Abstract
Thrombolytic treatment of ischemic stroke with tissue plasminogen activator (tPA) is markedly limited due to concerns for hemorrhagic complications and the requirement that tPA be administered within 3 h of symptoms. Here we report that tPA activation of latent platelet-derived growth factor-CC (PDGF-CC) may explain these limitations. Intraventricular injection of tPA or active PDGF-CC, in the absence of ischemia, leads to significant increases in cerebrovascular permeability. In contrast, co-injection of neutralizing antibodies to PDGF-CC with tPA blocks increased permeability, indicating that PDGF-CC is a downstream substrate of tPA within the neurovascular unit (NVU). These effects are mediated through activation of PDGF α-receptors (PDGFR-α) on perivascular astrocytes, and treatment of mice following ischemic stroke with the PDGFR-α antagonist Imatinib reduces both cerebrovascular permeability and hemorrhagic complications associated with late administration of thrombolytic tPA. These data demonstrate that PDGF signaling regulates blood-brain-barrier (BBB) permeability and suggest potential new strategies for stroke treatment.
Stroke is a leading cause of adult morbidity and mortality1. Ischemic stroke is the most common form of stroke and occurs when there is an abrupt interruption of blood flow to the brain2. The only drug currently approved by the FDA specifically for ischemic stroke is the thrombolytic agent tPA. However, there is a narrow window during which thrombolysis can be safely accomplished, and treatment of patients more than 3 h after the onset of symptoms is not recommended. This results in < 3% of potential patients currently receiving tPA1,3. The limited benefit of tPA may be due to its previously unidentified activities in the central nervous system (CNS) beyond its well-established thrombolytic role.
TPA is a highly specific serine protease that activates the zymogen plasminogen to the broad-specificity protease plasmin. Outside the CNS, tPA is primarily a thrombolytic enzyme, since plasmin’s principal substrate is fibrin. However, within the CNS the role of tPA is not well characterized, and its primary substrates are not known. A growing body of evidence indicates that tPA is involved in pathological events in the CNS including neurodegeneration, seizures and cerebral ischemia4–12. The role of endogenous tPA in cerebral ischemia is complex, with the potential for both beneficial and harmful effects6,13. Early experiments in animal models of stroke demonstrated that thrombolytic tPA reduced neurological damage when given within a few hours of the onset of ischemia14. Likewise, in humans, early tPA treatment of ischemic stroke results in a 30% increase in patients with minimal disability at three months15. In contrast, recent studies in animal models of transient and permanent middle cerebral artery occlusion (MCAO) demonstrated that both genetic deficiency6,7 and inhibition8,10,16 of tPA within the CNS are associated with improved stroke outcome. The mechanisms of tPA’s harmful effects in stroke are unknown, but it has been suggested that tPA within the CNS may intensify ischemia-induced excitotoxicity6. TPA can also directly affect the integrity of the BBB17–19, and in one study the late administration of thrombolytic tPA significantly increased BBB dysfunction 24 h after embolic stroke16. This suggests that some of tPA’s negative effects following stroke may be secondary to its role in ischemia-induced BBB dysfunction.
Previously we showed that tPA is both necessary and sufficient to induce opening of the BBB17. This effect required proteolytically active tPA, but was independent of plasminogen, implicating another substrate for tPA. Recently, we identified the dimeric growth factor PDGF-CC as a novel substrate for tPA20. The PDGF-C protein has a two-domain structure, and tPA cleavage of the amino-terminal CUB domains from latent PDGF-CC generates active PDGF-CC capable of triggering PDGFR-α signaling21. Therefore, we hypothesize that PDGF-CC is a candidate substrate for tPA that may regulate BBB integrity in the CNS.
RESULTS
TPA increases cerebrovascular permeability through PDGF-CC
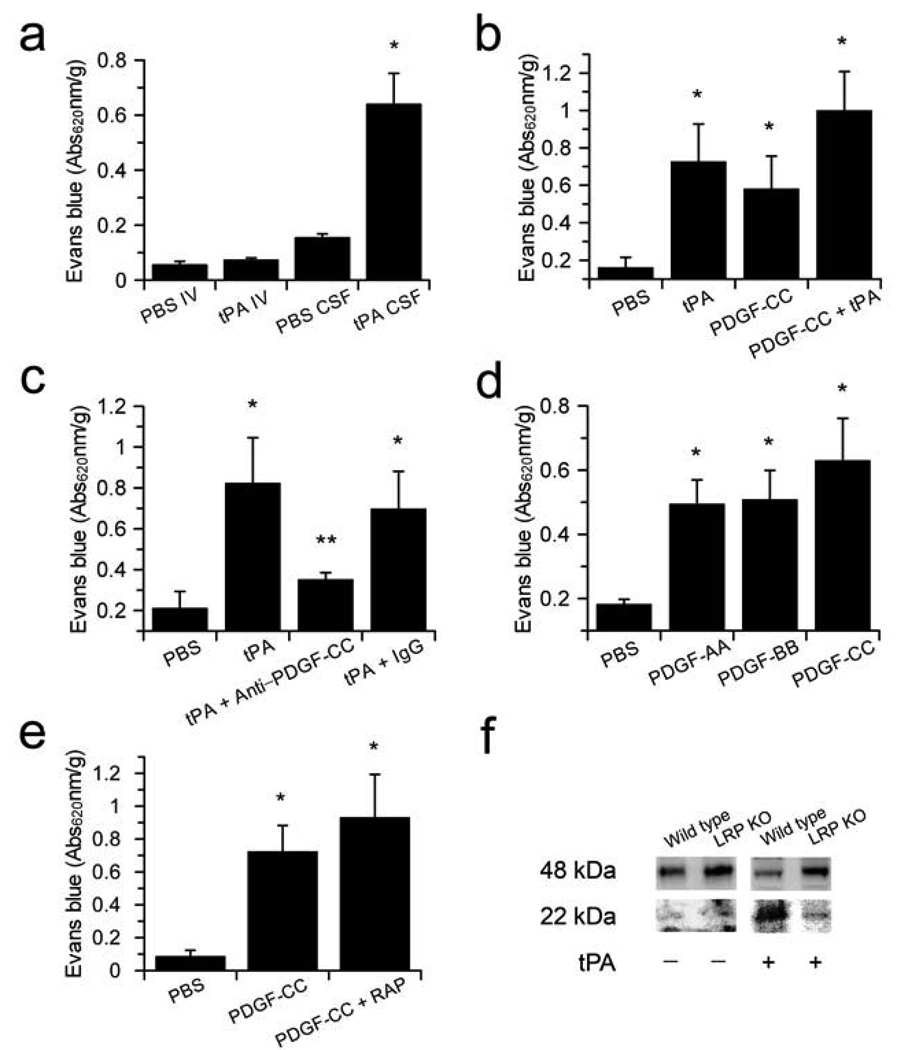
The demonstration that tPA injection into the cerebrospinal fluid (CSF) rapidly increases cerebrovascular permeability17 led us to propose that tPA regulates cerebrovascular permeability through a substrate-dependent receptor-mediated cell signaling event within the NVU. The NVU is composed of endothelial cells, astrocytes, microglia, neurons and smooth muscle cells or pericytes. To determine if tPA acts specifically on the CNS side of the NVU, we compared the effect of tPA injection into the CSF with tPA administered intravenously on Evans blue (EB) dye extravasation in nonischemic mice. This analysis revealed that tPA acts on the abluminal side of the NVU since increased cerebrovascular permeability was observed only when enzymatically active tPA was injected into the CSF, and not when administered intravenously (Fig. 1a, Supplementary Fig. 1 online). Since tPA activates latent PDGF-CC20,22, we examined if active PDGF-CC also induced cerebrovascular permeability. Like tPA, the intraventricular injection of active PDGF-CC significantly (p < 0.01) increased cerebrovascular permeability to an extent that was similar to that observed with tPA (Fig. 1b). The combined treatment with both tPA and PDGF-CC was not significantly different from either treatment alone indicating no additive or synergistic increase in cerebrovascular permeability with the two agents. This suggests that tPA and PDGF-CC may act within a common pathway.
Figure 1.
Active PDGF-CC mediates tPA-induced cerebrovascular permeability. (a) Comparison of EB extravasation 1 h following either intravenous injection (IV) with 10 mg/kg of tPA as a bolus (~250 µg/mouse) or intraventricular injection (CSF) with 585 ng of tPA. (b) EB extravasation 1 h after intraventricular injection of PBS (PBS), tPA (tPA), active PDGF-CC (PDGF-CC), or active PDGF-CC together with tPA (PDGF-CC+tPA). (c) EB extravasation 1 h after intraventricular injection of either PBS (PBS), active tPA (tPA), tPA with blocking antibodies to PDGF-CC (tPA+anti-PDGF-CC), or tPA together with control IgG (tPA+IgG). (d) EB extravasation 1 h after intraventricular injection of PBS (PBS), PDGF-AA (PDGF-AA), PDGF-BB (PDGF-BB), or active PDGF-CC (PDGF-CC). (e) EB extravasation 1 h after intraventricular injection with either PBS (PBS), active PDGF-CC (PDGF-CC), or active PDGF-CC together with the LRP antagonist RAP (PDGF-CC+RAP). For all injections into the CSF, 3 µl of 3 µM protein was used except for antibodies which were 0.4 mg/ml. Each group n = 8–10 and errors represent S.E.M. Single asterisks indicate p < 0.01 vs. PBS, and ** indicates p < 0.05 vs the IgG control. (f) PDGF-CC cleavage by tPA is impaired in Lrp−/− cells (LRP KO). Serum free medium from Lrp−/− MEFs and wild-type cells demonstrates that both cell lines express the 48 kDa full length PDGF-C, while the addition of exogenous tPA to the cells only generates the 22 kDa PDGF-C species in the presence of the wild type cells but not in Lrp−/− cells.
To test whether PDGF-CC was acting downstream of tPA, neutralizing antibodies against PDGF-CC (Supplementary Fig. 2 online) were co-injected with tPA. These data demonstrated that PDGF-CC-specific antibodies, but not pre-immune IgG, significantly (p < 0.05) inhibited the tPA-induced increase in cerebrovascular permeability (Fig. 1c). This indicates that PDGF-CC acts downstream of tPA and suggests that tPA activates latent PDGF-CC within the CNS.
PDGF-CC is a known agonist of the PDGFR-α21. Therefore, we tested whether two other PDGFR-α agonists, PDGF-AA and PDGF-BB23, also increased cerebrovascular permeability when injected into CSF. Both PDGF-AA and PDGF-BB increased EB extravasation significantly (p < 0.01) compared to the PBS-treated group, demonstrating a PDGFR-α dependent process (Fig. 1d). However, in contrast to PDGF-CC-specific antibodies, neutralizing PDGF-AB-specific antibodies had no effect on tPA-mediated enhancement of BBB permeability (Supplementary Fig. 3 online).
Several studies have shown that tPA-induced cerebrovascular permeability is inhibited by antagonists of members of the low-density lipoprotein receptor family (LDL-Rs)17,19,24. Therefore, we examined whether the LDL-R antagonist RAP also inhibited PDGF-CC-induced cerebrovascular permeability. In contrast to its inhibition of tPA-induced EB extravasation17, RAP had no effect on the ability of active PDGF-CC to increase cerebrovascular permeability (Fig. 1e). This implies that active PDGF-CC does not require LDL-Rs to mediate its effect on the NVU, suggesting that like tPA, the LDL-Rs act upstream of active PDGF-CC. Since both tPA and PDGF-CC bind to the low-density lipoprotein receptor related protein (LRP) (Supplementary Fig. 4 online), we speculated that LRP may facilitate activation of PDGF-CC by tPA. To test this we investigated tPA-mediated cleavage of PDGF-CC in the presence of mouse embryonic fibroblasts (MEF) that either express LRP or are genetically deficient in LRP25. These data demonstrated that both cell types express similar amounts of latent PDGF-CC, but that only cells expressing LRP stimulate efficient activation of PDGF-CC by tPA (Fig. 1f). This suggests that in vivo cell-associated LRP can markedly enhance PDGF-CC activation by tPA.
Analysis of vascular morphology by electron microscopy 1 h after intraventricular injection of either tPA or active PDGF-CC indicates that both proteins induced specific morphological changes in cerebral vessels compared to control injection (Fig. 2). In control tissue, vessels were intact and tightly associated with surrounding tissue (Fig. 2a,d). In contrast, arterioles from mice receiving intraventricular injections of tPA or PDGF-CC were surrounded by open areas of apparent fluid accumulation (Fig. 2b–c,e–f). This phenotype was observed in multiple arterioles in tPA and PDGF-CC-treated animals but not in brain capillaries (Fig. 2b–c). The similarity in morphological changes observed in tPA and PDGF-CC-treated animals along with the similar degree of increased cerebrovascular permeability induced by both agents suggests that PDGF-CC and tPA provoke comparable vascular changes that occur primarily in arterioles.
Figure 2.
TPA and PDGF-CC induce similar morphological changes in brain vasculature. (a–c) Light microscopy images of cerebral sections prepared for EM analysis but stained with toluene blue. (d–f) High magnification micrographs from electron microscopy of cerebral arterioles. Brains were harvested 1 h after intraventricular injection of either PBS (a and d), tPA (b and e) or PDGF-CC (c and f). Scale bar is 50 µM in a–c and 2 µM in d–f.
PDGF-CC, tPA and PDGFR-α in the NVU
Immunohistochemical staining demonstrated that PDGF-CC localization throughout the brain closely resembled the reported expression of tPA26, with the most abundant staining observed in cortex, striatum, and hippocampus (not shown). Higher resolution microscopy indicated that PDGF-CC was often closely associated with arterioles but not with capillaries, and was typically seen as a stained patch on one side of larger vessels (Fig. 3a). Immunohistochemical analysis of tPA demonstrated abundant vessel-associated tPA throughout the brain. In capillaries, the staining was largely confined to endothelial cells while in arterioles, tPA was also associated with perivascular cells (Fig. 3b). Thus, both PDGF-CC, and tPA were localized in the NVU of arterioles. The expression of PDGF-CC and its receptor PDGFR-α in brain was also investigated using mice doubly heterozygous for a nuclear-targeted histone H2B/green fluorescent protein (GFP) fusion protein inserted into the Pdgfrα locus27 (Supplementary Fig. 5 online), and for a lacZ-reporter inserted into the Pdgfc locus28. Brains from the Pdgfrα+/GFP/Pdgfc+/lacZ mice demonstrated both PDGFR-α and PDGF-CC expression in cells associated with arterioles (Fig. 3c, arrows). PDGF-CC expression was also seen in cells not directly associated with vessels (arrowheads).
Figure 3.
PDGF-CC, tPA and the PDGFR-α are expressed in the NVU. (a) Sections from normal mouse brains stained with antibodies to PDGF-CC show PDGF-CC staining in patches associated with arterioles (arrows), but not with capillaries (arrowheads). (b) Sections from normal mouse brains stained with antibodies to tPA show mainly perivascular tPA staining in association with an arteriole (arrows). (c–h) Micrographs showing PDGFR-α expression in mouse brain using a GFP reporter. (c) Sections of double heterozygous Pdgfrα+/GFP/Pdgfc+/lacZ mouse brains stained with the PDGF-C reporter Xgal and antibodies to the endothelial cell marker platelet/endothelial cell adhesion molecule-1 (PECAM red). The arrows indicate vessel associated PDGF-C expression and arrowheads indicate non-vessel associated expression. GFP and PECAM staining was visualized using fluorescence, whereas Xgal staining was viewed in bright field. (d,e) Whole mount immunofluorescence staining of GFP-positive vessel fragments stained for SMA (red) (d) confirmed that the GFP-positive vessel fragments are arterioles. The GFP-positive nuclei are mainly localized outside of the SMA-positive cells. In contrast, co-staining with GFAP (red) (e), an astrocyte marker, suggests that the GFP-positive cells are astrocytes. (f–h) Immunofluorescence staining of brain sections from Pdgfrα+/GFP mice stained with markers for astrocytes, GFAP (red) (f), vascular smooth muscle cells, SMA (red) (g), and endothelial cells, PECAM (red) (h). Similar to the isolated vessel fragments, co-localization of GFP expression with GFAP was abundant in the stained brain sections and produced a yellow color (g). Scale bar is 50 µm in a–b, d–h, and 20 µm in c.
To further characterize PDGFR-α-positive vessels, GFP-positive vessel fragments were isolated from brain. The most intensely GFP-positive vessels resembled arterioles and were covered with firmly attached GFP-expressing cells. Whole mount immunofluorescence staining of these vessel fragments for smooth muscle actin (SMA) revealed that the vessels were arterioles since they were strongly positive for SMA (Fig. 3d). However, the GFP-positive nuclei did not appear to directly co-localize with SMA and instead localized to the outside of the SMA-positive cells. In contrast, staining of the GFP-positive vessels for the astrocyte marker, glial fibrillar acidic protein (GFAP), showed that GFP-expressing nuclei appeared to be embedded in the GFAPpositive cells (Fig. 3e). To confirm these observations, intact brain sections from GFP-expressing mice were stained by immunofluorescence for GFAP, SMA, or endothelial cells (PECAM) and imaged together with GFP (Fig. 3f–h). These results showed that PDGFR-α largely co-localized with perivascular GFAP positive cells (Fig. 3f, yellow), and not in cells expressing SMA (Fig. 3g) or PECAM (Fig. 3h), demonstrating that perivascular astrocytes are the primary neurovascular cells expressing PDGFR-α. These data suggest that tPA, PDGF-CC and PDGFR-α are localized to arterioles in the CNS where they interact on the surface of perivascular astrocytes.
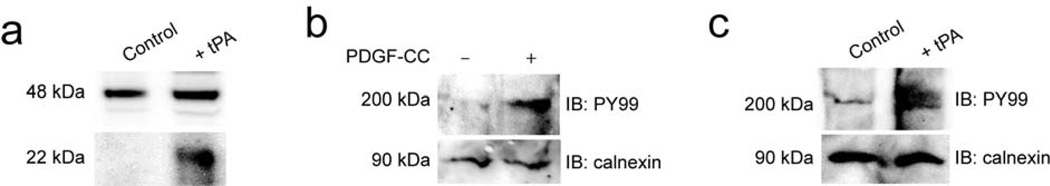
Since LRP is also known to be specifically associated with arteriolar perivascular astrocytes29, we tested whether type I fibrous astrocytes in culture could enhance the activation of PDGF-CC by tPA, and whether active PDGF-CC could stimulate PDGFR-α phosphorylation. These studies demonstrated that astrocytes in culture expressed latent PDGF-CC that was cleaved by added tPA (Fig. 4a). Active PDGF-CC also stimulated PDGFR-α phosphorylation in astrocytes (Fig. 4b), as did the addition of tPA, suggesting a possible autocrine effect of PDGF-CC on astrocytes (Fig. 4c).
Figure 4.
PDGF-CC is expressed by astrocytes in culture. (a–c) Immunoblot analysis of PDGFCC in astrocytes. (a) A 48 kDa band corresponding to full length PDGF-C is seen in control cell media whereas addition of tPA (tPA) to the cells prior to collection of the medium induces release of the active 22 kDa PDGF-C species. PDGFR-α expressed by astrocytes in culture is stimulated by addition of active PDGF-CC (b) or tPA (c) to the cells. Receptor tyrosine phosphorylation is determined by immunoblotting (IB) of cell lysates for phosphotyrosine. Immunoblotting calnexin is also shown as a loading control.
PDGFR-α regulates cerebrovascular permeability after stroke
To examine whether PDGFR-α was activated in a tPA-dependent manner during cerebral ischemia we developed a mouse photothrombotic model of stroke that is similar to a previously described model30,31. This model permits thrombolytic treatment and can be analyzed either with or without reperfusion (Supplementary Fig. 6 online). Six hours after unilateral MCAO in wild-type and tPA-deficient mice, isolated brains were divided into hemispheres, ipsilateral and contralateral to the MCAO, and each hemisphere was analyzed separately for PDGFR-α activation (Fig. 5a,b). Following stroke, PDGFR-α phosporylation was induced ~2-fold in the ipsilateral hemisphere of wild-type mice compared to the nonischemic hemisphere, while in tPA-deficient animals no induction of PDGFR-α activation was seen. These data suggest that PDGFR-α activation within the brain following ischemic stroke is tPA-dependent through PDGF-CC activation.
Figure 5.
Blocking PDGFR-α activation reduces cerebrovascular permeability and stroke volume after MCAO. (a) PDGFR-α activation following MCAO in wild-type mice 6 h after MCAO. Brains were divided into ipsilateral and contralateral hemispheres, detergent-solubilized and the lysates immunoprecipitated with PDGFR-α-specific antibodies followed by immunoblotting with phosphotyrosine-specific antibodies. As a loading control, the precipitated protein was visualized with PDGFR-α-specific antibodies. (b) Quantitation of PDGFR-α activation following MCAO in wild-type or tPA KO mice (n = 2 in each group and errors represent S.E.M). (c,d) Representative images of the ipsilateral hemisphere 24 h after MCAO from control (c) and Imatinib-treated mice (d) injected with EB 1 h before euthanasia. (e) Quantification of the EB extravasation 24 h after MCAO (n = 11 for each group and errors represent S.E.M.). (f) Quantification of the EB extravasation 24 h after MCAO in mice treated with intraventricular PBS, preimmune IgG or PDGF-CC-specific antibodies followed immediately by MCAO (n = 10 for each group and errors represent S.E.M). (g) Quantification of infarct size 72 h after MCAO in mice treated with either Imatinib or vehicle (n = 9 for control group and n = 11 for Imatinib group and errors represent S.E.M.). For all Imatinib studies mice were treated by gavage with either vehicle or 200 mg/kg Imatinib 1 h and 8 h after MCAO and twice daily for the duration of the experiment. In panels e–g the asterisks indicate p < 0.05 vs. control animals. Scale bar is 2 mm in c and d.
To test whether MCAO-induced activation of the PDGFR-α regulates the cerebrovascular response to stroke, we treated mice with the PDGFR-α inhibitor32 Imatinib mesylate 1 h after MCAO. Animals treated with Imatinib exhibited a 33% reduction in EB extravasation after MCAO compared to control mice (Fig. 5c–e), suggesting that PDGFR-α signaling regulates cerebrovascular permeability after stroke. To determine if this effect was due to activation of PDGFR-α by PDGF-CC, PDGF-CC-specific antibodies were injected intraventricularly into mice immediately after stroke induction, and EB extravasation was evaluated 24 h after MCAO (Fig. 5f). These data demonstrate that like Imatinib treatment, EB extravasation was significantly (p < 0.05) reduced in animals treated with PDGF-CC-specific antibodies but not in mice receiving preimmune IgG. This supports the hypothesis that endogenous PDGF-CC mediates the regulation of cerebrovascular permeability after stroke via activation of PDGFR-α.
Since reducing the extent of BBB dysfunction during stroke may impact infarct expansion and improve stroke outcome, we compared infarct volumes in mice treated with Imatinib to controls 72 h after MCAO. Imatinib significantly (p < 0.05) reduced lesion volume by 34% (Fig. 5g), suggesting that interfering with the PDGF-CC/PDGFR-α system may improve stroke outcome.
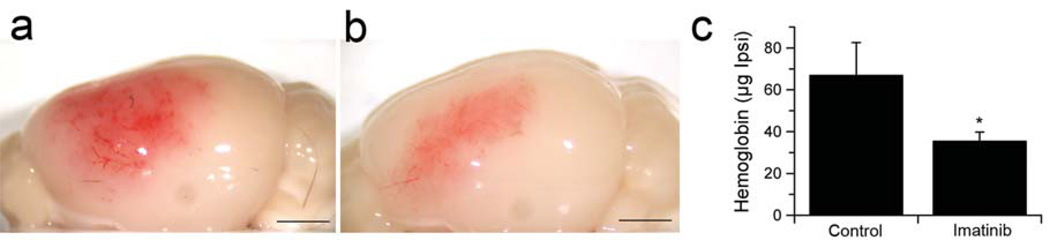
A major risk associated with the use of thrombolytic tPA in ischemic stroke is the occurrence of hemorrhagic complications33–36. Since bleeding represents an extreme example of loss of BBB integrity we examined whether inhibition of PDGFR-α with Imatinib administered 1 h after the onset of ischemia could reduce the extent of hemorrhagic complications associated with tPA-mediated thrombolysis initiated 5 h after the induction of MCAO. These data indicated that Imatinib treatment significantly (p < 0.05) decreased hemorrhage, reducing the amount of hemoglobin associated with the ischemic hemisphere by 50% compared to untreated mice (Fig. 6a–c). This suggests that the known association of thrombolytic tPA with hemorrhagic complications in some patients may be due in part to the activation of PDGF-CC by therapeutic tPA.
Figure 6.
Blocking the PDGF-CC/PDGFR-α pathway reduces intracerebral hemorrhage after MCAO. (a,b) Representative images of the intracerebral hemorrhage in the ipsilateral hemisphere 24 h after MCAO of animals treated with tPA 5 h after MCAO. Mice were treated by gavage with either vehicle (a) or 200 mg/kg Imatinib (b) 1 h and 8 h after MCAO. (c) Quantification of hemoglobin content in the ischemic hemisphere 24 h after MCAO (n = 10 for control group and n = 12 for Imatinib group and errors represent S.E.M). The asterisk indicate p < 0.05 vs. control animals. Scale bar is 2 mm in a and b.
DISCUSSION
Thrombolytic treatment only benefits a limited number of patients with ischemic stroke, and the development of improved therapies for stroke depends upon understanding the unique characteristics of the cerebrovasculature. The limitations of tPA appear to be due in part to unique activities that tPA has in the brain beyond its well established role in fibrinolysis9,17,19,37,38. While there are clear benefits to patients who receive early thrombolytic treatment3,15, the nonfibrinolytic effects of tPA suggest that there are unique challenges for the use of thrombolytic therapy in ischemic stroke. To understand these challenges it is necessary to understand the role that endogenous tPA plays in the CNS. It is clear that immediately after stroke onset, ischemia induces changes in cerebrovascular structures. We suggest that these changes include the release of tPA into perivascular tissue17. This release precedes the loss of BBB integrity and appears to be mechanistically important for the early loss of BBB function since mice lacking tPA are protected from the loss of barrier function17. The downstream target of tPA in this system is not plasminogen since mice lacking plasminogen exhibit similar levels of cerebrovascular permeability as wild type mice17. Other potential CNS targets include the NMDA receptor and LRP9,24,39. Experiments using antagonists of these receptors suggest that the NMDA receptor is not directly involved, whereas antagonists of LRP reduce cerebrovascular permeability after MCAO17,24. Our studies support this hypothesis, and also identify PDGF-CC as a specific substrate of tPA present within the NVU. PDGF-CC is a member of the PDGF family that binds to the PDGFR-α. Unlike PDGF-A or PDGF-B, PDGF-C contains an amino-terminal CUB domain that renders the dimer inactive. However, removal of the CUB domains by tPA20 generates active PDGF-CC which can then activate the PDGFR-α in the NVU.
The direct injection of active PDGF-CC into the CSF bypasses the requirement of activation and increases cerebrovascular permeability in non-ischemic brain. In contrast to tPA-induced vascular permeability17, the enhanced permeability of active PDGF-CC is independent of LRP, implying that both tPA and LRP act upstream of active PDGF-CC. Consistent with this, we show that cell-associated LRP enhances tPA-mediated activation of PDGF-CC. In the context of the NVU, LRP may be a necessary cofactor for efficient activation of latent PDGF-CC by tPA. We also show that PDGF-CC and PDGFR-α are expressed in cerebral arterioles, as is tPA40. The localization of tPA, LRP, PDGF-CC and the PDGFR-α within the NVU of arterioles may facilitate the response to ischemia through activation of the PDGFR-α.
Electron microscopy demonstrates that the development of edema in response to tPA or PDGF-CC is not due to gross destruction of vascular structures or the basement membrane. Instead, these data suggest that the development of edema may be occurring through a regulated process, and thus may represent an exaggeration of a normal vascular response. In pathological conditions such as stroke, neuronal depolarization associated with cerebral ischemia can result in a surge of local tPA activity17,41, which in turn could lead to continued production of active PDGF-CC, persistent activation of PDGFR-α in the NVU, and ultimately loss of BBB integrity.
The benefit of thrombolytic tPA administered within the first 3 h of stroke is likely dependent upon the early maintenance of BBB integrity. In this situation thrombolysis of the occluded vessel should rescue the affected ischemic zone and improve clinical outcome. However, administering tPA beyond the 3 h window increases the likelihood that changes in the cerebrovasculature impair BBB integrity to the point that thrombolytic tPA crosses into the perivascular tissue16 and interacts with the NVU. We postulate that this tPA may further increase activation of endogenous PDGF-CC, thus prolonging activation of the PDGFR-α. This leads to further deterioration of BBB integrity, and in extreme cases may lead directly to hemorrhagic complications.
Understanding this mechanism offers an opportunity to potentially extend the treatment window of tPA for stroke patients. Since blocking the PDGF-CC/PDGFR-α pathway is unlikely to disrupt tPA’s fibrinolytic function, then strategies specifically targeting the PDGF-CC/PDGFR-α pathway should maintain tPA’s beneficial thrombolytic activity while minimizing BBB dysfunction. Imatinib, also known as Gleevec or STI571, is an FDA-approved drug for the treatment of chronic myelogenous leukemia and other cancers, which binds to and inhibits several tyrosine kinases including PDGFR-α. In our study, Imatinib treatment reduced cerebrovascular permeability and stroke lesion volume as well as hemorrhagic complications associated with late thrombolysis, suggesting the possibility of an off-the-shelf adjunct therapy for use with thrombolytic tPA. Although Imatinib does not efficiently cross the BBB in healthy individuals, Imatinib is present in the CNS after oral administration42. In addition, dose and time requirements for using Imatinib in stroke should be very different than in anti-tumor applications. A transient high dose of Imatinib may be all that is required to extend the therapeutic window for tPA. However, further studies are needed to explore this possibility since it remains to be determined whether late administration of a combination therapy of Imatinib plus tPA can extend the standard 3 h treatment window of tPA, by reducing hemorrhagic complications and restoring neuroprotection. Toward this goal, a randomized controlled clinical trial is currently planned by the stroke research team at Karolinska University Hospital in Stockholm Sweden (Prof. N. Wahlgren, Department of Neurology, Karolinska University Hospital, personal communication). This trial is designed to evaluate the safety and feasibility of Imatinib, either alone or with tPA, for use within the first few hours after the onset of ischemic stroke.
In summary our data offer a new model for regulation of cerebrovascular permeability by endogenous tPA and PDGF-CC, and describe a potential novel interventional approach that may extend tPA’s therapeutic window in ischemic stroke. Future studies may also examine if other CNS activities affected by tPA, such as seizure spreading, susceptibility to drug addiction, or anxiety-like behaviors43, also involve tPA-mediated activation of PDGF-CC.
METHODS
Proteins and antibodies
The recombinant core domain of human PDGF-CC was produced in baculo-virus infected insect Sf9 cells and purified as described21. Blocking antibodies to active PDGF-CC (Supplementary Fig. 2 online) were affinity purified rabbit IgG isolated from rabbit no. 615 as described21. Preimmune IgG from the same animal was isolated on a protein A-Sepharose column (Amersham Biosciences). Mouse tPA and affinity purified antibodies to mouse tPA were from Molecular Innovations.
Cell culture, and immunoblotting
All cells used were maintained in Dulbecco’s Modified Eagle Medium (DMEM; 41965, Gibco) supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. The cells were cultured at 37°C in a humidified 5% CO2 atmosphere. The mouse astrocyte cell line C8-D1A was obtained from ATCC and grown in the presence of 1 mM pyruvate. Sub-confluent cultures of wild-type and Lrp−/− MEF cell lines25 were kept in serum-free DMEM over night with or without the addition of 1 µg/ml tPA. Conditioned serum-free medium was collected, and proteins precipitated using trichloroacetic acid (TCA) as described21. PDGF-C species were detected by immunoblotting using PDGF-CC-specific antibodies (no.615).
Intraventricular Injections
To perform intraventricular injection of tPA, active PDGF-CC, and PDGF-CC-specific antibodies, wild type C57BL/6J mice were anesthetized with chloral hydrate (450 mg/kg, IP), placed on a stereotactic frame, and injected at bregma – 2, mediolateral 0, and dorsoventral 217. Injections contained 3 ìl of either active tPA (3 µM), active PDGF-CC core protein (3 µM), PDGF-CC-specific antibodies (0.4 mg/ml), preimmune IgG (0.4 mg/ml) or PBS. Cerebrovascular permeability was then determined 1 h later from EB extravasation as described in Supplementary Methods 1 (online), except that brains were not divided into hemispheres. All animal experiments were approved by the Institutional Animal Care and Use Committee of Unit for Laboratory Animal Medicine at University of Michigan.
Electron Microscopic Analysis
One hour after intraventricular injection of either PBS, tPA or active PDGF-CC, C57BL/6J mice were perfused with PBS followed by 4% formaldehyde with 1% glutaraldehyde and fixed with the same solution for 24 h. The brain tissues were then rinsed with 5% sucrose phosphate buffer 3 times over 30 min, then post-fixed in 1% aqueous OsO4 for 1 h and then stained with 1% uranyl acetate for 1 h. After dehydration, the brain tissues were embedded in EPON (EMS), and ultra thin sections were cut and stained with uranyl acetate and lead citrate. Some sections were stained with toluene blue for analysis by light microscopy. For electron microscopic analysis, sections were examined with a Phillips CM12 transmission electron microscope.
Immunohistochemical localization
For immunohistochemistry, mice were perfused with 4% PFA in PBS, and the brains were post-fixed overnight in the same fixative, and then processed for paraffin-embedding and sectioned to 6 µm using standard protocols. Immunohistochemical staining of PDGF-CC and tPA expression was performed using affinity-purified rabbit IgG against human core PDGF-CC (10 µg IgG/ml) and affinity-purified rabbit antibody to mouse tPA (15 µg IgG/ml) respectively. Controls using normal rabbit IgG gave only background staining (data not shown). An Elite ABC Vectastain kit (Vector Laboratories) was used. Blocking of non-specific binding was performed using TNB blocking buffer (TSA indirect NEN Bioscience). For the tPA staining, antigen retrieval with 0.1 M citrate pH 3.0 at 95 °C for 20 min followed by treatment with 0.25% trypsin at 37 °C for 20 min was performed before the immunostaining as above.
Immunofluorescence localization
Details of the immunofluorescence localizations are presented in Supplementary Methods 2 online.
Analysis of receptor activation
PDGFR-α activation was determined essentially as described44. Briefly, for cell culture studies sub-confluent astrocyte cultures were stimulated with 100 ng/ml active PDGF-CC for 90 min on ice, or treated with tPA overnight, and then lysed as described21. Cell lysates were directly subjected to immunoblotting, and receptor tyrosine phosphorylation was determined using a phosphotyrosine-specific antibody (PY99, Santa Cruz). As a loading control immunoblotting of the endoplasmic reticulum integral membrane protein calnexin was also performed (sc-6465, Santa Cruz). For analysis of receptor activation after MCAO, both wild-type and tPA knockout mice (KO), male 10 weeks old, were subjected to MCAO. Six hours later, animals were perfused with PBS for 4 min and the brains were removed and separated into two hemispheres. Ipsilateral and contralateral hemispheres were then processed for PDGFR-α activation by immunoprecipitation of the PDGFR-α using a specific PDGFR-α antibody followed by immunoblotting for receptor tyrosine phosphorylation using the same phosphotyrosine-specific antibody as in the cell culture studies above. The amount of precipitated PDGFR-α was monitored by reprobing the blots with a PDGFR-α-specific antibodies (R&D Systems).
Mouse model of ischemic stroke
Details of the mouse model of ischemic stroke are presented in Supplementary Methods 1 and in Supplementary Figure 6 online.
Hemoglobin content assay
Twenty four hours after MCAO, animals were anesthetized with chloral hydrate (450 mg/kg, I.P., Morton Grove Pharmaceuticals, Inc.) and euthanized by exsanguination. Animals were then perfused with PBS for 4 min and the brains were removed and separated into hemispheres ipsilateral and contralateral to the MCAO. Each hemisphere was homogenized (Model T8, IKA Works, Inc) in 475 µl PBS at 25,000 rcf for 30 s on ice in a 1.5 ml microfuge tube. Twenty five µl 10% Triton X100 was added to the tube to make the final concentration of 0.5%. After thorough mixing, the tube was kept at room temperature for 5 min and then centrifuged at 25,000 rcf at 4 °C for 30 min (Eppendorf centrifuge, model 5417R). Fifty µl of the supernatant was read at A410nm (SpectraMax M5, Molecular Devices) and the hemoglobin content was quantified relative to a purified hemoglobin (Sigma-Aldrich) standard curve as described45.
Statistical Analysis
Data were analyzed using either a Wilcoxon Mann rank sum test or in cases where more than one group is compared, by ANOVA with repeated test (GraphPad, InStat). p values smaller than 0.05 were considered significant.
Supplementary Material
Acknowledgments
We want to thank P. Soriano for the PDGFR-α/GFP mice, A. Nagy for the PDGF-C knockout mice, M. Wang, G. Schielke and D. Lombardi for helpful discussions and critical reading of the manuscript; N. Gorlatova for surface plasmon resonance analysis; and S. Rezaian and M. Wahl for technical assistance. This work was supported by National Institutes of Health grants HL-55374, HL-55747, HL-54710, and HL-57346 (to D.A. Lawrence); HL50784 and HL54710 (to D. Strickland); NS49478 (to M. Yepes); and grants from Karolinska Institutet, Novo Nordisk Foundation, Swedish Research Council, Swedish Cancer Foundation, and the LeDucq Foundation (to U. Eriksson and C. Betsholtz).
Reference List
- 1.Thom T, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Hou ST, MacManus JP. Molecular mechanisms of cerebral ischemia-induced neuronal death. Int. Rev. Cytol. 2002;221:93–148. doi: 10.1016/s0074-7696(02)21011-6. [DOI] [PubMed] [Google Scholar]
- 3.Marler JR, Goldstein LB. Medicine. Stroke--tPA and the clinic. Science. 2003;301:1677. doi: 10.1126/science.1090270. [DOI] [PubMed] [Google Scholar]
- 4.Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- 5.Tsirka SE, Rogove AD, Bugge TH, Degen JL, Strickland S. An Extracellular Proteolytic Cascade Promotes Neuronal Degeneration in the Mouse Hippocampus. J. Neurosci. 1997;17:543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YF, et al. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 7.Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation. 1999;99:2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- 8.Yepes M, et al. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood. 2000;96:569–576. [PubMed] [Google Scholar]
- 9.Nicole O, et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat. Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 10.Cinelli P, et al. Neuroserpin, a neuroprotective factor in focal ischemic stroke. Mol. Cell Neurosci. 2001;18:443–457. doi: 10.1006/mcne.2001.1028. [DOI] [PubMed] [Google Scholar]
- 11.Yepes M, et al. Regulation of seizure spreading by neuroserpin and tissue-type plasminogen activator is plasminogen-independent. J. Clin. Invest. 2002;109:1571–1578. doi: 10.1172/JCI14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawlak R, Melchor JP, Matys T, Skrzypiec AE, Strickland S. Ethanol-withdrawal seizures are controlled by tissue plasminogen activator via modulation of NR2B-containing NMDA receptors. Proc. Natl. Acad. Sci. U. S. A. 2005;102:443–448. doi: 10.1073/pnas.0406454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabrizi P, et al. Tissue plasminogen activator (tPA) deficiency exacerbates cerebrovascular fibrin deposition and brain injury in a murine stroke model: studies in tPA-deficient mice and wild-type mice on a matched genetic background. Arterioscler. Thromb. Vasc. Biol. 1999;19:2801–2806. doi: 10.1161/01.atv.19.11.2801. [DOI] [PubMed] [Google Scholar]
- 14.Zivin JA, Fisher M, DeGirolami U, Hemenway CC, Stashak JA. Tissue plasminogen activator reduces neurological damage after cerebral embolism. Science. 1985;230:1289–1292. doi: 10.1126/science.3934754. [DOI] [PubMed] [Google Scholar]
- 15.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, et al. Adjuvant treatment with neuroserpin increases the therapeutic window for tissue-type plasminogen activator administration in a rat model of embolic stroke. Circulation. 2002;106:740–745. doi: 10.1161/01.cir.0000023942.10849.41. [DOI] [PubMed] [Google Scholar]
- 17.Yepes M, et al. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J. Clin. Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herz J. LRP: a bright beacon at the blood-brain barrier. J. Clin. Invest. 2003;112:1483–1485. doi: 10.1172/JCI20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, et al. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat. Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 20.Fredriksson L, Li H, Fieber C, Li X, Eriksson U. Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J. 2004;23:3793–3802. doi: 10.1038/sj.emboj.7600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, et al. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat. Cell Biol. 2000;2:302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- 22.Fredriksson L, Ehnman M, Fieber C, Eriksson U. Structural requirements for activation of latent platelet-derived growth factor CC by tissue plasminogen activator. J. Biol. Chem. 2005;280:26856–26862. doi: 10.1074/jbc.M503388200. [DOI] [PubMed] [Google Scholar]
- 23.Hart CE, et al. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988;240:1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- 24.Polavarapu R, et al. Tissue-type plasminogen activator-mediated shedding of astrocytic low-density lipoprotein receptor-related protein increases the permeability of the neurovascular unit. Blood. 2007;109:3270–3278. doi: 10.1182/blood-2006-08-043125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willnow TE, Herz J. Genetic deficiency in low density lipoprotein receptor-related protein confers cellular resistance to Pseudomonas exotoxin A. Evidence that this protein is required for uptake and degradation of multiple ligands. J. Cell Sci. 1994;107:719–726. [PubMed] [Google Scholar]
- 26.Yu H, et al. Control elements between −9.5 and −3.0 kb in the human tissue-type plasminogen activator gene promoter direct spatial and inducible expression to the murine brain. Eur. J. Neurosci. 2001;14:799–808. doi: 10.1046/j.0953-816x.2001.01700.x. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol. Cell Biol. 2003;23:4013–4025. doi: 10.1128/MCB.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding H, et al. A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nat. Genet. 2004;36:1111–1116. doi: 10.1038/ng1415. [DOI] [PubMed] [Google Scholar]
- 29.Wolf BB, Lopes MB, VandenBerg SR, Gonias SL. Characterization and immunohistochemical localization of alpha 2-macroglobulin receptor (low-density lipoprotein receptor-related protein) in human brain. Am. J. Pathol. 1992;141:37–42. [PMC free article] [PubMed] [Google Scholar]
- 30.Nagai N, et al. Tissue-type plasminogen activator has paradoxical roles in focal cerebral ischemic injury by thrombotic middle cerebral artery occlusion with mild or severe photochemical damage in mice. J. Cereb. Blood Flow Metab. 2002;22:648–651. doi: 10.1097/00004647-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Nagai N, Suzuki Y, Van HB, Lijnen HR, Collen D. Effects of plasminogen activator inhibitor-1 on ischemic brain injury in permanent and thrombotic middle cerebral artery occlusion models in mice. J. Thromb. Haemost. 2005;3:1379–1384. doi: 10.1111/j.1538-7836.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- 32.Capdeville R, Buchdunger E, Zimmermann J, Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat. Rev. Drug Discov. 2002;1:493–502. doi: 10.1038/nrd839. [DOI] [PubMed] [Google Scholar]
- 33.The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 34.Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32:438–441. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- 35.Thomalla G, et al. Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia: MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours. Stroke. 2007;38:313–318. doi: 10.1161/01.STR.0000254565.51807.22. [DOI] [PubMed] [Google Scholar]
- 36.Vora NA, et al. Factors predicting hemorrhagic complications after multimodal reperfusion therapy for acute ischemic stroke. AJNR Am. J. Neuroradiol. 2007;28:1391–1394. doi: 10.3174/ajnr.A0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yepes M, Lawrence DA. New functions for an old enzyme: nonhemostatic roles for tissue-type plasminogen activator in the central nervous system. Exp. Biol. Med. (Maywood. ) 2004;229:1097–1104. doi: 10.1177/153537020422901103. [DOI] [PubMed] [Google Scholar]
- 38.Yepes M, Lawrence DA. Tissue-type plasminogen activator and neuroserpin: a well-balanced act in the nervous system? Trends Cardiovasc. Med. 2004;14:173–180. doi: 10.1016/j.tcm.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Zhuo M, et al. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J. Neurosci. 2000;20:542–549. doi: 10.1523/JNEUROSCI.20-02-00542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hao Z, et al. New transgenic evidence for a system of sympathetic axons able to express tissue plasminogen activator (t-PA) within arterial/arteriolar walls. Blood. 2006;108:200–202. doi: 10.1182/blood-2005-12-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gualandris A, Jones TE, Strickland S, Tsirka SE. Membrane depolarization induces calcium-dependent secretion of tissue plasminogen activator. J. Neurosci. 1996;16:2220–2225. doi: 10.1523/JNEUROSCI.16-07-02220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breedveld P, et al. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 2005;65:2577–2582. doi: 10.1158/0008-5472.CAN-04-2416. [DOI] [PubMed] [Google Scholar]
- 43.Melchor JP, Strickland S. Tissue plasminogen activator in central nervous system physiology and pathology. Thromb. Haemost. 2005;93:655–660. doi: 10.1160/TH04-12-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponten A, et al. Transgenic overexpression of platelet-derived growth factor-C in the mouse heart induces cardiac fibrosis, hypertrophy, and dilated cardiomyopathy. Am. J. Pathol. 2003;163:673–682. doi: 10.1016/S0002-9440(10)63694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMahon GA, et al. Plasminogen activator inhibitor-1 regulates tumor growth and angiogenesis. J. Biol. Chem. 2001;276:33964–33968. doi: 10.1074/jbc.M105980200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.