Abstract
Human natural killer (NK) cells can be subdivided into different populations based on the relative expression of the surface markers CD16 and CD56. The two major subsets are CD56bright CD16dim/− and CD56dim CD16+, respectively. In this review, we will focus on the CD56bright NK cell subset. These cells are numerically in the minority in peripheral blood but constitute the majority of NK cells in secondary lymphoid tissues. They are abundant cytokine producers but are only weakly cytotoxic before activation. Recent data suggest that under certain conditions, they have immunoregulatory properties, and that they are probably immediate precursors of CD56dim NK cells. CD56bright NK cell percentages are expanded or reduced in a certain number of diseases, but the significance of these variations is not yet clear.
Keywords: CD56bright, immunoregulation, natural killer cells
Introduction
Natural killer (NK) cells have been the focus of interest of immunologists for almost two decades. The increasing knowledge of NK cell biology acquired throughout this period has led to a paradigm shift – for a long time NK cells were considered merely as relatively primitive killers but they are now seen not only as bona fide actors in innate immunity but also as important cells that shape and influence adaptive immune responses and are more and more being endorsed with an immunoregulatory role. However, NK cells are not a homogeneous cell population and several subtypes exist in both human and mouse.
In human peripheral blood, five NK cell subpopulations can be defined on the basis of the relative expression of the markers CD16 (or FcγRIIIA, low-affinity receptor for the Fc portion of immunoglobulin G) and CD56 (adhesion molecule mediating homotypic adhesion)1–3: (1) CD56bright CD16− (50–70% of CD56bright), (2) CD56bright CD16dim (30–50% of CD56bright), (3) CD56dim CD16−, (4) CD56dim CD16bright, and (5) CD56− CD16bright. In healthy individuals, populations (3) and (5) are numerically in the minority. Whereas the role of CD56dim CD16− cells is largely unknown, CD56− CD16bright NK cells are functional. They are often dramatically expanded in human immunodeficiency virus infection but are hyporesponsive under these conditions.2 The CD56dim CD16bright NK cells represent at least 90% of all peripheral blood NK cells and are therefore the major circulating subset.1,3 A maximum of 10% are CD56bright NK cells.1,3 The various subsets can easily be distinguished by flow cytometry as depicted in Fig. 1.
Figure 1.
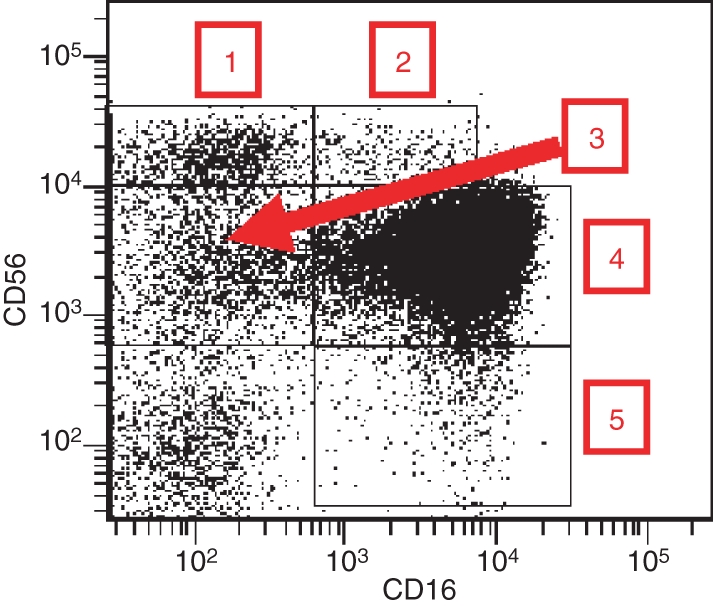
Natural killer (NK) cell subsets in human peripheral blood based on the relative expression of CD16 and CD56. Normal peripheral blood mononuclear cells (PBMC) were stained with anti-CD3, anti-CD14, anti-CD16, anti-CD19 and anti-CD56 antibodies conjugated to five different fluorochromes. A gate was set on CD3− CD19− cells (NK cells) and CD16 expression versus CD56 expression is shown. 1, CD56bright CD16−; 2, CD56bright CD16dim; 3, CD56dim CD16−; 4, CD56dim CD16+; 5, CD56− CD16+.
In this review, we will focus on the CD56bright NK cell population because important and interesting discoveries regarding this subset have been made in recent years. We will mostly review the literature but also present some data obtained in our laboratory. Mouse NK cells are excluded from the discussion because they do not express CD56. However, on the basis of the differential expression of the surface markers CD11b and CD27, three mouse NK cell subpopulations with different functional properties have been described.4 Recently, it has been suggested that mouse NK cells expressing the chemokine receptor CXCR3 might represent the murine equivalent to human CD56bright cells.5
CD56bright NK cells in peripheral blood
Historically, the first description of different NK cell subsets was given by Nagler et al.6 in 1989, and their data have been abundantly confirmed by other groups. Usually CD56bright NK cells were compared with CD56dim CD16bright NK cells in terms of phenotype and functions, and later also regarding their differential homing capacities.
Phenotype
The inhibitory receptors killer cell immunoglobulin-like receptors (KIR) and immunoglobulin-like transcript 2 (ILT2) are absent from CD56bright NK cells but are found on various (according to the receptors and the individuals) proportions of CD56dim cells.1,7 CD94/NKG2A, another major inhibitory receptor, is likewise expressed on a fraction of CD56dim cells in contrast to its expression at a higher density on all CD56bright NK cells.1 CD94 is a chaperone molecule that can also associate with NKG2C which is an activating receptor. Both isoforms (CD94/NKG2A and CD94/NKG2C) recognize the non-classical human leucocyte antigen (HLA) class I molecule HLA-E, which presents peptides derived from signal sequences of classical HLA class I molecules.1,3,8 Regarding the activating receptor NKp46, its density is usually highest on the CD56bright population.9 Only CD56bright cells express the haematopoietic stem cell marker CD117 (c-kit) and the high-affinity receptor for interleukin-2 (IL-2) and proliferate in response to picomolar concentrations of this cytokine.1 They also proliferate efficiently in response to IL-21.10 Other cytokine receptors, such as IL-1 receptor I (IL1RI) and IL18R are likewise expressed at a higher level by this subset (Table 1).1
Table 1.
Phenotypic comparison between CD56bright and CD56dim natural killer cells
| CD56bright | CD56dim | |
|---|---|---|
| CD56 | ++ | + |
| CD16 | ± | ++ |
| Inhibitory receptors | ||
| KIR | − | + |
| ILT2 | − | + |
| CD94/NKG2A | ++ | ± |
| Activating receptors | ||
| NKp46 | ++ | + |
| CD117(c-kit) | ++ | − |
| Cytokine receptors | ||
| IL2Rαβγ | + | − |
| IL2Rβγ | ++ | + |
| IL1RI | ++ | + |
| IL18R | ++ | + |
| Chemokine receptors | ||
| CCR7 | ++ | − |
| CXCR3 | ++ | ± |
| CXCR1 | − | ++ |
| CX3CR1 | − | ++ |
| Adhesion molecules | ||
| CD2 | ++ | ± |
| CD11c | ++ | ± |
| CD44 | ++ | + |
| CD49e | ++ | + |
| CD54 | ++ | ± |
| CD62L | ++ | ± |
| CD11a | + | ++ |
| Other molecules | ||
| CD57 | − | + |
| CD160 | − | ++ |
| CD55 | ++ | + |
| CD59 | ++ | + |
| HLA-DR | + | − |
++, strong expression (bright); +, weak expression (dim); ±, expression only on a subpopulation; −, no expression.
The chemokine receptor repertoire is completely different between the two populations. CD56bright cells are the only ones to express CCR7 and they bear CXCR3 at a much stronger density than CD56dim NK cells.1,11 In contrast, the latter cells express CXCR1 and CX3CR1 exclusively.1,11 Regarding the adhesion molecules, CD56bright NK cells display a stronger expression of CD2, CD11c, CD44, CD49e, CD54 and CD62L, whereas CD56dim NK cells express more CD11a (Table 1).1 The consequence of these different repertoires of chemokine receptors and adhesion molecules are divergent migratory properties: the CD56bright subset preferentially migrates to secondary lymphoid organs whereas the CD56dim cells migrate to acute inflammatory sites.1,11
In contrast to CD56dim cells, the CD56bright subset lacks expression of CD5712 and CD160,13 but displays the complement regulatory proteins CD55 and CD59 at a higher level.14 Also, the CD56bright subset is HLA-DR+ (Table 1).12
The phenotypic differences between both subsets have been confirmed and extended by gene chip arrays and protein arrays.15,16 Wendt et al.16 have shown that resting NK cell populations differ in 473 transcripts, 176 being exclusively expressed in CD56dim cells and 130 exclusively in CD56bright cells.
Functions
The cytotoxic activity of CD56dim NK cells is significantly higher than that of CD56bright cells (the CD56bright CD16− population being the less cytotoxic)1,6 and they contain much more perforin, granzymes and cytolytic granules.7 They also form more conjugates with K562 target cells.7 The high expression level of CD16 makes them efficient mediators of antibody-dependent cellular cytotoxicity, whereas CD56bright CD16dim NK cells perform antibody-dependent cellular cytotoxicity only weakly and CD56bright CD16− NK cells of course not at all.1,6 Upon stimulation with cytokines such as IL-2 or IL-12, the cytotoxic activity of all NK cell subsets dramatically increases.1,6
Regarding cytokine production, the situation is inverted. Indeed, CD56bright NK cells are the most efficient cytokine producers.1 The major cytokines released are interferon-γ (IFN-γ) (NK cells are one of the most important sources of this cytokine), tumour necrosis factor-α, granulocyte–macrophage colony-stimulating factor, IL-10 and IL-13, depending on the precise conditions of stimulation (for example, the combination of IL-12 and IL-18 is the best to induce a strong production of IFN-γ, which is 20–30 times higher in CD56bright than in CD56dim NK cells).17
CD56bright NK cells in secondary lymphoid organs
In the spleen, 7–50% of mononuclear cells are NK cells, mostly with a CD56dim CD16+ phenotype.18 They probably correspond to the same population in peripheral blood that is simply circulating through the red pulp.18
Among mononuclear cells in lymph nodes (LN), 1–5% are NK cells18,19 whose subset distribution is a mirror image of the one found in peripheral blood. Indeed, 75 to > 95% are of the CD56bright type.18,19 These cells are localized in parafollicular T-cell zones of the LN.19 Considering that LN contain 40% of all lymphocytes in the body (in contrast to 2% in peripheral blood), it appears that despite their low LN percentages, the biggest reservoir of NK cells is in the LN and not in blood and that there are many more CD56bright than CD56dim NK cells.20 The vast majority of tonsil NK cells18 are also CD56bright.
Phenotype
Lymph node CD56bright NK cells display a slightly different phenotype than their blood counterparts. They lack not only CD16 and KIR, but also CD8, CD62L, CCR7, the activating receptor NKp30 and HLA-DR. CD94/NKG2A and NKp46 are present only on a fraction of these cells.18,20
Tonsil CD56bright NK cells share the phenotype of LN NK cells. However, in inflamed tonsils, they become activated and NKp44+ CD69+ HLA-DR+.18,20 Before the discovery of this subtype, NKp44 expression had only been observed in vitro on NK cells activated with IL-2.21
As LN NK cells are in contact with T cells19 the effect of T-cell-derived IL-2 on these NK cells has been investigated. This cytokine upregulates or induces, respectively, the expression of CD16, KIR, NKp46 and NKp30 as well as of perforin, ending up with a phenotype close to that of peripheral blood CD56dim CD16+ cells.18
Functions
The phenotype of ex vivo isolated NK cells from secondary lymphoid organs suggests the absence of cytotoxic activity, and this is indeed the case.18 However, culture in IL-2 not only changes the phenotype (see above), but also induces an efficient cytotoxic activity.18 In addition, NK cells from inflamed tonsils produce IFN-γ and 35% of them proliferate upon culture in IL-2.18,20
CD56bright NK cells in the uterus
The endometrium contains a high number of NK cells that are almost exclusively CD56bright CD16−.22 The density of expression of CD56 is even higher on these cells than on peripheral blood CD56bright NK cells.23 Accounting for 20% of lymphocytes in the proliferative endometrium, they increase to 50% in the secretory phase and to 70–80% in early pregnancy decidua.22 A high percentage of these cells is proliferating, which suggests that local expansion could explain the dramatic increase in numbers. They play a role in implantation, angiogenesis and maintenance of pregnancy.22,23
Koopman et al.23 have compared uterine NK cells with the two major peripheral blood NK cell subsets by using gene microarrays. The profiles reveal that the uterine NK cells are different from the peripheral blood NK cells, and that they are more different from them than the two major peripheral blood NK cells are different from each other. They particularly over-express some tetraspanins and integrins, the activating receptors NKG2C and NKG2E, KIR, galectin-1 and granzyme A. CD9 and the immunosuppressive molecule PP14 (glycodelin A) are exclusive to uterine NK cells. Although they contain cytotoxic granules and express KIR, they are only weakly cytolytic ex vivo.23
Interactions of CD56bright NK cells with other cells of the immune system
Bidirectional interactions between NK cells and dendritic cells (DC) have been extensively investigated and reviewed. Regarding in particular CD56bright CD16− NK cells, Vitale et al.24 have shown that these cells preferentially proliferate in coculture with immature DC and lipopolysaccharide and abundantly produce IFN-γ. In vivo, CD56bright NK cells interact in LN with incoming DC from the periphery.20
In sites of peripheral inflammation, CD56bright NK cells represent 40–60% of all NK cells and display an activated phenotype (CD69+).25 Upon stimulation by monokines (IL-12, IL-15, IL-18), they produce IFN-γ and increase significantly the percentage of tumour necrosis factor-α-producing monocytes. In this type of bidirectional interaction, a direct physical contact between NK cells and monocytes is required for a maximal effect.25
It is possible that CD56bright NK cells also interact with T and B lymphocytes in a manner that is different from that of CD56dim NK cells, but this has, to the best of our knowledge, not been investigated in detail.
Relationship of CD56bright and CD56dim NK cells
According to Caligiuri,3 NK cell precursors leave the bone marrow, transit through peripheral blood and join the LN, where they differentiate into CD56bright NK cells under the influence of cytokines produced by stromal cells and DC.
A question that has been debated for a long time is the precise nature of the relationship between the two major NK cell subsets, CD56bright and CD56dim. Are the CD56bright NK cells precursors of CD56dim cells, or are they both finally differentiated cell types with no direct relation and arising from two different haematopoietic precursor cells?
In favour of the former hypothesis, it has been proposed that the phenotypic and functional properties of both subsets rather define CD56bright NK cells as immature precursors and CD56dim NK cells as finally differentiated cells. Maturation would be characterized by the downregulation of CD56, the acquisition of CD16 and of KIR, and CD56bright CD16dim cells would be an intermediate stage between the most immature (CD56bright CD16−) and the most mature (CD56dim CD16+) cell types.6,26
However, as CD56bright NK cells produce greater amounts of cytokines than CD56dim NK cells, and possess a completely different repertoire of adhesion molecules and chemokine receptors leading to different migration properties,1,11 the concept of two different terminally differentiated cell populations is likewise conceivable.
Three recent papers27–29 have finally shown, quite convincingly, that CD56bright NK cells are very likely precursor cells of the CD56dim subset. Indeed, CD56dim NK cells display shorter telomeres than CD56bright NK cells from peripheral blood and LN, which implies that the latter are less mature than the former. In addition, purified CD56bright CD16− NK cells cultured in the presence of synovial or skin fibroblasts can differentiate into CD56dim cells that have the characteristic phenotypic and functional features of peripheral blood CD56dim NK cells.27 A role for an interaction between CD56 and the fibroblast growth factor receptor-1 for this differentiation process has been demonstrated.27 As an argument for the differentiation of CD56bright NK cells into CD56dim NK cells in LN in vivo, Romagnani et al.28 show that efferent lymph and highly reactive LN contain a substantial proportion of CD56dim KIR+ NK cells.
In contrast, Keskin et al.30 demonstrate that upon culture in the presence of transforming growth factor-β, CD16+ NK cells convert into CD56superbright CD16− CD9+ CD103+ KIR+ NK cells. However, this phenotype corresponds to decidual NK cells and not to peripheral blood CD56bright NK cells, these populations being very different from each other.23 It could nevertheless be interesting to investigate if the transition from CD56dim CD16+ cells into CD56bright CD16− NK cells is possible in vitro, although this would go against the current paradigm.
CD56bright NK cell expansions and reductions in human pathology
In this field, several recent observations have been published. Some authors only investigate CD56bright CD16− NK cells, whereas others also include CD56bright CD16dim NK cells in their studies. We suggest that this point should be harmonized between different groups interested in the topic, and to follow the leader in the field, Michael A. Caligiuri1, who always considers both subpopulations together as CD56bright NK cells. Indeed, there is no obvious reason to ignore the CD56bright CD16dim subset.
Expansion of CD56bright NK cells
In healthy donors, not more than 10% of all peripheral blood NK cells are usually CD56bright. However, expansions above this low percentage have been described during reconstitution of the immune system after bone marrow transplantation, where the first lymphocytes to appear in blood are CD56bright NK cells.31 This population also increases in patients who are treated daily with a low dose of IL-2.31
Interestingly, there are increasing numbers of papers describing expansions of CD56bright NK cells in different diseases, and the list is probably far from being finished.
Our group32 has shown that in two cases of transporter associated with antigen processing (TAP) deficiency, which is characterized biologically by a very low amount of surface expression of HLA class I molecules, and clinically by chronic respiratory infections, bronchiectasis and deep skin ulcers, CD56bright NK cells represent 37% and 54%, respectively, of all peripheral blood NK cells. This was recently confirmed, although to a lesser extent, in a new case of TAP deficiency.33 Interestingly, asymptomatic TAP-deficient patients have normal percentages of CD56bright NK cells.32 In the first study, we compared the TAP-deficient patients with a small series of individuals with vasculitis or with respiratory diseases of other origins. The percentage of CD56bright NK cells was higher than 10% (12·60–25·52%) in approximately one-third of them.32 These data suggest that the expansion of CD56bright NK cells is in no way a hallmark of TAP deficiency, but can occur in several diseases of other origins.
In favour of this interpretation, Cac and Ballas34 present the observation of a woman with recalcitrant and treatment-resistant periungual warts. Flow cytometry revealed that 13·30% of her lymphocytes (and not of her NK cells, which makes the expansion quite dramatic) were CD56bright and only 0·83% of the lymphocytes were CD56dim. Not surprisingly, the patient had a severe reduction in NK cell-mediated cytotoxicity. The warts almost disappeared and NK cell cytotoxicity was restored upon treatment with IFN-α, whereas the proportion of CD56bright NK cells decreased. It is well known that IFN-α stimulates the cytotoxicity of NK cells, but maybe it also favours the maturation of NK cells towards the CD56dim stage. This process was probably blocked before the introduction of IFN-α, but for completely unknown reasons.
An inverse situation is described by Saraste et al.35 who treated a group of 11 multiple sclerosis patients with IFN-β and observed an expansion of CD56bright NK cells with a concomitant decrease of CD56dim cells after 12 months of treatment (14·7 ± 2·5% of all NK cells were CD56bright).
In another series of 22 multiple sclerosis patients36 a combined treatment with IFN-β and daclizumab was administered. Daclizumab is a humanized monoclonal antibody directed against the IL-2 receptor α chain (CD25) that was very efficient in stabilizing multiple sclerosis in clinical trials. Also in this case, an expansion of CD56bright NK cells, which correlated with a reduction in brain inflammation, was observed during treatment. The mechanism of action most likely corresponds to an upregulation of CD122 (β chain of the IL-2 receptor) with a better response to endogenous IL-2 as a consequence. In vitro, NK cells from the patients inhibited T-cell proliferation in a contact-dependent manner and are therefore considered as regulatory NK cells by the authors.
A study using daclizumab in five patients with active uveitis37 confirmed its therapeutic efficiency on the one hand and the selective expansion of CD56bright NK cells over time (it ranged from fourfold to 20-fold) on the other hand. The CD56bright NK cells produced high amounts of the immunosuppressive cytokine IL-10, and this molecule might be at the origin of the beneficial effect by downregulating the autoimmune response. It is tempting to speculate that CD56bright-mediated secretion of IL-10 explains the therapeutic effects in the three studies presented. Likewise, an efficient immune response against human papillomavirus-induced periungual warts in the case described by Cac and Ballas34 might have been precluded because of the IL-10 production by the abundant CD56bright NK cells. In this context, the concept of regulatory NK cells (‘NKreg’) is clearly emerging in the literature22,38 and deserves further investigation to check, for example, if the entire CD56bright population or only a subset have immunoregulatory properties.
In a case report of a human herpes virus type 6-associated acute necrotizing encephalopathy in a young child, Kubo et al.39 likewise noticed a dramatic expansion of CD56bright NK cells during the convalescent phase. Here, the authors speculate, without any demonstration, that the CD56bright NK cells produce high levels of inflammatory cytokines (indeed found in the serum of the patient) and that a high percentage of these cells is a risk factor for the development of encephalitis during infection with human herpes virus type 6.
A patient with X-linked severe combined immunodeficiency with Omenn syndrome-like manifestations40 had increased circulating NK cells (59·50% of lymphocytes), half of them (28·50%) being CD56bright CD16−. The skin, displaying marked thickening and severe erythema, was infiltrated predominantly by CD56bright CD16− NK cells expressing high levels of messenger RNA for not only inflammatory cytokines but also for IL-10.
In a cohort of female patients chronically infected with hepatitis C virus (HCV)41 compared with HCV resolvers and normal uninfected controls, total NK cell percentages among lymphocytes were reduced in the first group whereas the proportion of CD56bright cells among total NK cells was increased. These cells produced more IFN-γ than CD56bright NK cells from the other two groups, and overall NK cell cytotoxicity was not impaired.
Exactly the same picture, i.e. reduction of NK cell numbers but increased percentage of CD56bright NK cells, was found among individuals with a positive tuberculin skin test (TST+) compared with patients with overt tuberculosis and normal controls.42
The significance of the observations from these two studies is currently unclear. In the case of HCV infection, the authors41 speculate that CD56bright NK cells might contribute to T-cell polarization and liver damage, and that their expansion might be the result of a decreased rate of differentiation towards CD56dim cells. The TST+ subjects might be protected from active tuberculosis by the CD56bright NK cells secreting high amounts of IFN-γ.42
Schepis et al.43 describe an increased proportion of CD56bright NK cells in systemic lupus erythematosus regardless of disease activity. However, the authors do not consider nor do they investigate CD56bright CD16dim cells, their gating strategy does not exclude CD56dim CD16− cells, and finally, as most patients have one or more treatments at the time of blood drawing, the potential role of these treatments in the CD56bright NK cell expansion is not considered. Nevertheless, systemic lupus erythematosus might be added to the growing list of diseases characterized by an increased proportion of CD56bright NK cells, although this topic deserves further investigation.
Although they unfortunately do not discriminate between CD56bright and CD56dim NK cells, three papers44–46 describe a dramatic expansion of NKG2C+ NK cells in human infections caused by human immunodeficiency virus type 1 and cytomegalovirus. As a subset of CD56bright NK cells expresses NKG2C,47 this discrimination would be very interesting and important to make in future investigations.
Whereas the studies presented so far all deal with peripheral blood NK cells, others describe the presence of CD56bright NK cells in different tissues during disease states. CD56bright CD16− KIR– NK cells are found, among other cell types, within acute psoriatric plaques and abundantly produce IFN-γ upon stimulation.48 In rheumatoid arthritis, the proportions of different peripheral blood NK cell subsets are the same as in healthy donors, but the synovial fluid of the patients almost exclusively contains CD56bright KIR– NK cells.49 Likewise in sarcoidosis,50 no difference is observed between patients and normal controls in peripheral blood, whereas the former have more CD56bright NK cells in their bronchoalveolar lavage fluid.
Reduction of CD56bright NK cells
Interestingly, several recent observations show that in some diseases, the percentage of CD56bright NK cells is reduced. In patients with coronary heart disease,51 overall NK cell numbers are diminished as is the cytotoxic activity, and in addition, there is a tendency towards lower percentages of CD56bright NK cells in the patients compared with normal controls. The rationale of this study was the fact that infections are considered to be a risk factor for coronary heart disease and that NK cells participate in immune responses against viruses and bacteria.
By analysing a small series of patients with allergic rhinitis and/or asthma, Scordamaglia et al.52 found a significantly reduced percentage of CD56bright NK cells compared with non-allergic individuals. The consequences are a weak IFN-γ production and impaired interactions with DC, so that in allergic diseases, NK cells might be unable to sufficiently shape adaptive immunity in the T helper type 1 direction. All these data will have to be confirmed by larger series but they provide interesting indications.
In juvenile rheumatoid arthritis with systemic onset, not only is the NK cell cytotoxic activity strongly reduced, but in some patients, the total absence of CD56bright NK cells has been described.53 The same phenomenon had previously been found in patients with macrophage activation syndrome and haemophagocytic lymphohistiocytosis. The authors suggest, without demonstrating it, that the CD56bright NK cells have all been recruited to the sites of inflammation. It would be very interesting to check if in these patients, CD56bright NK cells are found in the LN or in the synovium for example. If not, one would have to ask the question how CD56dim NK cells can arise in the absence of CD56bright precursors.
Conclusion
CD56bright NK cells are currently extensively investigated and are no longer considered as just a minor subpopulation among total NK cells. As a result of their production of different cytokines, they might be important in early immune responses and in the shaping of the adaptive response (IFN-γ) as well as playing a role of regulatory NK cells (IL-10). This last point clearly deserves further studies.
Another interesting and emerging concept is the observation of increases or reductions, respectively, in the percentages of CD56bright NK cells in various diseases. Why are these cells expanded in several clinical conditions? What are the mechanisms leading to the expansion? One might suppose that CD56dim NK cells have a high turnover under these conditions and have to be replaced, and consequently their precursor cells (CD56bright) are released in high numbers from the bone marrow and/or the LN. On the other hand, CD56bright NK cells and their cytokine production might be important on their own in certain diseases and they would therefore selectively expand. Are these expansions a consequence of or a predisposing factor of the disease? Are they beneficial or deleterious for the host? The same questions of course also arise regarding the reductions or the absence of CD56bright NK cells.
Rapid progress in this field can be expected, and soon we will know much more about the true relevance of the CD56bright NK cell population in human health and disease.
References
- 1.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 2.Mavilio D, Lombardo G, Benjamin J, et al. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci USA. 2005;102:2886–91. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caligiuri M. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilk E, Kalippke K, Buyny S, Schmidt RE, Jacobs R. New aspects of NK cell subset identification and inference of NK cells’ regulatory capacity by assessing functional and genomic profiles. Immunobiology. 2008;213:271–83. doi: 10.1016/j.imbio.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143:3183–91. [PubMed] [Google Scholar]
- 7.Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, Sykora KW, Schmidt RE. CD56bright NK cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol. 2001;31:3121–6. doi: 10.1002/1521-4141(2001010)31:10<3121::aid-immu3121>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–93. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 9.Vitale M, Zimmer J, Castriconi R, et al. Analysis of natural killer cells in TAP2-deficient patients: expression of functional triggering receptors and evidence for the existence of inhibitory receptor(s) that prevent lysis of normal autologous cells. Blood. 2002;99:1723–9. doi: 10.1182/blood.v99.5.1723. [DOI] [PubMed] [Google Scholar]
- 10.Wendt K, Wilk E, Buyny S, Schmidt RE, Jacobs R. Interleukin-21 differentially affects human natural killer cell subsets. Immunology. 2007;122:486–95. doi: 10.1111/j.1365-2567.2007.02675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–82. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 12.Sedlmayr P, Schallhammer L, Hammer A, Wilders-Truschnig M, Wintersteiger R, Dohr G. Differential phenotypic properties of human peripheral blood CD56dim+ and CD56bright+ natural killer cell subpopulations. Int Arch Allergy Immunol. 1996;110:308–13. doi: 10.1159/000237321. [DOI] [PubMed] [Google Scholar]
- 13.Anumanthan A, Bensussan A, Boumsell L, et al. Cloning of BY55, a novel Ig superfamily member expressed on NK cells, CTL, and intestinal intraepithelial lymphocytes. J Immunol. 1998;161:2780–90. [PubMed] [Google Scholar]
- 14.Solomon KR, Chan M, Finberg RW. Expression of GPI-anchored complement regulatory proteins CD55 and CD59 differentiates two subpopulations of human CD56+ CD3− lymphocytes (NK cells) Cell Immunol. 1995;165:294–301. doi: 10.1006/cimm.1995.1217. [DOI] [PubMed] [Google Scholar]
- 15.Hanna J, Bechtel P, Zhai Y, Youssef F, McLachlan K, Mandelboim O. Novel insights on human NK cells’ immunological modalities revealed by gene expression profiling. J Immunol. 2004;173:6547–63. doi: 10.4049/jimmunol.173.11.6547. [DOI] [PubMed] [Google Scholar]
- 16.Wendt K, Wilk E, Buyny S, Buer J, Schmidt RE, Jacobs R. Gene and protein characteristics reflect functional diversity of CD56dim and CD56bright NK cells. J Leukoc Biol. 2006;80:1529–41. doi: 10.1189/jlb.0306191. [DOI] [PubMed] [Google Scholar]
- 17.Fehniger TA, Shah MH, Turner MJ, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–20. [PubMed] [Google Scholar]
- 18.Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA, Moretta A, Münz C. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–62. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 19.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–7. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 20.Ferlazzo G, Münz C. NK cell compartments and their activation by dendritic cells. J Immunol. 2004;172:1333–9. doi: 10.4049/jimmunol.172.3.1333. [DOI] [PubMed] [Google Scholar]
- 21.Vitale M, Bottino C, Sivori S, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187:2065–72. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito S, Nakashima A, Myojo-Higuma S, Shiozaki A. The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy. J Reprod Immunol. 2008;77:14–22. doi: 10.1016/j.jri.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Koopman LA, Kopcow HD, Rybalov B, et al. Human decidual natural killer cells are a unique NK subset with immunomodulatory potential. J Exp Med. 2003;198:1201–12. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vitale M, Della Chiesa M, Carlomagno S, Romagnani C, Thiel A, Moretta L, Moretta A. The small subset of CD56brightCD16− natural killer cells is selectively responsible for both proliferation and interferon-γ production upon interaction with dendritic cells. Eur J Immunol. 2004;34:1715–22. doi: 10.1002/eji.200425100. [DOI] [PubMed] [Google Scholar]
- 25.Dalbeth N, Gundle R, Davies RJ, Lee YC, McMichael AJ, Callan MF. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J Immunol. 2004;173:6418–26. doi: 10.4049/jimmunol.173.10.6418. [DOI] [PubMed] [Google Scholar]
- 26.André P, Spertini O, Guia S, et al. Modification of P-selectin glycoprotein ligand-1 with a natural killer cell-restricted sulphated lactosamine creates an alternate ligand for L-selectin. Proc Natl Acad Sci U S A. 2000;97:3400–5. doi: 10.1073/pnas.040569797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan A, Hong DL, Atzberger A, et al. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J Immunol. 2007;179:89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 28.Romagnani C, Juelke K, Falco M, et al. CD56brightCD16− killer Ig-like receptor− NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2008;178:4947–55. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 29.Ouyang Q, Baerlocher G, Vulto I, Lansdorp PM. Telomere length in human natural killer cell subsets. Ann N Y Acad Sci. 2007;1106:240–52. doi: 10.1196/annals.1392.001. [DOI] [PubMed] [Google Scholar]
- 30.Keskin DB, Allan DS, Rybalov B, Andzelm MM, Stern JN, Kopcow HD, Koopman LA, Strominger JL. TGFβ promotes conversion of CD16+ peripheral blood NK cells into CD16− NK cells with similarities to decidual NK cells. Proc Natl Acad Sci USA. 2007;104:3378–83. doi: 10.1073/pnas.0611098104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carson W, Caligiuri M. Natural killer cell subsets and development. Methods. 1996;9:327–43. doi: 10.1006/meth.1996.0038. [DOI] [PubMed] [Google Scholar]
- 32.Zimmer J, Bausinger H, Andrès E, Donato L, Hanau D, Hentges F, Moretta A, de la Salle H. Phenotypic studies of natural killer cell subsets in human transporter associated with antigen processing deficiency. PLoS ONE. 2007;2:e1033. doi: 10.1371/journal.pone.0001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villa-Forte A, de la Salle H, Fricker D, Hentges F, Zimmer J. HLA class I deficiency syndrome mimicking Wegener’s granulomatosis. Arthritis Rheum. 2008;58:2579–82. doi: 10.1002/art.23675. [DOI] [PubMed] [Google Scholar]
- 34.Cac NC, Ballas ZK. Recalcitrant warts, associated with natural killer cell dysfunction, treated with systemic IFN-α. J Allergy Clin Immunol. 2006;118:526–8. doi: 10.1016/j.jaci.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 35.Saraste M, Irjala H, Airas L. Expansion of CD56bright natural killer cells in the peripheral blood of multiple sclerosis patients treated with interferon-beta. Neurol Sci. 2007;28:121–6. doi: 10.1007/s10072-007-0803-3. [DOI] [PubMed] [Google Scholar]
- 36.Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2Rα -targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;103:5941–6. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Lim WK, Mahesh SP, Liu B, Nussenblatt RB. Cutting edge: in vivo blockade of human IL-2 receptor induces expansion of CD56bright regulatory NK cells in patients with active uveitis. J Immunol. 2005;174:5187–91. doi: 10.4049/jimmunol.174.9.5187. [DOI] [PubMed] [Google Scholar]
- 38.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 39.Kubo T, Sato K, Kobayashi D, Motegi A, Kobayashi O, Takeshita S, Nonoyama S. A case of HHV-6 associated acute necrotizing encephalopathy with increase of CD56bright NK cells. Scand J Infect Dis. 2006;38:1122–5. doi: 10.1080/00365540600740520. [DOI] [PubMed] [Google Scholar]
- 40.Shibata F, Toma T, Wada T, et al. Skin infiltration of CD56brightCD16− natural killer cells in a case of X-SCID with Omenn syndrome-like manifestations. Eur J Haematol. 2007;79:81–85. doi: 10.1111/j.1600-0609.2007.00874.x. [DOI] [PubMed] [Google Scholar]
- 41.Golden-Mason L, Madrigal-Estebas L, McGrath E, Conroy MJ, Ryan EJ, Hegarty JE, O’Farrelly C, Doherty DG. Altered natural killer cell subset distributions in resolved and persistent hepatitis C virus infection following single source exposure. Gut. 2008;57:1121–8. doi: 10.1136/gut.2007.130963. [DOI] [PubMed] [Google Scholar]
- 42.Barcelos W, Sathler-Avelar R, Martins-Filho OA, et al. Natural killer cell subpopulations in putative resistant individuals and patients with active Mycobacterium tuberculosis infection. Scand J Immunol. 2008;68:92–102. doi: 10.1111/j.1365-3083.2008.02116.x. [DOI] [PubMed] [Google Scholar]
- 43.Schepis D, Gunnarsson I, Eloranta MJ, Lampa J, Jacobson SH, Kärre K, Berg L. Increased proportion of CD56bright natural killer cells in active and inactive systemic lupus erythematosus. Immunology. 2009;126:140–6. doi: 10.1111/j.1365-2567.2008.02887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gumá M, Angulo A, Vilches C, Gómez-Lozano N, Malats N, López-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–71. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 45.Gumá M, Cabrera C, Erkizia I, Bofill M, Clotet B, Ruiz L, López-Botet M. Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1-positive patients. J Infect Dis. 2006;194:38–41. doi: 10.1086/504719. [DOI] [PubMed] [Google Scholar]
- 46.Mela CM, Burton CT, Imami N, Nelson M, Steel A, Gazzard BG, Gotch FM, Goodier MR. Switch from inhibitory to activating NKG2 receptor expression in HIV-1 infection: lack of reversion with highly active antiretroviral therapy. AIDS. 2005;19:1761–9. doi: 10.1097/01.aids.0000183632.12418.33. [DOI] [PubMed] [Google Scholar]
- 47.Kusumi M, Yamashita T, Fujii T, Nagamatsu T, Kozuma S, Taketani Y. Expression patterns of lectin-like natural killer receptors, inhibitory CD94/NKG2A, and activating CD94/NKG2C on decidual CD56bright natural killer cells differ from those on peripheral CD56dim natural killer cells. J Reprod Immunol. 2006;70:33–42. doi: 10.1016/j.jri.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Ottaviani C, Nasorri F, Bedini C, de Pita O, Girolomoni G, Cavani A. CD56brightCD16− NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. 2006;36:118–28. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- 49.Pridgeon C, Lennon GP, Pazmany L, Thompson RN, Christmas SE, Moots RJ. Natural killer cells in the synovial fluid of rheumatoid arthritis patients exhibit a CD56bright, CD94bright, CD158negative phenotype. Rheumatology. 2003;42:870–8. doi: 10.1093/rheumatology/keg240. [DOI] [PubMed] [Google Scholar]
- 50.Katchar K, Söderström K, Wahlstrom J, Eklund A, Grunewald J. Characterisation of natural killer cells and CD56+ T-cells in sarcoidosis patients. Eur Respir J. 2005;26:77–85. doi: 10.1183/09031936.05.00030805. [DOI] [PubMed] [Google Scholar]
- 51.Hak L, Mysliwska J, Wieckiewicz J, Szyndler K, Trzonkowski P, Siebert J, Mysliwski A. NK cell compartment in patients with coronary heart disease. Immun Ageing. 2007;4:3. doi: 10.1186/1742-4933-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scordamaglia F, Balsamo M, Scordamaglia A, Moretta A, Mingari MC, Canonica GW, Moretta L, Vitale M. Perturbations of natural killer cell regulatory functions in respiratory allergic diseases. J Allergy Clin Immunol. 2008;121:479–85. doi: 10.1016/j.jaci.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 53.Villanueva J, Lee S, Giannini EH, Graham TB, Passo MH, Filipovich A, Grom AA. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res Ther. 2005;7:R30–7. doi: 10.1186/ar1453. [DOI] [PMC free article] [PubMed] [Google Scholar]