A functional role for the p62–ERK1 axis in the control of energy homeostasis and adipogenesis
In this study, work from the Moscat group establishes genetically a direct and functional interaction between p62 and ERK1 in the control of obesity and insulin resistance in vivo. It is shown that the genetic inactivation of ERK1 in p62-/- mice reverses the increased adiposity and adipogenesis, lower energy expenditure, and insulin resistance observed in p62-/- mice. This suggests that inhibitors of ERK1 could be potential therapeutics in type 2 diabetes.
Keywords: p62, sequestosome, obesity, ERK, insulin
Abstract
In vivo genetic inactivation of the signalling adapter p62 leads to mature-onset obesity and insulin resistance, which correlate with reduced energy expenditure (EE) and increased adipogenesis, without alterations in feeding or locomotor functions. Enhanced extracellular signal-regulated kinase (ERK) activity in adipose tissue from p62-knockout (p62−/−) mice, and differentiating fibroblasts, suggested an important role for this kinase in the metabolic alterations of p62−/− mice. Here, we show that genetic inactivation of ERK1 in p62−/− mice reverses their increased adiposity and adipogenesis, lower EE and insulin resistance. These results establish genetically that p62 is a crucial regulator of ERK1 in metabolism.
Introduction
The signalling adapter p62, also known as sequestosome 1, has been implicated in crucial cellular processes owing to its ability to interact with important signalling intermediaries in biochemical assays (Moscat et al, 2006; Moscat & Diaz-Meco, 2009). The phenotypic analysis of genetically modified mice lacking p62 shows that, in fact, p62 regulates osteoclastogenesis and bone homeostasis through the E3 ubiquitin ligase TRAF6 by acting as an important intermediary of the RANK pathway in the activation of nuclear factor-κB (Duran et al, 2004). This is consistent with the finding that p62 mutations are associated with the Paget disease of bone, a genetic disorder characterized by aberrant osteoclastogenic activity (Laurin et al, 2002). Conversely, p62 levels are increased in human tumours and in cells transformed by the Ras oncogene, which is crucial for tumorigenesis (Duran et al, 2008). Indeed, a lack of p62 markedly inhibits Ras-induced cell transformation in cell cultures and in a mouse model of Ras-induced lung carcinogenesis that closely resembles human lung adenocarcinoma (Duran et al, 2008).
Together, these observations indicate that the loss of p62 at an organismal level has important consequences in normal physiology and in pathology. Another crucial feature of p62-deficient mice is that they develop late-onset obesity (Rodriguez et al, 2006). Thus, when p62-knockout (p62−/−) mice age they become obese and show impaired glucose tolerance and insulin resistance (Rodriguez et al, 2006). In addition, the metabolic rate of these mutant mice was reduced significantly even at an early age when they were not obese, suggesting a causative role for defects in energy expenditure (EE) in the obese phenotype (Rodriguez et al, 2006). The p62-deficient mice also showed genetic changes, suggesting that p62 promotes a negative energy balance by inhibiting adipogenesis and favouring energy combustion. Therefore, p62 emerges as a novel regulator of energy homeostasis by controlling resting adipogenesis and EE, without affecting locomotion or food intake (Rodriguez et al, 2006).
From a mechanistic perspective, it is most probable that p62 modulates adipogenesis through its ability to sequester extracellular signal-regulated kinase (ERK) into cytoplasmic aggregates that keep ERK activity low under basal conditions (Rodriguez et al, 2006). Consistent with this model, the basal activity of ERK was enhanced in fat from non-obese p62-deficient mice, and embryo fibroblasts (MEFs) from p62-deficient mice differentiated more efficiently into adipocytes than their wild-type controls (Rodriguez et al, 2006). This latter effect was abrogated by pharmacological inhibition of the ERK pathway (Rodriguez et al, 2006). Interestingly, p62 is induced during adipocyte differentiation, which suggests that the role of p62 is to provide a negative-feedback signal that keeps ERK activity in check to prevent excessive adipogenesis and adiposity. As p62 binds to several physiologically relevant molecules (Moscat et al, 2007), genetic evidence derived from in vivo systems is necessary to rigorously establish the various p62 partners as physiologically relevant, bona fide downstream targets of p62, as is the case for ERK in the control of adipogenesis, EE and obesity. Here we show for the first time to our knowledge, at the organismal level and through use of in vitro assays, that the role of p62 in the control of energy homeostasis and adipogenesis is mediated by ERK1.
Results
Loss of ERK1 reverts p62−/− adiposity
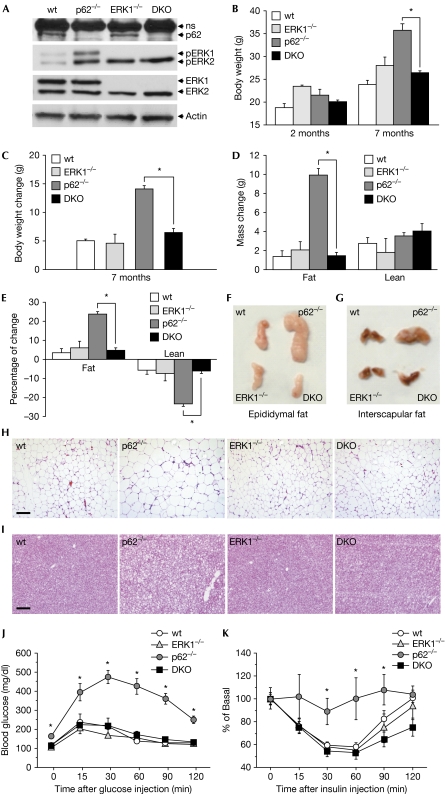
To investigate whether ERK1 deficiency decreases adiposity in p62−/− mice, we generated p62 and ERK1 double knockout (DKO) mice. Deletion of p62 and ERK1 was confirmed by western blotting of white adipose tissue (WAT) extracts (Fig 1A). As reported previously (Rodriguez et al, 2006), p62-deficient WAT showed increased ERK1 and ERK2 activities, as determined by phospho-ERK immunoblotting (Fig 1A). As expected, genetic inactivation of ERK1, either singly or concomitant with p62 ablation, led to complete inhibition of ERK1 activity in WAT. Notably, single inactivation of ERK1 led to compensatory activation of ERK2 (Fig 1A), which is not sufficient to drive the obesity phenotype of p62-deficient mice in the absence of ERK1 (see below). Eight-week-old wild-type, p62−/−, ERK1-knockout (ERK1−/−) and DKO mice were fed a normal chow diet, and body weight and fat and lean mass were monitored over 7 months. In agreement with data published previously, p62−/− mice gained significantly more weight than wild-type or ERK1-deficient mice (Fig 1B,C). Interestingly, Fig 1B and C show that weight gain was significantly lower in DKO mice compared with that in p62−/− mice. This indicates that ablation of the ERK1 gene is capable of reverting the enhanced weight gain of p62−/− mice. Consistent with this, fat gain was lower in DKO than in p62−/− mice, as determined by nuclear magnetic resonance-based body composition analysis (Fig 1D). Fat mass decreased by 70% in DKO mice compared with that in p62−/− mice (Fig 1E). The percentage of lean mass was decreased significantly in p62−/− mice and this effect was reversed completely in DKO mice (Fig 1E). This reversal was due to a primary decrease in fat mass (Fig 1E). Collectively, these findings showed that ERK1 deficiency is sufficient to chronically reverse the positive energy balance and prevent increased fat accruement of p62−/− mice. Morphological analyses of WAT (epididymal fat pads) and brown adipose tissue (interscapular depots) further supported the finding, with DKO mice showing decreased adipose tissue volume compared with p62−/− mice (Fig 1F,G). Histological analysis of WAT and brown adipose tissue with haematoxylin and eosin staining showed a decrease in cell size and lipid accumulation in DKO mice compared with p62−/− mice (Fig 1H,I).
Figure 1.
Decreased obesity and improved insulin action in DKO mice. Wild-type, p62−/−, ERK1−/− and DKO mice fed a normal chow diet were tracked for 7 months. (A) Western blot analyses of WAT. (B) Body weight at 2 and 7 months. (C) Changes in body weight over 5 months (from 2 months to 7 months of age). (D) Grams of fat and lean mass measured by nuclear magnetic resonance imaging (Whole Body Composition Analyzer, EchoMRI) at 7 months of age. (E) Fat and lean mass as the percentage of total body weight at 7 months of age. (A–E) Data are presented as mean±s.e. of 5–6 animals per group. *P<0.05 DKO compared with p62−/−. Different tissues were isolated from wild-type, p62−/−, ERK1−/− and DKO mice to show differences in tissue mass and cell density. (F) Epididymal adipose tissue. (G) Interscapular adipose tissue. (H) Histological analysis of epididymal adipose tissue and (I) histological analysis of interscapular adipose tissue. Sections obtained from three different animals were stained with haematoxylin and eosin. Scale bars, 200 μm. (J) Glucose-tolerance test and (K) insulin-tolerance test. Data are presented as mean±s.e. of 5–6 animals per group. *P<0.05 compared with wild type. DKO, double knockout; ERK, extracellular signal-regulated kinase; WAT, white adipose tissue; wt, wild type.
To further characterize the DKO phenotype, 8-week-old mice were fed on a high-fat diet for 2 months. Notably, even under high-fat diet feeding conditions, ERK1 deficiency inhibited the enhanced weight gain of p62−/− mice (supplementary Fig S1A,B online). Furthermore, the increase in fat mass under these conditions was less in DKO than in p62−/− mice (supplementary Fig S1C,D online). Morphological and histological studies showed increased adiposity in p62−/− mice, which was completely reversed in DKO mice (supplementary Fig S1E–H online). Together, these data show that deletion of ERK1 in p62−/− mice prevents the development of late-onset obesity in p62−/− mice and the enhanced obesity produced by high-fat diet feeding.
Increased insulin sensitivity in DKO mice
Insulin resistance is a main feature of the metabolic syndrome, in which obesity and lipid accumulation are responsible for altered insulin action, leading to development of glucose intolerance. Glucose-tolerance tests and insulin-tolerance tests were performed for 7-month-old animals, at which time the obese phenotype was well established in p62−/− mice. As reported previously, obese p62-deficient mice showed insulin resistance and glucose intolerance (Fig 1J,K). Importantly, we observed clear improvement in insulin action in DKO mice, compared with that in p62−/− mice and as reflected by normal glucose clearance in response to either glucose or insulin challenges (Fig 1J,K).
Increased EE in DKO mice
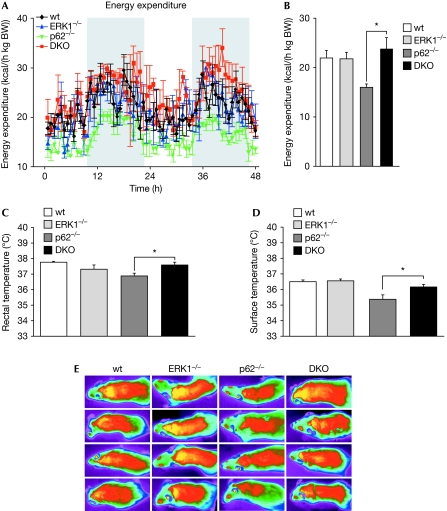
We reported previously that p62-deficient mice show increased adiposity as well as impaired EE (Rodriguez et al, 2006). To determine whether deletion of ERK1 in p62−/− mice would reverse the alteration in energy balance caused by a lack of p62, we quantified EE in 7-month-old mice. Interestingly, although the metabolic rate of p62−/− mice was decreased significantly compared with that of identically treated wild-type mice, that of DKO mice was increased compared with matched p62−/− mice. That is, DKO mice showed substantially higher oxygen consumption and EE than p62-deficent mice (Fig 2A,B). Thus, decreased adiposity in DKO mice when compared with that in p62−/− mice can be partly explained by the increase in EE.
Figure 2.
Increased energy expenditure in DKO mice. Metabolic rate was measured for two consecutive days by using a customised indirect calorimetric system. (A) Energy expenditure during light and dark phases. (B) Means of energy expenditure measurements. (C) Basal rectal temperature. (D) Surface temperature. (E) Heat production after cold exposure, measured and visualised with a high-resolution infrared camera. (A–E) Data are presented as mean±s.e. of 5–6 animals per group. *P<0.05 DKO compared with p62−/−. DKO, double knockout; ERK, extracellular signal-related kinase; wt, wild type.
Conversely, p62−/− and DKO mice showed similar body core and surface temperatures, compared with that of wild-type mice, when they were kept at an ambient temperature of 22°C (data not shown). However, basal rectal temperature was higher in DKO than in p62−/− mice (Fig 2C). Furthermore, the body temperature of p62−/− mice when exposed to a 4°C environment was significantly lower than that of wild-type and DKO mice (Fig 2D,E). Collectively, these results show that the obesity developed by p62-deficient mice is reversed in p62/ERK1 double-mutant mice. Therefore, we conclude that ERK1 inhibition by p62 under resting conditions is essential to maintain the organismal energy balance, indicating that ERK1 is a bona fide negative downstream target of p62.
Decreased adipocyte differentiation in DKO mice
Previous studies showed that a lack of p62 leads to a cell-autonomous increase in the ability of MEFs to undergo adipogenesis in vitro (Rodriguez et al, 2006). To determine whether the decreased adiposity observed in DKO mice in vivo might be due, at least in part, to a cell-autonomous decrease in adipocyte differentiation in the DKO cells, we studied adipogenesis in primary MEFs from mice of the four genotypes. Consistent with previous observations, we found that adipocyte differentiation was increased in p62−/− MEFs compared with that in wild-type MEFs. However, adipogenesis in DKO MEFs was comparable to that of wild-type and ERK1-deficient MEFs, indicating that ERK1 is required for enhanced adipogenesis of p62−/− cells (Fig 3A). Consistent with this idea, expression of peroxisome proliferator-activated receptor-γ (PPARγ), which is higher in p62-deficient MEFs, was diminished significantly in DKO MEFs during adipogenesis (Fig 3B). Furthermore, markers of matured adipocytes, fatty acid synthase and adipocyte fatty acid-binding protein (aP2), were also increased significantly in p62−/− MEFs (Fig 3B), concomitant with increased lipid accumulation (Fig 3A), but their levels were restored to wild type values in DKO MEFs (Fig 3B). These results indicate that the increased cell-autonomous adipogenesis triggered by a lack of p62 is reversed by the simultaneous genetic inactivation of ERK1.
Figure 3.
Decreased adipocyte differentiation in DKO MEFs. MEFs from wild-type, p62−/−, ERK1−/− and DKO embryos were grown to confluence and differentiated into adipocytes as described in Methods. (A) Macroscopic and microscopic images of MEFs at day 8 after induction of adipogenesis. Scale bar, 200 μm. Lipid droplets were stained with Oil-red O solution. (B) mRNA expression of PPARγ, fatty acid synthase and aP2 was measured by quantitative reverse transcription-PCR. Data are presented as mean±s.e. *P<0.05 DKO compared with p62−/−. aP2, adipocyte fatty acid-binding protein; DKO, double knockout; ERK, extracellular signal-regulated kinase; FAS, fatty acid synthase; MEF, mouse embryonic fibroblast; mRNA, messenger RNA; PPARγ, peroxisome proliferator-activated receptor-γ; wt, wild type.
Specific role of ERK1 in adipogenesis in p62-deficient cells
As simple genetic inactivation of ERK1 is sufficient to reverse the obesity phenotype of p62−/− mice and MEFs, we investigated whether p62 interacts preferentially, if not exclusively, with ERK1. Results in Fig 4 show that p62 interacts specifically with ERK1 and not with ERK2 both in vitro and in vivo. Thus, recombinant bacterially expressed maltose-binding protein (MBP)–p62 interacts selectively with recombinant glutathione-S-transferase (GST)–ERK1 but not with GST–ERK2 in pulldown experiments in vitro (Fig 4A). In addition, co-transfection experiments using HEK293 cells transfected with a GST p62 expression vector along with haemagglutinin (HA)–ERK1 or HA–ERK2 also showed preferential interaction of p62 and ERK1 compared with ERK2 (Fig 4B). To explore further the p62–ERK1 specificity in vivo, endogenous p62 was immunoprecipitated in wild-type or ERK1−/− MEFs treated with differentiation media to induce adipogenesis, and interaction with ERK1/2 was determined by immunoblotting. As shown in Fig 4C, p62 interacted selectively with ERK1 but not with ERK2 at 30 min after adipogenic differentiation, and this interaction was lost in ERK1−/− MEFs. Together, therefore, these results show a direct and specific p62–ERK1 interaction that probably underlies the molecular mechanism mediating p62 actions in adipogenesis and obesity.
Figure 4.
Selective physical and functional p62–ERK1 interaction. (A) Recombinant bacterially expressed GST–ERK1 and GST–ERK2 were incubated with MBP or MBP–p62 and the ability of ERK to bind directly to p62 was determined in pulldown experiments by using amylose beads. (B) HEK293 cells were transfected with GST or GST–p62 expression vector along with HA–ERK1 or HA–ERK2 and coprecipitation of ERK with p62 was determined by immunoblot analysis. (C) Wild-type or ERK1−/− MEFs were induced to differentiate into adipocytes for different durations, after which endogenous p62 was immunoprecipitated and ERK interaction was detected by immunoblot analysis. An irrelevant IgG was used as a negative control in immunoprecipitations performed in parallel. Results are representative of at least two further experiments with similar results. (D) Immunoblot analysis of wild-type or p62−/− MEFs showing effective lentiviral knockdown of ERK1/2 or negative controls (NT). (E) PPARγ, fatty acid synthase and aP2 mRNA expression in adipocyte-differentiated wild-type or p62−/− MEFs after ERK1/2 lentiviral knockdown. Data presented are mean±s.e. *P<0.05. aP2, adipocyte fatty acid-binding protein; ERK, extracellular signal-regulated kinase; FAS, fatty acid synthase; GST, glutathione-S-transferase; HA, haemagglutinin; IgG, immunoglobulin G; IP, immunoprecipitation; MBP, maltose-binding protein; MEF, mouse embryonic fibroblast; mRNA, messenger RNA; PPARγ, peroxisome proliferator-activated receptor-γ; WB, western blot; WCE, whole cell extract; wt, wild type.
To investigate further the functional relevance of the p62–ERK1-specific interaction in adipogenesis in vivo, p62−/− MEFs—in which ERK1 or ERK2 had been knocked down selectively by lentiviral infection of short-hairpin RNA (shRNA) interference vectors selective for each ERK gene—were fully differentiated to adipocytes and expression of adipogenic markers was determined by quantitative reverse transcription–PCR. Results in Fig 4D show that both ERKs were knocked down effectively by this strategy. Interestingly, selective knockdown of ERK1, but not ERK2, was sufficient to reverse the enhanced expression of PPARγ, fatty acid synthase and aP2 in the p62-deficient MEFs (Fig 4E). Collectively, these results suggest that a selective physical and functional p62–ERK1 interaction is instrumental in the role of p62 in adipogenesis.
Discussion
Our previous publication, in which we showed that the loss of p62 leads to increased adipogenesis and impaired EE, suggested that p62 is at least one of the crucial players in the finely tuned regulation of adipocyte differentiation and energy control that governs adiposity and obesity (Rodriguez et al, 2006). The fact that ERK activity is increased in the adipose tissue of even young, non-obese p62-deficient mice suggests that the role of p62 is to keep ERK under tight control (Rodriguez et al, 2006). Our data showing that MEFs from p62-deficient mice differentiated more efficiently into adipocytes in vitro, and that this effect was prevented by pharmacological inhibition of the ERK pathway (Rodriguez et al, 2006), suggested a cell-autonomous role for the p62–ERK complex in obesity and adipogenesis. However, rigorous in vivo and ex vivo genetic testing was necessary to prove this hypothesis.
Here, we present in vitro and in vivo genetic evidence that specific loss of ERK1 alone is capable of reversing the increased ex vivo adipogenesis, as well as the in vivo obesity phenotype, of p62-deficient mice. Interestingly, a lack of ERK1 in double-mutant mice also reduced the enhanced EE of p62-deficient mice. These results indicate that ERK1 can account for all metabolic actions that go awry in the absence of p62.
Genetic inactivation of ERK1 leads to compensatory activation of ERK2 (Fig 1A). The fact that ablation of ERK1 is sufficient to reduce the enhanced adipogenesis and obesity phenotype caused by p62 inactivation, further supports the notion that ERK1 is crucial for this function, irrespective of the activation state of ERK2. In keeping with this conclusion are our experiments in which selective inactivation of ERK1, but not ERK2, by shRNA knockdown markedly reduced the hyper-activation of the adipogenic gene programme activated by loss of p62 (Fig 4E). Collectively, these data strongly suggest that the p62–ERK1 complex is the crucial mediator of these processes.
Our observations are consistent with previous data implicating ERK as a positive regulator of adipogenesis and obesity (Bost et al, 2005; Kortum et al, 2005), but are at odds with data suggesting that ERK could be a negative regulator of these processes (Hu et al, 1996). In the light of these contradictory observations, there was a clear need for this demonstration that ERK1 is a bona fide downstream regulator of p62 during adipogenesis and obesity. Our results further establish the specificity of this pathway, as p62 interacts preferentially with ERK1, as opposed to ERK2, both in vitro and in vivo, and knockdown of ERK1 but not ERK2 inhibits the increased adipogenesis promoted by a lack of p62. However, loss of p62 leads to activation of both ERKs (Fig 1A; Rodriguez et al, 2006). This indicates that p62 inhibits ERK1 by direct interaction, whereas it inhibits ERK2 indirectly. As ERK2 activity is not necessary or sufficient to control adipogenesis in the context of p62 deficiency, we conclude that ERK2 might be mediating effects of p62 other than adipogenesis and energy homeostasis, and this idea would be addressed in future studies. Interestingly, activation of ERK during in vitro adipogenesis has been reported to be biphasic (Sale et al, 1995). Blockade of ERK with inhibitors of the upstream kinase MEK (MAP kinase kinase) ablates adipogenesis (Kortum et al, 2005; Kim et al, 2007). However, hyperactivation of ERK at later stages of the adipocyte differentiation process might be inhibitory for adipogenesis owing to the ability of ERK to inactivate PPARγ by direct phosphorylation (Hu et al, 1996). Interestingly, inhibition of the second ERK activity peak, but not the first, significantly enhances adipogenesis in 3T3-L1 cells (Kortum et al, 2005). The kinase RSK (ribosomal S6 kinase) has also been shown to be a crucial downstream target of ERK in adipogenesis (Kortum et al, 2005). However, our data show that, whereas RSK is hyperactivated in p62-deficient MEFs, its activity is not reduced by concomitant loss of ERK1 in DKO MEFs (supplementary Fig S2 online), although these DKO cells have reduced adipogenesis (Fig 3). These results can be interpreted to mean that, although RSK might be required for adipogenesis, it is the activity of ERK1 that controls this important function, at least in the context of p62 deficiency.
Collectively, these observations suggest that ERK1 has a crucial role during adipocyte differentiation, and that tight control of its activity is essential for adequate regulation of adipogenesis and obesity. Understanding this newly identified functional link between the two metabolic master regulators p62 and ERK1 is crucial for the identification of appropriate pharmacological targets for developing new and superior therapies for metabolic diseases.
Methods
Details of all materials and additional methods can be found in the supplementary information online.
Mice. Animals were maintained under conditions of controlled temperature (22.5°C) and illumination (12 h dark/light cycle). Eight-week-old genetically matched, and C57BL6 wild-type, ERK1−/−, p62−/− and p62/ERK1 DKO female mice were fed standard chow for 7 months or a high-fat diet (45 kcal % fat, 35 kcal % carbohydrates and 20 kcal % protein; diet number D1241; Research Diets, New Brunswick, NJ, USA) for 2 months. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati, in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Adipocyte differentiation of primary MEFs. Primary MEFs (passage 0 or 1) from 13.5 to 14.5 day embryos were grown to confluence (day 0) in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum. Adipocyte differentiation was induced for 3 days by differentiation medium supplemented with 500 μM 3-isobutyl-1-methylxanthine, 1 μM dexamethasone, 200 μM indomethacin and 1 μg/ml insulin. After this induction period, the medium was replaced with DMEM containing 10% fetal bovine serum and 1 μg/ml insulin, which was then changed every second day. After 5 days, the cells showed a fully differentiated phenotype with significant accumulation of multilocular lipid droplets. Differentiated adipocytes were stained with Oil-red O as described previously (Rodriguez et al, 2006).
Western blotting. Cell extracts for western blotting were prepared in radioimmunoprecipitation assay buffer (1 × phosphate-buffered saline, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate, 1 mM phenylmethylsulphonyl fluoride and protease inhibitors). Lysates were separated by SDS–polyacrylamide gel electrophoresis and transferred to Nitrocellulose-ECL membranes (GE Healthcare, Piscataway, NJ, USA) where the immune complex was detected by chemiluminescence (GE Healthcare).
Statistics. Data were analysed with the Statview software (Abacus, Berkeley, CA, USA) using one-factor analysis-of-variance analysis. Numerical data are expressed as mean±s.e. P-values<0.05 were considered statistically significant.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank M. Daston for editing the paper and G. Doerman for preparing the figures. This research was funded in part by National Institutes of Health grants R01-AI072581 (to J.M.), R01-CA132847 (to J.M.) and R01-CA134530 (to M.T.D-M).
Footnotes
The authors declare that they have no conflict of interest.
References
- Bost F et al. (2005) The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes 54: 402–411 [DOI] [PubMed] [Google Scholar]
- Duran A, Serrano M, Leitges M, Flores JM, Picard S, Brown JP, Moscat J, Diaz-Meco MT (2004) The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev Cell 6: 303–309 [DOI] [PubMed] [Google Scholar]
- Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, Moscat J (2008) The signaling adaptor p62 is an important NF-κB mediator in tumorigenesis. Cancer Cell 13: 343–354 [DOI] [PubMed] [Google Scholar]
- Hu E, Kim JB, Sarraf P, Spiegelman BM (1996) Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science 274: 2100–2103 [DOI] [PubMed] [Google Scholar]
- Kim KA, Kim JH, Wang Y, Sul HS (2007) Pref-1 (preadipocyte factor 1) activates the MEK/extracellular signal-regulated kinase pathway to inhibit adipocyte differentiation. Mol Cell Biol 27: 2294–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortum RL et al. (2005) The molecular scaffold kinase suppressor of Ras 1 (KSR1) regulates adipogenesis. Mol Cell Biol 25: 7592–7604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurin N, Brown JP, Morissette J, Raymond V (2002) Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am J Hum Genet 70: 1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT (2009) p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 137: 1001–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT, Albert A, Campuzano S (2006) Cell signaling and function organized by PB1 domain interactions. Mol Cell 23: 631–640 [DOI] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT, Wooten MW (2007) Signal integration and diversification through the p62 scaffold protein. Trends Biochem Sci 32: 95–100 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Duran A, Selloum M, Champy MF, Diez-Guerra FJ, Flores JM, Serrano M, Auwerx J, Diaz-Meco MT, Moscat J (2006) Mature-onset obesity and insulin resistance in mice deficient in the signaling adapter p62. Cell Metab 3: 211–222 [DOI] [PubMed] [Google Scholar]
- Sale EM, Atkinson PG, Sale GJ (1995) Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90 S6 kinase and for insulin or serum stimulation of DNA synthesis. EMBO J 14: 674–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.