Abstract
Exposure to psychostimulant drugs of abuse such as amphetamine can result in long-lasting “sensitization” of reward-directed behavior, such that subjects display enhancements in behavior directed by and toward rewards and reward-predictive cues (i.e. – “incentive sensitization”). The purpose of these experiments was to determine the degree to which such sensitization resulting from chronic amphetamine exposure influences both appetitive and consummatory food-motivated behavior. Adult male Long-Evans rats received daily i.p. injections of d-amphetamine (2.0 mg/kg) or saline vehicle for five consecutive days. This amphetamine exposure regimen produced lasting sensitization to the acute locomotor stimulant effect of the drug. One month after drug exposure rats were tested for instrumental responding (lever pressing) for food reward under various response schedules. Two months after drug exposure, rats were tested for food consumption in a discriminative Pavlovian context-potentiated eating task, involving pairings of one context with food and another context with no food. Amphetamine-exposed rats showed significantly greater instrumental responding for food reward than saline controls, particularly under conditions of high response ratios. In the potentiated eating task, testing under conditions of food satiation revealed that amphetamine-exposed rats ate significantly more than saline controls in the food-paired context. These experiments demonstrate that amphetamine exposure can cause enduring increases in both appetitive and consummatory aspects of natural reward-directed behavior. Such long-lasting incentive sensitization could account in part for the propensity for relapse in drug addiction, as well as for reported enhancements in non-drug reward-related behavior.
Keywords: Psychostimulant, amphetamine, instrumental responding, sensitization, incentive motivation
1. Introduction
Societal problems associated with addiction include costs of physical and psychological treatment, loss of productivity and social cohesion, crime, and accidents [1-3]. In addition, there is growing recognition that drug addiction can result in a range of cognitive and behavioral alterations that can far outlast the period of drug use and may contribute to relapse [4-9]. Animal models of addiction have shown that chronic administration of psychostimulant drugs in particular can cause a variety of long-lasting alterations in drug-related behaviors. For example, it is very well documented that repeated psychostimulant administration can lead to long lasting enhancements (sensitization) of the locomotor stimulant response to these drugs [10-14]. Repeated psychostimulant administration can also enhance, or sensitize, behaviors motivated by drug rewards (incentives). This “incentive sensitization” may contribute to addiction via exaggerated drug “wanting” and motivation to pursue and use drug rewards [15-17].
Chronic exposure to psychostimulants is also associated with long-lasting increases in behavior directed by and toward non-drug rewards, including sex [18-20], gambling [21, 22], and food [23-28]. However, with few exceptions [18] these effects of psychostimulant exposure have been on appetitive (reward “seeking”) rather than consummatory (reward “taking”) components of reward-directed behavior. Moreover, there are several reports that chronic psychostimulant exposure can result in decreases in reward-directed behavior or anhedonia [29-33]. The purpose of the experiments reported here was two-fold: first, to determine how amphetamine exposure would affect both appetitive and consummatory components of behavior, and second, to determine whether a regimen of exposure to the psychostimulant drug amphetamine would cause lasting increases or decreases in reward-directed behavior.
2. Materials and Methods
2.1 Subjects
The subjects were male Long-Evans rats weighing 250-275 g upon arrival (Charles River Laboratories, Wilmington, NC, USA). Rats were housed individually in a climate-controlled vivarium (25° C) in the Department of Psychology at Texas A&M University. Rats had food and water available ad lib (except as noted below) and were tested during the light cycle of a 12 hour light/dark schedule (lights on 0800-2000). Animal testing was conducted according to the “Principles of Laboratory Animal Care” (National Academy of Sciences, USA) and met all NIH and institutional animal care and use guidelines. Rats were allowed to acclimate to vivarium conditions for at least one week before the start of amphetamine exposure.
2.2 Behavioral Apparatus
2.2.1 Instrumental Responding Task
Instrumental responding was assessed in four identical standard rat behavioral test chambers (31 X 25 X 31 cm) with aluminum front and back walls, acrylic side walls, and a floor composed of steel rods (0.4 cm diameter) spaced 1.1 cm apart, located in sound-attenuating cubicles (Coulbourn Instruments, Whitehall, PA, USA). A recessed food delivery trough (4.1 X 3.2 cm) equipped with a photobeam to detect head entries was located in the center of the front wall of the chambers (2.2 cm above the floor). A standard response lever was placed above the food trough (9 cm above the floor). The chambers were interfaced with a computer running Graphic State 3.01 software (Coulbourn Instruments), which controlled stimulus deliveries and recorded data.
2.2.2 Potentiated-Eating Task
For the potentiated eating task, animals were exposed to two novel contexts located in separate rooms. Context “A” was a large opaque chamber (49 × 33 × 28 cm) and context “B” was a smaller translucent chamber (40 × 26 × 17 cm). Food pellets were presented in 50 mL glass jars that were placed inside the chambers. Note that although food consumption in rats is generally greater during the dark cycle, rats were tested during the light cycle to avoid disruption of circadian rhythms by removing animals from a darkened vivarium to lighted hallways and testing rooms [34, 35].
2.2.3 Locomotor assessment
Sensitization to the acute locomotor stimulant effects of amphetamine was tested in 8 identical activity-monitoring chambers (Versamax System, Accuscan Instruments, Columbus, OH, USA). Each chamber (40 × 40 × 30 cm) contained an array of photobeams raised 0.5 cm above the floor to detect movement in the horizontal plane. The activity chambers were connected to a computer running Versamap software (Accuscan Instruments), which recorded photobeam breaks.
2.3 Drugs
D-Amphetamine sulfate was kindly provided by the Drug Supply Program at the National Institute on Drug Abuse. Amphetamine was dissolved in 0.9% saline vehicle and all solutions were administered intraperitoneally at a volume of 1 ml/kg. For treatment, rats (n=8/group) were given injections of 2 mg/kg d-amphetamine (calculated as the weight of the salt) or saline vehicle once daily in their home cage for 5 consecutive days.
2.4 Procedures
2.4.1 Instrumental Responding
Rats were food restricted to 85% of their free feeding weight over the course of seven days prior to testing. One month after drug exposure rats were given a single day of magazine training, followed by lever press shaping in the test chambers. In these shaping sessions, rats were shaped to press the lever for immediate reward (a single 45 mg grain-based food pellet, TestDiet, Richmond, IN, USA) on an FR1 schedule until they reached a criterion of 100 lever presses in a 30 minute session. Following shaping, lever pressing was assessed using fixed ratio (FR) schedules (FR3, 10, 20, 40, 1, one schedule/day). Test sessions in the fixed ratio task were also 30 minutes in length. After testing with the fixed ratio schedules, instrumental responding was assessed using a progressive ratio schedule of reinforcement, on which the number of lever presses required to earn a reward increased with each successive reward earned (1, 4, 10, 20, 35, …) [36-38]. Rats were tested in the progressive ratio task for four consecutive sessions. These sessions varied in length, ending only after an hour with no reward delivery had passed (the breakpoint). Upon completion of these tasks, rats were returned to an ad lib feeding schedule.
2.4.2 Potentiated-Eating Task
Two months after amphetamine exposure, rats were once again food restricted to 85% of their free-feeding weight and trained in a discriminative Pavlovian context-potentiated eating task, involving food-paired and -unpaired contexts [35, 39]. The order in which the instrumental and potentiated eating tasks were conducted was based on availability of the experimental apparatus. Rats received eight daily 10 minute exposures to a paired context (which contained 7g of food in the form of the same 45 mg grain-based pellets used in the instrumental tasks) intermixed with eight equivalent exposures to an unpaired context (without food) in a semi-random order. The identities of the paired and unpaired contexts were counterbalanced across contexts A and B and drug condition. After returning the rats to their free-feeding weights over the course of 7 days, the ability of the paired and unpaired contexts to promote food consumption under satiated conditions was tested on two consecutive days in a counterbalanced order. For these test sessions, rats were placed in each context for a 10 minute free feeding session in the presence of 15 g of food. Food consumption was assessed by weighing the food remaining at the end of each session.
2.4.3 Locomotor assessment
To determine whether the amphetamine exposure regimen used in the previous experiments also produced locomotor sensitization, rats were treated with amphetamine or saline (n=4/group) using the same treatment protocol described above. One month after drug exposure, sensitization to the acute locomotor stimulant effects of amphetamine was tested. Under red light and white noise, rats were placed in the activity chambers for a 15 minute habituation period, after which they were briefly removed, injected with amphetamine (2 mg/kg), and returned to the chambers for an additional 30 minutes.
2.5 Data Analysis
Data analyses were conducted using SPSS 13.0. For instrumental responding, the number of lever presses and the number of rewards received were analyzed separately for each FR schedule using unpaired t-tests. The highest response ratio achieved in the progressive ratio task across the four sessions was analyzed using a two-factor repeated measures ANOVA (session X drug condition). During training and testing in the potentiated eating task, food consumption was analyzed using two-factor repeated measures ANOVAs (Training: session X drug condition; Testing: paired/unpaired context X drug condition). Performance in the instrumental task was compared to performance in the potentiated eating task using Pearson's partial correlations to factor out the effect of amphetamine exposure. In the test of locomotor sensitization in the activity chambers, horizontal activity (number of photobeam breaks) was collapsed into five minute bins across the 15 minutes before and 30 minutes after amphetamine injections. Baseline activity data for the five minute bins just prior to amphetamine exposure were analyzed using a two-tailed t-test. Data in each post-amphetamine bin were normalized to baseline activity by calculating the percent change from baseline. These normalized values were analyzed using a repeated measures ANOVA (bin X prior drug condition). In all cases p values less than .05 were considered significant.
3. Results
3.1 Instrumental Responding
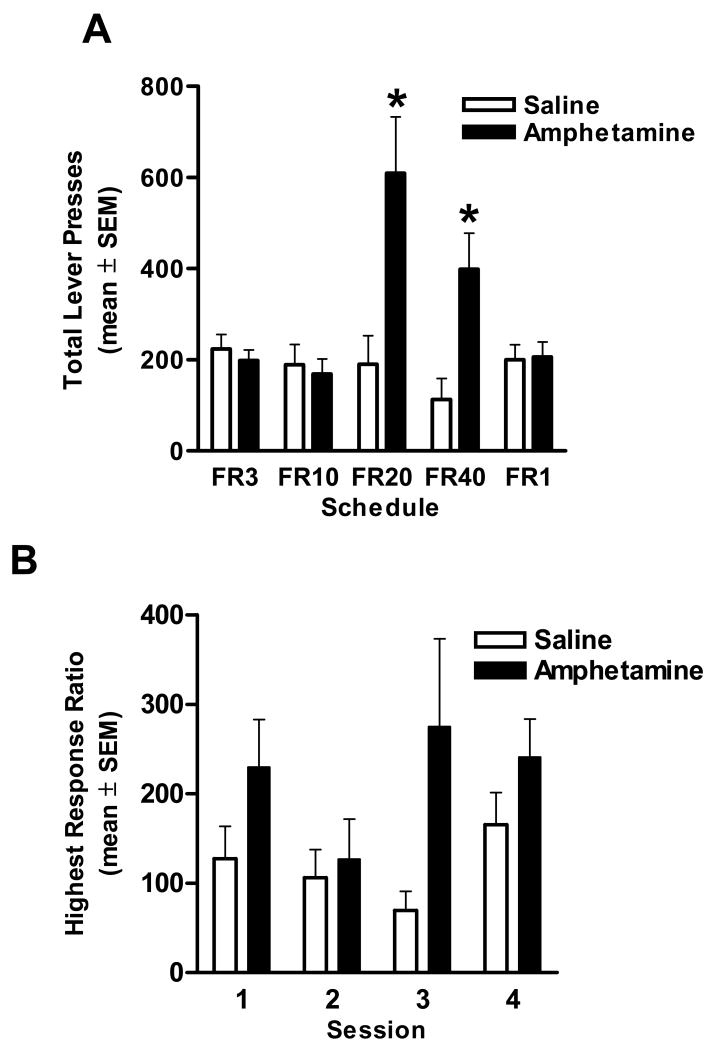
There were no differences in body weight between amphetamine and saline exposed rats either immediately prior to or during food restriction in the instrumental responding task (unpaired t-tests, ts(14) < .76, ps > .46). There was also no difference in the number of shaping session required by the two groups to reach criterion (t(1,14) = .37, n.s.). On the lower fixed ratio schedules of reinforcement (FR1, 3, and 10), amphetamine exposed rats did not differ from saline controls in either their number of total responses or rewards earned (ts(14) < 1.06, ps > .31). However, amphetamine exposed rats did show a robust increase in instrumental responding under the higher fixed ratio schedules, with greater numbers of lever presses on both the FR20 (t(14) = 3.04, p < .05) and FR40 (t(14) = 3.15, p < .01) schedules (Figure 1A). Similar results were observed in analyses of the number of rewards earned under these schedules (ts(14) > 2.65, ps < .02). On the progressive ratio schedule, a two-factor repeated measures ANOVA (session X drug condition) revealed a significant main effect of drug condition, such that across the four testing sessions amphetamine exposed rats achieved significantly higher response ratios compared to saline controls (F(1,14) = 5.15, p < .05; Figure 1B), but no main effect or interaction involving session (Fs < 1.44, ps > .24). As expected, similar results were obtained in comparisons of total lever presses (amphetamine M = 251.13, SE = 64.79; saline M = 140.72, SE = 35.68 across the 4 days of testing, F(1,14) = 4.43, p = .05) and number of rewards received (amphetamine M = 9.32, SE = 1.00; saline M = 7.38, SE = .93 across the 4 days, F(1,14) = 3.47, p = .08).
Fig. 1.
Effects of amphetamine exposure (2 mg/kg/day × 5 days) one month earlier on instrumental responding for food reward. (A) Effects of prior amphetamine exposure on instrumental responding for food under fixed ratio schedules. Bars indicate number of lever presses under each fixed ratio schedule. There were no differences in performance under the FR1, 3, and 10 schedules, but amphetamine exposed rats (filled bars) had significantly more lever presses under the higher ratio schedules (FR20 and FR40) than saline exposed controls (open bars). * p<.05 (B) Effects of prior amphetamine exposure on 4 sessions of testing under a progressive ratio of responding for food reward. Data points indicate the highest response ratio achieved on each day. Amphetamine exposed rats achieved significantly higher response ratios compared to saline controls.
3.2 Context Potentiated Eating
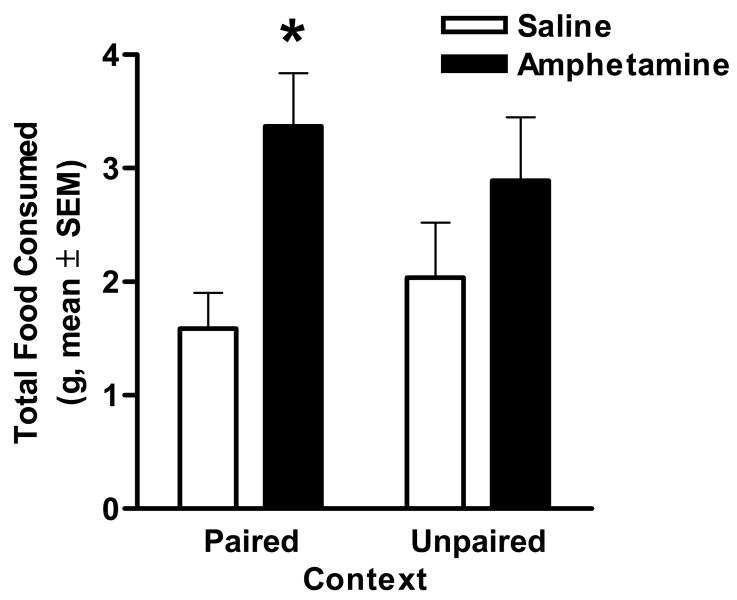
A 2-factor repeated measures ANOVA conducted on the data from training sessions (session X drug condition) revealed that both saline and amphetamine exposed rats increased the amount of food consumed across the 8 days of exposure to the paired context (F(1,7) = 13.77, p < .01), but there were no differences between groups in the amount of food consumed during these sessions (F(1,14) = .91, n.s.). In the test sessions, a two-factor repeated measures ANOVA (paired/unpaired context X drug condition) revealed no main effect of pairing (i.e.- across groups, rats did not eat more in the paired than the unpaired context) (F(1,14) = .003, n.s.). Importantly, however, there was a significant main effect of drug condition (F(1,14) = 4.78, p < .05), such that across both paired and unpaired contexts, amphetamine exposed rats ate significantly more than saline controls during testing (Figure 2). There was also a trend toward an interaction between context and drug condition (F(1,14) = 3.04, p = .10). Planned comparisons (both paired and unpaired t-tests) within pairing and drug conditions confirmed that although neither saline nor amphetamine exposed rats differed in their food consumption in the paired and unpaired contexts (ts(14) > 1.43, ps > .20), amphetamine exposed rats ate significantly more than saline controls in the paired (t(14) = 3.14, p < .01), but not unpaired (t(14) = 1.16, n.s.) context. Finally, significant partial correlations were obtained when comparing total food consumed across both paired and unpaired test days in the potentiated eating task and performance during sessions one (r = .53, p < .05) and two (r = .71, p < .01) of the progressive ratio task, such that rats that ate more in the potentiated eating task also had more lever presses in the instrumental tasks.
Fig. 2.
Effects of prior amphetamine exposure on context-potentiated eating. Bars indicate total food consumed during each test session. Amphetamine exposed rats (filled bars) ate significantly more than controls in the food-paired context. * p < .05.
3.3 Locomotor Sensitization Test
There were no activity differences between groups during the 5 minute baseline period prior to amphetamine administration (t(6) = 1.77, n.s.). Following acute amphetamine, a repeated measures ANOVA (time bin X prior drug condition) revealed both a main effect of time bin, such that activity increased across time (F(1, 6) = 26.55, p < .01), and a main effect of prior drug condition (F(1, 6) = 6.97, p < .05), such that rats that received prior amphetamine exposure displayed significantly greater activity compared to saline controls (Figure 3). These data indicate that the amphetamine exposure regimen used in these experiments is sufficient to induce locomotor sensitization.
Fig. 3.
Horizontal locomotor activity in amphetamine exposed and saline control rats (in 5 minute bins) during a 5 minute pre-drug baseline period (BL bin) and 30 minutes (bins 1-6) after an amphetamine challenge injection (2 mg/kg). Amphetamine exposed rats displayed significantly more locomotor activity in response to the amphetamine challenge than saline controls.
4. Discussion
The results of these experiments demonstrate that exposure to the psychostimulant drug of abuse amphetamine causes long lasting enhancements in both appetitive (“seeking”) and consummatory (“taking”) components of behavior directed toward a natural reward (food). Following one month of withdrawal, amphetamine exposed rats displayed higher levels of instrumental responding for food reward than saline exposed controls (specifically under high response ratio requirements), suggesting enhanced reward-directed motivation similar to that directed toward drug-related rewards following drug use [19, 26-28, 40-42]. Furthermore, under conditions of food satiation, amphetamine-exposed rats ate significantly more food than saline controls in an environment previously associated with food. These findings suggest that incentive sensitization resulting from repeated exposure to amphetamine similarly increases both appetitive (reward seeking) and consummatory (reward taking) behavior. It should be noted that the enhanced food consumption observed in the potentiated eating task was not likely due to enhanced “liking” of the food reward, as repeated drug exposure does not appear to enhance rewards' hedonic properties [25, 43]. Because enhanced consumption was only observed in the amphetamine exposed animals when tested in the food-paired context of the potentiated eating task under satiation conditions (and not during presentation of the food during the training sessions under conditions of food-restriction), it is perhaps more likely that this enhancement was due to enhanced control of motivation and behavior by the reward-associated context [23, 44]. Consistent with this idea, prior amphetamine exposure has been previously shown to have no effect on ad lib food consumption in a novel environment [27].
In a different group of rats, sensitization to the locomotor stimulant effects of acute amphetamine was observed 1 month following exposure to amphetamine using a regimen identical to that used for the instrumental and potentiated eating tasks. Although there was considerable variance associated with the small group sizes used for this experiment, we have shown previously in an experiment using larger group sizes that this amphetamine regimen induces locomotor sensitization [45]. Furthermore, although the experimental design did not afford the opportunity to directly address the relationship between incentive and locomotor sensitization in the current study, the fact that the amphetamine exposure regimen used was sufficient to induce both locomotor sensitization and enhanced instrumental responding for reward, at the same withdrawal timepoint [27, 45, 46], is consistent with the idea that both types of behavioral alterations were mediated by the same set of neural adaptations, likely within the mesoaccumbens dopamine pathway [46-49]. Because rats were tested in the potentiated eating task two months after amphetamine exposure, it is possible that amphetamine's effects on this task resulted from a separate set of neural alterations, as such alterations resulting from drug exposure are known to be time-dependent [13, 50, 51]. However, the fact that locomotor sensitization present at one month is reported to be stable for many months after [13], and the fact that performance across the instrumental and potentiated eating tasks was correlated, argues that amphetamine's effects on these two tasks were mediated through a common neural mechanism.
Some research has found that psychostimulant (including amphetamine) withdrawal is associated with decreased motivation for non-drug rewards, or anhedonia [29, 30, 32, 33, 36, 52]. However, we found no evidence for such decreased motivation resulting from amphetamine exposure. A resolution to this potential conflict may be found in the fact that anhedonia following drug administration is most commonly reported to occur within the first few days of withdrawal, whereas incentive sensitization effects are more frequently reported several weeks after drug exposure [10, 11, 13, 29, 31]. Notably, the decrease in motivation for non-drug rewards that can result from chronic amphetamine exposure appears to dissipate after a week of withdrawal [36, 52], whereas sensitization resulting from amphetamine exposure typically appears only after several weeks of withdrawal and may remain stable for weeks or even years thereafter [13, 51], suggesting that changes in reward motivation resulting from drug exposure follow a biphasic time course.
Amphetamine administration can produce reductions in food intake and subsequent weight loss, which might result in increased food intake during withdrawal [53]. However, it is unlikely that direct amphetamine-induced changes in food intake account for the present results as there were no differences in body weight between amphetamine exposed and control rats at the end of the one month amphetamine withdrawal period, and the two groups ate similar amounts of food during training sessions (when exposed to the paired context) in the potentiated eating task. It is also conceivable that the increase in reward-directed behavior in amphetamine exposed rats was a result of faster learning. Chronic amphetamine exposure can result in lasting increases in evoked dopamine release [18, 51], and increased activity at dopamine receptors can enhance memory through actions on consolidation processes [54, 55]. However, we found no evidence for faster acquisition of lever-pressing in amphetamine exposed rats, as they shaped at a rate similar to controls, and there were no differences in lever pressing under conditions of low response ratios. There was no comparable measure of acquisition in the potentiated eating task, and thus it is possible that amphetamine exposed rats ate more during the test sessions because they had more strongly acquired the association between the paired context and food availability. However, the fact that the context-potentiated eating effect was not significantly greater in the amphetamine exposed group argues against enhanced learning playing a significant role in amphetamine's effects in this task. It should be noted that in the potentiated eating task we did not observe a potentiating effect of the paired context on food consumption in either amphetamine exposed or saline control rats, suggesting that despite our efforts, the two contexts were insufficiently discriminable to obtain a reliable potentiated eating effect [35]. However, the fact that amphetamine exposure reliably enhanced food consumption only in the paired context suggests a possible interaction between the effects of food-paired contextual cues and amphetamine exposure.
The results of these experiments provide support for the concept of incentive sensitization, defined as an augmented assignment of motivational value to rewards and reward-related cues [14-16, 40]. Incentive sensitization has gained support as a mechanism that could explain the progression from casual to compulsive drug use. In concordance with this idea, sensitizing regimens of psychostimulant exposure result in subsequent increases in responding for drug rewards in animal models [40, 41, 56-58], and a similar relationship between prior stimulant exposure and subsequent drug use may exist in humans [59]. These experiments demonstrate that prior amphetamine exposure causes long-lasting enhancements in behavior directed by food reward. Amphetamine-induced neuroadaptations in brain systems underlying incentive motivation may cause rewards to gain enhanced incentive value (i.e. - exaggerated reward “wanting”, or incentive sensitization, perhaps equivalent to drug craving), leading to excessive reward pursuit and consumption. Such lasting incentive sensitization could account in part for the propensity for relapse in drug addiction, particularly in the presence of cues predictive of drug reward. Given the strong overlap between the neural circuitry mediating drug and non-drug rewards, it is not surprising that psychostimulant exposure results in similar increases in responding for non-drug rewards [18, 19, 23, 60]. Indeed, such effects may underlie some components of pathological non-drug reward-related behaviors frequently observed in addiction, such as excessive gambling [21, 22]. A better understanding of the development and expression of incentive sensitization could have considerable implications for the treatment of drug addiction and co-morbid behaviors.
Acknowledgments
We thank Nicholas Simon for his assistance in conducting these experiments and Dr. Gorica Petrovich for her suggestions regarding design of the potentiated eating experiment. Supported by the Department of Psychology at Texas A&M University (BS) and T32 MH65728 (IAM). We have no conflicts of interest regarding the contents of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behavior. Brain Research Reviews. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 2.Volkow N. The neuroscience of addiction. Nature Neuroscience. 2005;8(11):1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- 3.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nature Reviews Neuroscience. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 4.Di Sclafani V, et al. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug and Alcohol Dependence. 2002;66:161–171. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittenberg W, Motta S. Effects of chronic cocaine abuse on memory and learning. Archives of Clinical Neuropsychology. 1993;8:477–483. [PubMed] [Google Scholar]
- 6.Volkow ND, et al. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiology of Learning and Memory. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- 7.van Gorp WG, et al. Declarative and procedural memory functioning in abstinent cocaine abusers. Archives of General Psychiatry. 1999;56:85–89. doi: 10.1001/archpsyc.56.1.85. [DOI] [PubMed] [Google Scholar]
- 8.Berry J, et al. Neuropsychological deficits in abstinent cocaine abusers: preliminary findings after two weeks of abstinence. Drug and Alcohol Dependence. 1993;32(3):231–237. doi: 10.1016/0376-8716(93)90087-7. [DOI] [PubMed] [Google Scholar]
- 9.Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug and Alcohol Dependence. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 11.Pierce R, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Research. Brain Research Reviews. 1997;25(2):192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 12.Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behavioural Pharmacology. 1993;4(4):289–312. [PubMed] [Google Scholar]
- 13.Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology. 1991;103(4):480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research. Brain Research Reviews. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 15.Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 16.Berridge K. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 17.Vanderschuren L, Everitt B. Behavioral and neuronal mechanisms of compulsive drug seeking. European Journal of Neuroscience. 2005;526:77–88. doi: 10.1016/j.ejphar.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 18.Fiorino DF, Phillips AG. Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after d-amphetamine-induced behavioral sensitization. The Journal of Neuroscience. 1999;19:456–463. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nocjar C, Panksepp J. Chronic intermittent amphetamine pretreatment enhances future appetitive behavior for drug- and natural-reward: interactions with environmental variables. Behavioural Brain Research. 2002;128:189–203. doi: 10.1016/s0166-4328(01)00321-7. [DOI] [PubMed] [Google Scholar]
- 20.Levens N, Akins C. Chronic cocaine pretreatment facilitates Pavlovian sexual conditioning in male Japanese quails. Pharmacology, Biochemistry and Behavior. 2004;79:451–457. doi: 10.1016/j.pbb.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Toneatto T, Skinner W, Dragonetti R. Patterns of substance use in treatment-seeking problem gamblers: Impact on treatment outcomes. Journal of Clinical Psychology. 2002;58(7):853–859. doi: 10.1002/jclp.2011. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg MA, Kosten TA, Rounsaville BJ. Cocaine abuse and pathological gambling. American Journal of teh Addictions. 1992;1:121–132. [Google Scholar]
- 23.Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. The Journal of Neuroscience. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein ED, et al. Repeated cocaine experience facilitates sucrose-reinforced operant responding in enriched and isolated rats. Learning and Motivation. 2007;16(2):190–209. doi: 10.1016/j.lmot.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. The Journal of Neuroscience. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behavioural Pharmacology. 1998;9:299–308. [PubMed] [Google Scholar]
- 27.Nordquist RE, et al. Augmented reinforcer value and accelerated habit formation after repeated amphetamine treatment. European Neuropsychopharmacology. 2007;17(8):532–540. doi: 10.1016/j.euroneuro.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine, and 3,4-methylenedioxyamphetamine (“ecstasy”) Biological Psychiatry. 2001;50:137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- 29.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 30.Volkow ND, Li TK. Drug addiction: the neurobiology of behavior gone awry. Nature Reviews Neuroscience. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- 31.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 32.Koob GF, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neuroscience and Biobehavioral Review. 2004;27(8):739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278(5335):45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- 34.Petrovich G, et al. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. Journal of Neuroscience. 2002;22(19):8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrovich G, et al. Learned contextual cue potentiates eating in rats. Physiology & Behavior. 2007;90(23):362–367. doi: 10.1016/j.physbeh.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barr A, Phillips A. Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology. 1999;141:99–106. doi: 10.1007/s002130050812. [DOI] [PubMed] [Google Scholar]
- 37.Cetin T, et al. Dopamine in the orbitofrontal cortex regulates operant responding under a progressive ratio of reinforcement in rats. Neuroscience Letters. 2004;370:114–117. doi: 10.1016/j.neulet.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Kheramin S, et al. The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: implications for models of inter-temporal choice. Behavioural Brain Research. 2005;156(1):145–152. doi: 10.1016/j.bbr.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 39.Petrovich GD, Gallagher M. Control of food consumption by learned cues: A forebrain-hypothalamic network. Physiology & Behavior. 2007;91(4):397–403. doi: 10.1016/j.physbeh.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology. 1989;98:357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- 41.Valadez A, Schenk S. Persistence of the ability of amphetamine preexposure to facilitate acquisition of cocaine self-administration. Pharmacology, Biochemistry and Behavior. 1993;47:203–205. doi: 10.1016/0091-3057(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 42.Schenk S, Robinson B, Amit Z. Housing conditions fail to affect the intravenous self-administration of amphetamine. Pharmacology, Biochemistry and Behavior. 1988;31(1):59–62. doi: 10.1016/0091-3057(88)90311-5. [DOI] [PubMed] [Google Scholar]
- 43.Berridge K, Robinson T. Parsing Reward. Trends in Neuroscience. 2003;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 44.Taylor JR, Horger BA. Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology. 1999;142:31–40. doi: 10.1007/s002130050859. [DOI] [PubMed] [Google Scholar]
- 45.Simon N, Mendez I, Setlow B. Effects of prior amphetamine exposure on approach strategy in appetitive Pavlovian conditioning in rats. Psychopharmacology. doi: 10.1007/s00213-008-1353-y. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olausson P, et al. {Delta}FosB in the Nucleus Accumbens Regulates Food-Reinforced Instrumental Behavior and Motivation. J Neurosci. 2006;26(36):9196–9204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallace D, et al. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. The Journal of Neuroscience. 2008;28(41):10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–14. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 49.Taylor J, et al. Inhibition of Cdk5 in the nucleaus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. Proceedings of the National Academy of Sciences. 2007;104(10):4147–4152. doi: 10.1073/pnas.0610288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camp D, DeJonghe DK, Robinson TE. Time-dependent effects of repeated amphetamine treatment on norepinephrine in the hypothalamus and hippocampus assessed with in vivo microdialysis. Neuropsychopharmacology. 1997;17(3):130–140. doi: 10.1016/S0893-133X(97)00035-3. [DOI] [PubMed] [Google Scholar]
- 51.Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19(1):56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orsini C, Koob GF, Pulvirenti L. Dopamine partial agonist reverses amphetamine withdrawal in rats. Neuropscyhopharmacology. 2001;25(5):789–792. doi: 10.1016/S0893-133X(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 53.Wellman P. Overview of adrenergic anorectic agents. American Journal of Clinical Nurtrition. 1992;55(1):193S–198S. doi: 10.1093/ajcn/55.1.193s. [DOI] [PubMed] [Google Scholar]
- 54.Packard MG, McGaugh JL. Quinpirole and d-amphetamine administration posttraining enhances memory on spatial and cued discriminations in a water maze. Psychobiology. 1994;22:54–60. [Google Scholar]
- 55.Packard MG, White NM. Memory facilitation produced by dopamine agonists: role of receptor subtype and mnemonic requirements. Pharmacology Biochemistry and Behavior. 1989;33:511–518. doi: 10.1016/0091-3057(89)90378-x. [DOI] [PubMed] [Google Scholar]
- 56.Shippenberg TS, Heidbreder C. Sensitization to the conditioned rewarding effects of cocaine: pharmacological and temporal characteristics. The Journal of Pharmacology and Experimental Therapeutics. 1995;273:808–815. [PubMed] [Google Scholar]
- 57.Piazza PV, et al. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Research. 1990;514(1):22–26. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- 58.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neuroscience & Biobehavioral Reviews. 2004;27(8):827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101(5):713–725. doi: 10.1111/j.1360-0443.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- 60.Jentsch JD, Roth RH, Taylor JR. Role for dopamine in the behavioral functions of the prefrontal corticostriatal system: implications for mental disorders and psychotropic drug action. Progress in Brain Research. 2000;126:433–453. doi: 10.1016/S0079-6123(00)26028-7. [DOI] [PubMed] [Google Scholar]