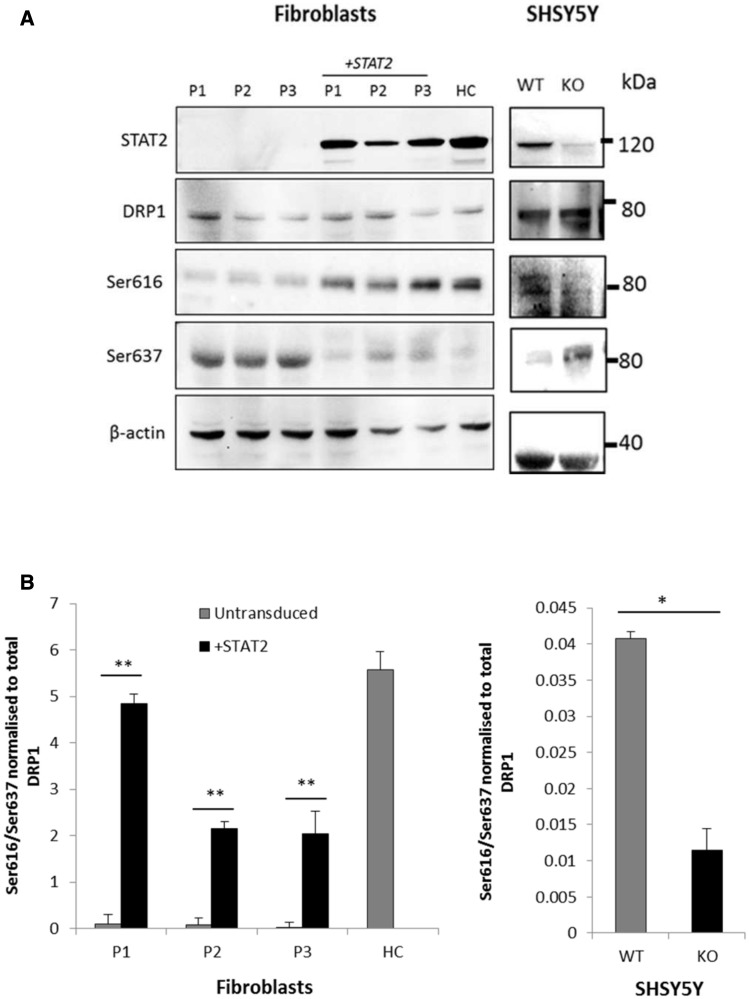
Figure 5.
DRP1 phosphorylation in patients and controls. (A) Protein samples (20 μg) from fibroblasts and SHSY5Y cells were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis and immunoblotted with the indicated antibodies. STAT2 protein was not detectable in the three patients (P1–3) confirming nonsense mediated decay; STAT2 levels were restored after lentiviral transduction with STAT2 (top). Total DRP1 protein levels remained constant throughout (second panel from top), but DRP1 phosphorylation at serine 616 was very low in STAT2 deficient cells and increased after lentiviral transduction with wild-type STAT2 (third panel), whilst phosphorylation at serine 637 reduced after STAT2 transduction (fourth panel). Actin was used as a loading control (bottom). (B) Quantitative analysis of phosphorylation at serines 616 and 637 of DRP1 in STAT2 transduced patient and control fibroblasts (left) and SHSY5Y wild-type and STAT2 knockout cells (right). Data were normalized to the total level of DRP1 and represent the mean ± SD of three independent experiments. *P < 0.05, **P < 0.005.