Summary
Mice lacking the chemokine receptor CCR5 are susceptible to mortality from a normally non-lethal influenza infection. Here we found that CXCR3-deficiency rescued CCR5-deficient mice from influenza-induced mortality. The number of mononuclear phagocytes in the airways was transiently increased in CCR5-deficient mice but not in CXCR3-CCR5 double-deficient mice. Antigen-specific CXCR3-CCR5 double-deficient CD8 effector cells were less efficient at entering the airways compared to wild-type or CCR5-deficient CD8 effector cells. The decrease in inflammatory cell infiltrates in CXCR3-CCR5 double-deficient infected mice correlated with a decrease in CCL2 and IFNγ production in the airways. Finally, CXCR3-CCR5 double-deficient mice that survived the primary viral challenge were protected from a lethal secondary challenge, indicating that T cell-mediated protective memory was not compromised in mice lacking these chemokine receptors. In conclusion, CXCR3 deficiency attenuated the lethal cellular immune response in CCR5-deficient influenza-infected mice without hindering viral clearance or long-term immunity.
Keywords: T cells, Virology, Chemokines, Lung inflammation
Introduction
Influenza infection of the respiratory tract remains a global health burden [1]. Effective immune response, viral clearance, and recovery from respiratory viral infections, such as influenza, are partly regulated by the migration of leukocytes into inflamed tissue. Chemokines produced at sites of inflammation are among the chemoattractants that control migration of leukocytes [2].
CCR5 is found on the surface of dendritic cells, macrophages, activated and memory T cells, as well as natural killer cells [2, 3]. CCR5 ligands -- CCL3 (MIP1α), CCL4 (MIP1β), and CCL5 (RANTES) -- are commonly expressed in lungs of mice infected with respiratory viruses, including influenza [4–6]. CCR5-deficient (CCR5−/−) mice are susceptible to a fatal pneumonitis after infection with influenza [4]. It has been suggested that increased early migration of mononuclear phagocytes [4] and increased susceptibility of macrophages to apoptosis [7] contribute to the increased pulmonary inflammation that has been associated with increased mortality of CCR5−/− mice following respiratory viral infection. However, the exact mechanism driving this increased inflammation and the participation of additional cell types in the increased morbidity of CCR5−/− mice are not fully understood.
CXCR3 is a chemokine receptor that is specific for CXCL9 (Mig), CXCL10 (IP-10), and CXCL11 (ITAC) and is expressed on effector Th1 and CD8 T cells, NK cells, and NKT cells [5, 6, 8–11]. CXCR3 and its ligands are expressed in a wide array of inflammatory and infectious diseases, including influenza [5, 6, 8–10]. CXCL10 mRNA levels are increased in the lungs of CCR5−/− mice infected with influenza compared to those of infected wild-type mice [4]. These findings suggest that there may be an increase in CXCR3-mediated migration of leukocytes in CCR5−/− mice. In contrast to the increased mortality seen with CCR5−/− mice, CXCR3-deficient (CXCR3−/−) mice infected with influenza exhibit a transient decrease in lymphocytes in the airways with no effect on survival or viral clearance [5]. Furthermore, pretreatment of T cells with the CCR5-specific ligand, CCL4, has been shown to inhibit CXCR3-mediated chemotaxis in vitro [12]. The interplay between CXCR3 and CCR5 in vivo remains poorly understood..
In the present study, we examined whether CXCR3-induced cellular migration contributed to the mortality of CCR5−/− mice during influenza infection. We analyzed the immune response of mice deficient in both CXCR3 and CCR5 (CXCR3−/−/CCR5−/−) and isolated the role of these receptors on CD8+ T cells by adoptive transfer experiments. Using these experimental systems, we found that CXCR3 deficiency decreased cellular infiltrates into the airways of mice infected with influenza, which led to an attenuation of inflammatory mediators and protection of CCR5−/− mice from mortality.
Results
CXCR3-deficiency rescues CCR5−/− mice from mortality after viral infection
To determine if CXCR3-mediated migration contributes to a lethal hyper-inflammatory response in CCR5−/− mice, we first monitored the survival and weight of wild-type mice, CXCR3−/−, CCR5−/−, and CXCR3−/−/CCR5−/− mice infected with a sublethal dose of influenza A/PR8/34 (PR8) (Fig. 1A,B). As previously reported, CXCR3-deficiency did not compromise the survival of mice infected with influenza, while CCR5−/− mice exhibited increased mortality, 89% (Fig. 1A) [4, 5, 7]. During the first week of infection, CCR5−/− mice lost weight with similar kinetics compared to wild-type mice (Fig. 1B). However, CCR5−/− mice continued to lose weight, while the weight of wild-type mice plateaued eight days after infection and then began to increase. Surprisingly, CXCR3−/−/CCR5−/− mice were not susceptible to mortality due to infection with influenza. In fact, similar to CXCR3−/− mice, CXCR3−/−/CCR5−/− mice exhibited a trend of less weight loss than the wild-type mice during the course of infection (Fig. 1B). This difference was significant when compared to weight loss in CCR5−/− mice. At day 7 post-infection, CCR5−/− mice had lost 25% of their body weight, whereas CXCR3−/−/CCR5−/− mice lost only 13% of their body weight (p=0.002) (Fig. 1C). Hence, CXCR3 deficiency protected CCR5−/− mice from mortality after influenza infection.
Figure 1. CXCR3 deficiency protects CCR5−/− mice from mortality and weight loss.
Six to nine week old mice were infected intranasally with a non-lethal dose of influenza PR8, 0.3 MLD50. (A) Mortality and (B,C) weight loss were monitored. (A) Kaplan Meier survival curves were generated and the curves were different by Logrank Test (X2=56.64, df=3, p<0.0001). Survival curves were generated from at least four independent experiments (n=13–27) (B) Weight loss is expressed as mean percentage of original weight ± SD. (C) Weight loss on Day 7 was compared using Students T test (n=13–27 per group; *p<0.01). (D) Viral clearance was determined by MDCK plaque assay of supernatants from lung homogenates acquired at different time points post-infection. Viral titers at each time point were compared using one-way non-parametric ANOVA and were not significantly different. The Day 9 time point represents a pool of samples from days 9 and 10 post-infection for all groups. Each bar represents mean plaque forming units per ml of supernatant derived from one lobe of the right lung ± SEM (n=8–14 mice per bar) from at least three independent experiments.
Influenza virus is cleared in infected wild-type and CXCR3−/− mice between 10–14 days after primary infection (Fig. 1D) [5]. We also found that CCR5−/− and CXCR3−/−/CCR5−/− mice did not differ from wild-type or CXCR3−/− mice in their viral burden on day 9 post-infection (Fig. 1D). Similar to wild-type and CXCR3−/− mice, viral titers were low or undetectable in CXCR3−/−/CCR5−/− mice on day 14 after infection (Figure 1D).
Cellular infiltration
CCR5−/− mice have been shown to have increased mononuclear phagocyte and lymphocyte infiltration into the lungs after Influenza A and Mycobacterium infection, respectively [4, 13]. After primary influenza infection, innate immune cells are the first inflammatory cell types that respond to the viral attack. This is followed by lymphocyte infiltration into the lungs five to seven days post-infection [14, 15].
The level of inflammation in hematoxylin and eosin-stained (H&E) lung sections was scored by an observer blinded to the genotypes (Fig. 2A,B). Three days post-infection, the lungs of infected mice were only mildly inflamed. Cellular infiltration, filling spaces around large airways and throughout the interstitium, increased by seven days post-infection and peaked nine days post-infection (Fig. 2A,B). Although there was a trend towards a greater inflammatory score in CCR5−/− mice compared to wildtype and CXCR3−/− mice seven days post-infection, the difference did not reach statistical significance. However, CXCR3−/−/CCR5−/− mice had less inflammation than CCR5−/− mice seven days post-infection (p=0.036).
Figure 2. CXCR3 deficiency attenuates immunopathology in CCR5−/− mice.
(A) Paraffin-embedded lung sections from Days 3, 7, 9, and 14 after infection were stained with H&E to assess the level of inflammation in the lungs. (B) An observer assessed and ranked the level inflammation in blinded samples. A score of 0 equals no inflammation and a score of 4 equals the highest level of inflammation. Each bar represents the mean score ± SD of sections from at least three independent experiments (n=6–22/group). Groups were then compared by Student’s T-test. CXCR3−/−/CCR5−/− mice (n=22) had significantly less inflammation on Day 7 than CCR5−/− mice (n=15) (*Students T-test, p=0.03). (C–D) The numbers of inflammatory cell infiltrates were also counted in cytospin preparations of BALF from 2–4 independent experiments at designated times post-infection. Each bar represents the number of cells ± SD (n=7–16 per group), and each time point was analyzed by one-way ANOVA and groups were compared by Student’s T-test. * indicates p values <0.05.
Next, we characterized the type of cells that entered the airways. Cytospin preparations of brochoalveolar lavage fluid (BALF) were obtained from mice at different times post-infection. There was an accelerated influx of mononuclear phagocytes in CCR5−/− mice, peaking at day 3 post-infection with twice as many mononuclear phagocytes compared to wild-type mice (p=0.0001) and CXCR3−/−/CCR5−/− mice (p=0.001) (Fig. 2C). The number of neutrophils was significantly increased in CCR5−/− and CXCR3−/−/CCR5−/− mice compared to wild-type mice seven days after infection (p<0.01) (Fig. 2D). CCR5−/− mice therefore had exacerbated mononuclear phagocytes infiltration into the airways that was not seen in CXCR3−/−/CCR5−/− mice, but the additional CXCR3 deletion did not impact neutrophil infiltration into the airways
Lymphocyte recruitment
Lymphocytes enter the airways five days post-infection, after being primed in the draining lymph nodes [15]. This time point is also when mice typically start losing weight after infection with influenza (Fig. 1B). Although T cells are essential for viral clearance, CD8 T cells have been strongly implicated in mediating immunopathological damage to the lungs [16–19]. We analyzed relative numbers of CD8+ and CD4+ T cells in the lungs and the airway by flow cytometry.
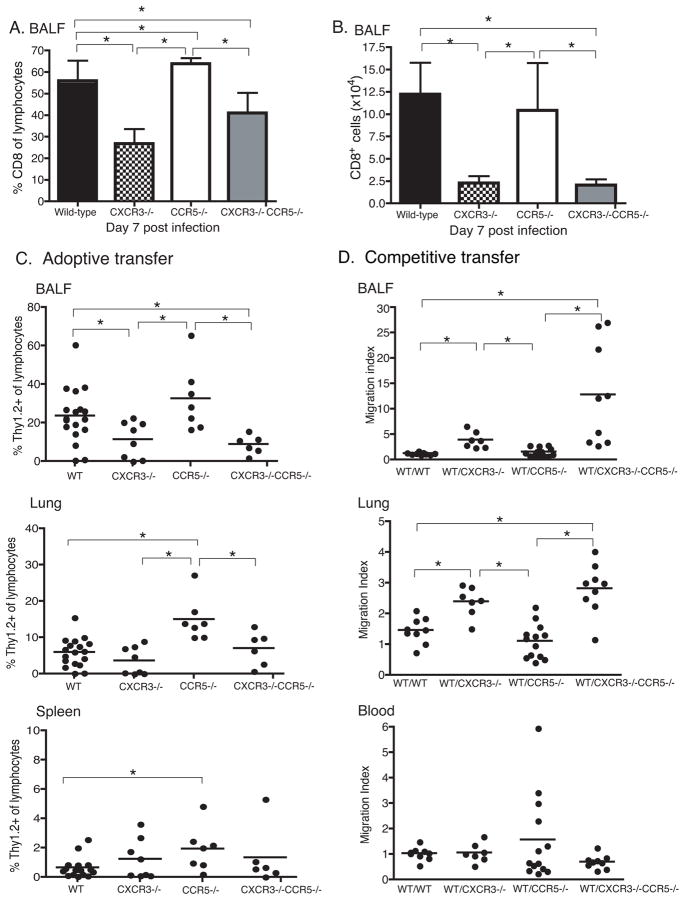
We first determined the percentage and number of CD8+ T cells seven days after infection with a non-lethal dose of PR8 (Fig. 3A,B), which is the day before most CCR5−/− mice become moribund (Fig. 1A). The percentage of CD8 T cells in the airways of CCR5−/− mice was increased compared to the percentages in wild-type (p=0.05), CXCR3−/− (p<0.0001), and CXCR3−/−/CCR5−/− (p=0.0032) mice (Fig. 3A). Although, there was no difference in the number of CD8 T cells between CCR5−/− and wild-type mice (Fig. 3B), CXCR3−/− and CXCR3−/−/CCR5−/− mice had lower percentages (p≤0.01) and numbers (p<0.01) of CD8+ T cells in the airways (Fig. 3A–B) compared to wild-type and CCR5−/− mice.
Figure 3. CXCR3−/− CD8 T cells are less efficient at entering the airways.
(A–B) Mice were infected with a non-lethal dose of influenza PR8. Seven days after infection, BALF samples were harvested and stained for CD8 surface markers. Each bar represents (A) the mean percent ((B) or number of CD8+ T cells in the lymphocyte gate ± SD from two independent experiments (n=8/group). The number and percentage of CD8 cells were significantly different (one-way ANOVA, p<0.0001 for (A) and p=0.0157 for (B)). Groups were then compared with Student’s T-Test (* indicates p≤0.05). A Welch’s correction was applied when the variance were different between the groups. (C) To determine the role of these chemokine receptors on CD8 effector T cell, 1.5×106 naive wild-type (WT), CXCR3−/−, CCR5−/−, or CXCR3−/−/CCR5−/− OTI cells were adoptively transferred into wild-type congenic mice that were infected with WSN-OVA the following day. BALF, Lung, and Spleen samples were harvested 8 days post-infection. Each dot represents the accumulation of donor cells in an individual recipient mouse pooled from three independent experiments (n=7–18/group). Results were analyzed first by one-way ANOVA (BALF p=0.0078; lung p<0.0001) and then followed by Students’ T-test; * indicates significance by Students’ Test p<0.05). (D) Competitive adoptive transfer assay was also performed in which naive 0.75×106 WT Thy1.1+ OTI cells were co-transferred with 0.75×106 WT, CXCR3−/−, CCR5−/−, or CXCR3−/−/CCR5−/− Thy1.2+ OTI cells into Thy1.1+/Thy1.2+ recipient mice. Each dot represents the migration index of cells isolated from individual recipient mice pooled from three independent experiments (n=7–13/group). Migration index = organ (%Thy1.1/%Thy1.2)/input (%Thy1.1/%Thy1.2). BALF and lung data were significant as determined by one-way ANOVA, p<0.0001. Each experimental group was also analyzed as compared to WT (Thy1.1) to WT (Thy1.2) transfers by Student’s T-test. (*=p<0.05).
In-vivo generation and trafficking of CD8+ effectors in response to virus infection
CXCR3 and CCR5 are expressed on several mononuclear cell populations. Thus, it is difficult to isolate the role of these receptors on CD8 T cells without the compounding effect on other cell populations. To determine whether CXCR3 and/or CCR5 are necessary for CD8 T cell migration to sites of pulmonary infection, we bred CXCR3−/− and CCR5−/− mice with OTI mice transgenic for a T cell receptor specific for OVA257–264. CD8+ cells were bead-purified from naive wild-type, CXCR3−/−, CCR5−/−, and CXCR3−/−/CCR5−/− OTI mice (all Thy1.2+). Transgenic T cells were then transferred intravenously into naive congenic (Thy 1.1+) mice. Recipient mice were infected with WSN-OVA, an influenza strain engineered to express OVA257–264 in the neuraminidase stalk protein of influenza A/WSN/33 [20]. CXCR3−/−, CCR5−/−, and CXCR3−/−/CCR5−/− OTI cells generate effector cells (CD44hiCD62LloCD69+) in vivo similar to wild-type OTI cells and expand to similar levels or slightly increased levels in the spleen and mediastinal lymph nodes (Fig. 3C and data not shown). On day 8 post-infection, the peak of the primary response to WSN-OVA [20], we harvested organs from recipient mice and analyzed the tissues for the presence of Thy1.2+ donor cells (Fig. 3C).
There was a 2.5-fold increase in the percentage of CCR5−/− T cells in the lungs compared to wild-type OTI cells (p=0.0002) (Fig. 3C). Even though CXCR3−/− OTI cells were able to migrate to the lung, they were two and 2.8-fold less efficient at entering the airways when compared to wild-type (p=0.04) and CCR5−/− (p=0.01) OTI cells, respectively (Fig. 3C). Similarly, the percentages of CXCR3−/−/CCR5−/− OTI cells found in the airways of recipient mice were one-third and one-fourth of the percentages observed when wild-type (p=0.001) or CCR5−/− OTI cells (p=0.0075) were adoptively transferred (Fig. 3C).
Competitive homing studies
To confirm our findings on the role of CCR5 and CXCR3 in T cell homing into the influenza infected lung and to better control for mouse-to-mouse variability, we performed competitive homing experiments, using CCR5−/−, CXCR3−/− and CCR5−/−/CXCR3−/− OTI CD8 T cells. We bred wild-type C57BL/6 (Thy 1.2) mice with a congenic strain, B6/PLThy1a (Thy1.1), to generate mice with endogenous Thy1.1+/Thy1.2+ T cells. We then transferred an equal number of naive wild-type CD8+ OTI Thy 1.1+ cells and naive CCR5−/−, CXCR3−/− and CCR5−/−/CXCR3−/− CD8+ OTI Thy 1.2+ cells into Thy1.1+/Thy1.2+ wild-type mice. To control for inter-assay variability, wild-type OTI Thy 1.2+ cells were co-transferred with wild-type OTI Thy1.1+ cells (Fig. 3D).
We first examined the efficiency of chemokine receptor-deficient OTI cells to generate effector cells in vivo and then migrate to the site of infection. The migration indices in the blood for the different combinations of cells were similar (Fig. 3D). CCR5 deficiency did not impair the ability of antigen-specific CD8 T cells to enter the site of infection (Fig. 3C–D). In contrast, antigen-specific CXCR3−/− and CXCR3−/−/CCR5−/− CD8 T cells were less efficient at entering the lung (CXCR3−/− p=0.001, CXCR3−/−/CCR5−/− p= 0.0004) and the airways (CXCR3−/− p=0.0002, CXCR3−/−/CCR5−/− p= 0.003) compared to wild-type CD8 T cells (Fig. 3D). Similarly, CXCR3−/− and CXCR3−/−/CCR5−/− CD8 T cells were less efficient at entering the lung (CXCR3−/− p<0.0001, CXCR3−/−/CCR5−/− p<0.0001) and the airways (CXCR3−/− p=0.008, CXCR3−/−/CCR5−/− p= 0.0097) compared to CCR5−/− OTI T cells (Fig. 3D).
Competitive migration of in-vitro generated effector cells
Since CXCR3 and CCR5 may influence the generation of functional effectors from naive T cells in vivo [13, 21, 22], we determined the influence of these chemokine receptors solely on the migration of effector CD8 T cells. To do so, we generated wild-type, CXCR3−/−, CCR5−/−, and CXCR3−/−/CCR5−/− OTI cells effector CD8 T cells in vitro and then adoptively transferred them into the same recipient mice for competitive homing studies. OTI T cells were stimulated in vitro with OVA-peptide pulsed APCs for five days to generate effector CD8 T cells [23]. Wild-type, CXCR3−/−, CCR5−/−, and CXCR3−/−/CCR5−/− OTI cells exhibited similar levels of proliferation as well as activation based on CD25, CD69, CD44, and CD62L expression (data not shown). Wild-type effector Thy1.1+ OTI cells were mixed with equal numbers of chemokine receptor deficient Thy1.2+ OTI effector cells and transferred intravenously into Thy1.1+/Thy1.2+ mice. The following day, mice were challenged with a sub-lethal dose of WSN-OVA, and organs were harvested four days later (Fig. 4).
Figure 4. Adoptively transferred CXCR3−/− CD8 effector T cells are less efficient at entering the airways.
Wild-type (Thy1.1 or Thy1.2) and chemokine receptor deficient (Thy 1.2) OTI cells were stimulated in vitro with OVA peptide-pulsed irradiated APCs in the presence of IL-2, Il-12, and anti-CD28 for 5 days. A total of 1.5 ×106 effector T cells were then transferred i.v. into wild-type recipient mice that co-express Thy 1.1 and Thy 1.2. The following day, recipient mice were infected with WSN-OVA for 4 days. Accumulation of effector cells in the spleen, lungs, and BALF are shown. Each dot represents the migration index of cells isolated from individual recipient mice pooled from three independent experiments (n=10–12/group). Migration index = organ (%Thy1.1/%Thy1.2)/input (%Thy1.1/%Thy1.2). The accumulation of effector cells in the BALF was different among the groups, ANOVA p=0.0004. Each experimental group was also analyzed as compared to WT (Thy1.1) to WT (Thy1.2) transfers by Student’s T-test. (*=p<0.05)
Similar to our observations using naive T cell competitive transfers (Fig. 3), CXCR3−/− CD8 T cells were less efficient at entering the airways compared to wild-type and CCR5−/− OT-I cells (Fig. 4). Specifically, wild-type Thy1.1 OTI cells were four-fold more efficient at migrating into the airways compared to CXCR3−/− OTI (p=0.0003) and CXCR3−/−/CCR5−/− OTI (p=0.0002) effector cells (Fig. 4). Also, in vitro-generated CXCR3−/− OTI effector cells (p=0.01) and CXCR3−/−/CCR5−/− OTI effector cells (p=0.009) were less efficient at migrating into the lung parenchyma compared to wild-type OTI effector cells (Fig. 4). Additionally, CXCR3−/− OTI effector cells (p=0.02) and CXCR3−/−/CCR5−/− OTI effector cells (p=0.02) were less efficient at migrating into the airways compared to CCR5−/− OTI effector cells, but they did not differ in their migratory efficiency into the lungs (Fig. 4).
CD4 T cell recruitment
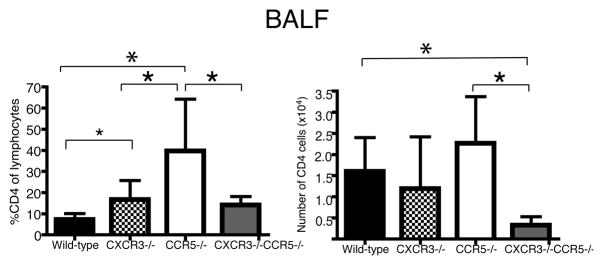
We also examined CD4+ T cell numbers in mice infected with influenza (Fig. 5). More so than what was observed for CD8 T cells, the percentage of CD4+ cells in the lymphocyte gate of the airways of CCR5−/− mice was 5.5-fold more than in wild-type mice (p=0.008), 2.4-fold more than in CXCR3−/− mice (p=0.03), and 2.8-fold more than in CXCR3−/−/CCR5−/− mice (p=0.03) (Fig. 5). The total numbers of CD4 cells, though, did not reach statistical significance between wild-type and CCR5−/− mice; however CD4+ cell numbers were reduced in CXCR3−/−/CCR5−/− mice compared to wild-type mice by fivefold (p=0.003) and CCR5−/− mice by seven-fold (p=0.01) (Fig. 5).
Figure 5. Impaired entry of CD4 T cells into the airways in CXCR3−/−/CCR5−/− mice.
Mice were infected with a non-lethal dose of influenza PR8, 0.3 MLD50. Seven days after infection, BALF samples were harvested and stained for CD4 surface markers. Each bar represents the mean percent or number of CD4+ T cells in the lymphocyte gate ± SD. The number and percentage of CD8 cells were significantly different (one-way ANOVA, p=0.0005 and p=0.008 for respectively). Panels are results from two independent experiments (n=6–8). Groups were then compared by Student’s T-test. *p value <0.05
Pro-inflammatory cytokine and chemokines in airways of infected mice
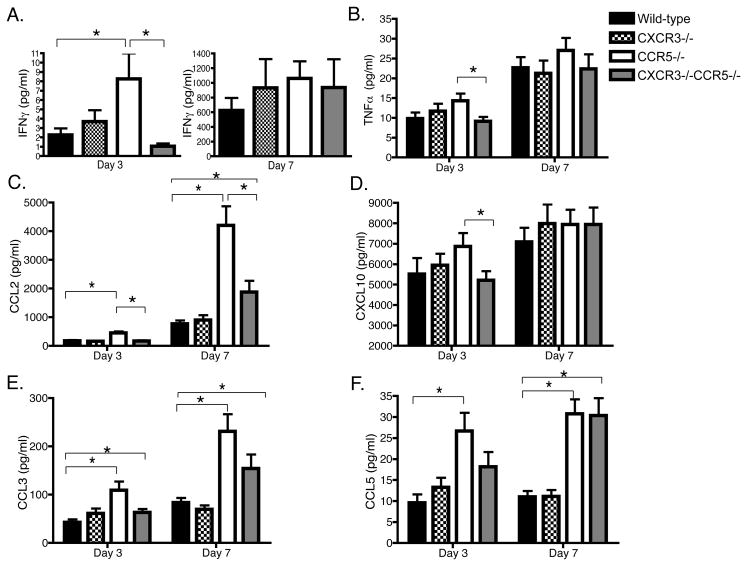
We measured the concentration of pro-inflammatory cytokines and chemokines in BALF samples from mice infected with influenza days 3 and 7 after infection (Fig. 6). Levels of IFNγ and TNFα were undetectable in uninfected mice (data not shown). CCR5−/− mice had an early burst of IFNγ production in the BALF three days post-infection that was reduced in CXCR3−/−/CCR5−/− mice (p=0.02). IFNγ levels in the BALF of CXCR3−/− mice were not different from that in wild-type mice (Fig. 6A). TNFα production in CXCR3−/−/CCR5−/− mice was also reduced compared to CCR5−/− mice three days after infection (p=0.03) (Fig. 6B). Seven days post-infection, IFNγ and TNFα levels increased but were not different between the genotypes (Fig. 6A–B).
Figure 6. Cytokines and chemokines increased in the airways of CCR5−/− mice are reduced in CCR5−/− CXCR3−/− mice.
Mice were infected with a sublethal dose of influenza PR8, 0.3 MLD50. (A) IFNγ, (B) TNFα, (C) CCL2, (D) CXCL10, (E) CCL3, and (E) CCL5 protein levels were measured in duplicate from supernatant of BALF samples obtained three and seven days after infection using Luminex multi-plex technology. Data in each bar graph represent 9–12 mice per group obtained from three independent experiments. Results are expressed as mean pg/ml ± SEM and analyzed by one-way ANOVA and Student’s T-test (*p < 0.05) (A–E).
Dawson et al. reported an increase in CXCL10, CCL2, and CCL5 mRNA expression in CCR5−/− mice compared to wild-type mice within 2–3 days after infection with influenza [4]. We therefore determined the protein levels of these chemokines in CCR5−/−, CXCR3−/− and CXCR3−/−/CCR5−/− mice three and seven days after infection. There was three-fold more CCL2 in the airways of CCR5−/− mice (~450 pg/ml) three days post-infection when compared to the other groups (152–165 pg/ml) (p=0.0002–0.0003) (Fig. 6C). By day 7 post-infection, the difference was even more pronounced. The concentration of CCL2 in the airways of CCR5−/− mice was >4 ng/ml; however, it was less than 1ng/ml in wild-type mice (p=0.0011) (Fig. 6C). Though the concentration of CCL2 was increased in CXCR3−/−/CCR5−/− mice compared to wild-type mice (p=0.03), the concentration of CCL2 was reduced by more than half compared to CCR5−/− mice (p=0.0058) (Fig. 6C).
CXCL10 levels were detectable in uninfected mice and did not differ between the groups (data not shown). CXCL10 levels increased sharply three days after infection and remained elevated for the next four days in a pattern similar to IFNγ production in all groups tested (Fig. 6D). Despite a slight trend towards decreased CXCL10 levels in CXCR3−/−/CCR5−/− mice compared CCR5−/− mice (p=0.052), the deficiency in CXCR3 and/or CCR5 did not influence CXCL10 levels compared to wild-type mice (Fig. 6D).
Similar to the levels of CCL2, CCL3 levels were 2–3 fold higher in the airways of CCR5−/− mice compared to wild-type mice three (p=0.009) and seven days post-infection (p=0.003)(Fig. 6E). There was a trend towards reduced levels of CCL3 in the airways of CXCR3−/− CCR5−/− mice compared to CCR5−/− mice, but this did not reach statistical significance (Fig. 6E). Similarly, the levels of CCL5 in CCR5−/− and CXCR3−/−/CCR5−/− mice were not different from each other but were elevated compared to wild-type and CXCR3−/− mice (p<0.001) (Fig. 6F).
Protection from secondary lethal challenge
Our study suggests that there may be therapeutic potential to inhibiting CXCR3 function in certain cases of influenza-induced lung immunopathology. However, CXCR3 has been implicated as a marker of CD8 memory cells that are more likely to be efficient mediators of recall responses [8], and CCR5 has been shown to promote CD8+ T cell memory generation and migration [22, 24]. We therefore wanted to determine whether inhibiting CXCR3 interactions would compromise long-term T-cell mediated immunity, particularly when CCR5 was also deficient.
We first challenged mice with a low dose of influenza x31, H3N2 subtype. Approximately 50% of CCR5−/− mice and 100% of the other groups survived the primary challenge (data not shown). Six to eight weeks later, mice were challenged with a lethal dose of influenza PR8 (H1N1). Since the mice are rechallenged with a virus of a different subtype, cross-reactive T cells and not antibodies mediate protection, a phenomenon called heterosubtypic immunity. Immune CXCR3−/−, CCR5−/−, and CXCR3−/−/CCR5−/− mice were all protected when rechallenged with a lethal dose of PR8 (Fig. 7).
Figure 7. CXCR3 deficiency does not compromise protective immunity.
Six to eight week old mice were primed with low dose x31 (H3N2) influenza (primed). Six to eight weeks later, mice that were previously primed or naive (unprimed) were challenged with a lethal dose of PR8 (H1N1) influenza virus. Weight loss and survival of mice were monitored for two weeks. Primed mice all survived the lethal secondary challenge. Unprimed mice became moribund between 7–11 days after infection. Kaplan Meier analysis was generated from results of three independent experiments with 12–18 mice per group.
Discussion
Our findings demonstrate a role for CXCR3 in amplifying hyper-inflammatory responses to influenza. CCR5−/− mice are susceptible to mortality from viral infection (Fig. 1)[4, 7]. Using various models of respiratory infection, others have reported that an early influx of mononuclear phagocytes [4], an increase in macrophage apoptosis [7], an increase in lymphocyte influx [13], and changes in dendritic cell numbers and migration in lungs [13] all contribute to hyper-inflammatory immune responses in CCR5−/− mice. Here we show that deleting CXCR3 decreased mononuclear cell migration into the airways and lung parenchyma (Fig. 2–5), which led to a decrease in the pro-inflammatory cytokine and chemokine mileu (Fig. 6). The decrease in inflammatory cells and mediators in CXCR3−/−/CCR5−/− correlated with their protection from morbidity after viral infection (Fig. 1A).
CXCR3−/−/CCR5−/− mice exhibited ameliorated clinical symptoms when compared to CCR5−/− mice after viral infection. CXCR3-deficiency reduced the weight loss and anorexia of infected CCR5−/− mice (Fig. 1B–C), which correlated with decreased CD8 migration into the lung airways (Fig. 3). As reported by other groups, we also observed accelerated mononuclear phagocyte infiltration into the airways three days after infection in CCR5−/− mice (Fig. 2C)[4, 7]. Mononuclear phagocytes contribute to lethal immunopathology in mice infected with a high dose of influenza [25]. We were surprised that a deficiency in CXCR3 would impact the migration of this population (Fig. 2C). It is not clear if the decrease in the numbers of mononuclear phagocytes in CXCR3−/−/CCR5−/− mice is a direct result of their inability to migrate into the lung parenchyma and airways via CXCR3. CXCR3 expression on macrophages has been reported and would support this hypothesis [26, 27]; however, it is also possible that another CXCR3+ population, e.g. NK cells, plasmacytoid dendritic cells or T cells, indirectly influenced the migration of this population in CCR5−/− mice.
Neutrophils were evident in the airways of infected mice within three days after infection, but persisted in CCR5−/− and CXCR3−/−/CCR5−/− mice until day 7 after infection. The increased number of neutrophils we observed in the airways of CCR5−/− and CXCR3−/−/CCR5−/− mice (Fig. 2D) could be attributed to the increased concentrations of CCL3 (Fig. 6E) or other murine neutrophil-active CCR1 or CXCR2 ligands [28]. Since, the neutrophils infiltration did not impact the survival of CXCR3−/−/CCR5−/− mice, we did not explore this further.
T-cell-mediated lung injury leads to morbidity within the first two weeks after infection, whereas T-cell deficient mice exhibit less lung immunopathology but become morbid at 3–4 weeks post-infection as a result of uncontrolled viral titers [29]. Since CCR5−/− mice became moribund between 8–10 days after infection, T-cell mediated immunopathology was implicated. Consistent with Dawson et. al’s observations, we did not find an increase in viral burden in CCR5−/− mice (Fig. 1D), making it unlikely that these mice were dying from lack of viral control. Since CXCR3 is predominantly expressed on activated T lymphocytes [30], we hypothesized the CXCR3 deletion inhibited lethal T-cell-mediated immunopathology in CCR5−/− mice.
Indeed, when cells in the airways of infected mice were analyzed by flow cytometry, it was evident that CXCR3−/− CD8 T cells were less able to enter the airways (Fig. 3), and this was due to a deficiency in the trafficking and not the generation of effector cells (Fig. 4). Similarly, CD4+ T cells were less able to enter the airways in infected CXCR3−/− and CXCR3−/−/CCR5−/− mice (Fig. 5). CCR5 deficiency did not seem to decrease efficiency by which CD8 effector cells enter the lung or the airways and may have increased the ability of cells to accumulate in the lung parenchyma (Fig. 3C) or airways (Fig. 3A). Galkina et al. previously reported that CCR5 regulates the homeostatic migration of effector cells from the vasculature into the lung parenchyma [31]. The role of CCR5 in effector CD8 migration into the lung parenchyma may be less important when the lung is inflamed after a viral infection because other chemokines are produced with infection.
It is unclear whether CCR5−/− T cells are inherently more pathogenic, or if T cells simply amplify a defect in the innate cell responders in CCR5−/− mice. Though not tested extensively, transferring CCR5−/− CD8 OTI cells into mice infected with WSN-OVA did not lead to increased lethality (data not shown). However, two independent groups have reported spontaneous production of IFNγ by CCR5−/− and CXCR3−/−/CCR5−/− CD8 T cells [13, 32]. Also, antigen-specific clonal expansion of T cells and T-cell mediated immunopathology was augmented in CCR5−/− mice infected with lymphocytic choriomeningitis virus (LCMV) [33, 34]. Yurchenko et al. found that CCR5−/− regulatory T cells (Treg) were less efficient than wild-type Treg at migrating to sites of infection, resulting in an increase in the magnitude of effector CD4 T cells at the infected site [35]. However, we did not find that transferring CD4+CD25+ T cells from naive wild-type protected CCR5−/− mice from mortality from influenza PR8 (data not shown).
In addition to decreased cellular infiltrates in the airway, CXCR3 deficiency resulted in decreased concentrations of IFNγ and TNFα during the early phase of the infection (Fig. 6A–B). The elevated concentrations of CCL2 in the airways of CCR5−/− mice seen in our study, as well as by Dawson et al.[4], were also reduced in CXCR3−/−/CCR5−/− mice (Fig. 6C). This may reflect the decreased recruitment of activated T cells into the airways of CXCR3-deficient mice, which are known to induce the production of CCL2 from airway epithelial cells [18, 36].
We did attempt to determine if a specific CXCR3+ cell population was responsible for the immunopathology seen in infected CCR5−/− mice using depleting mAbs for CD4+, CD8+, GR-1+ (including neutrophils and plasmacytoid DCs), and NK1.1+ cells individually before and during infection of CCR5−/− mice (Supplementary Fig. 1). All of these cell types have been reported to play a role in the inflammatory response to influenza [6, 19, 37, 38, 39]. However, depleting any one of these populations did not improve the survival of infected CCR5−/− mice. Thus it is apparent that the mechanism by which CXCR3 deficiency protects infected CCR5−/− mice is multi-factorial and not simply due to one cell type.
Nevertheless, CCR5 deficiency resulted in hyper-inflammatory responses after in our murine model of viral infection similar to that reported by other groups [4, 7, 33, 34]. It is not known whether humans with naturally occurring CCR5 null mutations have increased susceptibility to influenza as has been seen for other viral pathogens [40, 41], and this warrants further investigation. However, CCR5 inhibitors are now approved for treating HIV-1 infection [42], and if murine data extrapolates to humans, these individuals may have increased susceptibility to influenza. While inhibiting CXCR3 may not benefit most persons infected with influenza, our results suggest that blockade of CXCR3 may be therapeutic for those individuals on CCR5 inhibitors or with CCR5 null mutations that develop influenza-induced immunopathogenic injury as seen in CCR5-deficient influenza-infected mice.
Materials and methods
Mice
Wild-type C57BL/6 mice were purchased from National Cancer Institute (Rockville, MD). CXCR3−/− mice backcrossed ten generations into the C57BL/6 background were initially generated and provided by C. Gerard (Children’s Hospital, Boston, MA) [43] and then bred in our facility. CCR5−/− mice were generated as described [44] and backcrossed ten generations into the C57BL/6 background. To generate CXCR3−/−/CCR5−/− mice, CXCR3−/− female and CCR5−/− male mice were bred to each other in our facility. F1 offspring (CXCR3−/− CCR5+/− males and CXCR3+/− CCR5+/− females) were then bred to each other to generate mice deficient in both CXCR3 and CCR5 for further breeding. For adoptive transfer studies, OTI TCR transgenic mice, whose T cell receptor is specific for the peptide OVA257–264, was used to generate donor CD8 T cells. OTI mice in the C57BL/6-Thy1.1 background were provided by P. Shrikant (Roswell Park Cancer Institute, Buffalo, NY), and OTI mice in the C57BL/6-Thy1.2 background were obtained from Jackson Immunoresearch Laboratories and bred in our facility. CCR5−/− and female CXCR3−/− mice were then crossed with OTI mice to generate CCR5−/− OTI and CXCR3−/− OTI. To generate CXCR3−/−/CCR5−/− OTI mice, female CXCR3−/−/CCR5−/− mice were bred with CCR5−/− OTI male mice as done for the CXCR3−/−/CCR5−/− mice. CXCR3 and CCR5 deficiencies were confirmed by PCR for all mouse lines. Recipient congenic mice for adoptive transfer studies were purchased from Jackson Laboratories (B6Pl Thy1a; Thy1.1+) (Bar Harbor, ME) or bred in our facility (Thy1.1/Thy1.2). All protocols were approved by the Massachusetts General Hospital Subcommittee on Research and Animal Care.
Virus and infections
Influenza A/Puerto Rico/8/34 (PR8) was obtained from the American Type Culture collection (ATCC; VR-1469, Manassas, VA). The virus was grown in Madin Darby canine kidney cells (MDCK). Influenza A/HongKong/8/68-x31 (x31) was kindly provided by Dr. Troy Randall (Trudeau Institute) and grown in embryonated chicken eggs in our laboratory. The recombinant virus influenza A/WSN/33 OVAI containing the peptide OVA257–264 (WSN-OVA) was generated and kindly provided by Dr. David Topham (University of Rochester)[20]. WSN-OVA was then grown in MDCK cells in our laboratory. Virus pools titers were measured by MDCK plaque assay and appropriate infecting doses were determined by in vivo titration. MLD50 and MID50 were calculated using the Reed and Meunch method [45]. For the pools of PR8 virus used in these studies the MLD50 was 102.4–102.9
Mice were infected with influenza A at 6–9 weeks of age with a nonlethal dose of influenza (0.3 MLD50). Mice were anesthetized with ketamine (80mg/kg)-xylazine (12mg/kg). Viruses were diluted to the appropriate dose using sterile PBS. Twenty-30μl of virus was administered intranasally once mice were anesthetized. At certain time points post-infection, lungs were removed and snap frozen. Lung homogenates were prepared in 2mL PBS using a Polytron homogenizer, spun down, and aliquots of the supernatants were frozen for viral titer measurements by MDCK plaque assay.
MDCK plaque assay
MDCK cells were grown to confluency in six-well plates overnight. After washing the monolayers, dilutions of virus stock or supernatant from lung homogenates were added to the wells in duplicate and incubated for one hour at 37°C. The wells were then washed and an overlay of serum free Minimal essential media (Mediatech, Manassas, VA) plus 1.5% SeaKem agarose at a 1:1 ratio, and 0.5μg/ml TPCK-treated trypsin (Worthington Biochemical Corporation, Lakewood, NJ) was added to the monolayers. Plates were incubated at 37°C for 48 hours and then stained with 1% crystal violet. Viral titers were calculated as plaque forming units per mL (PFU/mL).
Histopathology
Lung lobes were inflated with 10% buffered formalin and then placed in formalin. Paraffin-embedded 4μm sections were prepared and stained with hematoxylin and eosin (H&E). A investigator blinded to the genotypes scored tissue sections at low power based on a global assessment of inflammation, defined by increased cellularity of leukocytes and loss of normal lung architecture. The scoring parameters were: 0 = normal lung, 1 = <25% of lung tissue with inflammation, 2 = 25–50% of lung tissue with inflammation, 3 = 50–75% of lung tissue with inflammation, and 4 = >75% of lung tissue with inflammation.
Cytospin
Mice were sacrificed at different time points post-infection. Bronchoalveolar lavage fluid (BALF) was obtained by infusing six 0.5 ml washes of cold PBS with 0.12% 2mm EDTA. Differential cells counts were obtained after spinning 0.75×106 cells onto slides and staining with Hema-3 staining protocol (Fisher Scientific). Differential counts were performed on at least 100 cells per slide.
Flow cytometry
Single-cell suspensions of spleen, blood, mediastinal lymph node, BALF, and lung were prepared, and red blood cells were lysed. To extract the leukocytes from lung tissue, lung lobes were removed, minced with scissors and then digested for 45 min in RPMI with 0.28 Wunsch U/ml Liberase Blendzyme (Roche) and DNase 30 U/ml (Sigma-Aldrich) at 37° C. The digested tissues were then strained through a 70μM filter prior to red blood cell lysis. Samples were blocked with purified CD16/CD32 monoclonal antibody (BD Biosciences), and then stained with fluorescently labeled antibodies to CD4, CD8, Thy1.1, and Thy1.2 (BD Biosciences). Flow cytometry was performed on a FACSCalibur analytical flow cytometer (BD Biosciences), and data were analyzed using FlowJo software (Tree Star).
Adoptive transfer
CD8+ cells were purified from spleens of OTI transgenic mice by MACS CD8α (Ly-2) magnetic microbeads (Miltenyi Biotec, Auburn, CA). A total of 1.5×106 OTI cells were transferred intravenously via the tail vein one day prior to infection. For competitive transfer studies, 0.75×106 wild-type OTI Thy1.1+ cells were co-transferred with 0.75×106 wild-type or chemokine receptor knockout OTI Thy1.2+ cells one day prior to infection. OTI cells were either transferred as naive cells or as in vitro-activated effector cells as previously described with a few modifications [23]. Briefly, naive CD8+OTI+ cells were placed in culture with OVA257–264-pulsed irradiated APCs, prepared from spleens of C57BL/6 mice, at a T cell/APC ratio of 1:10, in the presence of 2mg/ml anti-CD28 (BD Biosciences, San Diego, CA), 50U/ml mouse recombinant IL-2 (R&D Systems, Minneapolis, MI) and 10ng/ml recombinant mouse IL-12 (R&D Systems).
Cytokine Multiplex Assay
The supernatants of the first mL of BALFs obtained as described above were frozen for cytokine and chemokine analysis. Concentrations of IFNγ, TNFα, CCL2, CXCL10, CCL3, and CCL5 were measured using a LINCOplex six-plex mouse cytokine-chemokine kit as per manufacturer’s guidelines (Millipore, Billerica, MA) and read on a Luminex 100 (Luminex Corporation, Austin, TX). Results were analyzed using Beadview software (Upstate Cell Signaling Solutions, Temecula, CA).
Statistical analysis
Data are expressed as mean ± SD or SEM as indicated. Statistical analysis was performed using GraphPad Prism. A p value less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants to S.A.F. (F32AI063781) (5T32AR007238-30) and A.D.L. (RO1 CA069212-13). We would like to thank Dr. Fjoralba Kristo, Nicole Brousaides, Uwanda Coleman, and Carol Leary for technical assistance
Abbreviations
- CCR5−/−
CCR5-deficient
- CXCR3−/−
CXCR3-deficient
- CXCR3−/−/CCR5−/−
CXCR3 and CCR5 deficient
- PR8
Influenza A/PuertoRico/8/34
- x31
Influenza A/HongKong/8/68-x31
- WSN-OVA
Influenza A/WSN/33 OVAI
- MDCK
Madin Darby canine kidney cells
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Doherty PC, Turner SJ, Webby RG, Thomas PG. Influenza and the challenge for immunology. Nat Immunol. 2006;7:449–455. doi: 10.1038/ni1343. [DOI] [PubMed] [Google Scholar]
- 2.Viola A, Luster AD. Chemokines and their receptors: Drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol. 2008;48:171–197. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- 3.Lederman MM, Penn-Nicholson AP, Cho M, Mosier D. Biology of CCR5 and its role in HIV infection and treatment. JAMA. 2006;296:815–826. doi: 10.1001/jama.296.7.815. [DOI] [PubMed] [Google Scholar]
- 4.Dawson TC, Beck MA, Kuziel WA, Henderson F, Maeda N. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am J Pathol. 2000;156:1951–1959. doi: 10.1016/S0002-9440(10)65068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J Leukoc Biol. 2004;76:886–895. doi: 10.1189/jlb.1203644. [DOI] [PubMed] [Google Scholar]
- 6.Debes GF, Dahl ME, Mahiny AJ, Bonhagen K, Campbell DJ, Siegmund K, Erb KJ, Lewis DB, Kamradt T, Hamann A. Chemotactic responses of IL-4-, IL-10-, and IFN-gamma-producing CD4+ T cells depend on tissue origin and microbial stimulus. J Immunol. 2006;176:557–566. doi: 10.4049/jimmunol.176.1.557. [DOI] [PubMed] [Google Scholar]
- 7.Tyner JW, Uchida O, Kajiwara N, Kim EY, Patel AC, O’Sullivan MP, Walter MJ, Schwendener RA, Cook DN, Danoff TM, Holtzman MJ. CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat Med. 2005;11:1180–1187. doi: 10.1038/nm1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taqueti VR, Grabie N, Colvin R, Pang H, Jarolim P, Luster AD, Glimcher LH, Lichtman AH. T-bet controls pathogenicity of CTLs in the heart by separable effects on migration and effector activity. J Immunol. 2006;177:5890–5901. doi: 10.4049/jimmunol.177.9.5890. [DOI] [PubMed] [Google Scholar]
- 10.Thomas SY, Hou R, Boyson JE, Means TK, Hess C, Olson DP, Strominger JL, Brenner MB, Gumperz JE, Wilson SB, Luster AD. CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. Journal of Immunology. 2003;171:2571–2580. doi: 10.4049/jimmunol.171.5.2571. [DOI] [PubMed] [Google Scholar]
- 11.Medoff BD, Wain JC, Seung E, Jackobek R, Means TK, Ginns LC, Farber JM, Luster AD. CXCR3 and its ligands in a murine model of obliterative bronchiolitis: regulation and function. J Immunol. 2006;176:7087–7095. doi: 10.4049/jimmunol.176.11.7087. [DOI] [PubMed] [Google Scholar]
- 12.Rahangdale S, Morgan R, Heijens C, Ryan TC, Yamasaki H, Bentley E, Sullivan E, Center DM, Cruikshank WW. Chemokine receptor CXCR3 desensitization by IL-16/CD4 signaling is dependent on CCR5 and intact membrane cholesterol. J Immunol. 2006;176:2337–2345. doi: 10.4049/jimmunol.176.4.2337. [DOI] [PubMed] [Google Scholar]
- 13.Algood HM, Flynn JL. CCR5-deficient mice control Mycobacterium tuberculosis infection despite increased pulmonary lymphocytic infiltration. J Immunol. 2004;173:3287–3296. doi: 10.4049/jimmunol.173.5.3287. [DOI] [PubMed] [Google Scholar]
- 14.Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence CW, Braciale TJ. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J Immunol. 2004;173:1209–1218. doi: 10.4049/jimmunol.173.2.1209. [DOI] [PubMed] [Google Scholar]
- 16.Moskophidis D, Kioussis D. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J Exp Med. 1998;188:223–232. doi: 10.1084/jem.188.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiley JA, Cerwenka A, Harkema JR, Dutton RW, Harmsen AG. Production of interferon-gamma by influenza hemagglutinin-specific CD8 effector T cells influences the development of pulmonary immunopathology. Am J Pathol. 2001;158:119–130. doi: 10.1016/s0002-9440(10)63950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Small BA, Dressel SA, Lawrence CW, Drake DR, 3rd, Stoler MH, Enelow RI, Braciale TJ. CD8(+) T cell-mediated injury in vivo progresses in the absence of effector T cells. J Exp Med. 2001;194:1835–1846. doi: 10.1084/jem.194.12.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu L, Yoon H, Zhao MQ, Liu J, Ramana CV, Enelow RI. Cutting edge: pulmonary immunopathology mediated by antigen-specific expression of TNF-alpha by antiviral CD8+ T cells. J Immunol. 2004;173:721–725. doi: 10.4049/jimmunol.173.2.721. [DOI] [PubMed] [Google Scholar]
- 20.Topham DJ, Castrucci MR, Wingo FS, Belz GT, Doherty PC. The role of antigen in the localization of naive, acutely activated, and memory CD8(+) T cells to the lung during influenza pneumonia. J Immunol. 2001;167:6983–6990. doi: 10.4049/jimmunol.167.12.6983. [DOI] [PubMed] [Google Scholar]
- 21.Grayson MH, Ramos MS, Rohlfing MM, Kitchens R, Wang HD, Gould A, Agapov E, Holtzman MJ. Controls for lung dendritic cell maturation and migration during respiratory viral infection. J Immunol. 2007;179:1438–1448. doi: 10.4049/jimmunol.179.3.1438. [DOI] [PubMed] [Google Scholar]
- 22.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 23.Grabie N, Delfs MW, Westrich JR, Love VA, Stavrakis G, Ahmad F, Seidman CE, Seidman JG, Lichtman AH. IL-12 is required for differentiation of pathogenic CD8+ T cell effectors that cause myocarditis. J Clin Invest. 2003;111:671–680. doi: 10.1172/JCI16867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohlmeier JE, Miller SC, Smith J, Lu B, Gerard C, Cookenham T, Roberts AD, Woodland DL. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29:101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 26.Cantor J, Haskins K. Recruitment and activation of macrophages by pathogenic CD4 T cells in type 1 diabetes: evidence for involvement of CCR8 and CCL1. J Immunol. 2007;179:5760–5767. doi: 10.4049/jimmunol.179.9.5760. [DOI] [PubMed] [Google Scholar]
- 27.Menke J, Zeller GC, Kikawada E, Means TK, Huang XR, Lan HY, Lu B, Farber J, Luster AD, Kelley VR. CXCL9, but not CXCL10, promotes CXCR3-dependent immune-mediated kidney disease. J Am Soc Nephrol. 2008;19:1177–1189. doi: 10.1681/ASN.2007111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramos CD, Canetti C, Souto JT, Silva JS, Hogaboam CM, Ferreira SH, Cunha FQ. MIP-1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4. J Leukoc Biol. 2005;78:167–177. doi: 10.1189/jlb.0404237. [DOI] [PubMed] [Google Scholar]
- 29.Wells MA, Albrecht P, Ennis FA. Recovery from a viral respiratory infection. I. Influenza pneumonia in normal and T-deficient mice. J Immunol. 1981;126:1036–1041. [PubMed] [Google Scholar]
- 30.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galkina E, Thatte J, Dabak V, Williams MB, Ley K, Braciale TJ. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J Clin Invest. 2005;115:3473–3483. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Lemos C, Christensen JE, Nansen A, Moos T, Lu B, Gerard C, Christensen JP, Thomsen AR. Opposing effects of CXCR3 and CCR5 deficiency on CD8+ T cell-mediated inflammation in the central nervous system of virus-infected mice. J Immunol. 2005;175:1767–1775. doi: 10.4049/jimmunol.175.3.1767. [DOI] [PubMed] [Google Scholar]
- 33.Nansen A, Christensen JP, Andreasen SO, Bartholdy C, Christensen JE, Thomsen AR. The role of CC chemokine receptor 5 in antiviral immunity. Blood. 2002;99:1237–1245. doi: 10.1182/blood.v99.4.1237. [DOI] [PubMed] [Google Scholar]
- 34.Holst PJ, Orskov C, Qvortrup C, Christensen JP, Thomsen AR. CCR5 and CXCR3 are dispensable for liver infiltration, but CCR5 protects against virus-induced T-cell mediated hepatic steatosis. J Virol. 2007 doi: 10.1128/JVI.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yurchenko E, Tritt M, Hay V, Shevach EM, Belkaid Y, Piccirillo CA. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J Exp Med. 2006;203:2451–2460. doi: 10.1084/jem.20060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao MQ, Stoler MH, Liu AN, Wei B, Soguero C, Hahn YS, Enelow RI. Alveolar epithelial cell chemokine expression triggered by antigen-specific cytolytic CD8(+) T cell recognition. J Clin Invest. 2000;106:R49–58. doi: 10.1172/JCI9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakai S, Kawamata H, Mantani N, Kogure T, Shimada Y, Terasawa K, Sakai T, Imanishi N, Ochiai H. Therapeutic effect of anti-macrophage inflammatory protein 2 antibody on influenza virus-induced pneumonia in mice. J Virol. 2000;74:2472–2476. doi: 10.1128/jvi.74.5.2472-2476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 39.Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, Hanna J, Qimron U, Landau G, Greenbaum E, Zakay-Rones Z, Porgador A, Mandelboim O. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 40.Kindberg E, Mickiene A, Ax C, Akerlind B, Vene S, Lindquist L, Lundkvist A, Svensson L. A deletion in the chemokine receptor 5 (CCR5) gene is associated with tickborne encephalitis. J Infect Dis. 2008;197:266–269. doi: 10.1086/524709. [DOI] [PubMed] [Google Scholar]
- 41.Lim JK, Louie CY, Glaser C, Jean C, Johnson B, Johnson H, McDermott DH, Murphy PM. Genetic deficiency of chemokine receptor CCR5 is a strong risk factor for symptomatic West Nile virus infection: a meta-analysis of 4 cohorts in the US epidemic. J Infect Dis. 2008;197:262–265. doi: 10.1086/524691. [DOI] [PubMed] [Google Scholar]
- 42.Gulick RM, Su Z, Flexner C, Hughes MD, Skolnik PR, Wilkin TJ, Gross R, Krambrink A, Coakley E, Greaves WL, Zolopa A, Reichman R, Godfrey C, Hirsch M, Kuritzkes DR. Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-Infected, treatment-experienced patients: AIDS clinical trials group 5211. J Infect Dis. 2007;196:304–312. doi: 10.1086/518797. [DOI] [PubMed] [Google Scholar]
- 43.Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, Smiley ST, Ling M, Gerard NP, Gerard C. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–1520. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan IA, Thomas SY, Moretto MM, Lee FS, Islam SA, Combe C, Schwartzman JD, Luster AD. CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection. PLoS Pathog. 2006;2:e49. doi: 10.1371/journal.ppat.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1937;27:493–497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.