Abstract
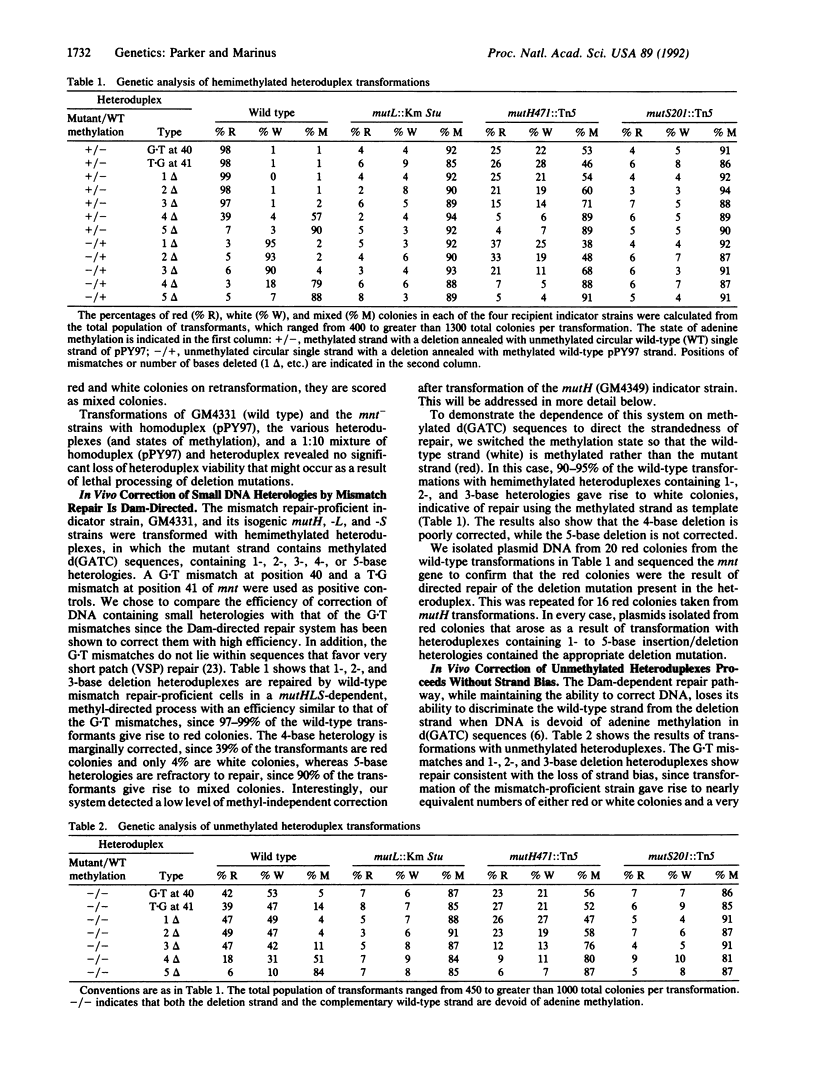
Plasmid heteroduplexes were constructed that contain 1, 2, 3, 4, or 5 unpaired bases within the mnt gene. These were used to assess the efficiency of repair of small heterologous sequences ("heterologies") in DNA by the Escherichia coli Dam-directed mismatch repair system. Heteroduplexes in defined states of methylation at d(GATC) sites were used to transform a repair-proficient indicator strain (which has a mnt-lac fusion coding for a nonfunctional mnt repressor) and its isogenic mutH, -L, and -S derivatives. Using this in vivo transformation system, we scored for repair on the basis of colony color: correction in favor of the strand bearing mnt+ coding information gives rise to colonies that are white, whereas correction on the opposite strand (mnt-) yields colonies that are red when grown on MacConkey agar. Failure to repair a heterology yields colonies that are both red and white ("mixed"). The correction efficiencies of two heteroduplexes, each containing a single G.T mismatch within mnt, were also monitored for purposes of comparison. Our results show that mutHLS-dependent, methyl-directed repair of heteroduplexes with 1-, 2-, and 3-base deletions is as highly efficient as the repair of G.T mismatches. Heteroduplexes with a 4-base deletion are marginally repaired and DNA with a 5-base deletion is not detectably repaired. In addition, we show that purified MutS protein from Salmonella typhimurium, which can substitute for E. coli MutS in vivo, binds to oligonucleotide duplexes containing 1, 2, 3, and 4 unpaired bases of a sequence identical with that used for the in vivo studies. Specific binding of MutS to homoduplex DNA and to DNA that had undergone a 5-base deletion was not observed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bebenek K., Kunkel T. A. The use of native T7 DNA polymerase for site-directed mutagenesis. Nucleic Acids Res. 1989 Jul 11;17(13):5408–5408. doi: 10.1093/nar/17.13.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. K., Andersen J., Kolodner R. D. Specificity of mismatch repair following transformation of Saccharomyces cerevisiae with heteroduplex plasmid DNA. Proc Natl Acad Sci U S A. 1989 May;86(10):3713–3717. doi: 10.1073/pnas.86.10.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway M., Rewinski C., Marinus M. G. Mutations produced by DNA polymerase III holoenzyme of Escherichia coli after in vitro synthesis in the absence of single-strand binding protein. Mol Microbiol. 1990 Oct;4(10):1645–1652. doi: 10.1111/j.1365-2958.1990.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Claverys J. P., Lacks S. A. Heteroduplex deoxyribonucleic acid base mismatch repair in bacteria. Microbiol Rev. 1986 Jun;50(2):133–165. doi: 10.1128/mr.50.2.133-165.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly D. M., Winkler M. E. Genetic and physiological relationships among the miaA gene, 2-methylthio-N6-(delta 2-isopentenyl)-adenosine tRNA modification, and spontaneous mutagenesis in Escherichia coli K-12. J Bacteriol. 1989 Jun;171(6):3233–3246. doi: 10.1128/jb.171.6.3233-3246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohet C., Wagner R., Radman M. Methyl-directed repair of frameshift mutations in heteroduplex DNA. Proc Natl Acad Sci U S A. 1986 May;83(10):3395–3397. doi: 10.1073/pnas.83.10.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishel R. A., Siegel E. C., Kolodner R. Gene conversion in Escherichia coli. Resolution of heteroallelic mismatched nucleotides by co-repair. J Mol Biol. 1986 Mar 20;188(2):147–157. doi: 10.1016/0022-2836(86)90300-1. [DOI] [PubMed] [Google Scholar]
- Gasc A. M., Garcia P., Baty D., Sicard A. M. Mismatch repair during pneumococcal transformation of small deletions produced by site-directed mutagenesis. Mol Gen Genet. 1987 Dec;210(2):369–372. doi: 10.1007/BF00325708. [DOI] [PubMed] [Google Scholar]
- Grilley M., Holmes J., Yashar B., Modrich P. Mechanisms of DNA-mismatch correction. Mutat Res. 1990 Sep-Nov;236(2-3):253–267. doi: 10.1016/0921-8777(90)90009-t. [DOI] [PubMed] [Google Scholar]
- Grilley M., Welsh K. M., Su S. S., Modrich P. Isolation and characterization of the Escherichia coli mutL gene product. J Biol Chem. 1989 Jan 15;264(2):1000–1004. [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Jiricny J., Su S. S., Wood S. G., Modrich P. Mismatch-containing oligonucleotide duplexes bound by the E. coli mutS-encoded protein. Nucleic Acids Res. 1988 Aug 25;16(16):7843–7853. doi: 10.1093/nar/16.16.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer B., Kramer W., Williamson M. S., Fogel S. Heteroduplex DNA correction in Saccharomyces cerevisiae is mismatch specific and requires functional PMS genes. Mol Cell Biol. 1989 Oct;9(10):4432–4440. doi: 10.1128/mcb.9.10.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahue R. S., Au K. G., Modrich P. DNA mismatch correction in a defined system. Science. 1989 Jul 14;245(4914):160–164. doi: 10.1126/science.2665076. [DOI] [PubMed] [Google Scholar]
- Learn B. A., Grafstrom R. H. Methyl-directed repair of frameshift heteroduplexes in cell extracts from Escherichia coli. J Bacteriol. 1989 Dec;171(12):6473–6481. doi: 10.1128/jb.171.12.6473-6481.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. L., Clark S., Modrich P. Methyl-directed repair of DNA base-pair mismatches in vitro. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4639–4643. doi: 10.1073/pnas.80.15.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Längle-Rouault F., Maenhaut-Michel G., Radman M. GATC sequences, DNA nicks and the MutH function in Escherichia coli mismatch repair. EMBO J. 1987 Apr;6(4):1121–1127. doi: 10.1002/j.1460-2075.1987.tb04867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P. DNA mismatch correction. Annu Rev Biochem. 1987;56:435–466. doi: 10.1146/annurev.bi.56.070187.002251. [DOI] [PubMed] [Google Scholar]
- Pang P. P., Lundberg A. S., Walker G. C. Identification and characterization of the mutL and mutS gene products of Salmonella typhimurium LT2. J Bacteriol. 1985 Sep;163(3):1007–1015. doi: 10.1128/jb.163.3.1007-1015.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukkila P. J., Peterson J., Herman G., Modrich P., Meselson M. Effects of high levels of DNA adenine methylation on methyl-directed mismatch repair in Escherichia coli. Genetics. 1983 Aug;104(4):571–582. doi: 10.1093/genetics/104.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposa S., Fox M. S. Some features of base pair mismatch and heterology repair in Escherichia coli. Genetics. 1987 Nov;117(3):381–390. doi: 10.1093/genetics/117.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewinski C., Marinus M. G. Mutation spectrum in Escherichia coli DNA mismatch repair deficient (mutH) strain. Nucleic Acids Res. 1987 Oct 26;15(20):8205–8215. doi: 10.1093/nar/15.20.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Kidd S., Kelley M. R. An improved filamentous helper phage for generating single-stranded plasmid DNA. Gene. 1986;45(3):333–338. doi: 10.1016/0378-1119(86)90032-6. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Dunn R. L. Spectra of spontaneous mutations in Escherichia coli strains defective in mismatch correction: the nature of in vivo DNA replication errors. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6220–6224. doi: 10.1073/pnas.84.17.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. C., Kamel F. Reversion of frameshift mutations by mutator genes in Escherichia coli. J Bacteriol. 1974 Mar;117(3):994–1001. doi: 10.1128/jb.117.3.994-1001.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. C., Wain S. L., Meltzer S. F., Binion M. L., Steinberg J. L. Mutator mutations in Escherichia coli induced by the insertion of phage mu and the transposable resistance elements Tn5 and Tn10. Mutat Res. 1982 Mar;93(1):25–33. doi: 10.1016/0027-5107(82)90122-1. [DOI] [PubMed] [Google Scholar]
- Skopek T. R., Hutchinson F. Frameshift mutagenesis of lambda prophage by 9-aminoacridine, proflavin and ICR-191. Mol Gen Genet. 1984;195(3):418–423. doi: 10.1007/BF00341442. [DOI] [PubMed] [Google Scholar]
- Sohail A., Lieb M., Dar M., Bhagwat A. S. A gene required for very short patch repair in Escherichia coli is adjacent to the DNA cytosine methylase gene. J Bacteriol. 1990 Aug;172(8):4214–4221. doi: 10.1128/jb.172.8.4214-4221.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S. S., Grilley M., Thresher R., Griffith J., Modrich P. Gap formation is associated with methyl-directed mismatch correction under conditions of restricted DNA synthesis. Genome. 1989;31(1):104–111. doi: 10.1139/g89-020. [DOI] [PubMed] [Google Scholar]
- Su S. S., Lahue R. S., Au K. G., Modrich P. Mispair specificity of methyl-directed DNA mismatch correction in vitro. J Biol Chem. 1988 May 15;263(14):6829–6835. [PubMed] [Google Scholar]
- Su S. S., Modrich P. Escherichia coli mutS-encoded protein binds to mismatched DNA base pairs. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5057–5061. doi: 10.1073/pnas.83.14.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh K. M., Lu A. L., Clark S., Modrich P. Isolation and characterization of the Escherichia coli mutH gene product. J Biol Chem. 1987 Nov 15;262(32):15624–15629. [PubMed] [Google Scholar]
- Youderian P., Vershon A., Bouvier S., Sauer R. T., Susskind M. M. Changing the DNA-binding specificity of a repressor. Cell. 1983 Dec;35(3 Pt 2):777–783. doi: 10.1016/0092-8674(83)90110-1. [DOI] [PubMed] [Google Scholar]