Blaise reaction
Appearance
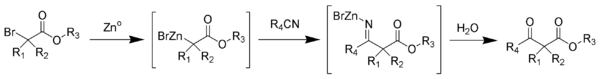
The Blaise reaction is a chemical reaction that forms a β-ketoester from the reaction of zinc metal with a α-bromoester and a nitrile. The final intermediate is a metaloimine, which is hydrolyzed to give the desired β-ketoester.
References
- Blaise, E. E.; Compt. Rend. 1901, 132, 478.
- Cason, J. et al.; J. Org. Chem. 1953, 18, 1594.
- Hannick, S. M.; Kishi, Y.; J. Org. Chem. 1983, 48, 3833.
See also