This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Light-activated ink developed to remotely control cardiac tissue to repair the heart
Researchers from Mass General Brigham and collaborating institutions have developed a non-invasive approach to manipulate cardiac tissue activity by using light to stimulate an innovative ink incorporated into bioprinted tissue. Their goal is to develop a technique that can be used to repair the heart. Their findings in preclinical models, published in Science Advances, show the transformative potential of non-invasive therapeutic methods to control electrically active tissues.
"We showed for the first time that with this optoelectronically active ink, we can print scaffolds that allow remote control of engineered heart tissues," said co-corresponding author Y. Shrike Zhang, Ph.D., of the Division of Engineering in Medicine at Brigham and Women's Hospital, a founding member of the Mass General Brigham health care system. "This approach paves the way for non-invasive light stimulation, tissue regeneration, and host integration capabilities in cardiac therapy and beyond."
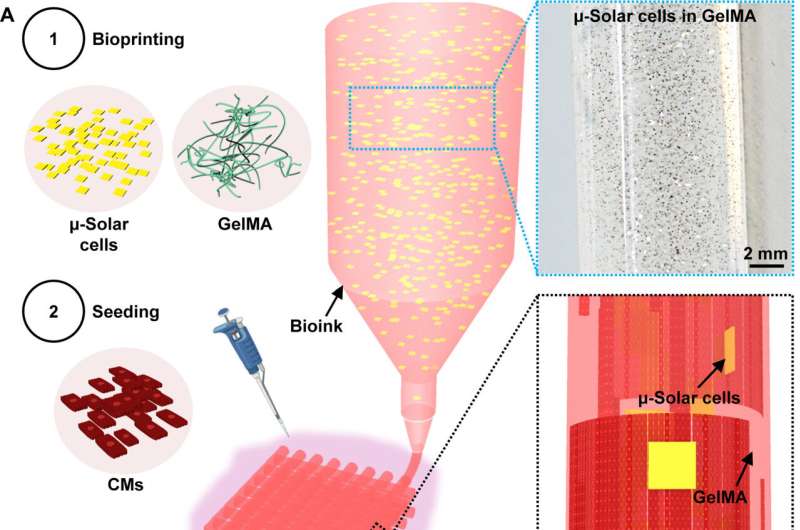
Three-dimensional bioprinted tissues composed of cells and other body-compatible materials are a powerful emerging tool to repair damaged heart tissue. But most bioprinted tissues cannot generate the necessary electrical activity for cellular function. They must instead rely on invasive wire and electrode placement to control heart activity, which can damage body tissues.
Zhang and his colleagues addressed this limitation by infusing the bioprinted tissue with the "optoelectronically active" ink that can be remotely stimulated by light to generate electrical activity in these tissues. The authors also showed that these new, dynamic engineered tissues can synchronize with and accelerate the heart rate when stimulated by light in preclinical models.
"Now that we have established the proof-of-concept for this technology, we are shifting our efforts towards understanding how it might promote long-term tissue regeneration and integrating it seamlessly within the heart's biology," said Zhang.
More information: Faheem Ershad et al, Bioprinted optoelectronically active cardiac tissues, Science Advances (2025). DOI: 10.1126/sciadv.adt7210