Abstract
This case series describes autopsy findings in 10 patients with proven severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection who died at a university medical center in Germany.
Approximately 15% of individuals affected by coronavirus disease 2019 (COVID-19) develop severe disease, and 5% to 6% are critically ill (respiratory failure and/or multiple organ dysfunction or failure).1,2 Severely ill and critically ill patients have a high mortality rate, especially with older age and coexisting medical conditions. Because there are still insufficient data on cause of death, we describe postmortem examinations in a case series of patients with COVID-19.
Methods
Between April 4 and April 19, 2020, we conducted serial postmortem examinations in patients with proven severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection who died at the University Medical Center Augsburg (Germany). Autopsies were conducted according to published best practice.3 Specimens from lung, heart, liver, spleen, kidney, brain, pleural effusion, and cerebrospinal fluid (CSF) were assessed. Postmortem nasopharyngeal, tracheal, bronchial swabs, pleural effusion, and CSF were tested for SARS-CoV-2 by reverse transcriptase–polymerase chain reaction. This study was approved by the local institutional review board, and written informed consent was obtained from next of kin.
Results
Of 12 consecutive patients with COVID-19 who died, postmortem examinations were conducted in 10. The median age was 79 years (range, 64-90 years); 7 patients were male. All cases tested positive for SARS-CoV-2 by nasopharyngeal swab at time of hospital admission. The median duration from admission to death was 7.5 days (range, 1-26 days). The most frequent initial symptoms included fever, cough, and dyspnea. In 9 patients, infiltrations with ground-glass opacity predominantly in middle and lower lung fields were detected by chest x-ray. Patients had a median of 4 known preexisting comorbidities (range, 0-6), with cardiovascular disease being most frequent (Table). Preexisting structural lung damage (eg, emphysema) was found in 2 patients. None of the patients had thromboembolic events in central vessels at autopsy or prior to death.
Table. Clinical Characteristics and Lung Pathology.
| Patient No.a | Known comorbidities | Symptom duration before admission, d | Time from admission to death, d | Duration of ventilator management, d | Stage of diffuse alveolar damageb | ||
|---|---|---|---|---|---|---|---|
| Acute | Organizing | End-stage | |||||
| 1 | Chronic myelomonocytic leukemia, hypothyroidism | 7 | 26 | 21 | − | + | ++ |
| 2 | Arteriosclerosis, arterial hypertension, atrial fibrillation, chronic lymphocytic leukemia, coronary artery disease | 14 | 15 | 14 | + | ++ | − |
| 3 | Arterial hypertension, arteriosclerosis, chronic obstructive pulmonary disease, diabetes, fatty liver disease | 7 | 7 | 6 | + | ++ | − |
| 4 | Arterial hypertension, atrial fibrillation, chronic kidney failure, dilated cardiomyopathy, hypothyroidism, morbid obesityc | 21 | 9 | 8 | + | ++ | − |
| 5 | Hypertrophic cardiomyopathy | 4 | 8 | NAd | ++ | + | − |
| 6 | Arterial hypertension, arteriosclerosis, atrial fibrillation | 6 | 3 | NAd | + | ++ | − |
| 7 | Adenocarcinoma of the lung (stage IV), arterial hypertension, chronic kidney failure, hyperthyroidism | 5 | 7 | NAd | ++ | + | − |
| 8 | Chronic obstructive pulmonary disease, chronic kidney failure, diabetes, morbid obesityc | 2 | 5 | NAd | ++ | + | − |
| 9 | Arterial hypertension, arteriosclerosis, atrial fibrillation, dementia | 3 | 1 | NAd | ++ | + | − |
| 10 | Arterial hypertension, arteriosclerosis | 2 | 8 | NAd | ++ | + | − |
Abbreviation: NA, not applicable.
Due to data privacy, patient numbers have been randomly assigned.
Scale for predominant stage of acute alveolar damage: ++, strong; +, moderate; and −, weak/absent.
Morbid obesity was defined as body mass index greater than 40 (calculated as weight in kilograms divided by height in meters squared).
No invasive mechanical ventilation.
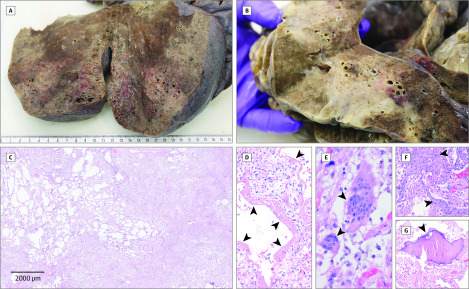
In all cases, including 6 patients who did not receive invasive ventilation, disseminated diffuse alveolar damage at different stages (the histopathological correlate of acute respiratory distress syndrome) was the major histologic finding. Diffuse alveolar damage was detectable in all lobes but appeared unevenly distributed with pronounced manifestation in middle and lower lung fields (Figure, A-B). Signs of exudative early-phase acute diffuse alveolar damage with hyaline membrane formation, intra-alveolar edema, and thickened alveolar septa with perivascular lymphocyte-plasmocytic infiltration were consistently found. Organizing-stage diffuse alveolar damage with pronounced fibroblastic proliferation, partial fibrosis, pneumocyte hyperplasia leading to interstitial thickening and collapsed alveoles, and patchy lymphocyte infiltration was the predominant finding. In areas of organizing diffuse alveolar damage, reactive osseous and squamous metaplasia were observed (Figure, C-G). Fully established fibrosis was most prominent in patient 1, ultimately leading to almost complete destruction of pulmonary parenchyma. In 5 patients, minor neutrophil infiltration was indicative of secondary infection and/or aspiration.
Figure. Macroscopic and Microscopic Findings in the Lung.
Macroscopic (A and B) and histologic (C) images of organizing and end-stage diffuse alveolar damage (hematoxylin-eosin staining) with hyaline membranes (D, arrowheads, ×100), multinucleated giant cells (E, arrowheads, ×400), and squamous/osseous metaplasia (F and G, arrowheads, ×200) in a patient with a fatal course of coronavirus disease 2019.
Mild lymphocytic myocarditis and signs of epicarditis were detectable in 4 and 2 cases, respectively. Liver histology showed minimal periportal lymphoplasmacellular infiltration and signs of fibrosis. There was no morphologically detectable pathology in other organs. Specifically, no signs of encephalitis or central nervous system vasculitis were found.
At time of autopsy, SARS-CoV-2 was still detectable in the respiratory tracts of all patients. Polymerase chain reaction testing was positive in pleural effusion but negative in all CSF samples.
Discussion
In this postmortem evaluation of 10 patients with COVID-19, acute and organizing diffuse alveolar damage and SARS-CoV-2 persistence in the respiratory tract were the predominant histopathologic findings and constituted the leading cause of death in patients with and without invasive ventilation. Periportal liver lymphocyte infiltration was considered unspecific inflammation. Whether myoepicardial alterations represented systemic inflammation or early myocarditis is unclear; criteria for true myocarditis were not met. Central nervous system involvement by COVID-19 could not be detected.
This study has limitations, including the small number of cases from a single center and missing proof of direct viral organ infection.
The pulmonary histologic characteristics of COVID-19 resembled those observed in diseases caused by other Betacoronavirus infections such as severe acute respiratory syndrome4 and Middle East respiratory syndrome.5
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, et al. . Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. Published online February 7, 2020. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanley B, Lucas SB, Youd E, Swift B, Osborn M. Autopsy in suspected COVID-19 cases. J Clin Pathol. 2020;73(5):239-242. doi: 10.1136/jclinpath-2020-206522 [DOI] [PubMed] [Google Scholar]
- 4.Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170(4):1136-1147. doi: 10.2353/ajpath.2007.061088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Brand JM, Smits SL, Haagmans BL. Pathogenesis of Middle East respiratory syndrome coronavirus. J Pathol. 2015;235(2):175-184. doi: 10.1002/path.4458 [DOI] [PMC free article] [PubMed] [Google Scholar]