Abstract
Rationale
Cocaine strengthens behaviors associated with its administration. The stress response by individuals that are defeated in a brief aggressive confrontation can also promote enduring behavioral consequences similar to those of stimulants.
Objectives
The study intends to find whether intermittent episodes of defeat promote cocaine's reinforcing effects by triggering N-methyl-D-aspartic acid (NMDA)-receptor-mediated plasticity in the ventral tegmental area (VTA).
Materials and methods
Long-Evans rats were investigated after four social defeats in three experiments. Two experiments examined systemic or intra-VTA antagonism of NMDA receptors during stress on the later expression of behavioral sensitization and cocaine self-administration during fixed and progressive ratio (PR) schedules of reinforcement (0.3 mg/kg/infusion), including a novel 24-h variable-dose continuous access binge (0.2, 0.4, and 0.8 mg/kg/infusion, delivered in an irregular sequence). Third, the expression of receptor proteins NR1 (NMDA) and GluR1 [alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)] were examined in VTA and nucleus accumbens.
Results
Intermittent defeats augment locomotor responses to cocaine and increase cocaine taking. Rates of responding during binges are increased after defeat stress. These effects are prevented when NMDA or AMPA receptor antagonists are administered before defeats. VTA infusions of the NMDA antagonist AP-5 (5 nmol/side) before stress prevents locomotor sensitization to cocaine and intensified responding for cocaine during a PR schedule or binge. Episodic defeats increase GluR1 AMPA subunit protein expression in the VTA.
Conclusions
Social stress stimulates NMDA receptors in the VTA, and this neural action of defeat may be essential for prompting a later increase in cocaine intake during binges.
Keywords: Cocaine, Self-administration, Ventral tegmental area, Glutamate, NMDA-AMPA, Progressive ratio schedule, Binge
Introduction
Many kinds of stressors, ostensibly aversive in nature, potently activate the mesocorticolimbic dopamine (DA) system (Abercrombie et al. 1989; Kalivas and Duffy 1995), and this activation shares similarities with drugs of abuse (Hurd et al. 1988; Hernandez and Hoebel 1988; Tidey and Miczek 1996, 1997). Intermittent exposures to threatening stimuli also augment behavioral responses to psychomotor stimulants for long periods of time (Marinelli et al. 1996; Prasad et al. 1998; Covington and Miczek 2005; but see Yap and Miczek 2007). Neuronal plasticity of the mesocorticolimbic DA system in response to brief aversive stressors provides a potential basis for elucidating where the convergence of diverse stimuli promotes the emergence of “escalated” cocaine taking (Goeders 2002; Marinelli and Piazza 2002).
Social defeat stress induces long-lasting adaptations in the VTA as measured by the expression of immediate early genes (Nikulina et al. 1998, 2004, 2005; Covington et al. 2005). Behaviorally, intermittent episodes of social defeat or amphetamine, and this particular exposure to social defeat also intensifies cocaine self-administration behavior during a progressive ratio schedule, and during conditions of extended binge-like access (Covington and Miczek 2001). The induction of locomotor sensitization to repeated psychomotor stimulant and opiate administrations depends on functional N-methyl-D-aspartic acid (NMDA) and AMPA receptors located on DA neurons in the ventral tegmental area (VTA; Karler et al. 1989; Zhang et al. 1997; for reviews see Wolf 1998; Vanderschuren and Kalivas 2000). The aims of the current study are based on the hypothesis that intermittent episodes of social defeat activate ionotropic glutamate receptors in the VTA, and this activation is necessary for the development of increased behavioral responses to cocaine. As revealed pharmacologically by the use of selective NMDA receptor antagonists, VTA NMDA receptor activation is frequently reported to be an essential process for many forms of behavioral adaptations to occur (Kalivas and Alesdatter 1993; Suto et al. 2003). Thus, the first specific aim of the current study was to further characterize the contribution of NMDA receptors (via systemic administration of the NMDA antagonist MK-801) and the specific role of VTA NMDA receptors (via direct infusions of AP-5) during episodes of social defeat stress on subsequent locomotor responses to cocaine. The systemic administration of NBQX was also examined for the possibility that AMPA receptors also mediate the effect of social stress on later behavioral responses to a cocaine challenge. Prolonged responding for intravenous (IV) cocaine infusions, in socially defeated rats, during 24 h of continuous access meets several criteria for out-of-control drug taking, most prominently after ca 16 h of cocaine availability, when the pattern of well-regulated cocaine self-administrations undergoes the transition to dysregulated (Kreek and Koob 1998; Tornatzky and Miczek 2000). In the phase of dysregulation, most animals cease self-administration behavior for an extended period of time (i.e., hours), whereas defeated rats continue beyond the routinely observed onset of this phase (Tornatzky and Miczek 2000; Covington and Miczek 2001). Protocols of self-administered cocaine binges have begun to identify the conditions that promote the transition from behavior that is maintained by circadian variables to compulsive behavior (Fowler et al. 2007). The second specific aim of the present study was to examine the role of VTA NMDA receptors during social defeat on intensified cocaine taking. Rats that were pretreated in the first experiment with either systemic, or intra-VTA NDMA receptor antagonists during social defeats were additionally studied in a second series of experiments allowing for IV access to cocaine self-administration. Specifically, each experimental group of rats was observed during a progressive ratio schedule of cocaine infusion deliveries, and during a novel 24-h binge protocol that utilizes several unit doses of cocaine within a 24-h interval of unlimited access (Gerber and Wise 1989), to capture features of the “dysregulated” component of cocaine taking in previously defeated rats.
The AMPA receptor subunit GluR1 and the NMDA receptor subunit NR1 are dynamically regulated in the VTA after intermittent exposures to psychomotor stimulants and environmental stressors (Fitzgerald et al. 1996). Therefore, a third experiment examined if changes in GluR1 or NR1 protein in the VTA or nucleus accumbens (NAc) are evident after episodic social defeats. Overall, if brief exposures to social defeat promote behavioral cross-sensitization to cocaine and strengthen self-administration of cocaine, in an NMDA dependent manner, then these findings would highlight the importance of glutamate-mediated plasticity due to stress experiences and provide insight into the paradoxical likeness of this aversive stimulus to positive reinforcers.
Materials and methods
Subjects
Male Long-Evans rats (Charles River Laboratories, Wilmington, MA) weighing 225-250 g were individually housed in custom-built acrylic chambers (30×30.5×24.5 cm) on arrival. Removable panels on each wall of the home cage were fitted with wire mesh (0.5 cm2 mesh holes). The floor of each cage was lined with Cellu-Dri™ pellet bedding (Shepherd Specialty Papers, Kalamazoo, MI) and maintained on a reversed light/dark cycle (lights on 2000-0800 hours), controlled temperature (21° C) and humidity (35-40%) with unlimited access to food (Purina laboratory chow) and water. Housing conditions remained consistent throughout all phases of each experiment (i.e., during the interval of social defeat stress, during cocaine challenge injections, and during intravenous (IV) self-administration of cocaine). All experimental procedures were approved by the Tufts Institutional Animal Care and Use Committee, following the principles of the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Social-defeat stress protocol
For all three social-defeat experiments, rats were first randomly assigned to two groups, defeat stressed or non-stressed controls, upon arrival to the laboratory. Subsequently, experimentally stressed and non-stressed control rats were assigned to pharmacological treatment conditions (see Fig. 1 for an outline of group assignments and experimental timelines). Experimentally stressed rats were submitted to a social defeat episode as described previously (Tornatzky and Miczek 1993; Covington and Miczek 2001) on days 1, 4, 7, and 10. Specifically, each episode of social defeat took place in an adjacent room to the housing location, while control animals remained in their home cages in the vivarium. Before each defeat, rats in the experimental and control groups were weighed and administered their assigned glutamate receptor antagonist, or saline. Fifteen minutes later, experimentally stressed rats (while remaining in their “protective” home cage) were placed inside a larger home cage of an aggressive “resident” male rat. After 10 min of social “threat” behind the shielding confines of the experimentally stressed rat's home cage, the experimental intruder was removed from its protective home cage, and an attack by the resident was permitted to occur. Defeat was defined as the display of 5 s of supine posture, typically in reaction to approximately four attack flurries by the resident, within maximally 5 min. Immediately after each defeat, the experimental rat was again placed back in its own protective home cage that was once again relocated inside the larger resident's cage, to expose it to the resident's threats for an additional 10 min. At the completion of the final phase of social threat, each experimentally stressed rat (while remaining in its home cage) was returned to the vivarium. Each experimental intruder was exposed to a different resident over the course of defeats. During each defeat episode, the attack latency, number of bites, and duration of each aggressive encounter were recorded.
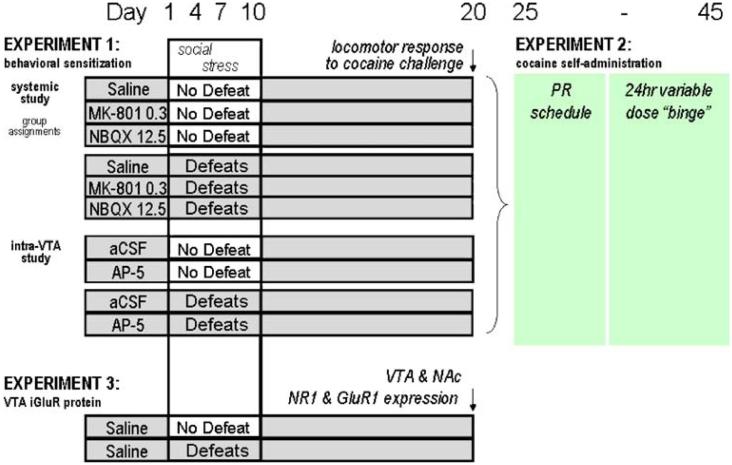
Fig. 1.
Separate groups of rats experienced four brief episodes of social defeat stress on experimental days 1, 4, 7, and 10 (once every 72 h). In the first experiment, one group of rats was intermittently stressed 15 min after receiving an injection of either saline, dizocilpine “MK-801” (0.3 mg/kg), or NBQX (12.5 mg/kg), and three similar groups of rats served as contemporary non-stressed controls (n=10/group). In addition, separate groups of rats were either handled or also socially defeated on days 1, 4, 7, and 10 with an infusion of AP-5 or aCSF just before each defeat. Ten days after the last defeat, all groups of rats were challenged with cocaine (10 mg/kg), and locomotor behavior was assessed in the home cage. In a continuation of experiment I, these same groups of rats were surgically implanted with intravenous catheters and examined for cocaine self-administration behavior in experiment II to observe their performance during a progressive ratio schedule of cocaine reinforcements and during a novel 24-h variable dose cocaine “binge.” Experiment III was conducted to examine the impact of the social defeat stress protocol on GluR1 and NR1 protein expression in the VTA and NAc. The assessments of dependent measures are indicated in italics
Residents
A separate group of 18 500-700 g male Long-Evans rats (Charles River Laboratories, Wilmington, MA) were housed with females in large stainless steel cages (45.7×71.1×45.7 cm) and served as aggressive stimulus rats (residents). Each resident was selected based on its consistent display of aggressive behavior during the assessment of regularly scheduled confrontations with an undefeated intruder rat (Miczek 1979).
Cocaine challenge: assessment of behavioral sensitization
Ten days after the fourth social defeat stress episode (i.e., experimental day 20), experimental and control rats were given a challenge injection of cocaine (10 mg/kg, i.p.), to assess behavioral cross-sensitization. While remaining in their home cage, each rat was moved into an adjacent procedure room and briefly removed from its cage to be weighed and injected with saline. After the saline injection, rats were placed back into their home cages, and video recordings monitored behavior for 15 min. Both groups were then injected with cocaine (10 mg/kg, i.p.) and behavioral samples were collected for 30 min.
A trained observer analyzed each video record using a custom keyboard and commercial software (The Observer Video-Pro© version 5.0, Noldus Information Technology, Wageningen, The Netherlands). The frequency and duration of rearing, walking, sniffing, grooming, digging, and inactivity were recorded. Five-minute samples were analyzed 5-10 min after saline injection (baseline) and 5-10 and 25-30 min after the cocaine challenge.
Surgical procedures
Intra-VTA cannula placement
The competitive NMDA receptor antagonist, AP-5, was infused directly into the VTA before social defeats, closely adhering to an intra-VTA infusion protocol reported previously that established a decisive role for VTA NMDA receptors during the emergence of amphetamine-induced behavioral responses in the rat (Vezina and Queen 2000). Rats examined for NMDA receptor activation in the VTA during social stress were surgically prepared for the delivery of AP-5 or aCSF (vehicle) microinjections. While under ketamine (100 mg/kg) and xylazine (6 mg/kg) anesthesia, rats were surgically implanted with bilateral guide cannulae (26 gauge stainless steel, 14 mm) aimed 1 mm above the VTA (AP -5.4; ML +1.6; DV -7.4, at a 14° angle relative to bregma (Paxinos and Watson 1997). Cannulae were cemented in place by affixing dental acrylic to three stainless steel screws anchored into the skull. Patency of the cannulae was maintained by the insertion of 15 mm obdurators (33 gauge) before and after microinfusions. Each infusion of AP-5 (5 nmol) or vehicle (aCSF) was delivered over 3 min in a volume of 0.5 μl using a CMA/100 microinfusion pump (CMA Microdialysis, Sweden). After each infusion, the injector cannula was left in place for one additional minute to allow for diffusion and prevent backflow. Intracerebral infusions were administered immediately before each of the four stress episodes (i.e., once every 72 h) or once every 72 h in the absence of social stress (as a control). After the completion of cocaine self-administration experiments, each cannula placement was verified by histological procedures. Specifically, 50-μm sections were mounted on slides that were subsequently stained with cresyl violet.
IV Catheter Surgery
Rats examined during cocaine self-administration studies were permanently implanted with indwelling catheters (Silastic® silicon tubing, ID 0.63 mm, OD 1.17 mm) into the right jugular vein (Covington and Miczek 2001; Remie et al. 1990) under a combination of ketamine (100 mg/kg) and xylazine (6 mg/kg) anesthesia. The catheter was passed subcutaneously to the rat's back where it exited through a small incision and affixed to a plastic pedestal (Plastics One, Roanoke, VA) mounted inside a harness system (Instech Laboratories, Plymouth Meeting, PA). After catheter surgery, each rat was allowed to recover for 5 days, and only body weight measurements were collected during this time.
Cocaine self-administration
After recovering from catheter surgery, rats remained in their home cage that was moved into a procedure room equipped for IV self-administration experiments (Miczek and Mutschler 1996). Catheters were flushed with heparinized saline (20 IU/ml) each morning, and during the light phase, 0.17 ml pulses of saline were delivered every 30 min.
Acquisition protocol
Initially, all rats were allowed to self-administer cocaine (0.75 mg/kg/infusion), without a priming infusion, and each lever press was reinforced with an IV infusion (fixed ratio; FR 1 schedule of reinforcement) followed by a 30-s time-out. Each daily session terminated after the delivery of 15 infusions or after 5 h of access. After reliable self-administration behavior was verified (two consecutive days of 15 infusions), the FR schedule progressively increased to every fifth response being reinforced (FR 5), over three to five additional days. Rats were maintained on an FR 5 schedule of limited access for at least five consecutive days (i.e., 15 infusions/day).
Progressive ratio schedule protocol
The equation for delivering each successive cocaine infusion was derived to escalate the response demand requirement quickly enough so that the rat would extinguish responding within 5 h (Richardson and Roberts 1996). The ratios are as follows, 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178,…, 402, 492, 603.... The session terminated when no cocaine infusion was delivered within a 60-min interval. The “breaking point” was defined by last completed ratio achieved. The average number of completed infusions for each group of rats was calculated as the dependent measure. Daily PR sessions alternated with daily maintenance sessions (i.e., 15 infusions, FR5, 0.75 mg/kg/infusion).
Twenty-four-hour binge protocol
During the binge, a variable dose protocol (Gerber and Wise 1989) was implemented throughout it to detect slight changes in each rat's controllability over cocaine's reinforcing effect, operationally defined by the consistency of timing the post-infusion interval that follows the delivery of each dose. A prolonged variable dose access protocol to three unit doses of cocaine (0.2, 0.4, 0.8 mg/kg/infusion) was implemented for a period of 24 h starting between 0900 and 1000 hours (ca the second hour after the start of the dark phase). Each dose of cocaine was delivered at random in separate blocks of 15 total infusions (five infusions at each dose), to ensure that the maximum number of possible repetitions of a single dose was limited to five. The dose of cocaine administered was adjusted according to the duration of the infusion because the concentration of cocaine solution remained constant. The amount of cocaine administered, number of infusions, post-infusion intervals for each unit dose, and patterns of responding were calculated for dependent measures.
GluR1 and NR1 protein expression
Immunoblot analysis
Immunoblots were processed as previously described in Rajadhyaksha et al. 2004. Briefly, brains were sectioned in a cryostat to the rostral end of the NAc (shell and core; spanning 1.2 to 1.7 mm relative to the bregma; Paxinos and Watson 1997) and a 1-mm bilateral punch was obtained with an 18-gauge stainless steel stylet. The same brain was sectioned to the rostral end of the VTA. A 1-mm midline punch of the VTA (spanning -5.20 to -6.04 mm relative to bregma; Paxinos and Watson 1997) was obtained with a 20-gauge stylet. Tissue samples were sonicated in sodium dodecyl sulfate (SDS) sample buffer (1% SDS in Tris-EDTA, pH 7.4) and protein concentration determined by the BCA Assay (Pierce, Rockford, IL). Two (to detect GluR1) or 10 μg (to detect NR1) of protein was loaded on a 10% SDS-polyacrylamide gel. Top halves of the blots were probed with 1:1,000 anti-rabbit GluR1 antibody (Upstate Biotechnology, Lake Placid, NY) or 1:1,000 anti-mouse NR1 antibody (Chemicon, Temecula, CA). To ensure equal protein loading, bottom halves were probed with 1:20,000 actin antibody (Chemicon). GluR1 blots were incubated with 1:10,000 goat anti-rabbit, and NR1 and actin blots with horse anti-mouse horseradish peroxidase-linked IgG. Protein bands were visualized by chemiluminescence. Kaleidoscope prestained standards (Bio-Rad) were used for protein size determination. GluR1 was detected at 108 kDa, NR1 at 120 kDa, and actin at 42 kDa. For quantitation the blots were scanned with an HP Scanjet 7,400 C scanner (Hewlett Packard, Palo Alto, CA). Intensity of the protein bands was measured as optical density, using the NIH Image program (National Institutes of Health, Bethesda, MD).
Experimental procedures
Experiment I. Intermittent social defeat: behavioral cross-sensitization to cocaine and the role of VTA IGluRs
Sixty rats were randomly distributed across two initial groups, defeat stressed (n=30), and non-defeated “handled” controls (n=30). Subsequently, experimentally stressed and control rats were assigned to one of three pharmacological treatment conditions (n=10/group): the NMDA receptor antagonist dizocilpine (0.3 mg/kg); the AMPA receptor antagonist NBQX (12.5 mg/kg); or the vehicle saline. Separate groups of non-stressed control rats received either dizocilpine (0.3 mg/kg), NBQX (12.5 mg/kg), or saline.
Forty-four additional rats were assigned to one of four treatment conditions to examine the specific contribution of VTA NMDA receptors during episodes of social defeat stress on subsequent behavioral responses to cocaine. Two groups of defeated rats were distributed across one of two prestress treatment conditions, including: VTA microinjections of AP-5 (n=10), or aCSF (n=12) as a control. Likewise, two groups of non-stressed control rats were divided across the two infusion conditions and surgically prepared to allow for local microinfusions of AP-5 (n=11) or aCSF (n=11).
All socially defeated rats (i.e., in both “the systemic study,” and “the intra-VTA study”) were submitted to a social defeat episode on days 1, 4, 7, and 10 (see Fig. 1 for an outline of experimental groups). On experimental day 20 (i.e., 10 days after the last social defeat episode or cocaine injection), all rats were administered the cocaine challenge (10 mg/kg, i.p.), and behavior was observed via video recordings in the home cage environment to examine the expression of behavioral sensitization to cocaine's psychomotor stimulant effects.
Experiment II. Intermittent social defeat: intense cocaine taking and the role of VTA NMDA receptors
In a progression from Experiment I, rats receiving four intermittent episodes of defeat stress were further examined for their intravenous cocaine self-administration behavior. Specifically, experimentally defeated rats comprising the systemic NMDA antagonist condition (0 or 0.3 mg/kg dizocilpine; n=10/group), and the two groups of non-defeated controls (0 or 0.3 mg/kg dizocilpine) were selected for examining NMDA receptor activation during each stress episode on subsequent cocaine taking. Also, to examine the specific role of NMDA receptor activation in the VTA during episodes of social defeat on cocaine taking, all 44 rats examined in experiment I that were administered VTA microinjections of AP-5, or alternatively aCSF, were also examined during intravenous cocaine self-administration.
Rats included in experiment II were implanted with IV catheters the day after assessing their expression of sensitization to a cocaine challenge. After the initial period of acquisition and maintenance, rats were examined during a PR schedule of cocaine reinforcement (0.3 mg/kg/infusion) every other day, with limited daily access on alternating days, until three PR sessions were completed. After assessing PR performance, the 24-h variable dose access binge was allowed to occur for each rat.
Experiment III. Intermittent social defeat: effects on VTA GluR1 protein expression
As in Experiments I and II, experimentally stressed rats were submitted to a social defeat episode on days 1, 4, 7, and 10. Ten days after the last social defeat episode (i.e., experimental day 20), experimentally stressed and non-stressed controls were rapidly decapitated, their brains were quickly removed (<45 s), and this tissue was immediately frozen in isopentane (-50°C), before being stored (-80°C) until immunoblots were performed. Western blots for GluR1 (n=4/group) and NR1 (n=7/group) were conducted on frozen tissue removed from the area of the VTA or the NAc for each brain by a trained experimenter, blind to experimental conditions, at Harvard Medical School, M.G.H. Charlestown Campus.
Statistics
Experiment I
Across the six groups of systemically treated defeated and non-defeated rats, the frequency/ min of each behavior in response to a cocaine challenge (10 mg/kg) was analyzed by a twoway between group analysis of variance (stress × drug treatment). Likewise, a twoway analysis of variance (ANOVA) was used to analyze walking behavior in response to a cocaine challenge (10 mg/kg) across groups of defeated and non-defeated rats receiving intra-VTA infusions of AP-5 or aCSF. Bonferroni post hoc analyses of all pair-wise comparisons were made for each study.
Experiment II
PR data obtained during IV self-administration were analyzed according to the average number of accumulated reinforcements over each trial. The average number of infusions delivered (corresponding to a specific ratio criterion) for all rats in the systemic study and the intra-VTA study were averaged for each group and compared to all other groups by two-way ANOVA (stress × drug treatment). Similarly, total binge intake (expressed as mg/kg) for each rat contributed to the group's average and was compared across groups by two-way ANOVA. Bonferroni post hoc analyses for all pair-wise comparisons were made for PR and binge intake data. The average post-infusion interval for each the three doses of cocaine delivered (i.e., 0.2, 0.4, and 0.8 mg/kg/infusion) was also averaged for each rat across the 24-h binge and then used to determine the group's average post-infusion interval for each dose of cocaine self-administered throughout the entire binge (any post-infusion interval that exceeded 30 min was not used in this average because this value exceeds the average post-infusion interval by at least 2 SDs for any given IV dose).
Experiment III
For immunoblot analyses, optical density values were analyzed by one-way ANOVA with all pair-wise post hoc comparisons made (Holm-Sidak method) between treatment groups.
Drug solutions
Cocaine hydrochloride was obtained from the Research Technology Branch of the National Institute on Drug Abuse (Rockville, MD) and was dissolved in sterile 0.9% saline. Dizocilpine and NBQX were purchased from Sigma-Aldrich (St. Louis, Mo, USA) and also dissolved in 0.9% saline. AP-5 was purchased from Sigma-Aldrich and dissolved in artificial cerebrospinal fluid (aCSF; 147 mM NaCl, 1.3 mM anhydrous CaCl2, 0.9 mM anhydrous MgCl2, 4.0 mM KCl in 250 ml, pH=6.7-7).
Results
Experiment I. Cocaine challenge
On experimental day 20 (i.e., 10 days after social defeat stress) no significant differences were found between groups with regard to the frequency of any behavior recorded after an initial saline challenge. However, the cocaine challenge increased locomotor activity in only those defeated rats given saline injections before each defeat (See Fig. 2a). After cocaine injection, a main effect of stress was found on the frequency of walking behavior in episodically stressed rats when compared to non-stressed controls (F1,54=16.8, p=0.0001), and a significant interaction between stress and drug treatment was also observed (F2,54=8.2, p=0.0008). Infusions of AP-5 into the VTA immediately before being socially defeated also reduced the later behavioral response to a cocaine challenge compared to aCSF (See Fig. 2b). As revealed in the frequency of walking, a significant main effect of stress (F1,32=18.7, p=0.0001) and an interaction between stress and the VTA infusion was observed (F1,32=9.2, p=0.004).
Fig. 2.
Effects of systemically administered glutamate receptor antagonists during episodic social defeat stress on the later behavioral response to cocaine. a portrays the mean frequency of walking behavior/min (±SEM) after a 10-mg/kg i.p. cocaine challenge for rats with or without prior defeat stress. The open bars represent non-defeated and defeated rats that were injected with saline during the social defeat phase of the experiment, the gray bars represent rats that received 0.3 mg/kg dizocilpine treatment, and the dark gray bars represent rats that received 12.5 mg/kg NBQX treatment. b portrays rats that received aCSF infusions (open bars) during the social defeat phase of the experiment, and the gray bars represent rats that received AP5 (5 nmol). The asterisks indicate groups of defeated rats that were significantly different from the non-defeated control's vehicle condition at the p<0.05 level
Experiment II. Cocaine self-administration
Progressive ratio schedule of cocaine reinforcement
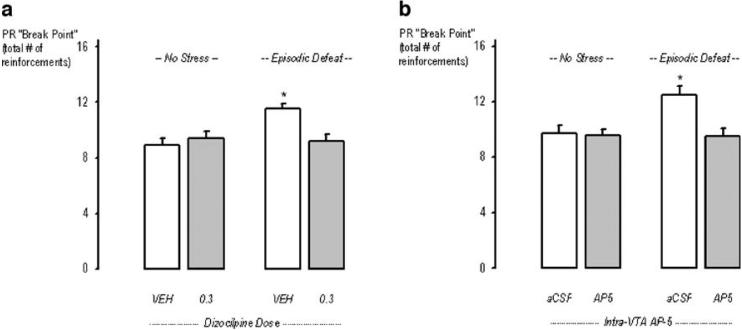
The “breaking point” of cocaine intake was significantly increased by social defeat stress in rats that received systemic administrations of saline before each stress episode. Specifically, across dizocilpine- and saline-treated defeated or control rats, a main effect of stress (F1,36=6.1, p=0.02) and an interaction between stress and drug treatment (F1,36=9.1, p=0.005) was observed for the averaged completed ratio (See Fig. 3a).
Fig. 3.
Effects of systemically administered glutamate receptor antagonists during episodic social defeat stress on subsequent cocaine self-administration: the “breaking point.” a portrays the mean (±SEM) total number of cocaine infusions achieved on a PR schedule by non-defeated rats (left bars) and episodically defeated rats (right bars). The open bars represent rats that received saline injections during the social defeat phase of the experiment and the gray bars represent rats that received 0.3 mg/kg dizocilpine. b portrays rats that received aCSF infusions (open bars) during the social defeat phase of the experiment, and the gray bars represent rats that received AP5 (5 nmol). The asterisks indicate groups of defeated rats that were significantly different from the non-defeated control's vehicle condition at the p<0.05 level
A significant role for VTA activation during social stress was revealed by the effects of AP-5 infusion before each defeat on subsequent cocaine taking during the progressive ratio schedule of cocaine reinforcements (See Fig. 3b). Specifically, responding during the PR schedule was significantly influenced by social defeat, as revealed by a main effect of stress (F1,32=5.5, p<0.02), a main effect of VTA infusion (F1,32=7.51, p<0.009) and an interaction between stress and AP5 infusion (F1,32=6.02, p<0.02).
Twenty-four-hour variable dose cocaine binge
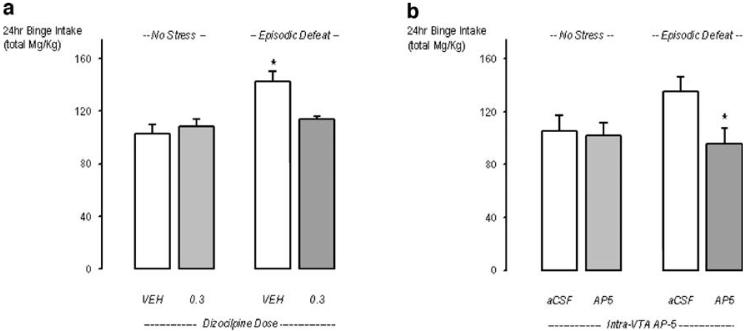
Just before the 24-h binge, two rats from the systemic dizocilpine study were excluded from the project due to catheter disruptions (resulting from damage to the tethered line secured to the catheter or a loss of catheter patency). As a result of the two losses, nine rats from the vehicle-treated non-stressed control group and another nine rats treated with dizolcilpine (0.3 mg/kg) before each social defeat contributed to the overall binge analyses for these two groups. During the binge, total intake was highest in stress-sensitized rats pretreated with saline (See Fig. 4a), which contributed to a significant main effect of stress (F1,34=13.54, p<0.0008) and an interaction between stress and drug treatment (F1,34=7.42, p<0.01]. Across the entire 24-h binge, each rat accumulated an equivalent number of each cocaine unit dose delivered in semi-random sequences of 15 total infusions. Overall, nonsensitized control rats waited longer after the delivery of each unit dose (i.e., 0.2, 0.4, and 0.8 mg/kg/inf) before resuming cocaine intake (2.3, 4.9, and 8.0 min) compared to stress-sensitized (2.1, 3.7, and 6.4 min) rats (see Fig. 5a). If the post-infusion intervals for highest dose of cocaine (0.8 mg/kg/infusion) are isolated a posteriori and compared between defeated and non-defeated control rats via t test, then the average post-infusion intervals were significantly different from each other (t17,=4.7, p<0.0002; see Fig. 5a), indicating that the interval observed in control rats was indeed longer than that observed in defeated rats. The clear demonstration of adjusting the time of each infusion delivery with the unpredictable delivery of each dosage is also interpreted to reflect a significant degree of control (or regulation) over cocaine reinforcement even after large amounts of cocaine intake.
Fig. 4.
Effects of systemically administered or local VTA infusion of NMDA receptor antagonists during episodic social defeat stress on subsequent cocaine self-administration: total cocaine intake during a 24-h variable dose “binge.” The mean (±SEM) total amounts of cocaine self-administered by non-defeated rats (left bars) and episodically defeated rats (right bars). a portrays rats that received systemic saline injections (open bars) during the social defeat phase of the experiment, and the gray bars represent rats that received 0.3 mg/kg dizocilpine. The asterisks indicate groups of defeated rats that were significantly different from the non-defeated control's vehicle condition at the p<0.05 level. b portrays rats that received aCSF infusions (open bars) during the social defeat phase of the experiment, and the gray bars represent rats that received AP5 (5 nmol). The asterisks indicate defeated rats treated with AP5 were significantly different from the control AP5 condition at the p<0.05 level
Fig. 5.
Patterns of cocaine self-administration during a 24-h binge: an analysis of the post-infusion intervals after variable deliveries of three unit doses of cocaine in previously defeat stressed and non-defeated control rats. a portrays the mean (±SEM) minute between successive infusions after 0.2, 0.4, and 0.8 mg/kg/infusion for rats with a history of repeated episodic defeat (filled circles), and non-defeated rats (open circles). The asterisk indicates significance between defeated and non-defeated controls at the highest dose of cocaine self-administered at the p<0.05 level. b portrays the mean (±SEM) post-infusion intervals after variable doses of cocaine for the non-stressed rats infused with aCSF (open circles) and defeated rats infused with AP-5 (open triangles) or aCSF (filled circles) during the binge. c shows individual cumulative records for cocaine intake by defeated rats receiving intra-VTA aCSF vehicle (left panel) or the NMDA receptor antagonist AP-5 (middle panel) and for non-stressed rats (right panel) receiving either the AP-5 (black lines) or aCSF (gray lines) infusions
Total intake was somewhat higher in previously defeated rats that received control infusions of aCSF into the VTA (See Fig. 4b), but this failed to reach significance (F=1.08, p=0.3). A planned t test was subsequently conducted between the two groups of socially defeated rats to reveal whether or not AP-5 protection during stress significantly influenced the amount of cocaine intake accumulated during the binge, as the effect size between these two groups was large. AP-5 infusions delivered into the VTA were confirmed to have significantly reduced the impact of social defeat stress on IV cocaine intake during the binge (t16=2.451, p=0.025).
Over a 24-h binge, the average post-infusion intervals for stressed and non-stressed rats that received AP-5 or aCSF are portrayed in Fig. 5b, effectively confirming the ability of these rats to regulate cocaine intake over the 24-h access period. Each individual rat's cumulative record, associated within each corresponding treatment group, is also portrayed in Fig. 5c. Although not significant, stress-sensitized rats tended to have shorter post-infusion intervals after the delivery of each dose throughout the binge (F2,24=1.73, p<0.19).
Experiment III. GluR1 protein expression
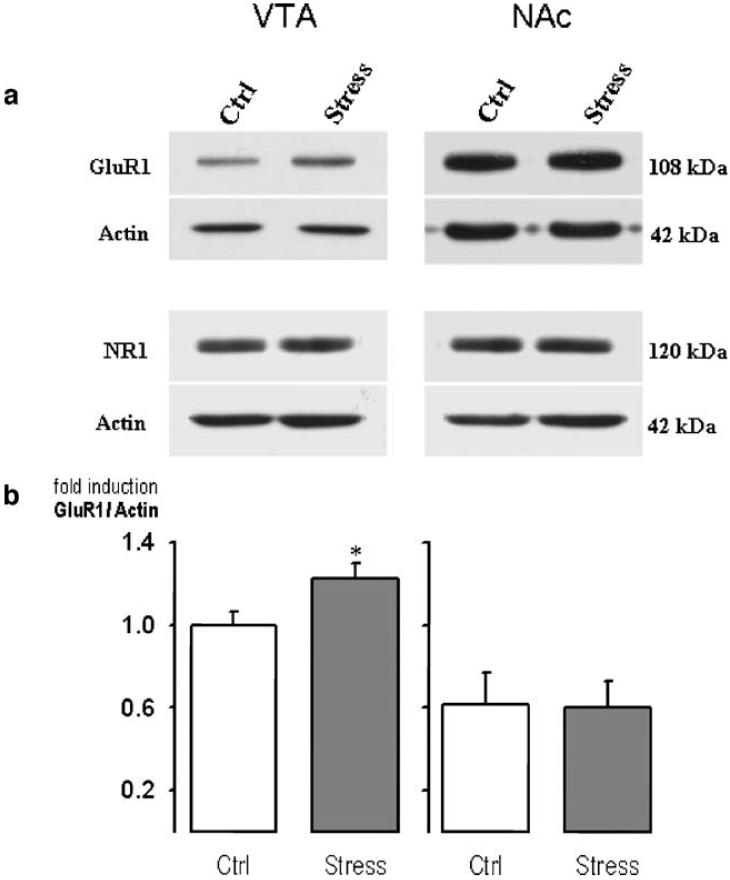
Four brief episodes of social defeat stress significantly increased GluR1 subunit protein in the VTA 10 days after the last defeat, compared to contemporary non-stressed controls (F1,6=5.37, p=0.04; see Fig. 6). In the NAc, this form of episodic social defeat stress did not alter the expression of GluR1 subunit protein (F1,6=0.006, p=0.94). NR1 subunit expression was not regulated by social defeat in areas of the VTA (F1,12=0.051, p=0.83), or NAc (F1,12= 0.751, p=0.4).
Fig. 6.
Quantification of protein for the GluR1 subunit of the AMPA receptor and the NR1 subunit of the NMDA receptor after episodic defeat stress. a represents a VTA (left panel) or NAc (right panel) image obtained from the blotting procedures for GluR1 or NR1 after episodic defeat stress compared to non-defeated control rats. b portrays the mean (±SEM) levels of GluR1 protein, expressed as a ratio of the control protein actin, in the VTA (left panel) and NAc (right panel) for defeated (filled bars) and control rats (open bars). The asterisk indicates significance compared to non-defeated controls at the p<0.05 level
VTA cannula placements
Out of the 44 rats implanted with bilateral cannulae directed at the VTA, 38 rats successfully completed all cocaine self-administration phases (due to three head mount complications and the development of three infections), and 36 of these rats who completed the binge were confirmed to have placements located within the VTA (see Fig. 7). Those two rats that did not have properly placed guide cannulae were excluded from behavioral analyses.
Fig. 7.
Left. Schematic portrayal of accurately placed intra-VTA injection sites. The figures correspond to coronal sections of the rat brain at -4.8 to -5.6 mm from bregma. Filled circles represent the average location of each pair of bilateral cannulae. The injection sites from the two rats with inaccurate placements are shown as gray circles. Right. Photomicrograph of an intra-VTA injection site from a rat that received four microinjections of AP-5 before each stress episode. Fifty-micrometer slices of midbrain tissue were taken from all rats studied for VTA infusions and stained with crysl violet for histological confirmation of the injection area
Discussion
Episodes of social defeat stress in rats can augment later behavioral effects of cocaine, and this behavioral plasticity is an NMDA and AMPA receptor-dependent process, closely similar to the long-lasting impact of intermittent amphetamine, cocaine, morphine, alcohol, or nicotine administrations (Wolf and Jeziorski 1993; Broadbent and Weitemier 1999; Shim et al. 2002; Yap et al. 2005). Systemic administrations of the NMDA receptor antagonist dizocilpine or the AMPA receptor antagonist NBQX was hereby shown to attenuate the social stress-induced behavioral response to a subsequent cocaine challenge. The augmented behavioral response to cocaine after episodic social defeat stress was accompanied by persistently elevated GluR1 AMPA receptor subunit protein in the VTA. Likewise, local antagonism of VTA NMDA receptors was also effective at preventing the social stress-induced behavioral response to cocaine.
Prior exposure to stimulant drugs influences subsequent drug taking behavior (Piazza et al. 1990; Pierre and Vezina 1998; Schenk and Partridge 2000; Mendrek et al. 1998; Vezina et al. 1999). The present experiments explored further how episodic social defeats impact the reinforcing effects of cocaine (e.g., Miczek and Mutschler 1996; Tidey and Miczek 1996). The currently observed cocaine self-administration progressive ratio and “binge” data after social defeat stress appear relevant to abusive or compulsive patterns of drug intake (O'Brien et al. 1998). Socially defeated rats persisted with cocaine self-administration during prolonged access, accumulating significantly more drug. Control rats tended to cease cocaine self-administration after 18-20 h, confirming previous reports that rats self-administering cocaine at moderate dose ranges typically stop responding after accumulating ca 120 mg/kg (Weiss et al. 1992; Tornatzky and Miczek 2000), often limiting experimental “binges” to 16 h or less (Markou et al. 1992; Mutschler and Miczek 1998; Mutschler et al. 2001). It is noteworthy that all experimental and control groups maintained rigorous regulation over responding under the control of different cocaine doses, adjusting the average post-infusion interval predictably according to the dose received. Rates of cocaine self-administration were higher in defeated rats at the largest unit dose, as indicated by faster intake after the delivery of 0.8 mg/kg dose infusions. The faster rates of intake may be a sensitive measure for predicting the emergence of “escalation” that occurs with cocaine taking during specific access conditions (Ahmed and Koob 1999). For example, when daily access to cocaine is extended to 6 h or more, rats shift “upwardly” to higher levels of intake after a few daily sessions, interpreted to reflect a change in the set point for cocaine's reinforcing effect (Ahmed and Koob 1998). However, upward shifts in the rate of responding for cocaine remain challenging to interpret (Zernig et al. 2004), and it is unclear from the results of the current experiment if dysregulation occurs with a shift to increased cocaine taking. In fact, defeated rats self-regulated cocaine intake and controls during the binge and perhaps better, as reflected by somewhat smaller variations in response rates across hourly intervals over the later hours of the binge (data not shown). In a similar progression, upon careful examination of patterns of responding over multiple prolonged cocaine binges, it becomes evident that the variability of response rates decreases systematically over successive hours of recurrent binges, as indicated by the root mean square of successive differences (RMSSDs) over accumulating post-infusion times (Mutschler et al. 2000). Hypothetically, it remains possible that intense cocaine taking is more readily achieved after establishing either greater control over its reinforcing effect, or tolerance to its disruptive effect, or both.
Episodic stress-induced behavioral plasticity
Evidence supports the hypothesis that environmental stressors enhance the incidence of psychiatric illnesses (for reviews see Yui et al. 1999; Sinha 2001; McEwen 2004; Sapolsky 2005). However, the intensity, controllability, and chronicity of exposure to a stressor are significant for understanding the ensuing behavioral and physiological outcomes. Individuals at risk for psychotic episodes, for instance, may be more likely to experience psychosis when exposed to minor stressors on a day-to-day basis as opposed to intense extraordinary types of stress (Myin-Germeys et al. 2005). Similarly, the multiphasic process of stress-induced behavioral sensitization is sensitive to stress exposure parameters including intensity, duration, and intermittency (Miczek et al. 2004).
Glutamate plays an important role in the induction and expression of behavioral sensitization to psychomotor stimulants and opiates (Wolf 1998; Vanderschuren and Kalivas 2000; Everitt and Wolf 2002). Of particular significance are glutamatergic afferents originating in DA terminal regions including the prefrontal cortex that descend and synapse with DA cells in the VTA (Kalivas et al. 1993; Westerink et al. 1998). This `feedback loop' is activated during the induction of behavioral sensitization, as pharmacological blockade of NMDA or AMPA receptors within the VTA prevents this process from emerging (Karler et al. 1989; Kalivas and Alesdatter 1993; Wolf et al. 1994; Zhang et al. 1997; Li et al. 1999). Based on the current results, enhanced behavioral responses to cocaine are likely to be the result of increased glutamatergic neurotransmission acting on VTA NMDA receptors during episodic social defeats, strengthening the hypothesis that diverse sensitizing stimuli can similarly facilitate synaptic plasticity in mesocorticolimbic structures (Saal et al. 2003). With regard to the present findings, this current study lacks an anatomical control for the VTA infusions, and the possibility that surrounding brain targets participate in the effects of defeat stress (in an NMDA-dependent manner) is also feasible. Moreover, glutamatergic afferents in the VTA originate from many areas throughout the brain (Geisler et al. 2007), so a diversity of cortical or subcortical structures may uniquely participate in the activation of VTA neurons in response to stress and drug administrations.
Episodic stress-induced neural plasticity
Environmental stress and psychomotor stimulants can transiently elevate GluR1 and NR1 protein subunits in the VTA (Fitzgerald et al. 1996), supporting the hypothesis that a common molecular process is activated by stressors, stimulants, or opiates. Intermittent social defeat stress is presently revealed to increase GluR1 (but not NR1) protein expression for up to 10 days after the last defeat. It is tempting to relate immediate activational effects of particular stress experiences, including rises in mescorticolimbic DA and glutamate release (Wang et al. 2005; Tidey and Miczek 1996; Takahata and Moghaddam 1998) and increased GluR1 expression (Fitzgerald et al. 1996; current results) to the emergence of presumably permanent neuronal changes (Yap et al. 2005).
Conclusion
The neural basis for the transition from initial drug use to escalated, or out of control drug taking, is a complex issue that likely incorporates neuronal plasticity subserving reward tolerance or learning and memory processes (Kalivas 2005; Hyman et al. 2006). Supporting the latter, particular neural adaptations emerge during repeated exposures to psychomotor stimulants that significantly impact on their reinforcing and locomotor-activating effects, at least in animal models (Nestler 2004). At least three conclusions can be submitted based on the present experimental results. First, the experience of uncontrollable episodic stress has profound effects on behaviors that may be relevant to the progression to compulsive cocaine taking expressed in cocaine-dependent individuals. Second, brief social stress episodes engender neural adaptations that are remarkably similar to those after the administration of psychomotor stimulants, by engaging glutamatergic modulation of the mesocorticolimbic DA system to promote cocaine's reinforcing effects (Yap et al. 2005). Third, cocaine “bingeing” is the intake schedule preferred by its heaviest users, the most severe form of cocaine taking, and experimentally, this form of cumulative intake during continuous cocaine access is intensified by prior stress experiences. All in all, the current experiments extend earlier findings that more intense cocaine taking emerges with a series of neural adaptations that strengthen behavioral responses leading to cocaine intake. Stress episodes are hereby shown to activate the mesocorticolimbic DA circuit via glutamate receptors, engaging neural mechanisms that are attributed to cocaine's reinforcing and psychomotor stimulant effects on behavior.
Acknowledgements
This research was supported by USPHS research grants DA02632 (KAM), KO1DA14057 (AMR), and KO2DA00354 (BEK).
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Weitemier AZ. Dizocilpine (MK-801) prevents the development of sensitization to ethanol in DBA/2J mice. Alcohol Alcohol. 1999;34:283–288. doi: 10.1093/alcalc/34.3.283. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology. 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology. 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: Common adaptions among cross-sensitizing agents. J Neurosci. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SC, Covington HE, III, Miczek KA. Stereotyped and complex motor routines expressed during cocaine self-administration: results from a 24-h binge of unlimited cocaine access in rats. Psychopharmacology. 2007;192:465–478. doi: 10.1007/s00213-007-0739-6. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber GJ, Wise RA. Pharmacological regulation of intravenous cocaine and heroin self-administration in rats: a variable dose paradigm. Pharmacol Biochem Behav. 1989;32:527–531. doi: 10.1016/0091-3057(89)90192-5. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988;42:1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Kehr J, Ungerstedt U. In vivo microdialysis as a technique to monitor drug transport: correlation of extracellular cocaine levels and dopamine overflow in the rat brain. J Neurochem. 1988;51:1314–1316. doi: 10.1111/j.1471-4159.1988.tb03103.x. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. How do we determine which drug-induced neuroplastic changes are important. Nat Neurosci. 2005;8:1440–1441. doi: 10.1038/nn1105-1440. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Alesdatter JE. Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;267:486–495. [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Sorg BA, Hooks MS. The pharmacology and neural circuitry of sensitization to psychostimulants. Behav Pharmacol. 1993;4:315–334. [PubMed] [Google Scholar]
- Karler R, Calder LD, Chaudhry IA, Turkanis SA. Blockade of “reverse tolerance” to cocaine and amphetamine by MK-801. Life Sci. 1989;45:599–606. doi: 10.1016/0024-3205(89)90045-3. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu XT, Berney TG, Vartanian AJ, Stine CD, Wolf ME, et al. Both glutamate receptor antagonists and prefrontal cortex lesions prevent induction of cocaine sensitization and associated neuroadaptations. Synapse. 1999;34:169–180. doi: 10.1002/(SICI)1098-2396(19991201)34:3<169::AID-SYN1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Le Moal M, Piazza PV. Acute pharmacological blockade of corticosterone secretion reverses food restriction-induced sensitization of the locomotor response to cocaine. Brain Res. 1996;724:251–255. doi: 10.1016/0006-8993(96)00309-5. [DOI] [PubMed] [Google Scholar]
- Markou A, Hauger RL, Koob GF. Desmethylimipramine attenuates cocaine withdrawal in rats. Psychopharmacology. 1992;109:305–314. doi: 10.1007/BF02245878. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Blaha CD, Phillips AG. Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology. 1998;135:416–422. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: Differential effects of d-amphetamine and cocaine. Psychopharmacology. 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology. 1996;128:256–264. doi: 10.1007/s002130050133. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Covington HE, Nikulina EA, Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from a self-administered or non-contingent cocaine binge: differences in ultrasonic distress vocalizations in rats. Psychopharmacology. 1998;136:402–408. doi: 10.1007/s002130050584. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA, Hammer RP., Jr Reduction of zif268 messenger RNA expression during prolonged withdrawal following “binge” cocaine self-administration in rats. Neuroscience. 2000;100:531–538. doi: 10.1016/s0306-4522(00)00298-0. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Covington HE, III, Miczek KA. Repeated self-administered cocaine “binges” in rats: effects on cocaine intake and withdrawal. Psychopharmacology. 2001;154:292–300. doi: 10.1007/s002130000646. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, Delespaul P, van Os J. Behavioural sensitization to daily life stress in psychosis. Psychol Med. 2005;35:733–741. doi: 10.1017/s0033291704004179. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. National Academy; Washington, DC: 1996. [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuro-pharmacology. 2004;47(Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Marchand JE, Kream RM, Miczek KA. Behavioral sensitization to cocaine after a brief social stress is accompanied by changes in fos expression in the murine brainstem. Brain Res. 1998;810:200–210. doi: 10.1016/s0006-8993(98)00925-1. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, III, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Miczek KA, Hammer RP., Jr Prolonged effects of repeated social defeat stress on mRNA expression and function of mu-opioid receptors in the ventral tegmental area of rats. Neuropsychopharmacology. 2005;30:1096–1103. doi: 10.1038/sj.npp.1300658. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion. J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd edn. Academic; San Diego: 1997. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, le Moal M, Simon H. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res. 1990;514:22–26. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. D1 dopamine receptor blockade prevents the facilitation of amphetamine self-administration induced by prior exposure to the drug. Psychopharmacology. 1998;138:159–166. doi: 10.1007/s002130050658. [DOI] [PubMed] [Google Scholar]
- Prasad BM, Ulibarri C, Sorg BA. Stress-induced cross-sensitization to cocaine: effect of adrenalectomy and corticosterone after short- and long-term withdrawal. Psychopharmacology. 1998;136:24–33. doi: 10.1007/s002130050535. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha A, Husson I, Satpute SS, Kuppenbender KD, Ren JQ, Guerriero RM, et al. L-type Ca2+ channels mediate adaptation of extracellular signal-regulated kinase 1/2 phosphorylation in the ventral tegmental area after chronic amphetamine treatment. J Neurosci. 2004;24:7464–7476. doi: 10.1523/JNEUROSCI.0612-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remie R, Coppes RP, Meurs H, Roffel AF, Zaagsma J. Characterization of presynaptic vascular muscarinic receptors inhibiting endogenous noradrenaline overflow in the portal vein of the freely moving rat. Br J Pharmacol. 1990;99:223–226. doi: 10.1111/j.1476-5381.1990.tb14684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Sensitization to cocaine's reinforcing effects produced by various cocaine pretreatment regimens in rats. Pharmacol Biochem Behav. 2000;66:765–770. doi: 10.1016/s0091-3057(00)00273-2. [DOI] [PubMed] [Google Scholar]
- Shim I, Kim HT, Kim YH, Chun BG, Hahm DH, Lee EH, et al. Role of nitric oxide synthase inhibitors and NMDA receptor antagonist in nicotine-induced behavioral sensitization in the rat. Eur J Pharmacol. 2002;443:119–124. doi: 10.1016/s0014-2999(02)01582-0. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse. Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Vezina P. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in an NMDA, AMPA/kainate, and metabotropic glutamate receptor-dependent manner. Neuropsychopharmacology. 2003;4:629–639. doi: 10.1038/sj.npp.1300075. [DOI] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Glutamatergic regulation of basal and stimulus-activated dopamine release in the prefrontal cortex. J Neurochem. 1998;71:1443–1449. doi: 10.1046/j.1471-4159.1998.71041443.x. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: An in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology. 1997;130:203–212. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Cocaine self-administration “binges” transition from behavioral and autonomic regulation toward homeo-static dysregulation in rats. Psychopharmacology. 2000;148:289–298. doi: 10.1007/s002130050053. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vezina P, Queen AL. Induction of locomotor sensitization by amphetamine requires the activation of NMDA receptors in the rat ventral tegmental area. Psychopharmacology. 2000;151:184–191. doi: 10.1007/s002130000463. [DOI] [PubMed] [Google Scholar]
- Vezina P, Pierre PJ, Lorrain DS. The effect of previous exposure to amphetamine on drug-induced locomotion and self-administration of a low dose of the drug. Psychopharmacology. 1999;147:125–134. doi: 10.1007/s002130051152. [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992;593:314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Enrico P, Feimann J, de Vries JB. The pharmacology of mesocortical dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and prefrontal cortex of the rat brain. J Pharmacol Exp Ther. 1998;285:143–154. [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Jeziorski M. Coadministration of MK-801 with amphetamine, cocaine or morphine prevents rather than transiently masks the development of behavioral sensitization. Brain Res. 1993;613:291–294. doi: 10.1016/0006-8993(93)90913-8. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, Hu XT. MK-801 prevents alterations in the mesoaccumbens dopamine system associated with behavioral sensitization to amphetamine. Neuroscience. 1994;14:1735–1745. doi: 10.1523/JNEUROSCI.14-03-01735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA. Social defeat stress, sensitization, and intravenous cocaine self-administration in mice. Psychopharmacology. 2007;192:261–273. doi: 10.1007/s00213-007-0712-4. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Covington HE, III, Gale MC, Datta R, Miczek KA. Behavioral sensitization due to social defeat stress in mice: antagonism at mGluR5 and NMDA receptors. Psychopharmacology. 2005;179:230–239. doi: 10.1007/s00213-004-2023-3. [DOI] [PubMed] [Google Scholar]
- Yui K, Ishiguro T, Goto K, Ikemoto S, Kamata Y. Spontaneous recurrence of methampetamine psychosis: increased sensitivity to stress associated with noradrenergic hyperactivity and dopaminergic change. Eur Arch Psychiatry Clin Neurosci. 1999;249:103–111. doi: 10.1007/s004060050073. [DOI] [PubMed] [Google Scholar]
- Zernig G, Wakonigg G, Madlung E, Haring C, Saria A. Do vertical shifts in dose-response rate-relationships in operant conditioning procedures indicate “sensitization” to “drug wanting”. Psychopharmacology. 2004;171:349–351. doi: 10.1007/s00213-003-1601-0. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther. 1997;281:699–706. [PubMed] [Google Scholar]