Abstract
Akt, a serine/threonine protein kinase, is constitutively phosphorylated and hyperactivated in multiple cancers, including acute myeloid leukemia. High levels are linked to poor survival and inferior responses to chemotherapy, making Akt inhibition an attractive therapeutic target. In this phase I/II study of TCN-PM, a small-molecule Akt inhibitor, TCN-PM therapy was well tolerated in patients with advanced hematological malignancies, and reduced levels of phosphorylation of Akt and its substrate Bad were shown, consistent with inhibition of this survival pathway and induction of cell death. Further investigation of TCN-PM alone or in combination in patients with high Akt levels is warranted.
Keywords: Akt, nucleoside analog, Triciribine, AML, phase I clinical trial
1. Introduction
Acute myeloid leukemia (AML) accounts for 80% of adult leukemias and is a genetically heterogeneous disease characterized by the proliferation and accumulation of myeloid blasts in the bone marrow that are blocked at various stages of their differentiation [1, 2]. Although cytotoxic chemotherapy is effective at inducing initial remissions in up to 70% of patients, the majority of patients relapse and develop refractory disease, which is associated with poor outcomes [3, 4].
Leukemic cells in AML patients are characterized by the activation of multiple receptor and non-receptor protein kinases [5, 6]. Although the upstream lesions may vary, they invariably converge on downstream effector pathways. One major pathway found to be constitutively activated is the phosphoinositol 3-kinase (PI3K)/Akt pathway [7, 8]. Both PI3K and Akt are kinases that are central to multiple oncogenic and tumor suppressor signaling networks [9]. Mechanistically, activation of Akt occurs when it interacts via its PH domain with phosphatidylinositol (3,4,5)-trisphosphate [10] to undergo translocation to the inner surface of the cell membrane along with its upstream kinases, which then phosphorylate Akt on Ser473 and Thr308 [11, 12]. Ser473 is primarily phosphorylated by the mammalian target of rapamycin [12], whereas Thr308 is phosphorylated by the PI3K-dependent kinase 1 [13]. Phosphorylation of Ser473 precedes and facilitates phosphorylation of Thr308, but both are required for the full activation of Akt [12]. Once activated, Akt phosphorylates a number of downstream substrates, such as BAD (BCL2-associated agonist of cell death) [14], caspase-9 [15], and the forkhead family (FOXO3A) of transcription factors [16]. Phosphorylation of these proteins by Akt suppresses their pro-apoptotic function, thus contributing to the potent pro-survival effects of Akt.
In patients with AML, 50-80% harbor activated Akt that is persistently phosphorylated on Ser473 and Thr308 [17-19]. High levels of phosphorylated Akt (pAkt) or its downstream substrates have been identified as adverse prognostic factors in AML [16, 20, 21]. Conversely, inhibition of Akt has been correlated with complete response to chemotherapy in AML [22]. Furthermore, the PI3K/Akt pathway appears to have a prominent role in promoting chemotherapeutic resistance in AML [23] via mechanisms that include dysregulation of adherence-mediated cytoprotection or upregulation of multidrug resistant protein-1 [24, 25]. Therefore, inhibition of Akt and/or its downstream targets in AML patients may represent an attractive target for anticancer therapeutics.
Triciribine (TCN) is tricyclic purine nucleoside analog that is metabolically activated inside cells by adenosine kinase to its mono-phosphate active analog TCN-P [26, 27]. Recently, TCN-P, but not TCN, was shown to interact with the PH domain of Akt and to interfere with its localization to the membrane, thereby preventing Akt phosphorylation and subsequent activation [28]. In early-phase I/II clinical trials with TCN-P conducted in patients with advanced solid tumors, a dose-intensive (35-40 mg/m2/day), 5-day continuous infusion schedule was used. Although TCN-P demonstrated some antitumor activity at these high concentrations based on its ability to inhibit DNA synthesis, therapeutic development of TCN-P has been hampered by dose-limiting toxicities (DLTs) at doses above 35-48 mg/m2, including hypertriglyceridemia, cardiac failure, hepatotoxicity, thrombocytopenia, and hyperglycemia [29-32]. Newer approaches have focused on the action of TCN-P on Akt activation [26, 33]. For instance, exposure of T-cell acute lymphocytic leukemia (ALL) cell lines to TCN inhibited Akt phosphorylation and its downstream signaling, inducing apoptosis in vitro at concentrations of 10 μM [28, 34]. Treatment with TCN has also inhibited tumor growth in xenograft tumor models that expressed high levels of Akt alone and in combination with other chemotherapy regimens [33, 35, 36]. Finally, in a recent dose-escalating trial in patients with advanced solid tumors that included 10 different solid neoplasms, TCN-PM (a TCN-P monohydrate formulation), administered weekly, resulted in inhibition of Akt in tumor cells from patient biopsies. More importantly, this intermittent dosing schedule was safe and well tolerated even at doses up to 45 mg/m2 [37].
In this study, we conducted a phase I dose escalation clinical trial of this small-molecule Akt inhibitor, TCN-PM, given on days 1, 8, and 15 of a 28-day schedule to patients with advanced hematological malignancies to assess its safety, tolerability, cellular pharmacology, and action on the Akt pathway in leukemic blasts. A secondary assessment was to evaluate its clinical activity.
2. Materials and Methods
2.1. Patients
Patients with histologically or cytologically confirmed refractory hematologic malignancies, including AML, ALL, chronic myeloid leukemia blast crisis, myelodysplastic syndrome (MDS), and chronic lymphocytic leukemia (CLL), were eligible for this trial. Patients were required to have an Eastern Cooperative Oncology Group performance status of 0-3. There were no limitations on the number of prior therapies, but active toxicities from these prior therapies must have resolved to grade <1 as defined by National Cancer Institute Common Toxicity Criteria version 2.0. Patients were also required to have normal levels of bilirubin, creatinine values less than or equal to 2.0 mg/dL, aspartate aminotransferase (AST) less than or equal to 3.0 × ULN, and alanine aminotransferase (ALT) less than or equal to 3.0 × ULN. All patients were informed of the investigational nature of this protocol in accordance with institutional policies. An Institutional Review Board-approved informed consent form was signed by all patients for both clinical and pharmacology studies; approval was obtained according to the Declaration of Helsinki. Exclusion criteria included heart disease, psychiatric illness that limited compliance with the study, and current enrollment on other standard or investigational therapies for leukemia.
2.2. Study drug
TCN-PM was supplied by Vioquest Pharmaceuticals as lyophilized powder in 50-mg vials. Before use, the drug was reconstituted with 2.5 mL of sterile water for a final concentration of 20 mg/mL per vial with a pH of 6.0 to 7.5. The study drug was then diluted in 500 mL isotonic saline solution and infused intravenously over 1 hour by means of a regulated infusion pump. A cycle of therapy was defined as three doses of TCN-PM administered on days 1, 8, and 15 every 28 days.
2.3. Trial design
TCN-PM was initiated at 15 mg/m2 weekly on days 1, 8, and 15 every 28 days based on previously conducted trials [37]. This weekly schedule was chosen based on the long half life of TCN (~89 hours) [26]. The escalation dose for new cohorts was planned as follows: 25, 35, 45, 55, and 65 mg/m2. After 65 mg/m2, and if no DLT was observed, further increments were to increase by 5 mg/m2 per cohort until DLT was observed. All adverse events were graded according to the NCI Common Terminology Criteria for Adverse Events version 3.0. A DLT was defined as any clinically significant grade 3-4 drug-related non-hematologic toxicity with the exception of grade 3 AST, ALT, or amylase/lipase elevations persisting for <7 days. Observations for DLT were performed during cycle 1 for a minimum period of 21 days. Hematologic DLT was defined as grade 3-4 neutropenia and/or thrombocytopenia (secondary to marrow hypoplasia and not leukemic burden) that did not recover to grade ≤ 2 by day 42.
The standard 3 + 3 escalation rule was used for this trial, and the recommended phase II dose was defined as the highest dose level at which no more than 1 of 6 patients experienced a DLT. The maximum tolerated dose (MTD) was defined as the highest dose level in which <2 patients of 6 developed DLT within the first cycle. At the MTD, up to 10 total patients could be accrued to further define the toxicities and response of the agent. All patients were considered to be evaluable for safety and toxicity if they received any dose of TCN-PM. Patients were considered to be evaluable for response if they received at least 1 full cycle of treatment and subsequent bone marrow biopsy or had progressive disease determined prior to completion of 1 full cycle. Patients who were removed from the study prior to day 22 for reasons other than DLT were replaced.
2.4. Response criteria
For acute leukemias and MDS, complete remission (CR) was defined as 5% or fewer leukemia cells in the normocellular or hypercellular marrow with absolute neutrophil count (ANC) equal to or more than 1.0 × 109/L and platelet count equal to or more than 100 × 109/L. A partial response was defined with the same hematologic parameters as for CR, but allowed for abnormal cells of 6% to 25% in the marrow or 50% reduction in marrow blasts. Hematologic improvement was defined as achievement of any of the following parameters: 1) increase in hemoglobin level by ≥ 1 g/dL if ≤ 11 g/dL prior to therapy and either independence or decrease from transfusion requirements by at least 50%; 2) increase in platelet count by 50% if below 100 × 109 prior to therapy, with a net increase ≥10 × 109/L; and 3) increase in ANC by 100% and to greater than 0.5 × 109/L if below this level prior to therapy. For CLL, CR was defined as 1) disappearance of all palpable lymph nodes, spleen, and liver without the appearance of new lesions along with <30% lymphocytes in normocellular marrow; if lymphoid nodules were seen, response was deemed as nodular CR; 2) absolute lymphocyte count (ALC) <4 × 109/L with hemoglobin >11 g/dL, ANC >1.5 × 109/L, and platelet count >100 × 109/L. Partial response was defined as 1) ALC reduced by >50% from pretreatment baseline value, hemoglobin >11 g/dL or 50% improvement over baseline without transfusions, ANC >1.5 × 109/L or 50% improvement over baseline, and platelet count >100 × 109/L or 50% improvement over baseline; and 2) when compared with pretreatment measurements, a reduction of >50% in measurable lesions without the appearance of new lesions.
2.5. Cell lines and TCN pharmacology
The ML-1 myeloid leukemia cell line was a gift from Dr. M. J. Kastan (Memphis, TN). OCI-AML3 cells were kindly provided by Dr. M. Minden (Ontario Cancer Institute, Toronto, ON, Canada). All cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum and 2 mM L-glutamine and maintained at 37°C in 5% CO2 in a fully humidified incubator. The cell doubling time was 24 hours under these conditions. Cells were incubated with various concentrations of TCN (Vioquest Pharmaceuticals) for 3 hours before being harvested to quantitate the cellular accumulation of TCN-PM by high-performance liquid chromatography (HPLC). In other experiments, cells were exposed to 1 and 10 μM TCN before being harvested to assess the levels of pAkt and cellular viability.
2.6. Samples for clinical pharmacology
Blood samples were collected at time 0 (prior to infusion) and as post-infusion samples of TCN-PM at 2 and 24 hours after completion of day 1 of cycle 1. Each blood sample (10 mL) was collected in a Vacutainer green top tube (heparin) and placed in an ice-water bath until transport to the laboratory.
2.7. Pharmacokinetics of TCN-PM
Blood samples were diluted with phosphate-buffered saline, and leukemia cells were isolated by Ficoll-Hypaque density gradient step-gradient centrifugation procedures. A Coulter channelyzer (Coulter Electronics, Hialeah, FL) was used to determine cell number and the mean cell volume. We used 1-2 × 107 cells to extract nucleotides including TCN-PM, after which nucleotides were separated and quantitated with HPLC using UV detection. The results were analyzed for the accumulation and retention of TCN-PM in leukemia blasts during therapy.
2.8. Western blotting
Cellular lysates were harvested at 0 and 24 hours after infusion of TCN-PM and immunoblotted with antibodies against pSer473Akt, pSer112Bad, total AKT and Bad (Cell Signaling Technology), or GAPDH (Upstate Biochemicals). Proteins were quantitated using the densitometry function on the Odessey-LiCor and expressed as a ratio of pAkt/Akt or pBad/Bad.
3. Results
Forty-three patients consented to this study, of which 41 were treated with at least one dose of TCN-PM. Two patients were removed from the study before treatment initiation, 1 due to protocol ineligibility and 1 due to voluntary consent withdrawal. Detailed patient characteristics are summarized in Table 1. The majority of patients were men (77%) with a median age of 70 years (range of 23-83). Thirty-six patients (84%) had AML, 3 had MDS/chronic myelomonocytic leukemia (CMML), 2 had CLL, and 2 had ALL. Patients had a median of 2 prior therapies (range of 0-11). The median number of TCN-PM cycles received was 1 (range of 0-3).
Table 1.
Patient characteristics
| Patients (n = 43) | |
|---|---|
| Median age, years (range) | 70 (25-83) |
| Sex, no. (%) of patients | |
| Male | 30 (70) |
| Female | 13 (30) |
| ECOG performance status, no. (%) of patients | |
| 0-1 | 31 (72) |
| ≥ 2 | 12 (28) |
| Diagnosis, no. (%) of patients | |
| AML | 36 (84) |
| MDS/CMML | 3 (7) |
| ALL | 2 (5) |
| CLL | 2 (5) |
| Median no. of prior chemotherapy regimens | 2 (0-11) |
3.1. Toxicity
The toxicity profile of TCN-PM is detailed in Table 2. Overall, TCN-PM was well-tolerated. DLTs included mucositis (1 patient at the 35 mg/m2 cohort), lipase elevation (2 patients at the 65 mg/m2 cohort), and triglyceride elevation (1 patient at the 65 mg/m2 cohort). DLTs were reversible in all patients following discontinuation of TCN-PM. Common treatment-emergent non-hematologic toxicities included pain, infection/febrile neutropenia, nausea, bleeding, mucositis, and diarrhea, with the large majority being grade 1-2. Due to concerns for metabolic toxicity, the MTD was declared at 55 mg/m2.
Table 2.
Treatment-emergent adverse events occurring in ≥ 10% of patients (n=43)
| Adverse Event | 15 mg/m2 (n=4) |
25 mg/m2 (n=4) |
35 mg/m2 (n=16) |
45 mg/m2 (n=3) |
55 mg/m2 (n=9) |
65 mg/m2 (n=7) |
Total (n=43) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1/2 |
Grade 3/4 |
Grade 1/2 |
Grade 3/4 |
Grade 1/2 |
Grade 3/4 |
Grade 1/2 |
Grade 3/4 |
Grade 1/2 |
Grade 3/4 |
Grade 1/2 |
Grade 3/4 |
Grade 1/2 |
Grade 3/4 |
|
| Pain | 0 | 0 | 1 | 0 | 7 | 0 | 1 | 0 | 3 | 0 | 2 | 0 | 14(33) | 0(0) |
| Infection/Febrile Neutropenia |
0 | 1 | 0 | 2 | 1 | 3 | 0 | 1 | 0 | 2 | 0 | 1 | 1(2) | 10(24) |
| Nausea | 0 | 0 | 2 | 0 | 4 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 9(21) | 0(0) |
| Bleeding | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 6(14) | 1(2) |
| Mucositis | 0 | 0 | 0 | 0 | 3 | 1* | 1 | 0 | 1 | 0 | 0 | 0 | 5(12) | 1(2) |
| Diarrhea | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 5(12) | 0(0) |
| Constipation | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 5(12) | 0(0) |
| Dehydration | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 5(12) | 0(0) |
| Elevated lipase | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2* | 2(5) | 2(5) |
3.2. Clinical response
Of the 41 treated patients, 32 were considered evaluable for response based on the criteria described above. Overall, there were no complete or partial responses. Of the 32 evaluable patients, 15 had progressive disease and 17 had stable disease following 1 cycle of treatment. Of the patients with stable disease, 3 patients with AML achieved ≥50% bone marrow blast reduction and a fourth patient with CMML had marked spleen reduction and resolution of leukocytosis. These patients are highlighted in Table 3. Eight patients received at least 2 cycles of TCN-PM.
Table 3.
Profile of patients with reduction in leukemic burden following TCN-PM
| Age, years |
Dose Level, mg/m2 |
Disease Status | Karyotype | Response |
|---|---|---|---|---|
| 71 | 25 | AML-refractory | Complex | > 50% marrow blast reduction after cycle 1 |
| 65 | 35 | AML - relapsed | Complex | > 50% marrow blast reduction after cycle 1 |
| 78 | 45 | AML - refractory | i17q | < 5% marrow blasts after cycle 2 WBC normalized; |
| 74 | 55 | CMML - refractory | Normal | reduction in spleen by 15 cm |
3.3. Pharmacokinetics of TCN-P in AML blasts
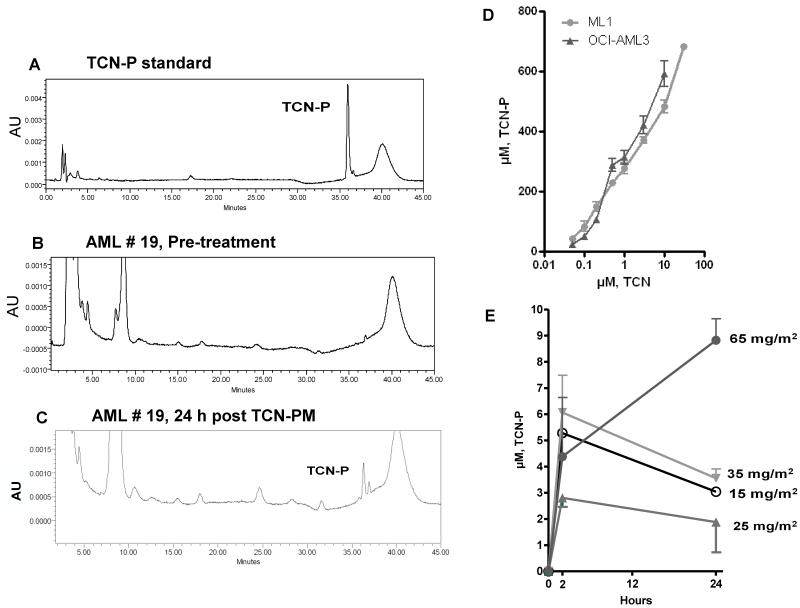
We initially determined the feasibility and reproducibility of detecting TCN-P by HPLC. To this end, a quality control standard was generated by adding a known amount of purified TCN-PM to an acid-soluble extract from AML blast cells. The standard curve indicated a well-defined TCN-P peak (Figure 1A). Next, we evaluated whether TCN-P could be detected in the leukemic cells from AML patients undergoing therapy with this Akt inhibitor. Our data demonstrate that TCN-P was absent in the leukemic blasts obtained before therapy (Figure 1B) but accumulated a discrete TCN-P peak 24 hours after TCN-PM infusion (Figure 1C).
Fig. 1.
Detection of TCN-P in AML blasts and dose-dependent accumulation of TCN-P in leukemia cell lines and AML blasts. A, Blood samples were obtained before therapy, after which normal nucleotides were extracted using perchloric acid. A known amount of TCN-PM was added to these cell extracts, and then normal and analog nucleotides were separated by HPLC to locate the peak for TCN-P. B and C, HPLC profiles from a representative patient before (B) and 24 hours after (C) TCN-PM therapy, demonstrating presence of a discrete TCN-P peak following therapy. AU, Arbitrary units. D, Ml-1 and OCI-AML3 cells were exposed to 0.05, 0.1, 0.2, 0.5, 1, 3, and 10 μM TCN for 3 hours before nucleotides were extracted and amounts of TCN-P were calculated. E, AML blasts were isolated at 2 or 24 hours after TCN therapy at dose levels of 15, 25, 35, and 65 mg/m2, after which nucleotides were extracted and levels of TCN-P were quantitated.
We then determined the pharmacokinetics of TCN-P in two leukemia cell lines (ML-1 and OCI-AML3), which were exposed to 0, 0.05, 0.1, 0.2. 0.5, 1, 3, and 10 μM TCN for 3 hours (TCN is metabolically converted to its active form TCN-P in cells) [26]. Our data demonstrate a dose-dependent accumulation of TCN-P in both leukemia cell lines. Cells exposed to <0.05 μM TCN accumulated between 20 and 40 μM of TCN-P, whereas exposure to 10 μM TCN resulted in the accumulation of over 500 μM of TCN-P (Figure 1D).
Next, leukemic blasts from 8 patients obtained at 0, 2, and 24 hours after start of TCN-PM therapy (day 1, cycle 1) were evaluated for the accumulation of TCN-P. Similar to the results obtained in the AML cell lines, primary AML blasts also demonstrated a dose-dependent accumulation of TCN-P, albeit to much lower concentrations (Figure 1E). In AML blasts, the peak level of TCN-P was achieved within 2 hours after the end of the infusion in the cohorts that received 15 (n=1), 25 (n=3), and 35 mg/m2 (n=2). AML blasts accumulated a median peak concentration of 4 μM (range of 2.1-7.5 μM) (Figure 1E). In patients who received 65 mg/m2 (n=2), the peak TCN-P levels occurred 24 hours after infusion (Figure 1E). Collectively, 4 of 6 samples in these cohorts retained at least 50% of their intracellular TCN-P after 24 hours (Figure 1E).
3.4. Pharmacodynamics of TCN-P in AML cell lines and primary leukemic blasts
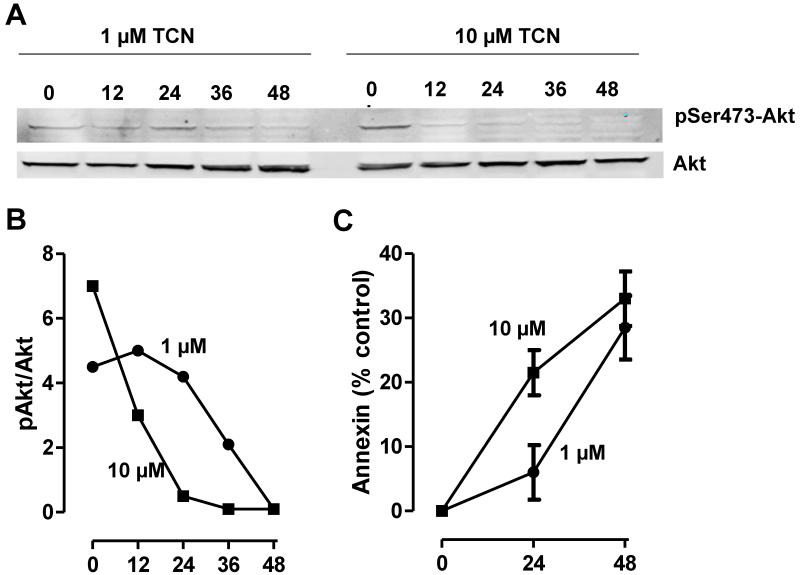
Both AML cell lines and AML blasts are known to express robust levels of Akt that is phosphorylated on Ser473 and Thr308 [38]. It is also established that exposure to chemotherapy is associated with decreased levels of pSer473 and pThr308 and increased levels of cell death in vitro and in primary AML blasts during therapy [38]. Because TCN inhibits Akt phosphorylation, we first evaluated the response of OCI-AML3 cells exposed to 1 or 10 μM TCN for 0, 12, 24, 36, and 48 hours. Our data show that the levels of pSer473Akt decreased in a time-dependent manner (Figure 2, A and B). Loss of pSer473Akt was associated with a reciprocal increase in the numbers of apoptotic cells, as measured by the appearance of annexin V positivity (Figure 2C).
Fig. 2.
Action of TCN-P on pSer473Akt levels and on cell death in OCI-AML3 cells. A and B, OCI-AML3 cells were exposed to 1 or 10 μM TCN for varying times, after which levels of pSer473Akt and total Akt were assayed as described under Materials and Methods. C, Percentage of annexin-positive cells.
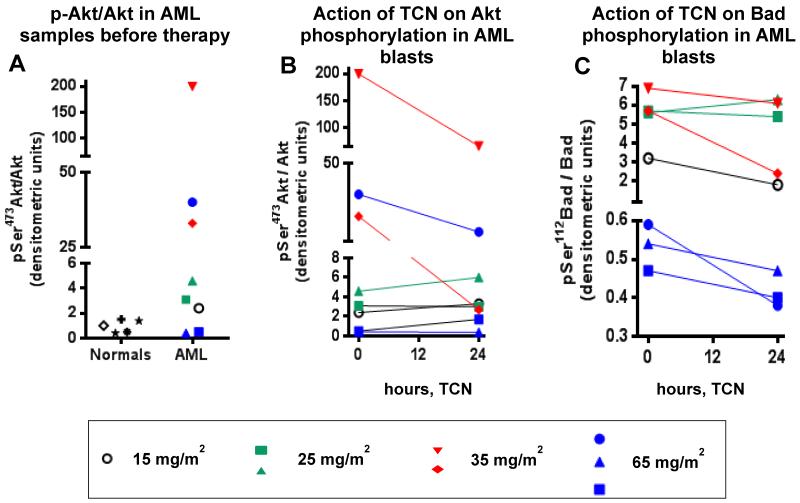
Because TCN-PM has been recently shown to inhibit Akt phosphorylation in tumor biopsies following treatment of patients whose tumors had high levels of activated Akt [37], we first compared the levels of pSer473Akt in the lymphocytes of normal donors and AML blasts from eight patients before therapy. Our data indicate that normal lymphocytes (n=5) express low levels of pSer473Akt, whereas AML blasts displayed variable levels of pSer473Akt (Figure 3A). Five samples expressed levels that were similar or 2-fold higher than the levels in normal lymphocytes, whereas three samples displayed between 30- and 200-fold higher expression of pSer473Akt, suggesting that these three patients were likely to have an activated Akt pro-survival signaling (Figure 3A). We then determined the levels of phosphorylation of Akt (pSer473) and its substrate Bad (pSer112) in AML blasts obtained before and 24 hours after TCN-PM infusion. Figure 3B shows that the pSer473Akt/Akt levels in the 5 patients whose tumors had low basal levels of phosphorylated Akt (1 treated with 15 mg/m2, 2 treated at 25 mg/m2, and 2 treated with 65 mg/m2) were little affected by TCN-PM treatment. In contrast, the three patients who had high basal levels of pAkt (2 treated at 35 mg/m2 and 1 at 65 mg/m2) demonstrated significant decreases in pSer473Akt/Akt levels. Similar results were observed with pSer112Bad/Bad levels, consistent with an inhibition of this survival pathway and the activity of TCN-P in this patient population (Figure 3C).
Fig. 3.
Effect of TCN-PM therapy on pSer473Akt and pSer112Bad levels. A, pSer473Akt levels in lymphocytes of normal donors and AML blasts. B and C, pSer473Akt (B) and pSer112Bad (C) levels in AML blasts during therapy.
4. Discussion
Early clinical studies of TCN-P followed the lead of multiple other trials, which have demonstrated the dramatic success of a variety of nucleoside analogs in treatment of patients with AML [39-42]. In these studies, TCN-P was administered under dose-intensive schedules, which induced a clinical response but produced DLTs [29, 30]. In a related trial conducted in patients with metastatic breast cancer, doses lower than 20 mg/m2 were ineffective, whereas doses over 40 mg/m2 were again associated with unacceptable toxicities, including mortality [31]. To circumvent the toxicities associated with the continuous infusion, a modified schedule that employed an intermittent, once per week dosing was evaluated in advanced solid cancers. Again, doses higher than 48 mg/m2 were associated with significant toxicity and no clinical benefit [32]. Because of these adverse events, further clinical trials with this agent would require re-evaluation of the safety and tolerability issues. Furthermore, the fact that some responses were seen suggested that a subset of patients may contain tumors with a molecular target that make them more likely to respond to TCN-P. Recently, TCN-P was shown to inhibit Akt phosphorylation and to suppress tumor growth in mice only in human tumor xenografts that express high levels of pAkt. This prompted a clinical trial that accrued patients with solid tumors that express high pAkt levels. In this trial, a dose escalation of TCN-PM on a weekly schedule in patients with a variety of advanced solid malignancies was well tolerated with acceptable toxicities and with decreased levels of pAkt in tumor biopsies from patients treated with 35 and 45 but not with 15 and 25 mg/m2 [37]. However, clear conclusions about the action of TCN on Akt were not feasible because of the heterogeneity in and small numbers of individual tumor types that were included in the study.
In this trial, we focused on advanced leukemias with the majority of the patients accrued having AML, thus allowing evaluations of the safety, tolerability, and action of TCN-PM largely in this tumor type. The first dose at which a single patient had a DLT was at 35 mg/m2; however, expansion of the cohort to 10 patients demonstrated that TCN-PM was safe at this dose. Further escalations were safe until 65 mg/m2 at which two patients had DLTs leading to the MTD being declared at 55 mg/m2. This dose is marginally higher than the previously declared dose of 48 mg/m2 [32]. However, it is important to point out that the 48 mg/m2 MTD was for administration of TCN-P on days 1, 8, 15, and 22 of a 42-day cycle [32], whereas our 55 mg/m2 MTD is for administration on days 1, 15, and 21 every 28 days. When we sought correlations between the dose of TCN-PM administered and the intracellular accumulation of the phosphorylated nucleoside analog, we found that, in two different AML cell lines (ML-1 and OCI-AML3), there was a linear dose-dependent increase between the amounts of pro-drug given and the accumulation of TCN-P where exposure to even as little as 0.1 μM TCN resulted in the accumulation of 50-75 μM TCN-P. However, when TCN-P levels were measured during therapy, we found that patients accumulated between 2 and 8 μM TCN-P over a wide range of doses (15-65 mg/m2). The low measurable levels of free TCN-P observed in our study support earlier observations that indicated that plasma measurements of TCN were complicated by its tendency to remain plasma bound as well as its long retention and repeated inter-conversion between TCN and TCN-P within cells [32].
Previous trials have indicated that therapy with TCN-PM is associated with inhibition of Akt activation [32]. In our study, out of eight evaluable samples, blasts from three patients had 30-to 200-fold higher levels of pSer473Akt compared to levels found in lymphocytes from normal donors. Each of these samples (2 treated with 35 mg/m2 and 1 treated with 65mg/m2) showed a robust decrease in pSer473Akt/Akt and pSer112Bad/Bad levels at 24 hours post-therapy. The two additional patients treated with 65 mg/m2 had tumors with very low basal levels of pAkt and therefore likely to be unaffected by Akt inhibition. These results are consistent with a recently published phase I clinical trial in advanced solid tumors where doses of 35 and 45 mg/m2 but not 15 and 25 mg/m2 decreased pAkt levels in patient biopsies [37]; nevertheless, the results are confounded by the small number of tumor biopsies analyzed. Therefore, evaluation of additional patients may be needed to establish the threshold of activated Akt that is needed prior to treatment for it to be inhibited by TCN-PM therapy.
Finally, although there were no objective responses, the findings that 3 patients had decreases in marrow blast counts, 1 had marked reduction in leukocytosis and spleen size, and 17 had stable disease with acceptable toxicities are encouraging and warrant further investigation of TCN-PM in patients who are prescreened for high levels of pAkt. Given the potential role of activated Akt in promoting chemotherapeutic resistance, combination trials are also worthy of exploration.
Acknowledgments
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
Funding Source
Supported in part by NCI Grant P50 CA100532 and VioQuest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
DS conducted experiments, analyzed data, and wrote the manuscript; WP analyzed data and wrote the manuscript; BJN conducted HPLC analysis; JL treated patients, analyzed data, and wrote the manuscript; FRK treated patients, analyzed data, and wrote the manuscript; SMS reviewed the data and contributed to the writing of the manuscript.
Disclosure of Potential Conflicts of Interest
Said M. Sebti is listed as a co-inventor in TCN patents.
ClinicalTrials.gov identifier: NCT00642031
References
- [1].O’Donnell MR, Abboud CN, Altman J, Appelbaum FR, Coutre SE, Damon LE, et al. Acute myeloid leukemia. J Natl Compr Canc Netw. 2011;9:280–317. doi: 10.6004/jnccn.2011.0027. [DOI] [PubMed] [Google Scholar]
- [2].Stone R, Sekeres M, Garcia-Manero G. Evolving strategies in the treatment of MDS and AML. Clin Adv Hematol Oncol. 2009;7:1–14. quiz 2 p following. [PubMed] [Google Scholar]
- [3].Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29:487–94. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- [4].Jabbour EJ, Estey E, Kantarjian HM. Adult acute myeloid leukemia. Mayo Clin Proc. 2006;81:247–60. doi: 10.4065/81.2.247. [DOI] [PubMed] [Google Scholar]
- [5].Stirewalt DL, Meshinchi S. Receptor tyrosine kinase alterations in AML - biology and therapy. Cancer Treat Res. 2011;145:85–108. doi: 10.1007/978-0-387-69259-3_6. [DOI] [PubMed] [Google Scholar]
- [6].Wiernik PH. FLT3 inhibitors for the treatment of acute myeloid leukemia. Clin Adv Hematol Oncol. 2011;8:429–36. 44. [PubMed] [Google Scholar]
- [7].Gilliland DG, Griffin JD. Role of FLT3 in leukemia. Curr Opin Hematol. 2002;9:274–81. doi: 10.1097/00062752-200207000-00003. [DOI] [PubMed] [Google Scholar]
- [8].Siendones E, Barbarroja N, Torres LA, Buendia P, Velasco F, Dorado F, et al. Inhibition of Flt3-activating mutations does not prevent constitutive activation of ERK/Akt/STAT pathways in some AML cells: a possible cause for the limited effectiveness of monotherapy with small-molecule inhibitors. Hematol Oncol. 2007;25:30–7. doi: 10.1002/hon.805. [DOI] [PubMed] [Google Scholar]
- [9].Testa JR, Tsichlis PN. AKT signaling in normal and malignant cells. Oncogene. 2005;24:7391–3. doi: 10.1038/sj.onc.1209100. [DOI] [PubMed] [Google Scholar]
- [10].Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997:275, 665–8. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- [11].Bayascas JR, Alessi DR. Regulation of Akt/PKB Ser473 phosphorylation. Mol Cell. 2005;18:143–5. doi: 10.1016/j.molcel.2005.03.020. [DOI] [PubMed] [Google Scholar]
- [12].Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- [13].Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–4. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- [14].Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- [15].Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–21. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- [16].Kornblau SM, Singh N, Qiu Y, Chen W, Zhang N, Coombes KR. Highly phosphorylated FOXO3A is an adverse prognostic factor in acute myeloid leukemia. Clin Cancer Res. 2011;16:1865–74. doi: 10.1158/1078-0432.CCR-09-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Grandage VL, Gale RE, Linch DC, Khwaja A. PI3-kinase/Akt is constitutively active in primary acute myeloid leukaemia cells and regulates survival and chemoresistance via NF-kappaB, Mapkinase and p53 pathways. Leukemia. 2005;19:586–94. doi: 10.1038/sj.leu.2403653. [DOI] [PubMed] [Google Scholar]
- [18].Min YH, Eom JI, Cheong JW, Maeng HO, Kim JY, Jeung HK, et al. Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: its significance as a prognostic variable. Leukemia. 2003;17:995–7. doi: 10.1038/sj.leu.2402874. [DOI] [PubMed] [Google Scholar]
- [19].Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–80. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- [20].Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M, et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108:2358–65. doi: 10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Min YH, Cheong JW, Kim JY, Eom JI, Lee ST, Hahn JS, et al. Cytoplasmic mislocalization of p27Kip1 protein is associated with constitutive phosphorylation of Akt or protein kinase B and poor prognosis in acute myelogenous leukemia. Cancer Res. 2004;64:5225–31. doi: 10.1158/0008-5472.CAN-04-0174. [DOI] [PubMed] [Google Scholar]
- [22].Brandwein JM, Hedley DW, Chow S, Schimmer AD, Yee KW, Schuh AC, et al. A phase I/II study of imatinib plus reinduction therapy for c-kit-positive relapsed/refractory acute myeloid leukemia: inhibition of Akt activation correlates with complete response. Leukemia. 2011;25(16):945–52. doi: 10.1038/leu.2011.34. [DOI] [PubMed] [Google Scholar]
- [23].Grandage VL, Gale RE, Linch DC, Khwaja A. PI3-kinase//Akt is constitutively active in primary acute myeloid leukaemia cells and regulates survival and chemoresistance via NF-kB, MAPkinase and p53 pathways. Leukemia. 2005;19:586–94. doi: 10.1038/sj.leu.2403653. [DOI] [PubMed] [Google Scholar]
- [24].Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nature Medicine. 2003;9:1158–65. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- [25].Tazzari PL, Cappellini A, Ricci F, Evangelisti C, Papa V, Grafone T, et al. Multidrug resistance-associated protein 1 expression is under the control of the phosphoinositide 3 kinase//Akt signal transduction network in human acute myelogenous leukemia blasts. Leukemia. 2007;21:427–38. doi: 10.1038/sj.leu.2404523. [DOI] [PubMed] [Google Scholar]
- [26].Powis G, Basseches PJ, Kroschel DM, Richardson RL, O’Connell MJ, Kvols LK. Disposition of tricyclic nucleoside-5′-monophosphate in blood and plasma of patients during phase I and II clinical trials. Cancer Treat Rep. 1986;70:359–62. [PubMed] [Google Scholar]
- [27].Schweinsberg PD, Smith RG, Loo TL. Identification of the metabolites of an antitumor tricyclic nucleoside (NSC-154020) Biochemical pharmacology. 1981;30:2521–6. doi: 10.1016/0006-2952(81)90577-3. [DOI] [PubMed] [Google Scholar]
- [28].Berndt N, Yang H, Trinczek B, Betzi S, Zhang Z, Wu B, et al. The Akt activation inhibitor TCN-P inhibits Akt phosphorylation by binding to the PH domain of Akt and blocking its recruitment to the plasma membrane. Cell Death Differ. 2010;17:1795–804. doi: 10.1038/cdd.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Feun LG, Blessing JA, Barrett RJ, Hanjani P, A Gynecologic Oncology Group Study A phase II trial of tricyclic nucleoside phosphate in patients with advanced squamous cell carcinoma of the cervix. Am J Clin Oncol. 1993;16:506–8. doi: 10.1097/00000421-199312000-00010. [DOI] [PubMed] [Google Scholar]
- [30].Feun LG, Savaraj N, Bodey GP, Lu K, Yap BS, Ajani JA, et al. Phase I study of tricyclic nucleoside phosphate using a five-day continuous infusion schedule. Cancer Res. 1984;44:3608–12. [PubMed] [Google Scholar]
- [31].Hoffman K, Holmes FA, Fraschini G, Esparza L, Frye D, Raber MN, et al. Phase I-II study: triciribine (tricyclic nucleoside phosphate) for metastatic breast cancer. Cancer Chemother Pharmacol. 1996;37:254–8. doi: 10.1007/BF00688325. [DOI] [PubMed] [Google Scholar]
- [32].Schilcher RB, Haas CD, Samson MK, Young JD, Baker LH. Phase I evaluation and clinical pharmacology of tricyclic nucleoside 5′-phosphate using a weekly intravenous regimen. Cancer Res. 1986;46:3147–51. [PubMed] [Google Scholar]
- [33].Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–9. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- [34].Evangelisti C, Ricci F, Tazzari P, Chiarini F, Battistelli M, Falcieri E, et al. Preclinical testing of the Akt inhibitor triciribine in T-cell acute lymphoblastic leukemia. J Cell Physiol. 2011;226:822–31. doi: 10.1002/jcp.22407. [DOI] [PubMed] [Google Scholar]
- [35].Balasis ME, Forinash KD, Chen YA, Fulp WJ, Coppola D, Hamilton AD, et al. Combination of farnesyltransferase and Akt inhibitors is synergistic in breast cancer cells and causes significant breast tumor regression in ErbB2 transgenic mice. Clin Cancer Res. 2011;17:2852–62. doi: 10.1158/1078-0432.CCR-10-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gloesenkamp CR, Nitzsche B, Ocker M, Di Fazio P, Quint K, Hoffmann B, et al. AKT inhibition by triciribine alone or as combination therapy for growth control of gastroenteropancreatic neuroendocrine tumors. Int J Oncol. 2012;40:876–88. doi: 10.3892/ijo.2011.1256. [DOI] [PubMed] [Google Scholar]
- [37].Garrett CR, Coppola D, Wenham RM, Cubitt CL, Neuger AM, Frost TJ, et al. Phase I pharmacokinetic and pharmacodynamic study of triciribine phosphate monohydrate, a small-molecule inhibitor of AKT phosphorylation, in adult subjects with solid tumors containing activated AKT. Invest New Drugs. 2011;29:1381–9. doi: 10.1007/s10637-010-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sampath D, Cortes J, Estrov Z, Du M, Shi Z, Andreeff M, et al. Pharmacodynamics of cytarabine alone and in combination with 7-hydroxystaurosporine (UCN-01) in AML blasts in vitro and during a clinical trial. Blood. 2006;107:2517–24. doi: 10.1182/blood-2005-08-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Giles FJ, Keating A, Goldstone AH, Avivi I, Willman CL, Kantarjian HM. Acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2002:73–110. doi: 10.1182/asheducation-2002.1.73. [DOI] [PubMed] [Google Scholar]
- [40].Kantarjian H, Gandhi V, Cortes J, et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–86. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- [41].Kantarjian HM, Estey EH, Keating MA. New chemotherapeutic agents in acute myeloid leukemia. Leukemia. 1996;10(Suppl 1):S4–6. [PubMed] [Google Scholar]
- [42].Keating MJ, O’Brien S, McLaughlin P, Dimopoulos M, Gandhi V, Plunkett W, et al. Clinical experience with fludarabine in hemato-oncology. Hematol Cell Ther. 1996;38(Suppl 2):S83–91. [PubMed] [Google Scholar]