanded the complexity of vertebrate cognition at the cost of susceptibility to mental illness.
Humans are thought to be different from other animals largely because of the far greater richness of their cognitive processes1. All animals draw upon attention, perception and simple forms of learning to adapt to changing environmental demands. Those species that have the capacity for more complex forms of associative learning and cognitive processing such as complex visual discrimination, visuo-spatial learning, and executive functioning (including cognitive flexibility and inhibitory response control) can adapt to even more complex and challenging environmental demands. These components of the cognitive repertoire are routinely assessed in humans using computerised touchscreen methods2, 3, which have proven useful in identifying specific cognitive impairments in patients with neurological and psychiatric diseases such as schizophrenia, autism, attention deficit hyperactivity disorder and Alzheimer’s disease, and recent reports show it is possible to use the same touchscreen approach to measure cognition in rodents4. Understanding how the vertebrate cognitive repertoire evolved and its underlying genomic mechanisms may lead to fundamental insights into the origins of our behavior and perhaps identify a basis for the cognitive disorders originating from disease mutations.
One approach, afforded by the touchscreen tests, is to compare the same cognitive abilities in animals and humans with similar genetic perturbations. This strategy allows the identification of cognition-essential genes in both species, which in the case of humans and mice would probe those mechanisms conserved since these species shared a common ancestor ~100 Million years ago (Mya). A related approach that probes an earlier vertebrate ancestry is the comparison of mutations in members of gene families (paralogs) that arose ~550 Mya from the two rounds of whole genome duplication (2R-WGD) at the base of chordates5. Genome duplications significantly shaped the evolution of most eukaryotes including fungi6, plants7 and vertebrates8 producing phenotypic novelty9. While it is widely considered that vertebrates have a greater cognitive repertoire with more complex behaviors than invertebrates10, it is unknown how this expansion in cognitive functions arose and whether the 2R-WGD that occurred in the vertebrate lineage was involved.
Here we address these issues with a focus on the role of the Discs Large Homolog (DLG) family of postsynaptic scaffold proteins, which bind neurotransmitter receptors and enzymes into signalling complexes found in the postsynaptic terminal of brain synapses11. Invertebrate genomes encode a single Dlg gene and following the 2R-WGD most vertebrates (including 40 mammalian genomes) retained four paralogs (Dlg1 [SAP-97, hDlg], Dlg2 [PSD-93, Chapsyn-110], Dlg3 [SAP-102] and Dlg4 [PSD-95/SAP-90]), which accumulated mutations diversifying their structure (Fig. 1a). Using deletion mutations in the family of Dlg proteins, we perform the first genetic dissection of the vertebrate cognitive repertoire using paralogous genes and a cross species comparison of homologous cognitive processes in both mice and humans.
Figure 1. Dissecting the role of Dlg paralogs in different components of cognition.
a. Comparison of invertebrate Dlg and 4 vertebrate paralogs (Dlg1-4). Two pairs of vertebrate Dlg genes can be identified reflecting their evolutionary origins in the two rounds of whole genome duplication (1R, 2R) at the base of chordates ~550 million years ago (Mya). Yellow box highlights the 4 vertebrate Dlg proteins showing high conservation of domain architecture.
b. A battery of rodent touchscreen tasks with increasing cognitive complexity was used to probe simple and complex forms of information processing. Seven tasks are grouped into 4 coloured boxes and representations of the stimuli displayed on the touchscreen are shown. Conditioning and simple forms of learning were measured using tests for operant and Pavlovian conditioning. More complex forms of learning (visual and visuo-spatial discrimination) and information processing (cognitive flexibility, inhibitory response control and attention) were measured using tests that involved more complex perceptual stimuli and/or required more complex response control.
RESULTS
Dlg paralogs confer differential capacities for simple forms of conditioning and associative learning
The first part of our strategy was to use mice to ask if the duplications and divergence of the 4 Dlg genes had conferred differences in their function. Heterozygous mice of the 4 knockout mouse lines were intercrossed, and consistent with published literature, Dlg1−/− homozygous mice were embryonic lethal in contrast to homozygous mutations of Dlg2, Dlg3 or Dlg4, which were viable12-14. Homozygous Dlg−/− mutations in Drosophila15 and C. elegans16 are lethal as are homozygous mutations of its murine ortholog Dlg1−/−17, suggesting vertebrate Dlg1 retained its ancestral roles whereas the function of Dlg2-4 diversified. At the level of protein sequence, the average similarity of the 4 paralogs is approximately 75% (in either mouse or human, Supplementary Fig. 1a-c). We proceeded to test the homozygous Dlg2, Dlg3 and Dlg4 mutant mice and heterozygous Dlg1+/− mice (as they were viable) in a battery of touchscreen tasks of increasing cognitive complexity (Fig. 1b). Across all the tasks we found that the presence of a single copy of the Dlg1 gene (Dlg1+/−) was sufficient for normal behavior (see Supplementary Fig. 2) and hereafter we will focus our data on the differential roles of Dlg2-4.
Two simple forms of associative learning are classical (Pavlovian) and operant (instrumental) conditioning where two or more events become linked or associated, such as two stimuli or a stimulus and a response. The cognitive tasks in the rodent touchscreen battery are built on the simple instrumental conditioned response of nose-poking a stimulus displayed on the touchscreen in order to obtain a reward. The first element of the battery was therefore the acquisition of this simple form of operant conditioning by training animals through several phases in the touchscreen (see methods for details). Dlg2−/− and Dlg3−/Y mice displayed normal rates of completing each training phase relative to wild-type (wt) littermate controls, indicating these genes were not essential for operant conditioning (Fig. 2a). In contrast, Dlg4−/− mice showed a marked deficit in acquisition of operant conditioning. They were able to successfully retrieve and eat reward pellets when delivery of the reward did not rely on a direct response (Phases 1 and 2, see methods), but were unable to complete the required trials when the reward was contingent on an instrumental operant response (i.e., touching the screen to attain a reward (Phase 3, see methods). To further investigate this phenotype in Dlg4−/− mice, we employed another simple associative learning task, a test of Pavlovian conditioned approach behavior (‘autoshaping’)18. In this task, a spatially localized conditioned stimulus (CS) reliably signals an appetitive unconditioned stimulus (US), a food reward. Mice were presented with a stimulus (white vertical rectangle) displayed on either the left or the right side of the screen (Fig. 2b) and when the stimulus was displayed on the left side for example, a food reward was delivered (CS+), whereas appearance of the stimulus on the right side was never rewarded (CS−). After repeated stimulus location-reward pairings, mice normally begin to display the Pavlovian conditioned response of approaching the CS+ more often than the CS−, with the number of discriminative approaches to the CS+ and CS− serving as a measure of how well the animals have learned the association between the CS+ and the reward. Rodents show this conditioned response behavior even though there is no contingency that requires the animal to approach the stimulus in order to receive the food reward. Wt mice robustly demonstrate associative learning and develop a strong conditioned response (making greater number of approaches to the CS+ and decreasing the number of approaches to the CS− with increased training sessions, as well as showing improved approach latencies to the CS+ compared to the CS−) (Fig. 2b). In contrast to wt mice, Dlg4−/− mice failed to demonstrate this discriminative capacity and showed equivalent approaches to the CS+ and CS− and no differences in response latencies to either the CS+ or CS−. These data so far highlight the divergence of Dlg paralogs in their contribution to simple forms of learning and information processing: unlike Dlg2 and Dlg3, Dlg4 is required for simple forms of associative learning. This requirement for Dlg4 was further highlighted in the next phase of testing where we examined all the Dlg mutant mice on a battery of tasks that involved more complex perceptual stimuli and/or required more complex response control. Dlg4−/− mice were incapable of performing the simple operant response underlying any of the more complex tasks, consistent with the view that simple forms of associative learning are a fundamental basis and prerequisite for at least some, more complex forms of cognition.
Figure 2. Distinct roles of Dlg paralogs in simple forms of conditioning and associative learning.
a. Mice were trained through several phases to nose-poke a stimulus displayed on the touchscreen to attain a reward (Operant conditioning). Animals were required to successfully complete and reach the set criterion at each phase before advancing to the next phase. Phase 1: animals acclimated for 20min on 2 days to the operant chamber and required to consume reward pellets freely available from the magazine. Phase 2: a single visual stimulus was displayed on the touchscreen after which, the disappearance of the stimuli coincided with delivery of a food reward, presentation of a tone and illumination of the pellet magazine. Phase 3: animals were required to nose poke a visual stimulus that appeared on the touchscreen in order to obtain a reward. Phase 4: animals were additionally required to initiate the commencement of a new trial with a head entry into the pellet magazine. Phase 5: In addition to that described for previous phases, responses at a blank part of the screen during stimulus presentation now produced a 5s time-out and were not rewarded. Dlg2−/− and Dlg3−/Y mice displayed normal acquisition rates relative to wt littermate controls. Dlg4−/− mice were able to successfully complete Phases 1 and 2 but were unable to complete trials during Phase 3, even after 20 sessions of training (*p<0.05).
b. Pavlovian conditioned approach. Number of approaches to CS+ and CS− (left graph). Wt (black) and Dlg4−/− mice (white). Wt: mixed between-within subjects ANOVA, main effect of genotype p<0.001, stimulus (CS+/ CS−) x session interaction p<0.001, post hoc paired samples t-test *p<0.001. Approach latency to CS+ and CS− (right graph). Wt: independent samples t-test, *p<0.05.
c. Visual discrimination learning. Total number of trials (left graph) (wt: 210.91±19.76, Dlg2−/−: 222.7±26.18) (wt: 243.46±18.25, Dlg3−/Y:173.38±10.06) and errors (right graph) (wt: 64.36±7.9, Dlg2−/−: 68.0±10.13) (wt: 81.54±8.60, Dlg3−/Y:57.46±5.27) to reach learning criterion on visual discrimination. Dlg3−/Y: independent samples t-tests, trials *p<0.005, errors *p<0.05.
d. Object-location paired-associates learning. L, left; C, centre; R, right. Percentage of correct responses across training sessions for Dlg2−/− (left graph) and Dlg3−/Y (right graph) mice.
Dlg2−/−: mixed between-within subjects ANOVA, main effect of genotype p<0.001, genotype x session interaction p<0.001, post hoc paired samples t-test *p<0.05.
All values reported represent mean ± SEM.
The first of these more complex tasks was a form of learning and memory that requires a choice based on perceptual visual discrimination. Mice were presented with two stimuli simultaneously on the screen and required to learn which one was correct (i.e., rewarded, the S+) and which was incorrect (i.e., unrewarded, the S−, Fig. 2c)19. In this task the learning rate of Dlg3−/Y mice was significantly faster than controls, requiring fewer trials and making fewer errors to learn the task (Fig. 2c). In contrast, the performance of Dlg2−/− mice was indistinguishable from that of wt mice. This result is interesting because it indicates not only are there differential functions of Dlg2 and Dlg3 in visual discrimination learning and that neither mutation impairs basic perceptual processing abilities, but it also indicates that the Dlg3 paralog restrains or attenuates a specific aspect of the cognitive repertoire.
We next increased the associative complexity of the task by incorporating spatial information in an object-location paired associates learning task. In this task the mice were required to learn and remember which of three objects (flower, plane, spider) was associated with one of three locations on the touchscreen (left, centre, right respectively) (Fig. 2d)20, 21. This task therefore requires animals to not only discriminate the objects, but also to learn the paired-association between the shape and the object’s location. On a given trial, only two different objects were presented; one displayed in its correct location (S+), the other in its incorrect location (S−), thereby allowing 6 possible trial types. Unlike the less complex visual discrimination task where the Dlg3−/Y mutants were faster, in this task they showed normal object-location associative learning and memory. In contrast, Dlg2−/− mice were significantly impaired and continued to perform at around 50% (chance level) (Fig. 2d). This double genetic dissociation indicates these two different forms of complex learning (visual and visuo-spatial learning) have distinct genetic regulation, each dependent on a different Dlg paralog.
Dlg2 and Dlg3 play opposing roles in cognitive flexibility, inhibitory response control and attentional processing
Complex environments confront animals not only with stable associative relationships between stimuli, responses, and outcomes, but with situations in which these relationships can change. To succeed in such environments, animals require greater response control to be able to adapt to such changes. The genes underlying such flexible behavior are unknown. Thus, having established that Dlg2−/− and Dlg3−/Y learn the visual discrimination task, we reversed the reward contingences so that the previously correct option was now incorrect and vice versa (S+ and S− were switched) (Fig. 3a) and thereby probed their ability to inhibit the established dominant or prepotent response and acquire the new association22. Dlg3−/Y mutants performed normally whereas Dlg2−/− mice showed significant impairments. When tested with simple visual stimuli, Dlg2−/− mice showed an impairment in the early trials (the ‘perseverative’ phase of reversal learning when correct responses are low due to continued responses at the previously rewarded stimulus22, 23. However, when challenged with more complex visual stimuli with greater perceptual demands, this impairment in reversal learning was more severe and found across all trials (Fig. 3b) while no differences were again observed in the initial discrimination learning (trials to criterion: Wt 502.36±58.69, Dlg2−/− 560.43±77.01). These results show a striking dichotomy of function of Dlg2 and Dlg3 in the acquisition and reversal learning of visual discrimination: Dlg3 regulates the acquisition of the discrimination (and the mutation amplifies the rate of learning) while Dlg2 regulates the reversal or flexibility of the learned information (and the mutation attenuates the rate of reversal learning).
Figure 3. Dlgs play distinct roles in cognitive flexibility and response inhibition.
a. Reversal learning. Percentage of correct responses across sessions for Dlg2−/− (left graph) and Dlg3−/− (right graph) mice. Dlg2−/−: mixed between-within subjects ANOVA sessions 1-8, main effect of genotype *p<0.05.
b. Dlg2−/− mice on reversal learning using complex perceptual stimuli. Percentage of correct responses across sessions on reversal learning. Dlg2−/−: mixed between-within subjects ANOVA, main effect of genotype *p<0.005.
c. Extinction learning. Percentage of responses made across sessions by Dlg2−/− (left graph) and Dlg3−/Y (right graph) mice. Dlg2−/−: mixed between-within subjects ANOVA, main effect of genotype p<0.05, genotype x session interaction p<0.005, post hoc paired samples t-test *p<0.05. Dlg3−/Y: mixed between-within subjects ANOVA, main effect of genotype *p<0.05.
All values reported represent mean ± SEM.
To examine if another form of behavioral flexibility has the same genetic requirements as reversal learning, we assessed another task for inhibitory response control using a test for extinction learning, which measure the ability to reduce responses when that response no longer results in a favourable outcome. In the touchscreen extinction task, mice are first trained to make a response to a simple visual stimulus (white square) and obtain a food reward, after which, extinction is tested by no longer rewarding the stimulus24. In the absence of reinforcement, normal mice rapidly decrease their responding (Fig. 3c). Both Dlg2−/− and Dlg3−/Y mice displayed normal rates of learning during the acquisition phase of the task (consistent with our earlier findings that these genes are not essential for simple operant learning (Supplementary Fig. 3). However, during the extinction phase, not only were there clear phenotypes for both Dlg2−/− and Dlg3−/Y mice but we found evidence of their opposing function: Dlg3−/Y mice showed faster extinction, whereas Dlg2−/− mutants showed slower extinction (sessions 4-6) (Fig. 3c).
The capacity to select optimally when confronted with several alternative choices can put a high premium on divided attentional processing. Attention is not a unitary process but subsumes several processes including constructs such as selective and sustained attention and speed of processing. Attentional capacities can be critical for how well an animal is able to adapt and learn information about the environment. The 5-choice serial reaction time task (5-CSRTT) measures sustained, divided attention since animals need to rapidly respond to a brief visual stimulus presented randomly in one of five spatial locations to obtain a reward (Fig. 4a, see methods for detailed description). Accurate responding requires attention in both the temporal and spatial domains and moreover, the 5-CSRTT measures abnormal responses such as premature or perseverative responses, which are thought to model impulsivity and compulsivity respectively25. We used a recently developed touchscreen version of this method26 where mice are first trained to respond to a stimulus displayed for a duration of 2s and after they acquire a stable performance level, the duration of the stimulus is decreased requiring greater attentional capacity to accurately detect it. In this task, we again observed opposing functions for Dlg2 and Dlg3. Dlg3−/Y mice acquired the stable level of performance at the same rate as controls (Supplementary Table 1), and as the stimulus duration decreased, they showed enhanced attentional selection (increased accuracy Fig. 4a), decreased premature responding (Fig. 4B) and a trend for decreased perseverative responses (Fig. 4c). In contrast, Dlg2−/− mutants took significantly longer to reach stable performance at a stimulus duration of 2s, as well as the less stringent condition of 4s stimulus duration (Supplementary Table 1a). With shorter stimulus durations, Dlg2−/− mice showed a significant impairment in accuracy, which was most pronounced at the shortest stimulus duration (0.2s, with the highest attentional load)(Fig. 4a) and made significantly more premature responses (Fig. 4b); however, perseverative responding was unaffected (Fig. 4c). These data show a remarkable divergence of function, with each of the two closely related Dlg2 and Dlg3 genes playing opposing roles on several measures of target detection and responding.
Figure 4. Dlgs are differentially involved in attentional processing and response control.
a-c. Performance on the 5-Choice serial reaction time task (5-CSRTT). See methods for detailed description of task. Dlg2−/− mice (graphs on left) and Dlg3−/Y mice (graphs on right).
a. Percentage accuracy (% correct responses).
b. Percentage of premature responses.
c. Number of perseverative responses.
Dlg2−/−: accuracy and premature responses, mixed between-within subjects ANOVA, main effects of genotype *p<0.01, genotype x session interaction p<0.05, post hoc paired samples t-test *p<0.05.
Dlg3−/Y: accuracy and premature responses, mixed between-within subjects ANOVA, main effects of genotype *p<0.05.
All values reported represent mean ± SEM.
Genetic dissection of cognition meta-analysis
The systematic quantitative comparison of Dlg paralogs provides a basis for asking general questions about the organisation of the behavioral measures with respect to their underlying genetic mechanisms. We can ask three questions: i) are specific genes required for specific components of the cognitive repertoire; ii) are there differences between simple and complex cognitive behaviors, and iii) do any cognitive measures share the same genetic regulation? Figure 5a compares the results of all the touchscreen testing in the 4 lines of mice with the tasks ordered from simple to complex using the organisational scheme in Figure 1b. Enhanced phenotypes are shown in green and attenuated phenotypes in red. This analysis shows that the Dlg family is involved in the majority (8/12) of the measures of simple and complex forms of cognition. The distinct pattern of gene-phenotype relationships shows that diversity in the regulation of cognitive responses in the mouse is conferred by the set of Dlg paralogs. A complementary way to view these data is shown in Figure 5b where the gene-phenotype relationships are clustered showing 4 groups of cognitive functions (referred to as Cognitive Clusters 1-4) where each cluster consists of the behavioral measures with the same gene dependencies. In Cluster 1, simple operant conditioning is characterised by a requirement for Dlg4. Cluster 2 (object-location paired-associates learning, reversal learning, acquisition of 5-CSRTT) only requires Dlg2. In comparison, three behaviors in Cluster 3 (extinction learning, accuracy and premature responding on the 5-CSRTT) are dependent on both Dlg2 and Dlg3, with each of these genes having opposing regulatory functions. Cluster 4 (visual discrimination) requires Dlg3. Thus different Dlg genes either alone or in combination regulate specific sets of cognitive functions.
Figure 5. Dlg paralogs have diversified to play distinct roles in different cognitive functions.
a. Summary of Dlg phenotypes in twelve measures within six cognitive tests. The cognitive repertoire is grouped into 4 boxes according to Figure 1b. Invertebrate Dlg mutants have lethal phenotypes as does mouse Dlg1−/− however, presence of a single copy of the Dlg1 gene (Dlg1+/−) was sufficient for these mice to perform normally across the different cognitive functions examined. Dlg4 was essential for simple forms of associative learning. Some cognitive functions were enhanced by a mutation in one Dlg gene (Dlg3) and attenuated or suppressed by a mutation in another Dlg gene (Dlg2), revealing that Dlg2 and Dlg3 play opposing regulatory roles in more complex cognitive processes.
b. Clustering of gene-phenotype relationships shows 4 groups of cognitive functions (Cognitive Clusters 1-4).
Conserved cognitive functions of DLG2 in humans
Since mice and humans diverged from a common ancestor 100 Mya, there has been strong conservation in the protein coding of Dlg orthologs (>95% similarity, Supplementary Fig. 1a-c) and other postsynaptic proteins27. To ask if there has also been conservation in gene expression in brain regions of mice and humans, we correlated mRNA levels of each of the four vertebrate Dlg paralogs in 17 brain regions in both species. This analysis showed Dlg2, Dlg3 and Dlg4 were significantly correlated (Pearson’s R=0.71, 0.68 and 0.53 respectively, all p<0.05, Supplementary Fig. 1d). Recent studies of the coexpression patterns of Dlg family proteins and mRNAs indicate their importance in human brain function28-30. While these data show conservation in protein sequence and gene regulation, it is unknown if the cognitive functions of Dlg genes are also conserved. Indeed, this has been a general problem in behavioural genetics. Although forms of learning and memory appear to be similar between humans and mice, the conservation of their genetic regulatory mechanisms has been difficult to assess in part because assessment of cognition in mice has mostly been restricted to spatial learning and memory, and there has been a limitation in the comparable nature of the cognitive tests available for rodents. Taking advantage of the fact that multiple aspects of cognition can be tested in humans (and other primates) and mice (and other rodents) in the touchscreen system using analogous tasks designed to probe the same cognitive processes, we can ask if the gene-phenotype relationships of the Dlg genes are conserved between species.
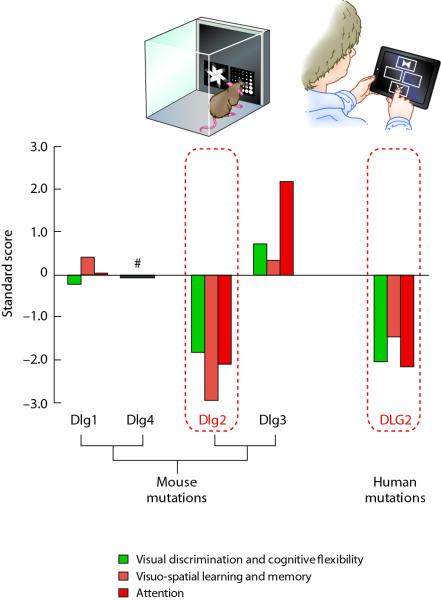
Humans carrying mutations disrupting the coding region of DLG2 have been reported31-34 and we assessed 4 of these individuals (see methods) on a set of cognitive tasks using a touchscreen test battery, the Cambridge Neuropsychological Test Automated Battery (CANTAB). We specifically analysed 3 tasks comparable to those within the rodent touchscreen battery: 1) Intra-extradimensional set-shifting (IED) to assess visual discrimination acquisition and cognitive flexibility (tested using the visual discrimination and reversal learning task in mice) (green bars, Fig. 6), 2) Paired Associates Learning (PAL) to examine visuo-spatial learning and memory (tested using the object-location paired-associates learning task in mice) (orange bars, Fig. 6) and 3) Rapid Visual Information Processing (RVP) to assess sustained attention (tested with the 5-CSRTT in mice) (red bars, Fig. 6). Consistent with results from Dlg2−/− mice, we found that humans with mutations in DLG2 made significantly more errors compared to controls in tests of visual discrimination acquisition/cognitive flexibility (total errors in IED: controls 27.13±1.52, DLG2 subjects 94.25±51.86, p<0.005) and visuo-spatial learning and memory (total errors in PAL: controls 16.68±0.68, DLG2 subjects 38.25±14.57, p<0.005). In addition, humans with mutations in DLG2 also showed decreased accuracy in responding compared to controls in a test for sustained attention (accuracy of target detection in RVP: controls 0.91±0.005, DLG2 subjects 0.8125±0.02, p<0.005), similar to the impaired response accuracy seen in Dlg2−/− mice. Using the highly comparable performance measurements derived from both the mouse and human touchscreen tests, we analysed the same performance parameter (for example, total errors made) from each test to calculate a standardised performance score (z-score) compared to controls, where a negative score indicates poorer than average performance. As shown in Figure 6, comparison of the profile of cognitive phenotypes observed in human DLG2 mutations shows a striking degree of similarity to that pattern of cognitive phenotypes seen in mice with Dlg2 mutations. This similarity in the human-mouse Dlg2 cognitive profile and its uniqueness compared to the three other Dlg genes is further reinforced by published and unpublished data from another 13 different genetically modified lines of mice tested in some of the same touchscreen tasks, which do not show the selective Dlg2 phenotype profile (data not shown).
Figure 6. Conservation of Dlg2 functions in mice and humans.
Using the Cambridge Neuropsychological Test Automated Battery (CANTAB), 4 individuals with mutations in DLG2 were assessed on 3 tasks comparable to those within the rodent touchscreen battery. The Intra-extradimensional set-shifting task (IED) was used to assess discrimination acquisition and cognitive flexibility, the Paired Associates Learning task (PAL) to assess visuo-spatial learning and memory and the Rapid Visual Information Processing task (RVP) to assess sustained attention.
Comparison of performance in touchscreen tasks for mice carrying mutations in Dlg1, Dlg2, Dlg3 or Dlg4 with humans carrying mutations in DLG2 (see methods). A standardised performance score compared to controls is shown, where a negative score indicates poorer than average performance. #Black bar denotes the lack of data for comparison due to the inability to test Dlg4 mutant mice on any of the three tasks represented.
DISCUSSION
Paralog diversification, cognitive complexity and disease
Our genetic dissection in mice suggests how different components of the cognitive repertoire are related at the genetic level, and how genome evolution produced the range of vertebrate behavioral responses. Our test battery comprised 7 tests (with 13 primary measures) and each of these required the function of at least one Dlg paralog, revealing a central role of this gene family across all aspects of cognition tested. Strikingly, each vertebrate paralog had a different phenotypic profile indicating each gene has evolved a unique contribution to the cognitive repertoire. A dramatic example of this divergence was the opposing direction of the phenotypes of Dlg2 and Dlg3 in complex cognitive behaviors. Moreover, while these two genes had no role in simple conditioning, Dlg4 in contrast was uniquely essential for simple forms of learning. A parsimonious model is that Dlg4 retained an ancestral (invertebrate) role in simple forms of learning, whereas the diversification of Dlg2 and Dlg3 provided novel regulation of complex cognitive processes arising in vertebrates. The grouping of different behaviours (Fig. 5b) according to their distinct genetic underpinnings shows it is possible to identify relationships between cognitive functions based on common and distinct genetic mechanisms, which is an approach that can extend previous studies based on neuroanatomy and pharmacology35, 36.
The reciprocal effects of Dlg2 and Dlg3 on complex behaviors reported here suggest these two genes play essential roles in balancing or tuning the synaptic signalling machinery. This is supported by electrophysiological studies of synaptic long-term potentiation (LTP) in CA1 synapses of the hippocampus, where Dlg2−/− mutants have reduced LTP37 and Dlg3−/Y mutants show enhanced LTP13. Dlg4−/− mutants show more severe LTP phenotypes14, 37 than Dlg2−/−37 or Dlg3−/Y13 mutants, which suggest a more severe disruption to activity-dependent synaptic strengthening is reflected in impairments in simple forms of learning. These differential roles likely reflect the distinct intracellular signaling functions mediated by Dlg proteins with their interacting proteins in the MAGUK Associated Signalling Complexes. In the accompanying manuscript38 differential association between Dlg paralogs and NMDA receptors (GluN2 subunits) is reported. Our data showing the conserved role of Dlg2 in cognition in mice and humans, together with the conservation in expression between brain regions and protein sequence, indicates that it is the conservation at the genomic level that maintains these functions between the two species.
Human mutations in DLG2 and DLG3 have been reported in psychiatric disorders31-34, 39 and mouse models of psychiatric diseases rely on conservation of mechanisms with humans. Rare human DLG2 mutations have been associated with schizophrenia in three independent studies of copy number variants31-34 and three of the four subjects in our study have this disease (the fourth subject is the youngest and at increased risk of developing the illness). The cognitive impairments observed in Dlg2−/− mice parallel those observed in schizophrenia patients, such as deficits in reversal learning40, 41, object-location paired-associates learning42, extinction43 and attentional function44. Cognitive impairments are also observed in humans with Dlg3 mutations39 and we found that Dlg3−/Y mutant mice displayed enhanced visual discrimination ability and augmented attentional function and response control. In humans, enhanced or superior performance in some cognitive domains, particularly those associated with perceptual processing is reported in autistic individuals45. It is noteworthy that Dlg proteins interact with Neuroligin, Shank, DLGAP2 and GluN2 proteins, which are mutated in autism46. Mutations in the Dlg family and their interacting proteins cause other diseases with a spectrum of cognitive and motor phenotypes27, 47.
Our data support the model that genome duplication and diversification at the base of chordates around ~550 Mya was a driver of the expansion in complexity of the cognitive repertoire of vertebrates. This genomic mechanism, known to be important in generating complexity in other vertebrate biological systems48,49, expanded the complexity of vertebrate synaptic signalling processes50 before the anatomical diversification in many brain regions and encephalisation that characterises the tetrapod brain. Evidence that expansion of vertebrate postsynaptic signalling proteins is a general mechanism driving vertebrate behavioural complexity is supported by a study of GluN2 paralogs38. Importantly, conservation of Dlg2’s role in human and mouse cognition over the ~90 My since these two mammals shared a common ancestor suggests that genomic mechanisms underpin these (disease relevant) behaviours despite 1000-fold differences in brain size. While on one hand these results show that genome duplication in Dlg and other postsynaptic gene families endowed vertebrates with an expanded and flexible set of cognitive functions, on the other hand it indicates that any benefits to the behavioral repertoire came at the price of susceptibility to mental illness because disease-causing mutations occur in these novel genes. Our comparative touchscreen approach also demonstrate the feasibility of co-clinical trials, using humans and mice carrying the same mutations, aimed at identifying treatments for these illnesses. Together with human genome sequencing, the quantitative testing of human cognitive functions using computerized touchscreen test batteries should aid in understanding the genetic basis of cognition and its diseases.
METHODS
Animals
Dlg1 heterozygous mice (+/−) and wt littermates were generated from Dlg1 heterozygous intercrosses and maintained on 129S5/SvEvBrd background. Homozygous knockout mice (denoted by −/− with the exception of male Dlg3 null mutants which are denoted by −/Y as Dlg3 is located on the X chromosome) and wt littermates were generated from heterozygous intercrosses of Dlg212, Dlg313 and Dlg414 mice and maintained on a C57BL/6J background. Male and female knockout mice from all lines developed normally to adulthood, exhibited normal body size and no gross abnormalities. Mice were housed in mixed groups of wt and knockouts on a 12h light/dark cycle and all behavioural testing conducted during the light phase of the cycle. Two separate cohorts of male mice (n=10-15 for each cohort) from each knockout line were used for cognitive testing on the touchscreen tasks. Mice were maintained on a restricted diet at or above 85% of their free-feeding body weight during behavioural testing. Water was available ad libitum throughout the experiment. All experimentation was conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act (1986).
Cognitive testing in the touchscreen operant system
Apparatus
Testing was conducted in a touchscreen-based automated operant system consisting of an operant chamber (21.6 × 17.8 × 12.7 cm) with clear Perspex walls and stainless steel grid floor, housed within a sound- and light-attenuating box (40 × 34 × 42 cm) (Med Associates, St Albans, VT). A dispenser delivering reward pellets (14mg, BioServ, Frenchtown NJ) into a magazine, a house light, and a tone generator were located at one end of the chamber, and at the opposite end was a flat-screen monitor equipped with an infrared touchscreen (16 × 21.2 cm) (Craft Data Limited, Chesham, UK). A black Perspex ‘mask’ with windows was positioned in front of the touchscreen allowing the presentation of stimuli to be spatially localized and prevented animals from accidentally triggering the touchscreen. Stimuli presented on the screen were controlled by custom software (“MouseCat,” L.M. Saksida; Carola Romberg) and responses made via nose-pokes at the stimuli were detected by the touchscreen and recorded by the software.
Pre-training – Operant Conditioning
Prior to testing, mice were first slowly reduced and then maintained at or above 85% free-feeding body weight. Animals were then trained through five phases for instrumental operant conditioning similar to that previously described24. Mice were required to successfully complete the set criterion at each phase before advancing to the next phase. Briefly, mice were habituated to the operant chamber and to eating reward pellets from the magazine by being placed in the chamber for 20min on 2 days and required to consume a set number of pellets that were freely available in the magazine (Phase 1). In Phase 2, a single visual stimulus was displayed on the screen for 30s, after which, disappearance of the stimuli coincided with delivery of a food reward, presentation of a tone and illumination of the pellet magazine (criterion: 30 trials in 60min). For phases 2-5, a trial did not advance until the pellet was consumed. Mice then learned to nose-poke visual stimuli that appeared on the screen to obtain a reward (Phase 3, criterion=30 trials in 60min) and to initiate each new trial with a head entry into the pellet magazine (Phase 4, criterion=30 trials in 60min). In the last pre-training phase (Phase 5), responses at a blank part of the screen during stimulus presentation produced a 5s time-out (signaled by extinction of the house light, magazine inactive) to discourage indiscriminate screen responding (criterion=21/30 correct responses in 60min on 2 consecutive days). All values reported represent mean ± standard error of the mean (SEM).
Pavlovian Conditioned Approach (Autoshaping)
Testing was carried out in the Campden Instruments Bussey-Saksida touchscreen chamber (Campden Instruments Ltd, UK). Mice were habituated to the chamber over two daily sessions. On the first habituation session, mice were placed in the chamber for 20 min and a single delivery of reward (70μl strawberry milkshake, Yazoo Campina Ltd) given at the beginning of the session. On the second habituation session, mice were placed in the chamber and following a variable interval (VI, mean 40s), delivery of a liquid reward (20μl) coincided with illumination of the magazine light and a tone. A nose-poke into the magazine was required before the VI restarted and commencement of another trial. Animals were observed during both habituation sessions to ensure animals successfully consumed all rewards and completed 40 trials in 60 min (on the second session) before progressing onto the task.
Mice were trained to associate the presentation of a white rectangle stimuli (10s duration) at a specific location with delivery of a reward. For example, if the stimulus was presented on the left side of the screen, it was designated the CS+ and signalled the delivery of a reward immediately after the offset of this stimulus; the other stimulus (i.e., presented on the right side of the screen) was designated the CS− and was never followed by reward delivery. Designation of the location of the CS+ (i.e., left or right) was counterbalanced between animals. Stimuli were presented on a VI (mean 40s) schedule and training consisted of 40 trials per session per day (20 presentations each of CS+ and CS−, presented in a pseudorandom order) for 4 sessions. To commence or initiate a trial, animals were required to nose-poke at the back of the chamber ensuring animals were centrally located at the rear of the chamber when stimuli were presented and eliminated chance approaches to the stimuli. Approaches to a stimulus were measured via an infrared beam detector. Group differences were analyzed using a mixed between-within subjects ANOVA with conditioned stimulus (CS+, CS−) as the between-subjects factor and session as the within-subjects factor. A paired samples t-test was used for post hoc analysis to assess significant between x within-subjects interaction effects. All values reported represent mean ± SEM.
Visual discrimination and reversal learning
Mice were trained to discriminate between two novel, approximately equiluminescent stimuli presented in a spatially pseudorandomized manner over 30-trial sessions24. Responses at one stimulus (S+, correct) resulted in a reward; responses at the other stimulus (S−, incorrect) resulted in a 5 s time-out followed by a correction trial (correction error), whereby the trial was repeated until a correct choice was made. Stimuli were displayed on the screen until a response was made. Acquisition criterion for visual discrimination was attaining 80% correct responses (excluding correction trials) on 2 consecutive days, following which, mice were moved onto the reversal phase on the next session where the designation of the same discriminated stimuli as correct versus incorrect was reversed. Reversal performance was tested over 30-trial sessions for 20 sessions.
Group differences were analyzed using an independent samples t-test or a mixed between-within subjects ANOVA with genotype as the between-subjects factor and session as the within-subjects factor. A paired samples t-test was used for post hoc analysis to assess significant between x within-subjects interaction effects. All values reported represent mean ± SEM.
Object-location Paired-Associates Learning
Mice were tested for the ability to associate between three objects (flower, plane, and spider) and three correct spatial locations on the touchscreen (left, centre, and right, respectively) 20, 21. For each trial, only 2 objects were presented; one object in its correct location (S+) and the other object in one of two incorrect locations (S−) therefore six possible trial types. A nose-poke to the correct S+ resulted in delivery of a reward and incorrect responses resulted in a 5 s time-out followed by correction trial. Nose-pokes to response windows in which no stimulus was presented were ignored. Mice were given 36 trials per session per day for 50 sessions. Group differences were analyzed using a mixed between-within subjects ANOVA with genotype as the between-subjects factor and session block as the within-subjects factor. A paired samples t-test was used for post hoc analysis to assess significant between x within-subjects interaction effects. All values reported represent mean ± SEM.
Extinction
To examine acquisition and extinction of an instrumental response, mice were required to respond to a stimulus (single white square) presented in the centre of the screen to obtain a reward. During acquisition, the stimulus remained on the screen until a response was made. The acquisition criterion was defined as completing 30 trials within 12.5min on each of five consecutive sessions. Following acquisition, extinction was assessed where responses to the stimulus were no longer rewarded. The stimulus was displayed for 10s and animals given 30 trials per session per day for 6 sessions. Group differences were analyzed using a mixed between-within subjects ANOVA with genotype as the between-subjects factor and session as the within-subjects factor. A paired samples t-test was used for post hoc analysis to assess significant between x within-subjects interaction effects. All values reported represent mean ± SEM.
5 Choice Serial Reaction Time Task (5-CSRTT)
The 5-CSRTT task procedure in the touchscreen was similar to that previously described26, 51. Mice were trained to respond to presentations of a white square box that was pseudorandomly displayed in one of five spatial locations on the touchscreen. Each trial commenced with the illumination of the magazine light. A nose-poke to the magazine initiated the commencement of a trial was and then a 5s fixed delay during which, if the animal prematurely touched the screen, the response was recorded as a premature response and a 5s time-out given, followed by a 5 s ITI. The stimulus is then displayed (initially for 4s) followed by a limited holding period. Responses during stimulus presentation were recorded either as correct (response to the stimulus window) or incorrect (response to any other window). A correct choice was signalled by a tone and delivery of reward pellet. An incorrect response was punished with a 5s time-out. A failure to respond to any window either during stimulus display or the limited hold period was recorded as an omission. Responses made during the limited hold period were recorded as perseverative responses.
Mice were required to complete 50 trials in a 60 min session. Once an animal reached a performance criterion (completed 50 trials, >80% accuracy, <20% omissions for 3 out of 4 consecutive days) at 4s stimulus duration, this was reduced to 2s until animals attained the performance criterion again. . Animals were then tested for two days at a 2s stimulus duration to attain the baseline performance rate, following which, the stimulus duration was reduced to 1.0, 0.8, 0.6, 0.4 and 0.2s. Animals were tested 2 consecutive days at a given stimulus duration, then re-baselined at 2s stimulus duration for at least 2 days or until the animal re-attained performance criterion (>80% accuracy, <20% omissions). Accuracy of responding was measured by calculating the percentage of correct responses/correct responses+incorrect responses × 100. Percentage of omissions = number of omissions/total number of trials × 100. Percentage of premature responses = number of premature responses/total number of trials × 100. Number of perseverative responses made, latency to respond (response latency) and collect rewards (reinforcer latency) were recorded. Group differences were analyzed using a mixed between-within subjects ANOVA with genotype as the between-subjects factor and stimulus duration as the within-subjects factor. A paired samples t-test was used for post hoc analysis to assess significant between x within-subjects interaction effects. All values reported represent mean ± SEM.
Human DLG2 analysis - Subjects and Experimental Procedure
Four individuals with copy number variations (CNVs) within DLG2 participated in the current study (see Supplementary Fig. 4). Initial discovery of 4 unrelated cases of DLG2 CNV carriers was made in the ISC GWAS 33 from 1115 Scottish schizophrenia cases (0.36%). From 978 Scottish control individuals screened, none were found to have this CNV. Expanding the pedigree of one of the individuals discovered in GWAS led to finding 2 more individuals within the same family with DLG2 CNVs (2 daughters; 1 diagnosed with Schizophrenia and 1 unaffected). To further explore the GWAS results, two different Multiplex Amplicon Quantification (MAQ) assays52 were used: the first assay included a number of chromosomal regions previously shown to contain CNVs associated with schizophrenia31, 32 and a second assay focused specifically on DLG2. Twelve target amplicons comprising exons of DLG2 and 11 reference amplicons were used (see Supplementary Table 2). The study was approved by the Multi-Centre Research Ethics Committee for Scotland and patients gave written informed consent for the collection of DNA samples for use in genetic studies.
CANTAB
Subjects were asked to perform a series of 4 computerized neuropsychological tests in the Cambridge Neuropsychological Test Automated Battery (CANTAB, Cambridge Cognition, Cambridge, UK). Following explanation and successful completion of a simple Motor screening task (touching the centre point of flashing crosses on the screen), subjects were given 4 tests in the following order: 1) Spatial working memory (SWM). 2) Intra/Extradimensional set-shift task (IED): a test of rule acquisition and reversal involving several stages of visual discrimination (in which one of two stimuli is correct) and attentional set-shifting (including stages of reversal where the contingencies change such that the previously correct becomes incorrect).3) Rapid Visual Information Processing (RVP): a test of sustained attention (similar to the Continuous Performance Task) which requires individuals to monitor the continuous presentation of strings of numbers and only respond when a target sequence is displayed. 4) Paired associate learning (PAL): a test of simple visual pattern and visuo-spatial associative learning. For analogous comparison with mouse data obtained from the touchscreen tasks, data from only 3 tests are presented in the current study. Detailed descriptions of the 3 CANTAB tests employed can be found on the Cambridge Cognition’s website: http://www.cantab.com/camcog/site/page.acds?instanceid=476151&context=474593
Data Analysis
Individual patient results were compared to the internal normative database of CANTAB (containing data from 3000 healthy volunteers) and matched for age (a range of 9-15 years) and gender. For IED and PAL, the measure of total errors (adjusted) was used. This is a measure of the subject’s efficiency in attempting the test. Thus, whilst a subject may pass all stages, a substantial number of errors may be made in doing so. It is crucial to note that subjects failing at any stage of the test by definition have had less opportunity to make errors. Therefore, this adjusted score is calculated to take into account each stage not attempted due to failure. For RVP, A’ was used, which is the signal detection measure of sensitivity to errors, regardless of error tendency (range 0.00 to 1.00; bad to good). In essence, this metric is a measure of how good the subject is at detecting target sequences.
For transformation of the mouse data for comparison, mean group standard z-scores were calculated for each Dlg mutant line for each task using the following measures: visual discrimination and reversal (total errors made across all sessions), object-location paired-associate learning (total errors made across all sessions), 5-choice serial reaction time (average % accuracy for 0.2s stimulus duration).
Supplementary Material
ACKNOWLEDGEMENTS
J.N., N.H.K., L.N.L. and S.G.N.G. supported by The Wellcome Trust, Genes to Cognition Program, The Medical Research Council (MRC) and EU programs (Project GENCODYS No. 241995, Project EUROSPIN No. 242498 and Project SYNSYS No. 242167). MJ supported by grants from RS Macdonald Charitable Trust and AMS/The Wellcome Trust. We thank K. Elsegood, D. Fricker for mouse husbandry and genotyping, T.W. Robbins for advice with CANTAB, J. Barnett for assistance with CANTAB control data and T.W. Robbins and T.J. O’Dell for comments on the manuscript. Figure illustration contribution by D.J. Maizels.
Footnotes
COMPETING FINANCIAL INTERESTS: T.J.B. and L.M.S. consult for Campden Instruments.
REFERENCES
- 1.Gregory RL, editor. The Oxford Companion to the Mind. Oxford University Press; USA: 1987. [Google Scholar]
- 2.Fray PJ, Robbins TW. CANTAB battery: proposed utility in neurotoxicology. Neurotoxicol Teratol. 1996;18:499–504. doi: 10.1016/0892-0362(96)00027-x. [DOI] [PubMed] [Google Scholar]
- 3.Barnett JH, et al. Assessing cognitive function in clinical trials of schizophrenia. Neurosci Biobehav Rev. 2010;34:1161–1177. doi: 10.1016/j.neubiorev.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Bussey TJ, et al. New translational assays for preclinical modelling of cognition in schizophrenia: The touchscreen testing method for mice and rats. Neuropharmacology. 2011;62:1191–1203. doi: 10.1016/j.neuropharm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van de Peer Y, Maere S, Meyer A. The evolutionary significance of ancient genome duplications. Nat Rev Genet. 2009;10:725–732. doi: 10.1038/nrg2600. [DOI] [PubMed] [Google Scholar]
- 6.Kellis M, Birren BW, Lander ES. Proof and evolutionary analysis of ancient genome duplication in the yeast Saccharomyces cerevisiae. Nature. 2004;428:617–624. doi: 10.1038/nature02424. [DOI] [PubMed] [Google Scholar]
- 7.Adams KL, Wendel JF. Polyploidy and genome evolution in plants. Curr Opin Plant Biol. 2005;8:135–141. doi: 10.1016/j.pbi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Dehal P, Boore JL. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 2005;3:e314. doi: 10.1371/journal.pbio.0030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrad B, Antonarakis SE. Gene duplication: a drive for phenotypic diversity and cause of human disease. Annu Rev Genomics Hum Genet. 2007;8:17–35. doi: 10.1146/annurev.genom.8.021307.110233. [DOI] [PubMed] [Google Scholar]
- 10.Moore BR. The evolution of learning. Biol Rev Camb Philos Soc. 2004;79:301–335. doi: 10.1017/s1464793103006225. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery JM, Zamorano PL, Garner CC. MAGUKs in synapse assembly and function: an emerging view. Cell Mol Life Sci. 2004;61:911–929. doi: 10.1007/s00018-003-3364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGee AW, et al. PSD-93 knock-out mice reveal that neuronal MAGUKs are not required for development or function of parallel fiber synapses in cerebellum. J Neurosci. 2001;21:3085–3091. doi: 10.1523/JNEUROSCI.21-09-03085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuthbert PC, et al. Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies. J Neurosci. 2007;27:2673–2682. doi: 10.1523/JNEUROSCI.4457-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Migaud M, et al. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- 15.Woods DF, Hough C, Peel D, Callaini G, Bryant PJ. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol. 1996;134:1469–1482. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bossinger O, Klebes A, Segbert C, Theres C, Knust E. Zonula adherens formation in Caenorhabditis elegans requires dlg-1, the homologue of the Drosophila gene discs large. Dev Biol. 2001;230:29–42. doi: 10.1006/dbio.2000.0113. [DOI] [PubMed] [Google Scholar]
- 17.Caruana G, Bernstein A. Craniofacial dysmorphogenesis including cleft palate in mice with an insertional mutation in the discs large gene. Mol Cell Biol. 2001;21:1475–1483. doi: 10.1128/MCB.21.5.1475-1483.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bussey TJ, Everitt BJ, Robbins TW. Dissociable effects of cingulate and medial frontal cortex lesions on stimulus-reward learning using a novel Pavlovian autoshaping procedure for the rat: implications for the neurobiology of emotion. Behavioral neuroscience. 1997;111:908–919. doi: 10.1037//0735-7044.111.5.908. [DOI] [PubMed] [Google Scholar]
- 19.Morton AJ, Skillings E, Bussey TJ, Saksida LM. Measuring cognitive deficits in disabled mice using an automated interactive touchscreen system. Nat Methods. 2006;3:767. doi: 10.1038/nmeth1006-767. [DOI] [PubMed] [Google Scholar]
- 20.Talpos JC, Winters BD, Dias R, Saksida LM, Bussey TJ. A novel touchscreen-automated paired-associate learning (PAL) task sensitive to pharmacological manipulation of the hippocampus: a translational rodent model of cognitive impairments in neurodegenerative disease. Psychopharmacology. 2009;205:157–168. doi: 10.1007/s00213-009-1526-3. [DOI] [PubMed] [Google Scholar]
- 21.Bartko SJ, Vendrell I, Saksida LM, Bussey TJ. A computer-automated touchscreen paired-associates learning (PAL) task for mice: impairments following administration of scopolamine or dicyclomine and improvements following donepezil. Psychopharmacology. 2011;214:537–548. doi: 10.1007/s00213-010-2050-1. [DOI] [PubMed] [Google Scholar]
- 22.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behavioral neuroscience. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- 24.Brigman JL, et al. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learning and memory. 2008;15:50–54. doi: 10.1101/lm.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 26.Romberg C, Mattson MP, Mughal MR, Bussey TJ, Saksida LM. Impaired attention in the 3xTgAD mouse model of Alzheimer’s disease: rescue by donepezil (Aricept) J Neurosci. 2011;31:3500–3507. doi: 10.1523/JNEUROSCI.5242-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayes A, et al. Characterization of the proteome, diseases and evolution of the human postsynaptic density. Nature neuroscience. 2011;14:19–21. doi: 10.1038/nn.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayes A, et al. Comparative study of human and mouse postsynaptic proteomes finds high compositional conservation and abundance differences for key synaptic proteins. PloS one. 2012;7:e46683. doi: 10.1371/journal.pone.0046683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawrylycz MJ, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konopka G, et al. Human-specific transcriptional networks in the brain. Neuron. 2012;75:601–617. doi: 10.1016/j.neuron.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu B, et al. Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet. 2008;40:880–885. doi: 10.1038/ng.162. [DOI] [PubMed] [Google Scholar]
- 32.Walsh T, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 33.International Schizophrenia Consortium Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirov G, et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Molecular psychiatry. 2011;17:142–153. doi: 10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robbins TW. Animal models of neuropsychiatry revisited: A personal tribute to Teitelbaum. Behavioural brain research. 2012;231:337–342. doi: 10.1016/j.bbr.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 37.Carlisle HJ, Fink AE, Grant SG, O’Dell TJ. Opposing effects of PSD-93 and PSD-95 on long-term potentiation and spike timing-dependent plasticity. The Journal of physiology. 2008;586:5885–5900. doi: 10.1113/jphysiol.2008.163469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan TJ, et al. Genetic exchange of evolutionarily derived GluN2A and GluN2B cytoplasmic domains identifies shared and unique contributions to vertebrate behavior and synaptic plasticity. Nature neuroscience. 2012 [Google Scholar]
- 39.Tarpey P, et al. Mutations in the DLG3 gene cause nonsyndromic X-linked mental retardation. Am J Hum Genet. 2004;75:318–324. doi: 10.1086/422703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leeson VC, et al. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biological psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophrenia research. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnett JH, et al. Visuospatial learning and executive function are independently impaired in first-episode psychosis. Psychological medicine. 2005;35:1031–1041. doi: 10.1017/s0033291704004301. [DOI] [PubMed] [Google Scholar]
- 43.Holt DJ, et al. Extinction memory is impaired in schizophrenia. Biological psychiatry. 2009;65:455–463. doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luck SJ, Gold JM. The construct of attention in schizophrenia. Biological psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- 46.van de Lagemaat LN, Grant SG. Genome variation and complexity in the autism spectrum. Neuron. 2010;67:8–10. doi: 10.1016/j.neuron.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez E, et al. Targeted tandem affinity purification of PSD-95 recovers core postsynaptic complexes and schizophrenia susceptibility proteins. Mol Syst Biol. 2009;5:269. doi: 10.1038/msb.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conant GC, Wolfe KH. Turning a hobby into a job: how duplicated genes find new functions. Nat Rev Genet. 2008;9:938–950. doi: 10.1038/nrg2482. [DOI] [PubMed] [Google Scholar]
- 49.Carroll SB. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell. 2008;134:25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 50.Emes RD, et al. Evolutionary expansion and anatomical specialization of synapse proteome complexity. Nature neuroscience. 2008;11:799–806. doi: 10.1038/nn.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartko SJ, et al. Intact attentional processing but abnormal responding in M1 muscarinic receptor-deficient mice using an automated touchscreen method. Neuropharmacology. 2011;61:1366–1378. doi: 10.1016/j.neuropharm.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suls A, et al. Microdeletions involving the SCN1A gene may be common in SCN1A-mutation-negative SMEI patients. Hum Mutat. 2006;27:914–920. doi: 10.1002/humu.20350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.