Abstract
The extracellular signal-regulated kinase (ERK) is a component of the mitogen-activated protein kinase cascade. Exon 2 of erk2 was deleted by homologous recombination and resulted in embryonic lethality at embryonic day 6.5. erk2 mutant embryos did not form mesoderm and showed increased apoptosis but comparable levels of BrdUrd incorporation, indicating a defect in differentiation. erk2 null embryonic stem (ES) cells exhibited reduced total ERK activity upon serum stimulation, augmented ERK1 phosphorylation, and decreased downstream p90Rsk phosphorylation and activity; yet ES cell proliferation was unaffected. Mutant ES cells were capable of forming mesoderm; however, treatment of mutant ES cells with the mitogen-activated protein kinase kinase inhibitor PD184352 decreased total ERK activity and expression of the mesodermal marker brachyury, suggesting that ERK1 can compensate for ERK2 in vitro. Normal embryos at embryonic day 6.5 expressed activated ERK1/2 in the extraembryonic ectoderm, whereas erk2 mutant embryos had no detectable activated ERK1/2 in this region, suggesting that activated ERK1 was not expressed, and therefore cannot compensate for loss of ERK2 in vivo. These data indicate that ERK2 plays an essential role in mesoderm differentiation during embryonic development.
The extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) cascade is a key signaling pathway controlling cell proliferation, differentiation, and apoptosis. Two members of this pathway, ERK1 and ERK2, share an overall 75% identity at the amino acid level and up to 90% identity at the C terminus (1), present the same in vitro substrate specificity, and are ubiquitously expressed. The classic MAPK cascade involves sequential activation of the serine/threonine kinase Raf, the dual-specificity MAPK kinase (MEK) and the MAPK ERK upon stimulation by growth factors, serum, or phorbol esters. Activated ERK phosphorylates numerous substrates on (S/T) P sites in different cellular compartments (reviewed in ref. 2), leading to increased nucleotide synthesis, activated transcription and translation, and enhanced cell cycle progression (reviewed in refs. 3 and 4). It has been shown that the duration and strength of ERK activation can gate cells to antagonistic fates such as proliferation or differentiation (5), cell cycle entry or G1 arrest (6), and apoptosis or survival (7, 8). Thus, the ERK pathway must be tightly controlled to ensure proper outcome of such integrated biological responses.
The identification of the exclusive MEK1/ERK1 scaffold protein MEK Partner 1 (MP1) indicates that ERK1 and ERK2 likely have different roles (9). This view is supported by genetic ablation studies in mice. erk1-deficient mice are viable and fertile, but have defective thymocyte maturation (10). These mice also have enhanced long-term memory and are able to up-regulate ERK2 signaling in response to glutamate (11). These results suggest that ERK2 cannot compensate for all of the functions mediated by ERK1. ERK2 itself may also possess distinct biological functions in vivo.
To dissect ERK2 functions in vivo, we deleted exon 2 of the erk2 gene by homologous recombination in embryonic stem (ES) cells. The resulting erk2 mutants were embryonic lethal at embryonic day (E) 6.5. There was no evidence of mesoderm formation in mutant embryos. In concert with this finding, sustained activation of ERK was observed during a 5-day in vitro differentiation process of WT ES cells, and the inhibition of ERK activation by the MEK inhibitor PD184352 abolished expression of brachyury, a mesodermal marker. However, erk2–/– ES cells without MEK inhibitor treatment were able to differentiate into mesodermal cells, indicating that although expressed at a lower level, ERK1 can compensate for the loss of ERK2 in vitro. Interestingly, erk2 mutant embryos incorporated BrdUrd, and erk2 null ES cells proliferated at a normal rate even after remaining phosphorylated ERK1 (p-ERK1) was depleted by treatment with PD184352, a finding consistent with previous observations (12). Although previous pharmacological data has shown that ERK1 and ERK2 are important for cell proliferation in various cell types, our results indicated that ERK1 and ERK2 are dispensable for cell proliferation during early embryonic development and in ES cells.
Materials and Methods
ES Cell Lines and Targeting of ERK2. ERK2-specific primers (forward 5′-TTA TTT ACT CTA CAA AGT GAC CAA GC-3′ and reverse 5′-AGG TTC AGC ATC TGC TGC TAC TTT AC-3′) were used to amplify a 400-bp probe from EST 709653 that was used to screen a lambda FixII 129Sv genomic library (Stratagene). A 5.2-kb BamHI/KpnI fragment and a 4.5-kb XbaI/XbaI fragment were cloned into TK2-PKJ1 as 5′ and 3′ arms of homology, respectively, to create the targeting construct. Alternatively, the PGK-neo cassette was removed and replaced with PGK-hygro for targeting the second erk2 allele for the creation of erk2 null ES lines.
The TC1 ES line (gift of Philip Leder, Harvard University, Cambridge, MA) was maintained in ES medium (13) and electroporated with 25 μg of linearized construct. Electroporated cells underwent selection in ES medium supplemented with G418 (Geneticin G418 sulfate, GIBCO) and ganciclovir. Genomic DNA was isolated, digested with AflII, run on a 0.8% agarose gel, and blotted onto nylon membranes. Blots were probed with a 1-kb 3′ mouse erk2 XbaI–EgrI or a neo probe. Hybridization with the 3′ probe resulted in a band of 9.2 kb for the WT erk2 allele and 11.4 kb for the mutated allele. Targeted clones that were identified by Southern hybridization with the 3′ probe were confirmed by Southern hybridization of AflII-digested DNA probed with a neo-specific probe.
Two correctly targeted ES lines were injected into C57BL/6 or BALB/c blastocysts and transferred into Swiss–Webster female mice that had been mated to vasectomized males. Chimeras were generated from BALB/c blastocyst injections and backcrossed onto the BALB/c strain. The Vertex Pharmaceutical Animal Care and Use Committee approved all animal protocols.
Northern Analysis of Mouse Embryonic and Adult Tissues. Mouse embryo and adult tissue blots were purchased from Clontech. Blots were hybridized according to the manufacturer's instructions. An erk2-specific probe of ≈500 bp was generated by digesting EST clone 709653 with NcoI and XhoI. After probing for erk2, the blots were stripped and hybridized with a β-actin probe (Clontech) for normalization.
Histologic and Immunohistochemical Analysis of Embryos. Embryos from erk2 heterozygous intercrosses were fixed overnight in 4% paraformaldehyde in PBS and processed (Leica tissue processor) through alcohols and embedded in paraffin. Sections of 5 μm were cut (Leica RM2135), stained with hematoxylin and eosin, and examined by light microscopy. Alternatively, slides were incubated with antibodies against doubly phosphorylated ERK1/2 (dp-ERK1/2, 1:100, Cell Signaling Technology, Beverly, MA) or subjected to terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining (Serologicals, Clarkston, GA). Secondary reagents and substrate kits were purchased from Vector Laboratories and used according to the manufacturer's instructions.
CD-1 embryos were isolated at E6.5 and E.7.5 and fixed for whole-mount immunohistochemistry in 8% paraformaldehyde overnight at 4°C. Fixed embryos were stained for dp-ERK1/2 according to the Rossant Lab protocol (www.mshri.on.ca/rossant/protocols/DpERK%20Immunohistochem.html). Secondary antibodies and ABC reagent were from the Vector Elite ABC peroxidase kit (Vector Laboratories).
BrdUrd Incorporation in Vivo. Female erk2 heterozygous mice that had been mated to heterozygous erk2 males were checked for the presence of a vaginal plug. Plug date was designated as E0.5. On E6.5, 100 mg/kg of BrdUrd (Zymed) was injected s.c. and allowed to incubate for 2 h. After 2 h, mice were killed, and deciduae were collected and processed for immunohistochemistry with an anti-BrdUrd antibody (Zymed).
Differentiation of ES Cells and Growth Curve. For all cultures, mouse embryonic fibroblast-free ES cells were used. For serum stimulation, ES cells were seeded onto gelatin-treated 60-mm plates at a density of 2 × 106 per plate in ES cell medium. The next day, medium was removed, and cells were washed once with PBS and then fed with ES medium lacking serum. Twenty to 24 h later, cells were treated with ES medium containing 15% FBS. For MEK inhibitor treatment, PD184352 (Calbiochem) was added to serum-free ES medium at a final concentration of 5 μM. ES cells were pretreated with inhibitor for 30 min before a change to ES cell medium with 15% serum and 5 μM PD184352.
For mesodermal differentiation in a monolayer culture, 5 × 104 cells [for basic fibroblast growth factor (bFGF) treatment] or 104 cells (for serum treatment) were seeded on 60-mm gelatin-coated dishes in ES cell medium and incubated overnight for optimum attachment. To induce differentiation, cells were washed twice with chemically defined medium (CDM) (14, 15) and cultured in CDM/5 ng/ml bFGF/5 μg/ml heparin with or without 1 or 5 μM PD184352 or CDM/10% FBS. Cell cultures were maintained for 5 days; media were changed on days 2, 3, and 4.
For the growth curve assay, 2 × 104 cells were seeded in triplicate in 60-mm gelatin-coated plates in 4 ml of ES medium or ES medium with 5 μM PD184352 and cultured overnight. Cell number was counted the next day, which was considered as day 0. Medium was changed, and cell numbers were counted daily from day 2 onward.
Western Blotting. Cells were harvested by scraping in ice-cold 1× cell lysis buffer (Cell Signaling Technology) supplemented immediately before use with 1 mM PMSF. The cell suspension was then subjected to sonication (3 × 5-sec bursts) followed by centrifugation at 14,000 rpm at 4°C for 15 min. The supernatants containing soluble proteins were collected and kept at –80°C until use.
For Western analysis, 20 μg of protein was loaded on each well of a 10% Novex Tris-glycine polyacrylamide gel (Invitrogen) and subjected to electrophoresis at 120 V. Blotting to a nitrocellulose membrane was performed at 35 V for 1.5 h. The membrane was then blocked in TBST (10 mM Tris·HCl, pH 8.0/150 mM NaCl/0.1% Tween 20) supplemented with 5% dry milk for 1 h followed by incubation with a primary antibody at 4°C overnight with gentle rocking. The membrane was then washed 3 × 10 min in TBST and incubated in the secondary antibody (1:3,000, Cell Signaling Technologies) for 1 h. After 3 × 15-min washes, blots were developed in SuperSignal West Dura Extended Duration Substrate (Pierce) solution for 5 min, and images were captured with the Fluor-S MAX MultiImager System (Bio-Rad).
Immunoprecipitation and Kinase Assays. Cell lysates were prepared in 1× cell lysis buffer. dp-ERK1/2 antibody (Cell Signaling Technology) was used for the ERK1/2 assay and anti-Rsk1 antibody (Upstate Biotechnology, Lake Placid, NY) was used for the p90Rsk kinase assay. Briefly, 4 μg of each antibody was mixed with 200 μg of cell lysate and incubated at 4°C overnight, followed by 1-h incubation with 20 μl of 50% protein G agarose beads and precipitation. Pellets were washed three times in 1× cell lysis buffer and twice in 1× kinase buffer (Cell Signaling Technology) and then subjected to kinase reaction by using the p42/44 MAP Assay Kit (Cell Signaling Technology) or the S6 Kinase Assay Kit (Upstate Biotechnology). quantity one software (Bio-Rad) was used to quantitate the phospho-Elk1 signals on the Western blot from the ERK1/2 kinase assay, and a liquid scintillation analyzer (Packard) was used to detect a labeled p90Rsk peptide substrate.
RT-PCR of erk2-Deficient Embryos and ES Lines. RNA was isolated by using the RNeasy mini kit (Qiagen, Chatsworth, CA) and treated with DNaseI (Invitrogen), and RT-PCR was performed with the One-Step RT-PCR kit (Invitrogen) according to the manufacturer's instructions. Gene-specific primers were as published (16).
Results
To determine the role of ERK2 in vivo we deleted the region of erk2 that codes for the TEY activation motif by homologous recombination in ES cells (see Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org). Two targeted ES lines were used for injection into C57BL/6 or BALB/c blastocysts to create chimeras. Both lines generated germ-line chimeras in BALB/c mice and gave similar results. Heterozygous mice were viable, fertile, and phenotypically normal. However, no homozygous mice were recovered among the 181 mice genotyped. Thus, heterozygous mice were mated, and resulting embryos were examined at stages E6.5–E9.5. Approximately 25% of all of the embryos examined exhibited abnormal morphology and were negative for dp-ERK1/2 (see Table 1, which is published as supporting information on the PNAS web site). These presumptive mutant embryos were recovered up to E8.5, but were small and undergoing the process of resorption. In phenotypically normal embryos at E6.5, there was elongation of the egg cylinder, and gastrulation, the generation of the three embryonic germ layers, was clearly confirmed (Fig. 1A). In contrast, erk2 presumptive mutant embryos were small, and there was no evidence of mesoderm formation at E6.5 (Fig. 1B). Visceral endoderm was present, overlying the embryonic ectoderm. By E7.5, gastrulation had occurred in normal embryos and the egg cylinder had elongated (Fig. 1C). At this stage, erk2 presumptive mutant embryos slightly increased in size and their epiblasts appeared disorganized (Fig. 1D). At E8.5, normal embryos had turned and somite and cardiac development was evident (Fig. 1E). erk2 presumptive mutant embryos at E8.5 were disorganized and there was no evidence of differentiation (Fig. 1F). By E9.5, erk2 presumptive mutants were almost completely resorbed. Consistent with the morphological analysis of erk2 presumptive mutants, RT-PCR analysis of embryonic RNA isolated at E7.5 revealed that expression of the mesodermal marker brachyury and the transforming growth factor type β family member nodal, was present in WT, but not detected in erk2 mutant embryos (data not shown). Northern blot analysis with specific probes for erk1 and erk2 revealed that transcripts for both genes were present at all WT embryonic stages (E7, E11, E15, and E17) examined (see Fig. 6, which is published as supporting information on the PNAS web site). Subsequent whole-mount immunohistochemistry using an antibody against activated, dp-ERK1/2 identified a zone of expression in the extraembryonic ectoderm overlying the epiblast at E6.5 (see Fig. 7, which is published as supporting information on the PNAS web site). Expression was also seen in the chorion and parts of the ectoplacental cone. To confirm and examine this staining pattern more closely, paraffin-embedded sections of E6.5 and E7.5 embryos were stained with the antibody against dp-ERK1/2. The zone of expression seen in the extraembryonic ectoderm was also seen in sectioned embryos (Fig. 2A). By E7.5, expression of dp-ERK1/2 was restricted to parts of the chorion and ectoplacental cone (data not shown). In addition, occasional cells or the entire epiblast were stained faintly for dp-ERK1/2. However, erk2 mutant embryos at E6.5 had no detectable activation of dp-ERK1/2 (Fig. 2B), suggesting that dp-ERK2 predominates in the embryo at the time of gastrulation.
Fig. 1.
Histological analysis of normal and presumptive mutant embryos. Hematoxylin and eosin staining of phenotypically normal (A, C, and E) and erk2 presumptive mutant (B, D, and F) embryos between E6.5 and E8.5. The normal embryo elongates and has formed the primitive streak (C, arrow) by E7.5. erk2 presumptive mutants at E7.5 are smaller than normal embryos at E6.5 and E7.5. They increase slightly in size by E7.5 and show no evidence of primitive streak formation (D). At E8.5 normal embryos have turned and cardiac development (arrow) is present (E), whereas presumptive erk2 mutants have not increased in size and appear disorganized (F). Normal embryos have distinguishable neural and cardiac development at E9.5, whereas erk2 presumptive mutants do not increase in size and are resorbed by E9.5 (data not shown). * denotes either + or – for genotype. (Scale bars = 200 μM.)
Fig. 2.
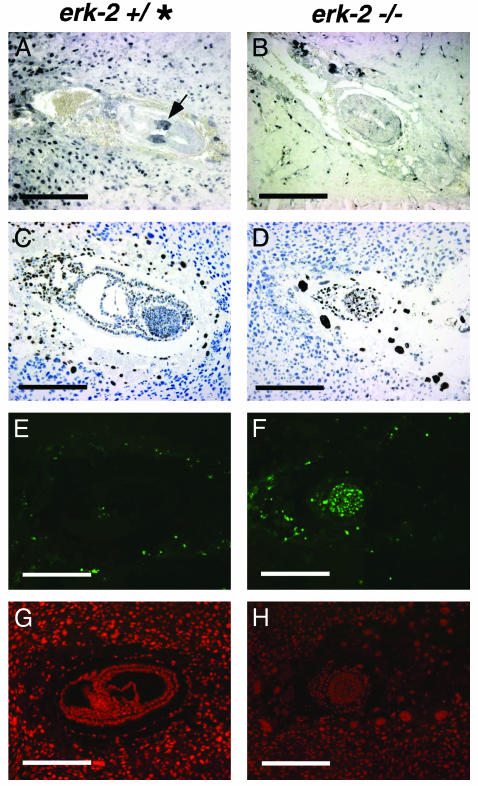
Immunohistochemical analysis of normal and mutant embryos. Activated, dp-ERK1/2 immunohistochemistry of E6.5 phenotypically normal (A) and presumptive mutant (B) embryos. Phenotypically normal embryo has strong dp-ERK1/2 staining in a zone of expression in the proximal extraembryonic ectoderm, whereas presumptive mutant embryo does not have this extraembryonic expression. BrdUrd labeling of E7.5 phenotypically normal (C) and presumptive mutant (D) embryos. Both embryos show incorporation of BrdUrd. TUNEL analysis of phenotypically normal (E) and presumptive mutant (F) E7.5 embryos. High levels of TUNEL staining are present in mutant embryo (F) whereas very low levels of TUNEL staining are found in a phenotypically normal embryo (E). Propidium iodide-counterstained phenotypically normal (G) and presumptive mutant (H) embryos at E7.5. * denotes either + or – for genotype. (Scale bars = 200 μM.)
The embryonic phenotype seen in erk2 mutant embryos could be caused by a defect in either proliferation or differentiation. To examine potential differences in embryonic proliferation, Br-dUrd incorporation into erk2 mutant embryos was assessed by s.c. injection of BrdUrd into pregnant (E6.5 and E7.5) erk2 heterozygous mice. Labeling of embryonic cells was determined by immunohistochemical staining of sectioned embryos with an antibody against BrdUrd. Our results showed that presumptive erk2 mutant and phenotypically normal embryos both incorporated BrdUrd at similar levels, suggesting that there was not a defect in embryonic proliferation (Fig. 2 C and D). Next, we examined apoptosis by performing TUNEL staining of sectioned erk2 presumptive mutant and phenotypically normal embryos (Fig. 2 E and F). Embryos were counterstained with propidium iodide (Fig. 2 G and H). In contrast to phenotypically normal embryos that showed very low levels or no apoptosis, presumptive erk2 mutants stained intensely, suggesting that their decreased size could be attributed to an increase in cell death, possibly because of lack of proper mesoderm induction, a differentiation defect.
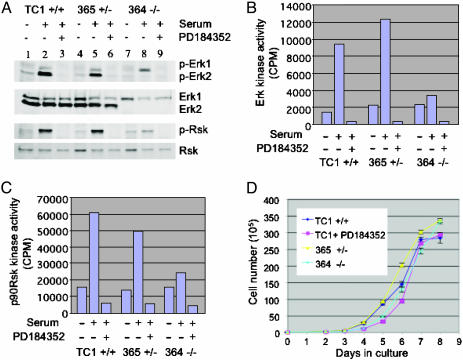
To further characterize the differentiation defect in erk2 mutant embryos, we deleted exon 2 of the remaining WT erk2 locus in heterozygous ES lines by using a targeting construct containing the hygromycin resistance gene (see Fig. 5). Two homozygous mutant ES lines were generated and used for in vitro analysis. Both null ES lines showed similar characteristics; hence, results for one of the null lines, line 364, are described as representative. Homozygous mutant (line 364), heterozygous (line 365), and WT (TC1) ES cell lines were cultured in the absence of serum overnight and then stimulated with 15% serum for 15 min. Total cell lysates were examined by Western analysis for total ERK1/2 and dp-ERK1/2. The mutant ES line, 364, did not have any detectable ERK2 protein (Fig. 3A), and all serum-starved cells had very low levels of dp-ERK1/2. Upon serum stimulation in WT ES cells, both dp-ERK1 and dp-ERK2 were up-regulated, with dp-ERK2 being predominant. As expected, the homozygous mutant ES line did not induce dp-ERK2. However, dp-ERK1 levels in these mutant cells were increased compared with WT and heterozygous ES lines (Fig. 3A). Functional analysis of ERK was conducted by performing an ERK assay. Both serum-stimulated TC1 WT and 365 heterozygous ES cells displayed 4- to 5-fold increased ERK activity when compared with serum-starved ES lines (Fig. 3B). In contrast, line 364 had a baseline level of ERK activity, attributed to ERK1, which was only marginally increased upon serum stimulation.
Fig. 3.
Analysis of erk2-deficient and WT ES cells. (A) ES cells in response to serum stimulation for 15 min in the absence or presence of the PD184352 MEK inhibitor. Western analysis examining ERK and Rsk levels in WT (TC1, lanes 1–3), heterozygous (365, lanes 4–6), and homozygous mutant (364, lanes 7–9). Cells were serum-starved for 20–24 h before serum stimulation. (B) Kinase activity assays for ERK showing relative kinase activity for serum-starved (lanes 1, 4, and 7), serum-stimulated (lanes 2, 5, and 8), or PD184352-treated (lanes 3, 6, and 9). (C) Kinase activity assays for p90Rsk for serum-starved (lanes 1, 4, and 7), serum-stimulated (lanes 2, 5, and 8), or PD184352-treated (lanes 3, 6, and 9) cells. (D) Growth curves for WT, heterozygous, and homozygous mutant ES lines. ES cells were seeded onto gelatin-coated dishes at a density of 1 × 104 per 60-mm dish. The day after plating is day 0. Cells were counted every day over the next 8 days. Cell numbers were plotted as total cell number compared with days in culture. WT (TC1, dark blue line), heterozygous (365, yellow line), homozygous mutant (364, light blue line), and PD184352-treated WT (pink line).
p90Rsk (Rsk1) is a well known downstream substrate for ERKs. Upon serum stimulation, phosphorylation of p90Rsk at Ser-369 (corresponding to Ser-380 of human p90Rsk) was detected in all ES lines examined (Fig. 3A). However, induction of p90Rsk phosphorylation was greatly reduced in the mutant ES cells. A functional analysis, with an in vitro kinase assay, showed a significant increase in p90Rsk kinase activity both in the TC1 and 365 lines upon serum stimulation and a negligible effect in the mutant line (Fig. 3C) over a baseline level of p90Rsk kinase activity. Treatment with the MEK inhibitor PD184352 abolished serum-stimulated dp-ERK1/2 and activity of ERKs and further reduced the basal level of p90Rsk phosphorylation and kinase activity, indicating that in the null ES cell line the augmented ERK1 activity is responsible for the baseline pRsk90 activity. Interestingly, there was no difference in the rate of cell proliferation between WT, heterozygous, and null erk2 ES lines when cells were cultured on gelatinized dishes for 8 days (Fig. 3D). Moreover, the MEK inhibitor did not change the kinetics of ES cell growth. Taken together, these results suggest that ERK activity is not essential for proliferation of progenitor cells in early embryonic development.
Mesodermal differentiation, as measured by expression of brachyury, has been shown in ES cells differentiated for 5 days in CDM supplemented with growth factors, such as bFGF, or serum (14). To assess ERK activation and its signaling during in vitro differentiation, WT (TC1) and erk2 null (364) ES cells were maintained as undifferentiated and exponentially growing or were differentiated for 5 days in CDM with serum or bFGF in the presence or absence of PD184352. Lysates were prepared, and total cellular protein was subjected to Western analysis of ERK, p90Rsk, and pIκBα (Fig. 4A). As a control, we examined the phosphorylation of IκBα on Ser-32 that was equally induced in all lines upon differentiation. In the WT ES cell line, phosphorylation of ERK was induced upon differentiation in the presence of bFGF or serum (Fig. 4A), suggesting a role of ERK in ES cell differentiation. PD184352 inhibited p-ERK induction in a dose-dependent manner in TC1 ES cells grown in CDM supplemented with serum. TC1 ES cells grown in CDM supplemented with bFGF did not survive when treated with 5 μM PD184352; however, these cells did survive in 1 μM PD184352 and induction of p-ERK was inhibited (Fig. 4A). In 364 ES cells, a similar effect on p-ERK induction was observed except that, as expected, p-ERK2 was not present. Interestingly, levels of p-ERK1 in the 364 ES line were consistently higher than p-ERK1 levels in TC1 ES cells, suggesting a compensatory up-regulation of p-ERK1 in response to the loss of p-ERK2.
Fig. 4.
In vitro differentiation of erk2-deficient ES cells. (A) Effects of differentiation on Rsk and ERK phosphorylation in ES cells. ES cells were differentiated in CDM in the presence of serum or bFGF and treated with 1 μM or 5 μM PD184352 or vehicle. Lysates from WT TC1 and 364 homozygous mutant ES cells were analyzed by Western blot for p-ERK1/2 and p-Rsk expression. U, undifferentiated, exponentially growing ES cells. p-ERK1/2 and p-Rsk S369 levels decreased upon treatment with PD184352. (B) WT and homozygous mutant ES lines are capable of forming mesoderm in vitro. ES cells were differentiated for 5 days in CDM supplemented with serum and 0, 1, or 5 μM PD184352. RT-PCR analysis for erk2, T (brachyury), and L7 was performed on WT TC1 and mutant 364 ES lines. TC1 ES cells treated with PD184352 have decreased levels of T after 5 days of differentiation in vitro, whereas 364 mutant cells after 5 days of differentiation in the presence of 1 or 5 μM PD184352 have barely detectable levels of T (B Top). Experiments were done in duplicate.
To investigate ERK signaling in TC1 and 364 ES cells under these differentiation conditions, phosphorylation of p90Rsk at Ser-369 was examined. There was a tight correlation between levels of p-Rsk and p-ERK in both ES cell lines (Fig. 4A). p-Rsk levels were induced significantly upon differentiation in both bFGF and serum containing media. The results shown in Fig. 4A strongly suggested that normal ERK signaling could be maintained in the absence of ERK2 during ES cell differentiation.
In a parallel experiment, we differentiated TC1 or 364 ES cells in CDM in the presence of serum to examine expression of a mesodermal marker. After 5 days of differentiation, RNA was isolated from cultures and RT-PCR was performed by using primers for brachyury, erk2, or the housekeeping marker L7. Both WT and mutant ES lines expressed brachyury, suggesting that erk2 mutant ES lines can differentiate into mesoderm in vitro (Fig. 4B). Because erk2 mutant ES cells still express ERK1, and ERK1 is phosphorylated in these cells in response to serum or differentiation in vitro (Figs. 3A and 4A), it is possible that ERK1 compensates for the loss of ERK2 and transduces signals necessary for mesoderm induction. To eliminate the potential compensation by ERK1 in vitro, mutant ES cells were cultured in the presence of PD184352. PD184352 treatment significantly reduced the amount of phosphorylated ERK in the TC1 and 364 ES cells (Figs. 3A and 4A) and the amount of brachyury mRNA expressed (Fig. 4B). When 364 mutant ES cultures were differentiated in the presence of serum and PD184352, brachyury was barely detectable (Fig. 4B). Interestingly, the treatment of erk2 null ES cells with 1 or 5 μM PD184352 in CDM supplemented with serum resulted in maintenance of a morphologically undifferentiated ES cell phenotype (data not shown). These data are consistent with previous reports indicating that ERK1 compensates for ERK2 in ES cells in vitro and underscore the role of ERK in mesoderm differentiation.
Discussion
The classical MAPK cascade is an essential component of the well characterized FGF signaling pathway for induction of mesoderm in Xenopus embryos. Xenopus explants treated with FGF activate MAPK and the downstream factor p90Rsk (17), and this activation is necessary for induction of mesoderm as shown by expression of mesodermal markers such as Xenopus brachyury (Xbra). In addition, expression of MAPK phosphatase can suppress FGF-induced mesoderm induction (18). Activation of MAPK can also be achieved by constitutively expressing the FGF receptor 1 in explants (19). Conversely, expression of a dominant negative FGF receptor 1 in Xenopus explants and embryos causes defects in gastrulation (20) and inhibits expression of mesodermal markers such as Xbra (21).
The role of MAPK in embryonic proliferation and gastrulation has not been previously studied in mammals. However, knockouts of other ERK pathway members have been performed that have suggested that the MAPK pathway may play a role in mesoderm induction at the time of gastrulation. Serum response factor (SRF), a downstream substrate of p90Rsk, has been targeted by homologous recombination in ES cells (22, 23). SRF mutant embryos die at E6.0 and exhibit decreased proliferation, increased apoptosis, and an inability to form mesoderm (22). When the differentiation of srf null ES cells was examined in vitro, the mutant cells were not able to form mesoderm under most conditions. Treatment of srf null ES cells with retinoic acid allows for a delayed, yet decreased expression of mesodermal markers such as brachyury (23). The described phenotype of ERK2-deficient embryos is very similar to that of embryos lacking SRF. SRF is a MADS box-containing transcription factor activated by phosphorylation. MAPKapk-2, CaM kinase II, and p90Rsk rather than MAPKs are shown to directly phosphorylate SRF (24–26). Given dramatically reduced p90Rsk phosphorylation and activity in ERK2-deficient ES cells, especially upon stimulation, it is reasonable to think that erk2 null embryos have low p90Rsk activity, resulting in diminished SRF transcriptional activity. Collectively, these data strongly suggest a role for the MAPK pathway in mesoderm differentiation during gastrulation in the mouse embryo.
Here, we show that targeted disruption of the erk2 gene results in embryonic lethality at the time of gastrulation caused by a defect in mesodermal differentiation and an increase in apoptosis without affecting proliferation of the epiblast cell population. Mutant ES cells deficient in ERK2 have impaired ERK signaling as shown by reduced levels of activated p90Rsk, but are still capable of mesoderm differentiation in vitro because of residual ERK1 activity. Treatment with the MEK inhibitor PD184352 further reduces ERK activity and results in a lack of mesodermal marker expression. However, ERK1 is not able to rescue the early embryonic phenotype because it is not activated in the mutant embryos (Fig. 2B). It has been previously reported that mice deficient in ERK1 have no obvious developmental defect except thymocyte maturation (10). Although ERK2 is clearly phosphorylated upon anti-CD3 plus phorbol 12-myristate 13-acetate stimulation, proliferation of thymocytes is decreased by 60–70% compared with normal thymocytes, indicating that ERK2 is not able to compensate for the deficiency of ERK1 activity. One explanation for the difference could be the existence of specific scaffold molecules linking ERK1 or ERK2 to distinct sets of downstream substrates. For example, the scaffold protein MP1 only binds to ERK1 and MEK1 and can enhance activation of ERK1 and a reporter driven by the transcription factor Elk1 (9). Perhaps ERK2 is the only MAPK connected to upstream activators and downstream regulators by a yet-unidentified scaffold molecule during mesoderm induction.
Taken together, our data underscore the importance of the classical MAPK pathway in mesoderm induction in mammalian development. Although MAPKs, especially ERK1 and ERK2, are implicated in regulating cell cycle progression, our results indicate that ERK activity is dispensable for proliferation of the stem cell (epiblast) population in embryos and ES cells. It is important to note that those pluripotent stem cells exhibit distinct characteristics in cell cycle, which may be attributed to the dispensability of ERK activity. For example, ES cells have a very short G1 phase (≈1.5 h) and virtually little hypophosphorylated retinoblastoma protein (27). In addition, SRF and related MADS box transcription factors primarily regulated by MAPKs have been implicated in transcriptional regulation of genes expressed in postmitotic cells undergoing differentiation (28). Thus, ERK activity is more important for regulation of genes related to differentiation than to proliferation during early development. The identification and characterization of an ERK2-specific scaffold molecule and the generation of conditional knockout mice for ERK2 would shed more light on physiological roles of ERK2 in mammalian development after gastrulation and potential etiological roles of ERK2 in diseases such as cancer.
Supplementary Material
Acknowledgments
We thank Ms. Lina Du for blastocyst injection, Mr. Mark Sun for help with initial RT-PCR analysis, Ms. Heidi Cyr for animal facility maintenance, and Dr. Philip Leder for the TC1 ES cells.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ERK, extracellular signal-regulated kinase; p-ERK1/2, phosphorylated ERK1/2; dp-ERK1/2, doubly phosphorylated ERK1/2; MAPK, mitogen-activated protein kinase; MEK, MAPK kinase; En, embryonic day n; FGF, fibroblast growth factor; bFGF, basic FGF; CDM, chemically defined medium; SRF, serum response factor; ES, embryonic stem; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
References
- 1.Sugiura, N., Suga, T., Ozeki, Y., Mamiya, G. & Takishima, K. (1997) J. Biol. Chem. 272 21575–21581. [DOI] [PubMed] [Google Scholar]
- 2.Lewis, T. S., Shapiro, P. S. & Ahn, N. G. (1998) Adv. Cancer Res. 74 49–139. [DOI] [PubMed] [Google Scholar]
- 3.Lavoie, J. N., Rivard, N., L'Allemain, G. & Pouyssegur, J. (1996) Prog. Cell Cycle Res. 2, 49–58. [DOI] [PubMed] [Google Scholar]
- 4.Whitmarsh, A. J. & Davis, R. J. (2000) Nature 403 255–256. [DOI] [PubMed] [Google Scholar]
- 5.Traverse, S., Gomez, N., Paterson, H., Marshall, C. & Cohen, P. (1992) Biochem. J. 288 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahan, C., Seuwen, K., Meloche, S. & Pouyssegur, J. (1992) J. Biol. Chem. 267 13369–13375. [PubMed] [Google Scholar]
- 7.Le Gall, M., Chambard, J. C., Breittmayer, J. P., Grall, D., Pouyssegur, J. & Van Obberghen-Schilling, E. (2000) Mol. Biol. Cell 11 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zugasti, O., Rul, W., Roux, P., Peyssonnaux, C., Eychene, A., Franke, T. F., Fort, P. & Hibner, U. (2001) Mol. Cell. Biol. 21 6706–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaeffer, H. J., Catling, A. D., Eblen, S. T., Collier, L. S., Krauss, A. & Weber, M. J. (1998) Science 281 1668–1671. [DOI] [PubMed] [Google Scholar]
- 10.Pages, G., Guerin, S., Grall, D., Bonino, F., Smith, A., Anjuere, F., Auberger, P. & Pouyssegur, J. (1999) Science 286 1374–1377. [DOI] [PubMed] [Google Scholar]
- 11.Mazzucchelli, C., Vantaggiato, C., Ciamei, A., Fasano, S., Pakhotin, P., Krezel, W., Welzl, H., Wolfer, D. P., Pages, G., Valverde, O., et. al. (2002) Neuron 34 807–820. [DOI] [PubMed] [Google Scholar]
- 12.Jirmanova, L., Afanassieff, M., Gobert-Gosse, S., Markossian, S. & Savatier, P. (2002) Oncogene 21 5515–5528. [DOI] [PubMed] [Google Scholar]
- 13.Robertson, E. (1987) in Teratocarcinomas and Embryonic Stem Cells: A Practical Approach, ed. Robertson, E. (IRL, Oxford), pp. 71–112.
- 14.Wiles, M. V. & Johansson, B. M. (1999) Exp. Cell Res. 247 241–248. [DOI] [PubMed] [Google Scholar]
- 15.Proetzel, G. & Wiles, M. (2002) Methods Mol. Biol. 185 17–26. [DOI] [PubMed] [Google Scholar]
- 16.Brown, D., Wagner, D., Li, X., Richardson, J. A. & Olson, E. N. (1999) Development (Cambridge, U.K.) 126 4317–4329. [DOI] [PubMed] [Google Scholar]
- 17.Graves, L. M., Northrop, J. L., Potts, B. C., Krebs, E. G. & Kimelman, D. (1994) Proc. Natl. Acad. Sci. USA 91 1662–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotoh, Y., Masuyama, N., Suzuki, A., Ueno, N. & Nishida, E. (1995) EMBO J. 14 2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umbhauer, M., Penzo-Mendez, A., Clavilier, L., Boucaut, J. & Riou, J. (2000) J. Cell Sci. 113 2865–2875. [DOI] [PubMed] [Google Scholar]
- 20.Amaya, E., Musci, T. J. & Kirschner, M. W. (1991) Cell 66 257–270. [DOI] [PubMed] [Google Scholar]
- 21.Amaya, E., Stein, P. A., Musci, T. J. & Kirschner, M. W. (1993) Development (Cambridge, U.K.) 118 477–487. [DOI] [PubMed] [Google Scholar]
- 22.Arsenian, S., Weinhold, B., Oelgeschlager, M., Ruther, U. & Nordheim, A. (1998) EMBO J. 17 6289–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinhold, B., Schratt, G., Arsenian, S., Berger, J., Kamino, K., Schwarz, H., Ruther, U. & Nordheim, A. (2000) EMBO J. 19 5835–5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fluck, M., Booth, F. W. & Waxham, M. N. (2000) Biochem. Biophys. Res. Commun. 270 488–494. [DOI] [PubMed] [Google Scholar]
- 25.Heidenreich, O., Neininger, A., Schratt, G., Zinck, R., Cahill, M. A., Engel, K., Kotlyarov, A., Kraft, R., Kostka, S., Gaestel, M. & Nordheim, A. (1999) J. Biol. Chem. 274 14434–14443. [DOI] [PubMed] [Google Scholar]
- 26.Rivera, V. M., Miranti, C. K., Misra, R. P., Ginty, D. D., Chen, R. H., Blenis, J. & Greenberg, M. E. (1993) Mol. Cell. Biol. 3 6260–6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savatier, P., Huang, S., Szekely, L., Wiman, K. G. & Samarut, J. (1994) Oncogene 9 809–818. [PubMed] [Google Scholar]
- 28.Ghosh, A. & Greenberg, M. E. (1995) Science 266 239–247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.