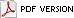
|

Fig. 1. Bacterial leaf scorch of oak (Quercus rubra). Look for a pronounced marginal discoloration with a dull red or yellow halo between scorched and green tissues. (Courtesy A. B. Gould)
| |
Bacterial leaf scorch (BLS) affects many different shade tree species such as American elm, red maple, sweet gum, sycamore and London plane, and a number of species of oak (Fig. 1) (Table 1) (11,13,20,34,35). The disease has been identified in the urban forest (landscapes, street plantings, and small woodlots) throughout the eastern United States and as far west as Texas.
BLS is one of a group of diseases caused by
Xylella fastidiosa. This bacterial pathogen is distributed throughout the Western Hemisphere, has a very wide host range, and causes diseases with two basic types of symptomology (leaf scorch or stunt) in a number of economically important hosts (Table 2).
X. fastidiosa also resides in "alternative" hosts, many of them common landscape ornamentals and weeds, where no discernible symptoms of disease may occur (Table 3) (19). As its name suggests,
X. fastidiosa lives in the xylem tissues of host plants, and the bacterium is transmitted (or vectored) by insects that feed on xylem fluid, such as leafhoppers or sharpshooters (17).
Table 1. Shade tree hosts affected by BLS (25,33).
|
Scientific name |
Common name |
|
Acer sp. | |
| A. rubrum | Red maple |
| A. negundo | Boxelder |
| A. saccharum | Sugar maple |
|
Cornus florida | Flowering dogwood |
|
Celtis occidentalis | Hackberry |
|
Liquidambar stryraciflua | Sweet gum |
|
Morus alba | White mulberry |
|
Platanus sp. | |
| P. occidentalis | American sycamore |
| P. x acerifolia | London plane |
|
Quercus sp. | |
| Q. velutina | Black oak |
| Q. incana | Bluejack oak |
| Q. macrocarpa | Bur oak |
| Q. prinus | Chestnut oak |
| Q. laurifolia | Laurel oak |
| Q. virginiana | Live oak |
| Q. rubra | Northern red oak |
| Q. palustris | Pin oak |
| Q. stellata | Post oak |
| Q. coccinea | Scarlet oak |
| Q. imbricaria | Shingle oak |
| Q. shumardii | Shumard oak |
| Q. falcata | Southern red oak |
| Q. bicolor | Swamp white oak |
| Q. laevis | Turkey oak |
| Q. nigra | Water oak |
| Q. alba | White oak |
| Q. phellos | Willow oak |
|
Ulmus americana | American elm |
Table 2. Some of the economically important diseases caused by
Xylella fastidiosa characterized by the primary symptom expressed.
|
Leaf scorch | Almond leaf scorch (Prunus amygdalus) |
| Bacterial leaf scorch of shade trees |
| Coffee leaf scorch (Coffea arabica) |
| Oleander leaf scorch (Nerium oleander) |
| Pear leaf scorch (Pyrus pyrifolia) |
| Pecan leaf scorch (Carya illinoinensis) |
| Pierce’s disease of grapevine (Vitis spp.) |
| Plum leaf scald (Prunus domestica,
P. salicina) |
|
Stunt | Alfalfa dwarf (Medicago sativa) |
| Citrus variegated chlorosis (Citrus spp.) |
| Phony peach disease (Prunus persica) |
| Periwinkle wilt (Catharanthus roseus) |
Table 3. Some alternative hosts of
Xylella fastidiosa.1
|
Scientific name |
Common name |
|
Aesculus x hybrid | Buckeye |
|
Ampelopsis arborea | Peppervine |
|
Ampelopsis brevipedunculata | Porcelain berry |
|
Artemisia spp. | Mugwort |
|
Baccharis halimifolia | Eastern baccharis |
|
Callicarpa americana | American beautyberry |
|
Celastrus orbiculata | Oriental bittersweet |
|
Cynodon dactylon | Bermuda grass |
|
Fagus crenata | Japanese beech bonsai |
|
Fragaria californica | Wild strawberry |
|
Hedera helix | English ivy |
|
Montia linearis | Miner’s lettuce |
|
Parthenocissus quinquefolia | Virginia creeper |
|
Parthenocissus tricuspidata | Boston ivy |
|
Paspalum dilatatum | Dallis grass |
|
Rhus sp. | Sumac |
|
Rubus procerus | Blackberry |
|
Sambucus canadensis | American elder |
|
Solidago fistulosa | Goldenrod |
|
Sorghum halapense | Johnson grass |
|
Trifolium repens var.
latum | Landino clover |
|
Vitis sp. | Wild grape |
1 For a more complete list of alternative hosts, refer to the
Xylella fastidiosa web site.
This article will describe and illustrate symptoms of BLS, discuss the unique bacterium that causes the disease, and explain disease development. It will finish with a discussion on disease management and the historical and current significance of
X. fastidiosa.
Leaf Scorching in Shade Trees
Leaf scorch in plants can be attributed to biotic agents or abiotic stresses (10). Abiotic (or environmental) stresses that can cause leaves to scorch include moisture extremes, wind, salt, air pollutants, toxic metals, and nutrient extremes. In most cases, this type of scorching is fairly uniform around leaf edges, affects newer leaves as well as older leaves, will appear on vast expanses of the canopy, and may also develop soon after a known stress (such as drought or a salt application) occurs (Fig. 2).
| |

Fig. 2. Leaf scorch of weeping beech caused by abiotic (environmental) stress. Note that most leaves are affected in a uniform pattern. (Courtesy A. B. Gould) | |
Plant infection by living or biotic agents (such as fungi, bacteria, nematodes, and viruses) can also result in leaf scorching, but this type of scorching is not clearly defined on the plant. Symptoms on leaves are often irregular in shape and, as is the case for BLS, may include a yellow or red "band" between green and scorched tissues (Fig. 3). In addition, symptoms may appear first on leaves of one or more branches, and then, over time, appear on other parts of the tree.
| |

Fig. 3. Leaf scorch of elm caused by
Xylella fastidiosa. Note that symptoms are irregular in shape, and a bright yellow "band" appears between green and scorched tissues. (Courtesy J. L. Sherald, APS Woody Ornamentals Digital Image Collection) | |
Symptoms
Symptoms of BLS vary by shade tree host (Fig. 4), but in most cases the disease is identified by a characteristic marginal leaf scorch (13). Symptoms first appear in late summer to early fall. In trees with determinate growth, such as oak, the scorching appears on leaves of all ages at about the same time. In trees with indeterminate growth, such as sycamore and elm, symptoms progress from older to younger leaves. Affected leaves may curl and drop prematurely, and as the disease progresses over several years, branches die and the tree declines. Elms may be killed outright by the disease; other affected species eventually decline to the point where the dead branches pose a risk and the tree must be removed. The process of tree decline may occur quickly or slowly, perhaps depending on the tree and the environment. Epicormic sprouts may be prominent on severely diseased trees, and scale insects, borers, Armillaria root rot, and other biotic diseases may be present as secondary pests.

A | |

B |

C | |

D |
|
Fig. 4. Symptoms of bacterial leaf scorch on: (A) red maple,
Acer rubrum (Courtesy J. L. Sherald, APS Woody Ornamentals Digital Image Collection); (B) white mulberry (Morus alba) (Courtesy J. L. Sherald, APS Woody Ornamentals Digital Image Collection); (C) shingle oak (Quercus imbricaria) (Courtesy A. B. Gould); and (D) sweet gum (Liquidambar stryraciflua) (Courtesy J. R. Hartman). |
The symptoms and distribution of several important shade tree hosts affected by BLS are described below.
Elm. Leaves of elm (Ulmus americana) affected by BLS have an irregular pattern of necrosis (tissue death) along the margin that is often accompanied by a chlorotic (yellow) halo (Fig. 3). This irregular scorch is quite different from the uniform pattern caused by environmental stress (such as drought). Symptoms progress from older to younger leaves (Fig. 5) (13).
| |

Fig. 5. Leaf scorch caused by
Xylella fastidiosa on American elm (Ulmus americana). Symptoms progress from older to younger leaves; leaves at the tip of the branch do not appear scorched. (Courtesy J. L. Sherald, APS Woody Ornamentals Digital Image Collection) | |
Elms affected by BLS are also very susceptible to
Dutch elm disease (DED), which is the usual reason such trees die and are removed. DED differs from BLS, however, in that elms with DED have leaves that flag and curl (not scorch) earlier in the growing season, and xylem discoloration, which is a diagnostic characteristic for DED, does not occur in trees affected by BLS. BLS of elm has been detected as far north as the Niagara Peninsula (7) and is particularly troublesome in the mid-Atlantic United States. For example, in 2001, 30% of 3000 elm trees planted in the monumental core in Washington, D.C. were affected by the disease (21).
Sycamore. BLS of sycamore (Platanus occidentalis) is a chronic disease, and it may be years before trees affected by the disease die. Symptoms appear late in the season as a papery, interveinal leaf scorch with a narrow chlorotic halo (Fig. 6). As in elm, the disease progresses from older to younger leaves (Fig. 7) (34). BLS of sycamore may be confused with sycamore anthracnose, which appears earlier in the growing season and tends to affect tissue along the veins instead of between them.
| |
 | |
 | |
| |
Fig. 6. A sycamore leaf (Platanus occidentalis) affected by leaf scorch. (Courtesy R. K. Jones, APS Woody Ornamentals Digital Image Collection) |
|
Fig. 7. Foliar symptoms of bacterial leaf scorch of sycamore (Platanus occidentalis). Older leaves on the branch are scorched and curled. (Courtesy J. L. Sherald, APS Woody Ornamentals Digital Image Collection) | |
BLS of sycamore is most common in street trees in the southeastern and mid-Atlantic United States. In East Potomac Park, Washington, D.C., 80% of trees were affected by the disease in 2001. BLS has also been detected in sycamore silage plantations and natural stands in the southeastern United States (2,21).
Oak. As mentioned previously, BLS affects many species of oak. Symptoms of BLS on the leaves of northern red oak (Quercus rubra) appear as a pronounced, marginal discoloration with a dull red or yellow halo between scorched and green tissues (Fig. 1). Due to oak’s determinate growth habit, most, if not all, of the leaves on an affected branch will be scorched (Fig. 8). In the early stages of this disease, portions of the tree remain unaffected, while other branches exhibit typical leaf scorch symptoms (13). As the disease progresses, more branches develop symptoms (Fig. 9). Within populations of trees, disease incidence usually appears randomly; trees neighboring severely affected trees are often asymptomatic (Fig. 10).
| |

Fig. 8. Due to the determinate growth habit of oak, most leaves on a branch affected by
Xylella fastidiosa will exhibit scorch. (Courtesy A. B. Gould) | |
| |
 | |
 | |
| |
Fig. 9. As bacterial leaf scorch of oak progresses, more branches develop symptoms. About 60% of the crown of this tree is affected by the disease. (Courtesy A. B. Gould) | |
Fig. 10. Within plantings, incidence of bacterial leaf scorch usually appears randomly; trees neighboring severely affected trees are often not symptomatic. (Courtesy A. B. Gould) | |
Leaf symptoms in pin oak (Q. palustris) are not as distinct (Fig. 11) and can be easily confused with abiotic stresses, but the distribution of the disease within the canopy and among trees is the same as for red oak.
| |

Fig. 11. Bacterial leaf scorch of pin oak (Quercus palustris). Leaf symptoms in pin oak are not as striking as those evident in red oak (Quercus rubra). (Courtesy A. B. Gould) | |
BLS of oak may be confused with oak wilt, another vascular disease. Like BLS, initial symptoms of oak wilt appear as scorched leaves. Unlike BLS, however, scorching appears in spring to early summer, and trees defoliate and die within several months after symptoms appear (33).
BLS of oak is has been reported from New Jersey, as far south as Tallahassee, Florida, the mid-western states, and in Texas. In some New Jersey municipalities, BLS is known to affect up to 35% of oaks planted as street trees and in landscapes (8,21).
BLS Pathogen
Xylella fastidiosa is a Gram-negative, rod-shaped bacterium that lacks flagella for motility and requires oxygen for respiration. The bacterial cells often possess a rippled (undulating) cell wall (Fig. 12) and terminal fimbriae (surface structures that are shorter than flagella and help to anchor the cells together in the xylem stream) (Fig. 13) (39).
X. fastidiosa, as the name suggests, has fastidious nutrient requirements and can be difficult to grow in culture. The bacterium grows slowly on selective medium to form small colonies that appear white to yellow (Fig. 14). The entire genome of a
X. fastidiosa strain isolated from citrus has been recently sequenced, the first plant pathogen for which this has been performed (36). Based on what is known in other microbes, the
X. fastidiosa genome encodes for proteins involved in cell to cell interaction, degradation of plant cell walls, synthesis of toxins, and plant pathogenicity.

A | |

B |
Fig. 12. (A) The undulating cell wall typical of
Xylella fastidiosa. (B) A dividing cell of
Xylella fastidiosa.
(Courtesy J. R. Hartman)
 | |
 |
Fig. 13. Cells of
Xylella fastidiosa from grape. Note the terminal fimbriae (or pili) (arrows). (Photo from
H. Feil, et al., Phytopathology 93:675-682.) |
|
Fig. 14. Colonies of
Xylella fastidiosa from oak on periwinkle wilt agar medium (Courtesy H. Staniszewska) |
Disease Diagnosis
Xylella fastidiosa was not recognized as a pathogen of landscape trees until the early 1980s. Since its symptoms are very similar to those caused by abiotic stresses, it is not surprising that the disease is frequently overlooked or misdiagnosed.
Preliminary diagnosis of BLS is made by interpreting the symptoms described above in late-summer and early fall. Especially useful diagnostic criteria include leaf scorch, premature leaf drop (Fig. 15), the random distribution of affected branches around the canopy, thinning of the crown (Fig. 16), and the random appearance of the disease within populations of trees.
| |
 | |
 | |
| |
Fig. 15. Premature leaf drop of infected oak is common. (Courtesy A. B. Gould) |
|
Fig. 16. A thinning silhouette is a characteristic common to many trees affected by bacterial leaf scorch. (Courtesy A. B. Gould) | |
Definitive diagnosis is most often made in the laboratory using a selective antibody technique known as enzyme-linked immunosorbent assay (ELISA) on symptomatic leaves (Fig. 17). Other techniques used to detect
X. fastidiosa include light and electron microscopy, standard PCR techniques, and immunomagnetic separation followed by PCR (27). Among these methods, ELISA remains the most rapid and cost-effective method to detect the bacterium in symptomatic tissues. The molecular techniques are more sensitive, however, and are helpful for detecting low populations of bacterial cells in infected, asymptomatic hosts or in insect vectors. Due to its fastidious nutritional requirements,
X. fastidiosa is difficult to culture on standard media, and selective media must be used (5,6).
| |

Fig. 17. A microtiter plate used to test plant samples for
Xylella fastidiosa using enzyme-linked immunosorbent assay (ELISA). Each well represents one tree sample; a color change from clear to yellow indicates a test positive for the pathogen. (Courtesy A. B. Gould) | |
Host Specificity
When the bacterium was first described in 1987, 25 bacterial strains isolated from 10 different hosts were included as a single species (39). Strains differ, however, in characteristics such as host range, the ability to cause disease (pathogenicity), nutritional requirements, and genetic homology. In 2004, several new subspecies of
Xylella fastidiosa were proposed based on pathogenicity, phylogenetic characteristics, and DNA relatedness. Placed in the
X. fastidiosa subsp.
multiplex taxon are strains of
X. fastidiosa that affect elm, sycamore, oak (31), and maple (25). Even within the same subspecies, however, differences in host range exist. For example, strains isolated from elm are not pathogenic to sycamore, and vice versa (32).

Fig. 18. The glassy-winged sharpshooter (Homalodisca coagulata), known vector of Pierce’s disease and phony peach disease. | |
Disease Cycle
Xylella fastidiosa is spread primarily by leafhopper insects (subfamily Cicadellidae) known as sharpshooters, and to a lesser extent, spittlebugs (family Cercopidae) (17,28). These insects have piercing-sucking mouthparts and subsist on xylem fluid. Both adult and immature (nymph) stages acquire the bacterium when feeding on succulent tissues of infected hosts. As xylem fluid is drawn into the insect, bacterial cells attach to the cibarial pump and the lining of the esophagus (collectively known as the foregut of the insect). There, the bacterium multiplies and forms a biofilm, where it becomes encased and protected in a bacterial "glycocalyx" (made of polysaccharide and protein), extracting nutrients from xylem fluid as it is pumped through the insect (3).
Once an insect acquires the bacterium, transmission to a new host can begin within 1 to 2 hours. In the early stages of feeding, bacterial cells become dislodged and are pumped directly into the xylem. An adult can transmit the bacterium for the remainder of its life, whereas nymphs, which shed the foregut during molting, can do so only until the next molt (29).
Xylem-feeding insects, particularly leafhoppers, can be polyphagous (i.e., they feed on many different hosts within a single season) (21). Many of the alternative (non economically important) hosts of
X. fastidiosa mentioned earlier serve as a food source for potential leafhopper vectors, and many leafhoppers overwinter as adults on these alternative hosts. Alternative hosts may be the source of a substantial amount of inoculum that is transmitted to economically important hosts by vectors that feed on both types of hosts (30). The insects [e.g., species of the leafhoppers
Graphocephala (Fig. 17),
Homalodisca (Fig. 18), and
Oncometopia] that vector some economically important diseases such as Pierce’s disease of grapevine and phony peach disease are known (Table 4), and their role in the disease process is well characterized (1,37).
Table 4. Some known leafhopper vectors of
Xylella fastidiosa.
|
Host |
Insect vector |
| Citrus (Brazil) |
Acrogonia terminalis
Dilobopterus costalimai
Oncometopia fascialis
Oncometopia nigricans |
| Grape |
Carneocephala fulgida
Draeculacephala minerva
Graphocephala atropunctata
Homalodisca coagulata
Oncometopia nigricans |
| Oleander |
Homalodisca coagulata
Homalodisca lacerta |
| Peach, plum |
Graphocephala versuta
Homalodisca coagulata
Homalodisca insolita
Oncometopia orbona |
Insects that vector BLS in shade trees, however, have yet to be identified, nor is it yet known what role alternative hosts play in the disease process for shade tree hosts. Current research on BLS in oak and other shade tree hosts indicates that several known vectors of other diseases caused by
X. fastidiosa are present in shade trees during the growing season (e.g.,
Graphocephala and
Oncometopia species). Their role in the transmission of BLS has yet to be confirmed.
In some hosts, such as alfalfa, citrus, grape, and peach,
X. fastidiosa is known to spread through root grafts and by budding (12,14,38). In citrus, there is some evidence that the pathogen can colonize fruit tissues and pass from seed to seedling (22). The importance of these methods of transmission in shade trees is not known. Since disease development within populations of trees is most often random, direct tree-to-tree spread is unlikely. It is not known, however, how long urban forest trees remain asymptomatic following infection, thus other methods of transmission may indeed occur.
Mechanism of Disease Development
Xylella fastidiosa lives and multiplies within the tracheary elements, tracheids, vessels, and intercellular spaces of xylem tissue (26) (Fig. 19), and populations of bacterial cells fluctuate seasonally. In hosts where leaf scorch is a primary symptom, such as BLS of shade trees, bacterial populations are greatest in the veins and petioles of symptomatic leaves. In diseases where stunting is a primary symptom, such as phony peach disease and alfalfa dwarf, bacteria congregate in the roots. Compared to phloem, xylem fluid is nutritionally poor but does consist of amino, organic, and inorganic acids (9). These compounds, especially the amino acids glutamine and asparagine, are used by both the bacterium and the insect vector for growth. The quality and composition of xylem fluid varies both between hosts and within a host by season, time of day, and with the health and age of the host plant. This affects the feeding behavior of the vector; insects move from plant to plant to obtain the right combinations of nutrients needed for different stages of growth. Those insects that harbor
X. fastidiosa transmit the pathogen to new hosts as they search for suitable sources of nutrients (21).

A | |

B |
Fig. 19. (A) Cells of
Xylella fastidiosa within the xylem tissue of a pin oak petiole. (B) Note the close-up of a bacterial cell in the bordered pit of a vessel element. (Courtesy J. R. Hartman)
Cells of
X. fastidiosa attach to xylem vessel walls as well as to the foregut of insect vectors by producing biofilms (23) (Fig. 20). Bacterial cells aggregate and are encased in a self-produced matrix of polysaccharide. The biofilm protects the cells and is thought to enhance nutrient uptake and pathogenicity. Terminal fimbriae (also called type IV pili) are important for biofilm formation. In addition, although the bacterium lacks flagella for motility, terminal fimbriae aid in a type of incremental movement called "twitching motility," which enables bacterial cells to move against the xylem stream (24).

A | |

B |
Fig. 20. (A) Biofilm of
Xylella fastidiosa on Chard2 medium and (B) close-up of individual bacterial cells.
(Used by permission of L. Marques; Leite et al.,
Phytopathology 95:963)
Symptoms of water stress (evident as leaf scorch) result from high populations of bacterial cells in xylem tissues as well as overproduction of defense compounds, such as pectins and tyloses, produced by the host plant in response to infection. Embolisms (or air bubbles) eventually form and help to plug affected xylem vessels, leading to reduced xylem function and water stress. When these stresses are prolonged, tissue available for photosynthesis is reduced and starch reserves are depleted, resulting in leaf scorching and premature senescence.
Disease Management
Historically, management of the many diseases caused by
Xylella fastidiosa has encompassed many strategies, such as reducing host stress, planting hosts not known to harbor the bacterium, and removing infected hosts, vectors, and alternative hosts (33). In shade trees, it is not known whether therapeutic pruning (removing infected branches as they become symptomatic) or removing infected trees stops the spread of the disease, both within a tree or within populations of trees. Indeed, the random incidence of BLS within a tree planting leads one to suspect that
X. fastidiosa also does not spread through root grafts, but this method of transmission has yet to be investigated. Management of BLS is further made difficult because it is not known how long hosts may remain asymptomatic prior to the first expression of symptoms.
The use of the antibiotic oxytetracycline to control
X. fastidiosa has been attempted on hosts such as grape, plum, and shade trees. Such trunk injections only provide temporary relief from symptoms, do not work well in trees with advanced disease, and must be repeated annually to be effective. Further studies are needed to determine whether such antibiotic injections are effective long-term or whether these compounds may be phytotoxic to trees treated for long periods. Management of the diseases caused by
X. fastidiosa through vector eradication has been attempted, but even with Pierce’s disease, where disease vectors are known, results are inconclusive. Trials evaluating the systemic insecticide imidacloprid are underway in infected shade trees. This compound is applied in a non-invasive manner to trees as a soil injection and is retained in the canopy for a 3-year period. Ultimately, vector eradication may be useful only to prevent or reduce the rate of spread to adjacent plantings. More novel means for control of
X. fastidiosa in grapes, such as biological control (16) or biofilm disruption, are currently under study, but use of such techniques for BLS of shade trees may not be feasible for years to come.
In summary, there are few cost-effective methods for the management of BLS in landscape plantings. Current management options include:
Maintain plant vigor. The best management tool for this disease is to maintain tree vigor. The development of BLS is enhanced by other diseases, insects, and environmental stresses such as drought. BLS may also predispose infected plants to other disease and insect problems.
Practice sanitation. Branches that have died due to BLS should be routinely removed. Infected trees that are in a severe state of decline should also be removed.
Use tolerant plants. In areas where BLS occurs, avoid planting highly susceptible trees, and design new tree plantings with a diverse complement of tree species. Management of BLS in many regions of the eastern United States may ultimately depend on the identification of germ plasm tolerant to the disease.
| |

Fig. 21. Newton B. Pierce. (Courtesy National Archives.) |
Significance of Xylella fastidiosa and BLS
In 1892, Newton B. Pierce (Fig. 21), a bacteriologist and state plant pathologist of California, examined grapevines (Vitis vinifera) with a scorch and decline of unknown cause. At that time, the problem was known as California vine disease (Table 5). Although the disease was eventually named for him, Pierce could neither isolate nor culture the causal agent, and he ended his career suspecting that a "minute microorganism" was involved (30). About the same period, peach growers in Georgia observed that many trees were stunted ("pony trees") and the fruit they produced was undersized and unmarketable due to a disease now known as "phony peach disease." It would be many years before scientists could prove that the causal agent of these two seemingly unrelated maladies was related.
Table 5. Discovery of the identity of
Xylella fastidiosa.
|
1890s | California vine disease (now known as Pierce’s disease) and phony peach disease observed and studied in different parts of the United States; As Newton B. Pierce states, a "minute microorganism" may be involved. |
1936 to
1959 | Root graft, budding, and/or insect transmission of Pierce’s disease, alfalfa dwarf, and elm scorch; causal agent considered to be a virus. |
|
1971 | Tetracycline antibiotic suppresses development of Pierce’s disease; causal agent now considered to be a mycoplasma-like organism (MLO). |
|
1973 | Electron microscopy reveals a "Rickettsia-like bacterium" in xylem tissues associated with Pierce’s disease and phony peach disease; elm leaf scorch still considered to be caused by a virus. |
|
1978 | Selective media were defined and a bacterium isolated from infected grapevine xylem tissues. |
|
1980 | A xylem-limited bacterium was associated with leaf scorch of elm. |
|
1987 |
Xylella fastidiosa described as a new bacterial species. |
|
2004 | New subspecies for
Xylella fastidiosa described based on pathogenicity, phylogenetic characteristics, and DNA relatedness. |
Clues to the etiology of what would become known as "Xylella-associated diseases" began in 1936 when both Pierce’s disease and alfalfa dwarf were transmitted through root grafts and by insects (14,15,38). Because of these properties, the causal agent was considered to be viral in origin until 1971, when antibiotics applied to grapevines with Pierce’s disease suppressed symptom development (18). Since viruses are not subject to control by antibiotics, the organism was reclassified as a mycoplasma-like organism. In 1973, electron microscopy revealed an organism in the xylem vessels of infected grapevine leaves and peach roots that had a rippled cell wall and could not be cultured on standard culture media. This "rickettsia-like" bacterium was subsequently found in many hosts, and the list of these "Xylella-associated diseases" grew.
Study of these diseases was significantly advanced in 1978 when a culture medium sufficient to isolate and grow the bacterium in vitro was developed (4). In 1987 this organism was properly described as a bacterium closely related to the bacterial plant pathogen
Xanthomonas, and was given the name
Xylella fastidiosa ("Xylella" because it grows in the xylem; "fastidiosa" because the organism is fastidious and difficult to grow in culture) (39).
Xylella fastidiosa is one of several "xylem-limited bacteria." More information on this type of pathogen can be found at the
APSnet Education Center.
Today, diseases caused by
X. fastidiosa continue to increase in importance as entire crops, such as grapes, citrus, and coffee, in certain locations are threatened.
The incidence of BLS in shade trees is equally important from both economic and aesthetic points of view. For example, in some communities in New Jersey (Fig. 22), BLS affects as many as 35% of street and landscape oaks. Current loss of value plus replacement costs for older trees affected by this disease is estimated at $8,000 per tree. Landowners and tree care professionals in these locations must plan for the loss of property values and high costs of replacement as shade trees in landscapes, wood lots, and golf courses affected by BLS decline and must be removed.
| |

Fig. 22. Spread of bacterial leaf scorch of oak in New Jersey from first detection (ca. 1985) to 2005. (Courtesy A. B. Gould) | |
Literature Cited
1. Adlerz, W. C., and Hopkins, D. L. 1979. Natural infectivity of two sharpshooter vectors of Pierce's disease of grape in Florida. J. Econ. Entomol. 72:916-919.
2. Britton, K. O., Leininger, T., and Chang, C. J. 1999. Sycamore dieback in the southeastern United States. Phytopathology 89:S9.
3. Brlansky, R. H., Timmer, L. W., French, W. J., and McCoy, R. E. 1983. Colonization of the sharpshooter vectors,
Oncometopia nigricans and
Homalodisca coagulata, by xylem-limited bacteria. Phytopathology 73:530-535.
4. Davis, M. J., Purcell, A. H., and Thomson, S. V. 1978. Pierce's disease of grapevines: Isolation of the causal bacterium. Science 199:75-77.
5. Davis, M. J., Purcell, A. H., and Thomson, S. V. 1980. Isolation medium for the Pierce's disease bacterium. Phytopathology 70:425-429.
6. Davis, M. J., French, W. J., and Schaad, N. W. 1981. Axenic culture of the bacteria associated with phony disease of peach and plum leaf scald. Curr. Microbiol. 6:309-314.
7. Goodwin, P. H., and Zhang, S. 1997. Distribution of
Xylella fastidiosa in southern Ontario as determined by the polymerase chain reaction. Can. J. Plant Path. 19:13-18.
8. Gould, A. B., Hamilton, G., Vodak, M., Grabosky, J., and Lashomb, J. 2004. Bacterial leaf scorch of oak in New Jersey: Incidence and economic impact. Phytopathology 94:S36.
9. Gould, A. B., French, W. J., Aldrich, J. H., Brodbeck, B. V., Mizell, R. F., III, and Andersen, P. C. 1991. Rootstock influence on occurrence of
Homalodisca coagulata, peach xylem fluid amino acids, and concentrations of
Xylella fastidiosa. Plant Dis. 75:767-770.
10. Hammerschlag, R., Sherald, J., and Kostka, S. 1986. Shade tree leaf scorch. J. Arboric. 12:38-43.
11. Hartman, J. R., and Jarlfors, U. E. 1996. First report of bacterial leaf scorch caused by
Xylella fastidiosa on sugar maple and sweetgum. Plant Dis. 80:1302.
12. He, C. X., Li, W. B., Ayres, A. J., Hartung, J. S., Miranda, V. S., and Teixeira, D. C. 2000. Distribution of
Xylella fastidiosa in citrus rootstocks and transmission of citrus variegated chlorosis between sweet orange plants through natural root grafts. Plant Dis. 84:622-626.
13. Hearon, S. S., Sherald, J. L., and Kostka, S. J. 1980. Association of xylem-limited bacteria with elm, sycamore, and oak leaf scorch. Can. J. Bot. 58:1986-1993.
14. Hewitt, W. B. 1939. A transmissible disease of grapevines. Phytopathology 29:10.
15. Hewitt, W. B., Houston, B. R., Frazier, N. W., and Freitag, J. H. 1946. Leafhopper transmission of the virus causing Pierce's disease of grape and dwarf of alfalfa. Phytopathology 36:117-128.
16. Hopkins, D. 2005. Biological control of Pierce's disease in the vineyard with a benign strain of
Xylella fastidiosa. Phytopathology 95:S44.
17. Hopkins, D. L. 1989.
Xylella fastidiosa: Xylem-limited bacterial pathogen of plants. Annu. Rev. Phytopathol. 27:271-290.
18. Hopkins, D. L., and Mortensen, J. A. 1971. Suppression of Pierce's disease symptoms by tetracycline antibiotics. Plant Dis. Reptr. 55:610-612.
19. Hopkins, D. L., and Adlerz, W. C. 1988. Natural hosts of
Xylella fastidiosa in Florida. Plant Dis. 72:429-431.
20. Kostka, S. J., Tattar, T. A., Sherald, J. L., and Hurtt, S. S. 1986. Mulberry leaf scorch, new disease caused by a fastidious, xylem-inhabiting bacterium. Plant Dis. 70:690-693.
21. Lashomb, J., Iskra, A., Gould, A. B., and Hamilton, G., eds. 2002. Bacterial Leaf Scorch in Amenity trees: A Wide-Spread Problem of Economic Significance to the Urban Forest. USDA Forest Service NA-TP-01-03.
22. Li, W.-B., Pria, W. D., Jr., Lacava, P. M., Qin, X., and Hartung, J. S. 2003. Presence of
Xylella fastidiosa in sweet orange fruit and seeds and its transmission to seedlings. Phytopathology 93:953-958.
23. Marques, L. L. R., Ceri, H., Manfio, G. P., Reid, D. M., and Olson, M. E. 2002. Characterization of biofilm formation by
Xylella fastidiosa in vitro. Plant Dis. 86:633-638.
24. Meng, Y., Li, Y., Galvani, C. D., Hao, G., Turner, J. N., Burr, T. J., and Hoch, H. C. 2005. Upstream migration of
Xylella fastidiosa via pilus-driven twitching motility. J. Bacteriol. 187:5560-5567.
25. Mundell, J. N. 2005. Phylogenetic analysis of Kentucky strains of
Xylella fastidiosa. M.S. Thesis. Department of Plant Pathology, University of Kentucky, Lexington.
26. Nyland, G., Goheen, A. C., Lowe, S. K., and Kirkpatrick, H. C. 1973. The ultrastructure of a rickettsialike organism from a peach tree affected with phony disease. Phytopathology 63:1275-1278.
27. Pooler, M. R., Myung, I. S., Bentz, J., Sherald, J. L., and Hartung, J. S. 1997. Detection of
Xylella fastidiosa in potential insect vectors by immunomagnetic separation and nested polymerase chain reaction. Lett. Appl. Microbiol. 25:123-126.
28. Purcell, A. H. 1979. Leafhopper vectors of xylem-borne plant pathogens. Pages 603-625 in Leafhopper Vectors and Plant Disease Agents, edited by K. Maramorosch and K. F. Harris. Academic Press, New York.
29. Purcell, A. H., Finlay, A. H., and McLean, D. L. 1979. Pierce's disease bacterium: Mechanism of transmission by leafhopper vectors. Science 206:839-841.
30. Raju, B. C., and Wells, J. M. 1986. Diseases caused by fastidious xylem-limited bacteria and strategies for management. Plant Dis. 70:182-186.
31. Schaad, N. W., Postnikova, E., Lacy, G., Fatmi, M. B., and Chang, C.-J. 2004.
Xylella fastidiosa subspecies:
X. fastidiosa subsp.
piercei supsp. nov.,
X. fastidiosa subsp.
multiplex subsp. nov., and
X. fastidiosa spbsp.
pauca subsp. nov. System. Appl. Microbiol. 27:290-300.
32. Sherald, J. L. 1993. Pathogenicity of
Xylella fastidiosa in American elm and failure of reciprocal transmission between strains from elm and sycamore. Plant Dis. 77:190-193.
33. Sherald, J. L. 2001.
Xylella fastidiosa, a bacterial pathogen of landscape trees. Pages 191-202 in Shade Tree Wilt Diseases, edited by C. L. Ash. American Phytopathological Society, St. Paul, MN.
34. Sherald, J. L., Hearon, S. S., Kostka, S. J., and Morgan, D. L. 1983. Sycamore leaf scorch: Culture and pathogenicity of fastidious xylem-limited bacteria from scorch-affected trees. Plant Dis. 67:849-852.
35. Sherald, J. L., Wells, J. M., Hurtt, S. S., and Kostka, S. J. 1987. Association of fastidious, xylem-inhabiting bacteria with leaf scorch in red maple. Plant Dis. 71:930-933.
36. Simpson, A. J. G., and et. al. 2000. The genome sequence of the plant pathogen
Xylella fastidiosa. Nature 406:151-157.
37. Turner, W. F., and H. N. Pollard. 1959. Insect transmission of phony peach disease. USDA. Tech. Bull. 1193.
38. Weimer, J. L. 1936. Alfalfa dwarf, a virus disease transmissible by grafting. J. Agric. Res. 53:333-347.
39. Wells, J. M., Raju, B. C., Hung, H.-Y., Weisburg, W. G., Mandelco-Paul, L., and Brenner, D. J. 1987.
Xylella fastidiosa gen. nov., sp. nov: Gram-negative, xylem-limited, fastidious plant bacteria related to
Xanthomonas spp. Int. J. Syst. Bacteriol. 37:136-143.
