Abstract
Heat shock protein (Hsp) 90 is an ATP-dependent molecular chaperone exploited by malignant cells to support activated oncoproteins, including many cancer-associated kinases and transcription factors, and is essential for oncogenic transformation. Originally viewed with skepticism, Hsp90 inhibitors are now actively pursued by the pharmaceutical industry, with 17 agents having entered clinical trials. Hsp90’s druggability was established using the natural products geldanamycin and radicicol which mimic the unusual ATP structure adopted in the chaperone’s N-terminal nucleotide-binding pocket and cause potent and selective blockade of ATP binding/hydrolysis, inhibit chaperone function, deplete oncogenic clients, and demonstrate antitumor activity. Preclinical data with these natural products have heightened interest in Hsp90 as a drug target, and 17-allylamino-17-demethoxygeldanamycin (17-AAG, tanespimycin) has demonstrated clinical activity (as defined by RECIST criteria) in HER2+ breast cancer. Many optimized synthetic small molecule Hsp90 inhibitors from diverse chemotypes are now in clinical trials. We review the discovery and development of Hsp90 inhibitors and assess their future potential. There has been significant learning from experience in both the basic biology and the translational drug development around Hsp90, enhanced by the use of Hsp90 inhibitors as chemical probes. Success will likely lie in treating cancers addicted to particular driver oncogene products, such as HER2, ALK, EGFR and BRAF, that are sensitive Hsp90 clients, as well as in malignancies, especially multiple myeloma, where buffering of proteotoxic stress is critical for survival. We discuss approaches to enhancing the effectiveness of Hsp90 inhibitors and highlight new chaperone and stress response pathway targets, including HSF1 and Hsp70.
Introduction
Heat shock protein (Hsp) 90 is an ATP-dependent molecular chaperone that regulates late stage maturation, activation, and stability of a diverse range of ‘client’ proteins (defined as proteins with demonstrated binding to Hsp90 whose steady-state level declines upon Hsp90 inhibitor treatment, usually as a result of proteasome-mediated degradation; see http://www.picard.ch/downloads for a curated list) many which are involved in signal transduction and other key pathways that are especially important in malignancy (1). Although it is highly expressed in normal cells where it helps to maintain protein homeostasis, Hsp90 is exploited by cancer cells for at least two purposes: 1) to support the activated or metastable (e.g., labile) forms of oncoproteins, including many kinases and transcription factors, that are mutated, translocated, amplified or overexpressed in malignancy; and 2) to buffer cellular stresses induced by the malignant lifestyle (Figure 1) (2, 3). Hsp90 is itself often overexpressed (4) and present in an activated multichaperone complex in cancer cells (5), and it is now regarded as essential for malignant transformation and progression (2, 3).
Figure 1.
Hsp90 buffers cancer cells from the many environmental stresses that they must endure and overcome. To accomplish this, the molecular chaperone regulates numerous signaling proteins and pathways (shown on the right).
When the concept of targeting Hsp90 in cancer was first promulgated in the early 1990s, it was viewed with considerable skepticism by the pharmaceutical industry. This was primarily because it was unprecedented to propose targeting a housekeeping protein that is abundantly expressed in normal cells and there was perceived risk that Hsp90 inhibition might therefore generate unacceptable toxicity. Thus, the early clinical development of Hsp90 inhibitors was undertaken by the U. S. National Cancer Institute, a small number of academic non-profit groups, and a few small biotechnology companies. The degree to which opinion has changed is shown by the fact that Hsp90 is now one of the most actively pursued cancer drug targets by big pharma, with 17 agents having entered clinical trials (6). There has also been impressive growth in interest in Hsp90 in both the academic and patent literature. Although there are currently no Hsp90-targeting agents approved for clinical use, clinical activity has been achieved with several drugs in multiple tumor types, and potential routes to regulatory approval are becoming apparent.
In parallel with recent preclinical and clinical therapeutic developments, there has been considerable progress in understanding the molecular, cellular and organismal contributions of Hsp90 (1-3). Experience gained over the last several years in both the basic biology and the translational drug development around Hsp90, enhanced by the use of Hsp90 inhibitors as chemical probes, has helped us to understand how best to achieve clinical success through inhibition of the molecular chaperone. As part of a CCR Focus on Drug Development: What Experience Has Taught Us (7-10), we provide here an update on both therapeutic and relevant fundamental Hsp90 research, we illustrate productive synergies between the two, and we examine future prospects for Hsp90 inhibitors in the clinic, as well as highlighting new follow-on targets. We conclude that future success will most likely come from the use of chemically optimized Hsp90 inhibitors – from the many that are now available – to treat cancers that especially depend on particular driver oncogene products that are sensitive Hsp90 clients, as well as those malignancies, best exemplified by multiple myeloma, where buffering of proteotoxic stress is critical for survival. We also discuss approaches to enhance the effectiveness of Hsp90 inhibitors, and we highlight new chaperone and stress response pathway targets, including HSF1 and Hsp70.
Target validation, chemical tools, drug discovery and development
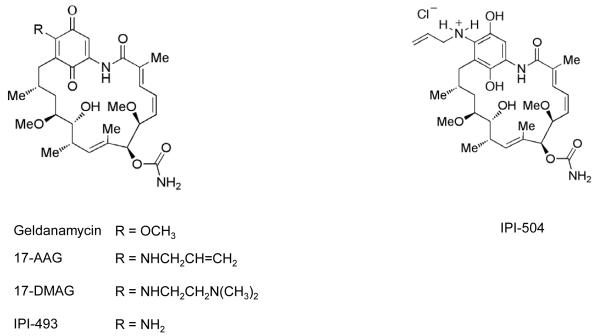
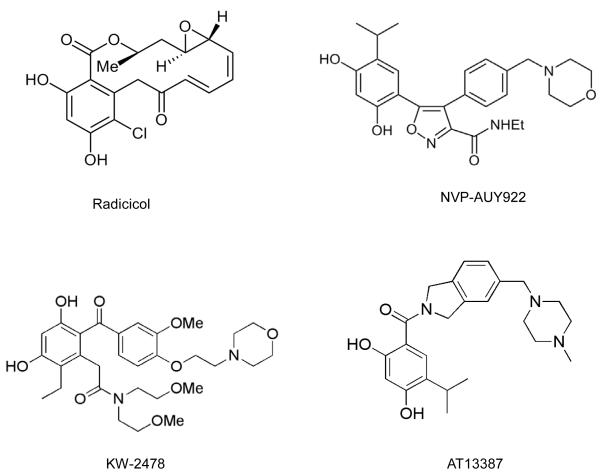
The druggability of Hsp90 was established using the natural products radicicol and geldanamycin (Figure 2). These were isolated in 1953 and 1970, respectively, and were shown to have biologic activity by a then unknown mechanism (11). A critical observation for the Hsp90 field was the demonstration in 1994 that Hsp90 was the molecular target of geldanamycin and that Hsp90 inhibition by this agent prevented formation of the complex between Hsp90 and its client protein SRC, resulting in SRC destabilization and explaining reversal of SRC transformation (12). Several years later, radicicol was also shown to target Hsp90 (13, 14). Another breakthrough was the discovery in the late 1990s that geldanamycin and radicicol mimicked the relatively unusual structure that ATP adopts in the deep, N-terminal nucleotide-binding pocket of Hsp90, exploiting the topologically distinct Bergerat fold that is characteristic of the small GHKL (Gyrase, Hsp90, Histidine Kinase, MutL) subgroup within the ATPase/kinase superfamily, thereby leading to potent and selective inhibition of ATP binding and hydrolysis (15, 16) and in turn to the depletion of oncogenic clients through ubiquitin-mediated proteasomal degradation (17, 18).
Figure 2.
Chemical structures of selected Hsp90 inhibitors discussed in this article.
The natural products provided important chemical probes that proved invaluable to the Hsp90 field, enabling its function to be queried in detail and validating Hsp90 as a druggable target (19). Moreover, although geldanamycin and radicicol proved too toxic and unstable/reactive for clinical use, they each provided the chemical basis for drugs that subsequently entered the clinic. The first to progress to clinical trials was the better tolerated geldanamycin analog 17-allylamino-17-demethoxygeldanamycin (17-AAG, KOS-953, tanespimycin; Figure 2). In addition, radicicol’s resorcinol Hsp90-binding unit was recapitulated in many subsequent small molecule Hsp90 inhibitors (see below). Thus, as is often the case in biomedical research, Hsp90 inhibitors owe a deep dept to the world of natural products.
In Phase I studies, tanespimycin showed proof-of-mechanism for target inhibition using a validated pharmacodynamic biomarker signature of client protein depletion and HSF1-dependent Hsp induction (20, 21). It also exhibited evidence of clinical activity in various malignancies, most impressively to date in Phase I and II studies in HER2+, trastuzumab-refractory breast cancer where objective RECIST responses were seen on a weekly schedule of 450 mg/m2 (22-24). This activity is attributed to the extreme sensitivity of HER2 as an Hsp90 client and its role as a driver oncoprotein in this patient population. Most common side effects with the weekly and other schedules were predominantly grade 1 or 2 diarrhea, fatigue, nausea, headache, neuropathy, and transaminitis, and were readily managed with pre-/supportive medications.
However, despite the promising results seen in HER2+ breast cancer and also in multiple myeloma, where encouraging activity was seen in combination with the proteasome inhibitor bortezomib (25, 26), tanespimycin’s development was subsequently discontinued. It has been speculated that termination by the company supplying tanespimycin (Bristol-Myers Squibb) may have been related to production/formulation and patent expiry concerns (24, 27) (http://www.myelomabeacon.com/news/2010/07/22/tanespimycin-development-halted/). Although prolonged disease stabilization was achieved in Phase I studies of tanespimycin in various additional tumor types expressing particular Hsp90 client proteins, neither complete nor partial tumor responses were seen (28, 29). This limited activity has been attributed to suboptimal inhibition of the target client proteins, most likely owing to insufficient drug dose or frequency of administration. In fact, consistent with the clinical data, inspection of animal model studies in relevant tumors – e.g. ovarian, colon, breast, melanoma, prostate and lung with appropriate client proteins and addictions – shows a similar pattern of growth inhibition or cytostatic arrest rather than tumor regression in response to single agent tanespimicin (21, 30-34). This is also consistent with cell cycle arrest predominating over apoptosis induction in cell culture models (35). Another limitation with tanespimicin is the requirement for reductive metabolism of the benzoquinone by NQO1/DT-diaphorase: this increases the drug’s inhibitory potency but is hypothesized to contribute to the observed liver toxicity as well as clearly allowing a mechanism of intrinsic and acquired drug resistance caused by low NQO1 expression (30, 36, 37).
Another geldanamycin analog (17-DMAG, 17-dimethylaminoethylamino-17-demethoxygeldanamycin, alvespimycin; Figure 2) is much less sensitive to, and dependent on, NQO1 and also has improved formulation and pharmacokinetic properties. Of interest, a recent study with alvespimycin reported one complete response in castrate-refractory prostate cancer (CRPC), seen by prostate specific antigen levels and confirmed on CT, as well as stable disease in other patients with CRPC, chondrosarcoma, and renal cancer (38). Activity in CRPC was likely related to depletion of the androgen receptor, an Hsp90 client protein, as seen in preclinical models (33). There appears to be no further development of either 17-DMAG or of IPI-493 (the 17-amino analog and metabolite of tanespimycin) which is similarly less dependent on NQO1 (39) (Figure 2). Still in clinical development, however, is the soluble stabilized hydroquinone form of 17-AAG, IPI-504 (retaspimycin hydrochloride, Figure 2). Its evaluation in gastrointestinal stromal tumor (GIST), usually driven the Hsp90 client protein KIT, was stopped because of higher than expected hepatic toxicity, probably due to the combined effects of drug and metastatic liver disease (39). However, encouraging activity has been seen in non-small cell lung cancer (NSCLC) patients with oncogenic anaplastic lymphoma kinase (ALK) gene rearrangements, the products of which are Hsp90 clients, and ongoing clinical evaluation in this setting includes genetic stratification (40).
The trailblazing proof-of-concept work with geldanamycin analogues stimulated the race to discover synthetic small molecule Hsp90 inhibitors that would overcome some or all of the limitations of this class, including the ability to use doses and schedules that would deliver sufficiently sustained client depletion while sparing the liver toxicity, hypothesized to be caused by quinone metabolism (41, 42), as well as avoiding P-glycoprotein-mediated efflux seen with tanespimycin (30). A large number of new Hsp90 inhibitors that do not suffer from these constraints are now in clinical development. Examples of disclosed chemical structures are shown in Figure 2.
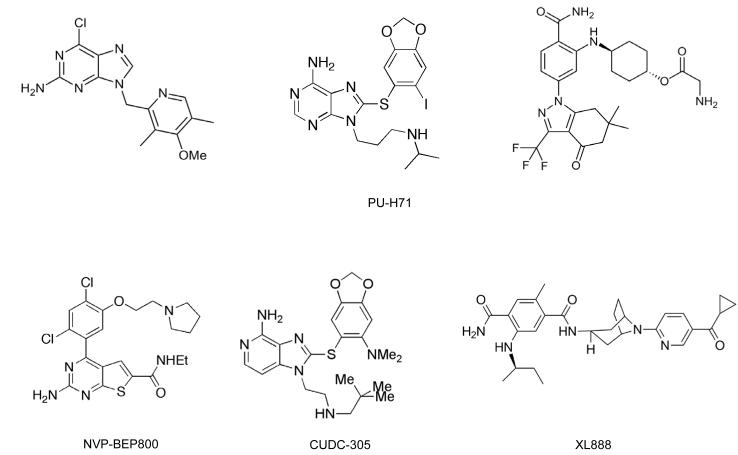
Success in discovering these new Hsp90 drug candidates has benefited greatly from structure-based design using available X-ray crystal structures of Hsp90, with initial chemical matter frequently emerging from high-throughput, fragment or virtual screening (39, 43). The two initial examples of success were with: 1) the purine scaffold series, based initially on PU3 (44, 45), leading to the clinical candidates BBIIB021 (CNF-2024) and BIIB028 (structure not disclosed), as well as PU-H71, now in phase 1 clinical trial (46); and 2) the resorcylic pyrazoles and isoxazoles (based on CCT018159) (47) leading to NVP-AUY922/VER52296 (48), the resorcylic dihydroxybenzamide AT13387 (49), and the structurally related KW-2478 (50). A diverse range of chemical scaffolds has since emerged, as illustrated by the publication of 40-70 patents per year from 2005-2010, covering purines and resorcinols as well as pyrimidines, aminopyridines, azoles and other chemotypes (Figure 2) (51). These include: SNX-5422 which is a prodrug of the active benzamide SNX-2112, the precursor of which was identified through a purine-based proteomic screen (52); the orally active thienopyrimidine NVP-BEP800/VER-82576 which was derived using a combined fragment-based and in silico approach (53); the 8-arylthiopurine CUDC-305 which is orally bioavailable, blood-brain barrier-permeant and active in an orthotopic brain tumor model (54); and the N-aryltropane XL888 (55). Another promising agent currently in multiple clinical trials is STA-9090 (ganetespib) (56). Although its full structure is undisclosed, it is thought to be a resorcinol-containing triazole, for which a phosphate prodrug is also being developed (39, 56).
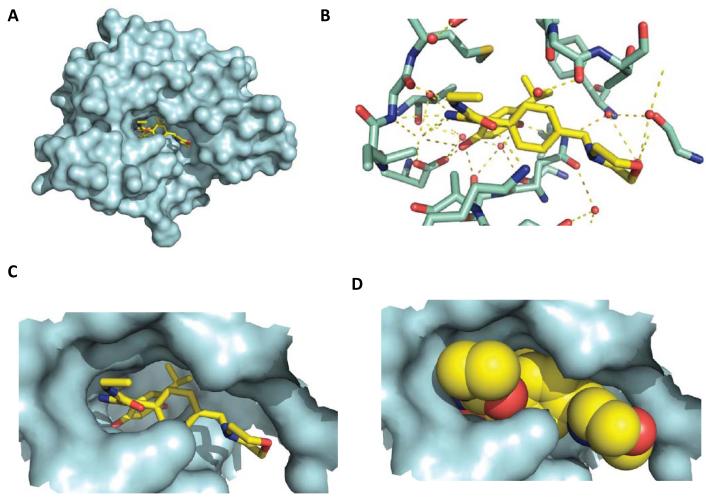
Importantly, where co-crystal structures of drug bound to the N-terminal domain of Hsp90 have been determined these inhibitors all seem to exploit the same core network of water-mediated hydrogen bonding interactions exploited by geldanamycin and radicicol (as well as ATP/ADP) to anchor the drugs into the base of the N-terminal nucleotide-binding pocket of the chaperone (16, 57, 58). This is illustrated in Figure 3 for the current clinical drug NVP-AUY922. The various new agents have the potential to be administered more frequently and to achieve a higher maximum dose (and hence better/more prolonged target inhibition), in some cases with oral administration and blood-brain barrier penetration. New drugs also lack the significant hepatotoxicity that was limiting for members of the geldanamycin chemotype, consistent with this side effect being related to the quinone in those agents (see earlier). Encouraging early clinical data have been reported concerning these agents’ pharmacodynamic and antitumor activity in diverse malignancies, again with the expected client protein and genetic profiles, including breast, non-small cell lung and rectal cancer, as well as melanoma and leukemia (40, 59-65).
Figures 3.
X-ray co-crystal structure of the clinically evaluated resorcylic isoxazole drug NVP-AUY922 bound to the N-terminal domain of human Hsp90α. The structure was obtained at 2.0 A°. PDB code 2VCI (48)
- A view of the surface of the entire human N-terminal domain of Hsp90α (in blue) with the resorcylic isoxazole inhibitor NVP-AUY922 (see Figure 2 for chemical structure) bound in the deep ATP pocket.
- A more detailed view of NVP-AUY922 in the ATP pocket. The same core network of hydrogen bonding interactions that are exploited by ATP/ADP, geldanamycin and radicicol, including water-mediated ones, are used to anchor this drug and other new synthetic inhibitors into the base of the N-terminal nucleotide-binding pocket. The core structure of the drug is shown in yellow and protein residues are in green. Hydrogen bonding interactions are shown as dotted yellow lines, some of which involve water molecules shown as red spheres. In this view the resorcinol ring is on the left-hand side pointing deep into the base of the pocket, and the two resorcinol hydroxyls groups make key hydrogen bond interactions. The isoxazole ring and morpholine ring (the later on the far right) are viewed side on. The amide substitution on the isoxazole ring extends out of the plane towards the viewer. All of these make additional hydrogen-bonding interactions in the ATP pocket.
- The position of the drug is the same as in (b) but the hydrogen bonding interactions have been removed and the protein surface is included, colored light blue. The way the resorcinol ring (on the left, with the two hydroxyl group oxygen atoms colored red) binds deep into the base of the nucleotide binding pocket is clearly visible, and the isopropyl group (on the resorcinol ring and pointing to the right) can be seen binding in a shallow hydrophobic pocket. It can also be seen that the morpholine ring points out of the solvent channel on the right-hand side. Again, the isoxazole ring amide projects out of the plane. Also clear in this representation is that the NVP-AUY922 molecule does not bind in the flat orientation that is depicted in the chemical structure representation (Figure 2). Rather, it adopts a twisted conformation which fits the unusual topology of the binding pocket. These features are generally characteristic of Hsp90 N-terminal inhibitors and they combine to give high levels of potency and selectivity for the target.
- The solvent accessible surface of the drug is shown to illustrate how effectively the drug fills the nucleotide binding pocket.
Biological insights and therapeutic implications
As more is learned about the role of Hsp90 in modulating signaling networks and about the sensitivity of various client proteins to Hsp90 inhibition, a better understanding of Hsp90 biology has educated and will continue to inform the ongoing clinical development of Hsp90 inhibitor-based therapy, in part by supporting the correct choice of tumor types and revealing additional molecular targets whose inhibition synergizes with Hsp90 inhibition, as discussed below.
Hsp90 helps to coordinate cellular and organismal responses to environmental stresses. For the fruit fly D. melanogaster, this may involve the ability to survive in a habitat where temperature fluctuations are common. For the protozoan parasite P. falciparum, this certainly involves successful adaptation to a life cycle that includes more than one host, as well as dramatically different local environments and temperatures. For cancer cells, unavoidable environmental perturbations include nutrient stress, proteotoxic stress, hypoxia, inherent genetic instability/aneuploidy, and even the necessity to avoid attack by the host immune system (Figure 1). Hsp90 is able to buffer these stresses, thereby allowing cancer cells to survive and indeed to flourish in an inhospitable environment. As described above, it does this by modulating the stability and/or activity of numerous proteins comprising nodal points in multiple signaling pathways that foster survival (3).
While specific Hsp90-dependent pathways that promote unlimited growth, survival in low oxygen conditions, escape from apoptosis, and overcoming nutrient deprivation by fostering angiogenesis have been known for some time, data have been reported more recently to suggest that Hsp90 inhibition promotes enhanced host natural killer cell-mediated tumor killing (66). Further, the receptor tyrosine kinase ephrin receptor A2 (EphA2) was recently identified to be an Hsp90 client (67). EphA2 is abundantly expressed in a broad range of cancers and is recognized as a ‘self’ protein by the host, thereby limiting the ability of CD8+ T cells to recognize and kill the tumor. Hsp90 inhibitor-dependent degradation of tumor cell EphA2 significantly improves the in vitro anti-tumor activity of host CD8+ T cells, suggesting that Hsp90 inhibition may have a beneficial impact on host tumor surveillance (68). It remains to be explored whether Hsp90 inhibitors might enhance the activity of therapeutic vaccines that rely on activating the host immune system to recognize and attack cancer cells.
Cancer cells are inherently genetically unstable and aneuploidy is a hallmark of cancer. Recently, data were reported identifying the Hsp90 inhibitor tanespimycin to be among a small group of compounds demonstrating enhanced efficacy toward aneuploid cancer cell lines in vitro (69). Thus, several colorectal cancer cell lines with high-grade aneuploid karyotypes were significantly more sensitive to the Hsp90 inhibitor than were colorectal cell lines with near euploid karyotypes. Similar differential sensitivity was seen in aneuploid lung cancer cell lines. Importantly, sensitivity of these cells to Hsp90 inhibition was independent of p53 status, although in some cellular contexts p53 status has been reported to influence response (70).
The enhanced sensitivity of aneuploid cancer cells to Hsp90 inhibition may reflect enhanced proteotoxic stress associated with aneuploidy, in part as a consequence of accumulating abundant misfolded proteins (69). Thus, it is not surprising that Hsp90 inhibitors synergize with proteasome inhibitors in multiple myeloma, a cancer in which the proteasome degradation machinery is taxed to the utmost. As described earlier in this review, combination of tanespimycin and the proteasome inhibitor bortezomib has been associated with durable responses in heavily pretreated multiple myeloma patients, including those with bortezomib-refractory disease (25, 26, 71, 72). Additional Hsp90 inhibitors are also being evaluated in this setting (50, 73, 74).
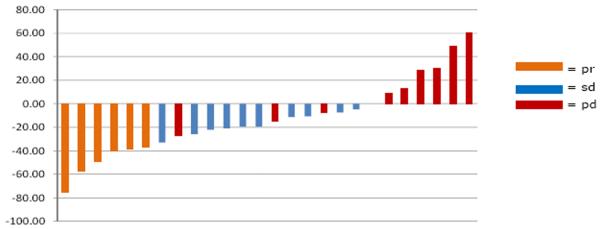
Some Hsp90 clients are known tumor driver proteins. Would tumors ‘addicted’ to (i.e., dependent on) these clients be most likely to show clinical responses to Hsp90 inhibitors? The strong relationship between client driver protein dependence on Hsp90 and potential clinical efficacy of Hsp90 inhibition is demonstrated by two powerful examples. The first is the tyrosine kinase receptor HER2, which was reported 15 years ago to be an Hsp90 client that is extremely sensitive (compared to most other clients) to chaperone inhibition (17, 75). Numerous pre-clinical data have shown Hsp90 inhibitors to be efficacious in HER2+ breast cancer xenograft studies (76). It is worth re-emphasizing that Hsp90 inhibitors combined with trastuzumab demonstrate clinical activity in HER2+ breast cancer patients for whom tumor progression was seen on trastuzumab alone. In the first phase II study to definitively show RECIST-defined responses for this drug in solid tumors, the overall response rate was 22% and the clinical benefit rate (complete response + partial response + stable disease) was 59% (Figure 4) (22-24).
Figure 4.
Patients with metastatic HER2+ breast cancer whose disease had previously progressed on trastuzumab received weekly treatment with tanespimycin at 450 mg/m2 intravenously and trastuzumab at a conventional dose. Therapy was continued until disease progression. The primary endpoint was response rate by RECIST criteria. The overall response rate in evaluable patients was 22% and the clinical benefit rate (CR + PR + SD) was 59%. Data are depicted as a waterfall plot with best response (%) indicated on the y-axis. Partial response (pr) is depicted by purple bars, stable disease (sd) is depicted by blue bars, and disease progression (pd) is depicted by red bars. The data are taken, with permission, from Modi et al. (19).
The second example is the mutated (rearranged) tyrosine kinase ALK, perhaps the only Hsp90 client that is as sensitive or more sensitive to chaperone inhibition than is HER2 (77). ALK rearrangements, particularly the EML4-ALK fusion protein, are found in a small subset (approximately 4%) of NSCLC patients. In a recent, non-randomized phase II study of the Hsp90 inhibitor retaspimycin in patients with molecularly defined NSCLC, an overall response rate of 7% was observed. However, of the three patients in the study whose tumors harbored ALK rearrangements, two had partial responses and the third experienced prolonged stable disease (40). Results of an open-label phase II study of a second Hsp90 inhibitor, ganetespib (STA-9090), in a similar patient population (advanced NSCLC) were reported at the ASCO 2011 Annual Meeting (65). Of 62 patients on study, 8 had tumors with ALK rearrangements. Of these, 4 patients had durable, objective responses.
These two examples suggest that client protein sensitivity to Hsp90 inhibition may be a key contributor to Hsp90 inhibitor clinical efficacy, but only in cases where the tumor is ‘addicted to’ the client. Hsp90 inhibition may also represent an effective strategy to overcome or prevent tyrosine kinase inhibitor (TKI) resistance (78-81). A recent study reported acquired resistance to the ALK inhibitor crizotinib in an NSCLC patient whose cancer relapsed after five months of treatment. Molecular analysis of resistant tumor cells revealed two novel mutations in the EML4-ALK gene, one of which – L1196M – conferred resistance to crizotinib. The study showed that these crizotinib-resistant tumor cells remained addicted to ALK signaling but retained sensitivity to the Hsp90 inhibitor tanespimycin (82). Indeed, the mutated ALK from these cells was efficiently depleted by tanespimycin. Similarly, in an NSCLC patient whose tumor initially responded to crizotinib and who remained on the drug for one year before progressing, a further tumor response was noted after treatment with ganetespib (65).
Chronic myelogenous leukemia in patients treated with the Abl TKI imatinib eventually becomes resistant to the drug due to development or outgrowth of Bcr-Abl drug resistant mutations, the most common being T315I. This Bcr-Abl mutant is resistant to all first- and second-line TKIs tested but remains sensitive to Hsp90 inhibition (Bcr-Abl is a highly dependent Hsp90 client) (83). These examples suggest that, at least in certain circumstances, combination of an Hsp90 inhibitor with a TKI may suppress or delay the occurrence of drug resistant mutations.
In addition to the relevance of amplification of – and dependence on – client proteins such as HER2 or rearranged ALK as predictors of tumor response to Hsp90 inhibitors, overexpression of Hsp90 itself has been identified as an independent prognostic factor in breast cancer (4). This may represent a clinical example of non-oncogene addiction to stress protection pathways and it may explain the Hsp90 inhibitor efficacy of triple negative breast cancer in preclinical models (46). Hsp90 expression levels are also inversely related to long-term survival in NSCLC patients (84), suggesting that stratification of patients’ tumors based on Hsp90 expression, as well as on expression of oncogenic clients, may select for a population that might be more responsive to Hsp90 inhibitors.
Future prospects: What next?
Several approaches are being investigated to enhance cancer cell sensitivity to Hsp90 inhibitors, including targeting Hsp90 co-chaperone proteins, HSF1 and its transcriptional targets, and post-translational modifiers of Hsp90. Modulating co-chaperone expression affects cancer cell sensitivity to Hsp90 inhibitors. For example, deletion of p23 in yeast results in hypersensitivity to the Hsp90 inhibitors geldanamcyin and radicicol, while p23 over-expression protects yeast from these agents (85). As this co-chaperone is reported to be over-expressed in cancer (86), pharmacologic inhibition of p23 may increase cancer cell sensitivity to Hsp90-targeted drugs. Similarly, silencing of the co-chaperones Aha1 and Cdc37, which are also over-expressed in cancer, sensitizes cancer cells to tanespimycin (87-90). Therefore, targeting these proteins, or their interaction with Hsp90, may be of therapeutic benefit when combined with Hsp90 inhibitors (90, 91).
Activation of the heat shock transcription factor HSF1 occurs uniformly in response to the Hsp90 inhibitors currently under clinical evaluation. Although useful to provide pharmacodynamic biomarkers, when this occurs in tumor cells it is generally considered to limit the activity of Hsp90 inhibitors because HSF1-dependent transcriptional induction of Hsp70, Hsp27, and to some degree Hsp90 itself, protects cancer cells from apoptosis. Indeed, cells in which HSF1 has been knocked out are much more sensitive to Hsp90 inhibitors than are their wild type counterparts (92). Likewise, silencing of either Hsp70 or Hsp27 has been shown to dramatically increase cancer cell sensitivity to Hsp90 inhibition and to induction of apoptosis (93, 94). Efforts are underway to identify and validate inhibitors of HSF1, Hsp70, and Hsp27, and to explore their combination with Hsp90 inhibitors (95-99).
Hsp90 is subject to several post-translational modifications that affect chaperone function. For example, the tyrosine kinase WEE1, an Hsp90 client, directly phosphorylates the chaperone on a conserved tyrosine residue in Hsp90’s N-terminal domain (100). Phosphorylation at this site positively affects the ability of Hsp90 to chaperone a number of cancer-related kinases, including HER2, SRC, CRAF, CDK4, and WEE1 itself. Importantly, WEE1 phosphorylation of Hsp90 negatively affects tanespimycin binding, and silencing or pharmacologic inhibition of WEE1 sensitizes prostate and cervical cancer cells to Hsp90 inhibition. These data suggest that a more detailed understanding of Hsp90 phosphorylation, and perhaps additional post-translational modifications, could provide new strategies to enhance the efficacy of Hsp90 inhibitors while at the same time revealing novel resistance mechanisms (please see (101) for a thorough review of the latest information on this topic).
Although to date the vast majority of drug development efforts have focused on targeting the N-domain ATP binding site of Hsp90, a second druggable site has been identified in the C-domain of the protein (102, 103). Coumarin antibiotics, such as novobiocin, are the prototypic inhibitors that interact at this site. Recently, significant advances have been made in improving the affinity of these compounds for Hsp90, and their ability to induce apoptosis in cancer cells, in some cases with superior efficacy to tanespimycin, has been demonstrated (104, 105). One potential benefit of these drugs is that some of the C-terminal inhibitors appear to be associated with significantly less robust HSF1 activation than is characteristic of N-terminal inhibitors (106). Existing data strongly support further medicinal chemistry optimization and pre-clinical evaluation of C-terminal Hsp90 inhibitors.
The relative importance in cancer of the four Hsp90 isoforms (Hsp90α and Hsp90β in the cytoplasm and the nucleus, GRP94 in the endoplasmic reticulum, and TRAP1 in the mitochondria) remains poorly understood. Although current inhibitors most potently interact with Hsp90α and β isoforms (e.g. see ref 41), the impact of isoform selectivity on the therapeutic index and toxic effects of these inhibitors should be explored in greater detail.
The critical role of Hsp90 in promoting cellular and organismal survival in response to environmental stress cannot be disputed. Thus although the protein has been validated as a bona fide molecular target in cancer and the inhibitors are generally well tolerated in the clinic, it should nonetheless be remembered that this chaperone also helps to maintain normal cellular homeostasis and has certain functions in specific cellular contexts that one would not want to inhibit, at least on a long-term basis. For example, prolonged Hsp90 inhibition may have a deleterious effect on DNA mutation frequency in germ cells, where Hsp90 is required for suppression of transposon activity (107, 108). Hsp90 inhibition may increase transposon mobility via loss of activity of the arginine methyltransferase PRMT5, an Hsp90 client (109).
Hsp90 also positively regulates at least one transcription factor with tumor suppressor activity, namely interferon regulatory factor 1 (IRF1). Loss of IRF1 cooperates with Ras mutation to transform cells, and IRF1 is deleted in certain cancers (110). Hsp90 inhibition is associated with inhibition of IRF1 transcriptional activity and IRF1 protein degradation (111).
LATS1 and LATS2 kinases are both clients of Hsp90 and are degraded upon Hsp90 inhibition (112). Both kinases positively regulate the Hippo tumor suppressor pathway, and LATS-deficient mice are prone to develop ovarian cancers and sarcomas (113, 114). LATS1 activity was disrupted in Hsp90 inhibitor-treated ovarian cancer cell lines in vitro, and in ovarian cancer xenograft tumors obtained from mice treated with the Hsp90 inhibitor tanespimycin (112).
Finally, low-penetrant mutations of the retinoblastoma (RB) tumor suppressor gene give rise to RB proteins that retain approximately 50% of wild type tumor suppressor activity. However, these mutant RB proteins interact with and depend on Hsp90 for stability, and they are inactivated and destabilized in cells exposed to the Hsp90 inhibitor geldanamycin (115). Thus, Hsp90 inhibitor treatment of patients harboring these low-penetrant RB mutant alleles, which normally are characterized by reduced or absent tumor formation, could phenocopy RB null mutations that are invariably associated with early onset multifocal retinoblastoma.
Taken together, these data demonstrate that several tumor suppressor pathways may be deregulated following Hsp90 inhibition, and they emphasize that the cumulative impact of an Hsp90 inhibitor on both the individual and the cancer cell is multi-factorial, and will almost certainly be influenced by the duration of treatment, the disparate sensitivity to Hsp90 inhibition of the various client proteins present in normal and cancer cells, the dependence of the particular cancer on the continued expression of one or more of these clients, and the local environmental context in which Hsp90 inhibition occurs. Nonetheless, with these caveats in mind, the promising clinical responses that continue to be seen with several Hsp90 inhibitors in a number of molecularly defined cancers certainly support the continued therapeutic development of these agents.
Concluding remarks
Considerable progress has been made both in understanding the basic structure-function biology of Hsp90 and in translating this knowledge into anti-cancer drug therapies. Tanespimycin showed proof-of-mechanism for Hsp90 inhibition in the clinic and proof-of-concept for therapeutic activity, most impressively in breast cancer. The view has been expressed that termination of development of this drug was premature and was not a result of scientific/clinical considerations (24, 27). Additional promising signs of activity have been seen in various molecularly relevant tumor types – including in prostate cancer where ongoing addiction to the androgen receptor client protein is clear, and also in other cancers in which Hsp90 client kinases are key drivers. On the other hand, no Hsp90 inhibitor has yet received marketing approval.
So are we there yet? While successfully targeting Hsp90 in cancer patients to achieve a significant therapeutic benefit is still a work in progress, we have certainly learned from experience. A number of highly potent and pharmaceutically improved Hsp90 inhibitors, lacking some of the drawbacks of the first generation inhibitors as discussed above, are now in clinical trial. In the near term, success will most likely lie in using these enhanced agents to treat cancers that are addicted to particular amplified, mutated or translocated driver oncogene products that are highly sensitive client proteins of Hsp90, such as HER2, ALK, EGFR, BRAF, and perhaps also androgen and estrogen receptors. Additional cancers that will likely prove sensitive to Hsp90 inhibitors include those malignancies, exemplified by multiple myeloma, in which buffering of proteotoxic stress is essential for cancer cell survival. In the long term, reaching the full therapeutic potential of Hsp90 inhibitors may require concomitant inhibition of Hsp70 isoforms or blockade of HSF1. In addition, tumor stratification by client protein status and Hsp90 expression level, more complete genetic profiling of tumors as a basis for patient selection, and judicious exploration of biologically informed drug combinations – including Hsp90 inhibitors combined with agents that directly block the function of a given Hsp90 oncoprotein client – will help guide the field to more efficacious use of these molecularly intriguing and therapeutically promising drugs.
Acknowledgments
We thank the Neckers and Workman lab members and many collaborators for valuable discussions. We thank Dr. Mehdi Mollapour for Figure 1, Professor Keith Jones for help with Figure 2, and Dr Nathan Brown for help with Figure 3. LN is supported by funds from the Intramural Research Program of the National Cancer Institute. PW is supported by Cancer Research UK program grant C309/A8274, is a Cancer Research UK Life Fellow, and acknowledges NHS funding to the NIHR Biomedical Research Centre. We apologize to those authors whose excellent work could not be cited because of space constraints.
Footnotes
Conflict of interest Paul Workman is an employee of The Institute of Cancer Research, which has a commercial interest in Hsp90 inhibitors and which operates a reward to inventors scheme. Intellectual property on Hsp90 inhibitors developed at The Institute of Cancer Research was licensed to Vernalis and Novartis. Paul Workman has also been a consultant to Novartis, and is a scientific founder of Chroma Therapeutics and current Chairman of Chroma Therapeutics Scientific Advisory Board.
References
- 1.Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J. 2008;410:439–53. doi: 10.1042/BJ20071640. [DOI] [PubMed] [Google Scholar]
- 2.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–16. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 3.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–49. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, et al. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007;67:2932–7. doi: 10.1158/0008-5472.CAN-06-4511. [DOI] [PubMed] [Google Scholar]
- 5.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–10. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, Alarcon SV, Lee S, Lee MJ, Giaccone G, Neckers L, et al. Update on Hsp90 inhibitors in clinical trial. Curr Top Med Chem. 2009;9:1479–92. doi: 10.2174/156802609789895728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alcalá A Marzuka, Flaherty K. BRAF-inhibitors for the treatment of metastatic melanoma: clinical trials and mechanisms of resistance. Clin Cancer Res. 2012;18 doi: 10.1158/1078-0432.CCR-11-0997. xx-xx. [DOI] [PubMed] [Google Scholar]
- 8.Pollak M. The insulin – insulin-like growth factor receptor family as a therapeutic target in oncology. Clin Cancer Res. 2012;18 doi: 10.1158/1078-0432.CCR-11-0998. xx-xx. [DOI] [PubMed] [Google Scholar]
- 9.Komlodi-Pasztor E, Sackett D, Fojo T. Mitotic kinase inhibitors: A tale of how great drugs against a promising target were brought down by a flawed rationale. Clin Cancer Res. 2012;18 doi: 10.1158/1078-0432.CCR-11-0999. xx-xx. [DOI] [PubMed] [Google Scholar]
- 10.Bates SE, Amiri-Kordestani L, Giaccone G. Drug development: portals of discovery. Clin Cancer Res. 2012;18 doi: 10.1158/1078-0432.CCR-11-1001. xx-xx. [DOI] [PubMed] [Google Scholar]
- 11.Uehara Y. Natural product origins of Hsp90 inhibitors. Curr Cancer Drug Targets. 2003;3:325–30. doi: 10.2174/1568009033481796. [DOI] [PubMed] [Google Scholar]
- 12.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91:8324–8. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma SV, Agatsuma T, Nakano H. Targeting of the protein chaperone, HSP90, by the transformation suppressing agent, radicicol. Oncogene. 1998;16:2639–45. doi: 10.1038/sj.onc.1201790. [DOI] [PubMed] [Google Scholar]
- 14.Schulte TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D, et al. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones. 1998;3:100–8. doi: 10.1379/1466-1268(1998)003<0100:arbttn>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grenert JP, Sullivan WP, Fadden P, Haystead TA, Clark J, Mimnaugh E, et al. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem. 1997;272:23843–50. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 16.Roe SM, Prodromou C, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42:260–6. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 17.Mimnaugh EG, Chavany C, Neckers L. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J Biol Chem. 1996;271:22796–801. doi: 10.1074/jbc.271.37.22796. [DOI] [PubMed] [Google Scholar]
- 18.Schneider C, Sepp-Lorenzino L, Nimmesgern E, Ouerfelli O, Danishefsky S, Rosen N, et al. Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci U S A. 1996;93:14536–41. doi: 10.1073/pnas.93.25.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Workman P, Collins I. Probing the probes: fitness factors for small molecule tools. Chem Biol. 2010;17:561–77. doi: 10.1016/j.chembiol.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerji U, O’Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol. 2005;23:4152–61. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 21.Banerji U, Walton M, Raynaud F, Grimshaw R, Kelland L, Valenti M, et al. Pharmacokinetic-pharmacodynamic relationships for the heat shock protein 90 molecular chaperone inhibitor 17-allylamino, 17-demethoxygeldanamycin in human ovarian cancer xenograft models. Clin Cancer Res. 2005;11:7023–32. doi: 10.1158/1078-0432.CCR-05-0518. [DOI] [PubMed] [Google Scholar]
- 22.Modi S, Stopeck AT, Gordon MS, Mendelson D, Solit DB, Bagatell R, et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J Clin Oncol. 2007;25:5410–7. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- 23.Modi S, Sugarman S, Stopeck A, Linden H, Ma W, Kersey K, et al. Phase II trial of the Hsp90 inhibitor tanespimycin (Tan) + trastuzumab (T) in patients (pts) with HER2-positive metastatic breast cancer (MBC) [abstract] J Clin Oncol. 2008;26:1027. [Google Scholar]
- 24.Modi S, Stopeck AT, Linden HM, Solit DB, Chandarlapaty S, Rosen N, et al. HSP90 Inhibition is Effective in Breast Cancer: A Phase 2 Trial of Tanespimycin (17AAG) plus Trastuzumab in Patients with HER2-Positive Metastatic Breast Cancer Progressing on Trastuzumab. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- 25.Richardson PG, Chanan-Khan AA, Lonial S, Krishnan AY, Carroll MP, Alsina M, et al. Tanespimycin and bortezomib combination treatment in patients with relapsed or relapsed and refractory multiple myeloma: results of a phase 1/2 study. Br J Haematol. 2011;153:729–40. doi: 10.1111/j.1365-2141.2011.08664.x. [DOI] [PubMed] [Google Scholar]
- 26.Richardson PG, Mitsiades CS, Laubach JP, Lonial S, Chanan-Khan AA, Anderson KC. Inhibition of heat shock protein 90 (HSP90) as a therapeutic strategy for the treatment of myeloma and other cancers. Br J Haematol. 2011;152:367–79. doi: 10.1111/j.1365-2141.2010.08360.x. [DOI] [PubMed] [Google Scholar]
- 27.Arteaga CL. Why is this effective HSP90 inhibitor not being developed in HER2+ breast cancer? Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-11-1218. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacey S, Gore M, Chao D, Banerji U, Larkin J, Sarker S, et al. A Phase II trial of 17-allylamino, 17-demethoxygeldanamycin (17-AAG, tanespimycin) in patients with metastatic melanoma. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9493-4. [DOI] [PubMed] [Google Scholar]
- 29.Solit DB, Osman I, Polsky D, Panageas KS, Daud A, Goydos JS, et al. Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma. Clin Cancer Res. 2008;14:8302–7. doi: 10.1158/1078-0432.CCR-08-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelland LR, Sharp SY, Rogers PM, Myers TG, Workman P. DT-Diaphorase expression and tumor cell sensitivity to 17-allylamino, 17-demethoxygeldanamycin, an inhibitor of heat shock protein. J Natl Cancer Inst. 1999;91:1940–9. doi: 10.1093/jnci/91.22.1940. [DOI] [PubMed] [Google Scholar]
- 31.Basso AD, Solit DB, Munster PN, Rosen N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene. 2002;21:1159–66. doi: 10.1038/sj.onc.1205184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger AM, Fiebig HH, Stinson SF, Sausville EA. 17-(Allylamino)-17-demethoxygeldanamycin activity in human melanoma models. Anticancer Drugs. 2004;15:377–87. doi: 10.1097/00001813-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Solit DB, Zheng FF, Drobnjak M, Munster PN, Higgins B, Verbel D, et al. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8:986–93. [PubMed] [Google Scholar]
- 34.Sawai A, Chandarlapaty S, Greulich H, Gonen M, Ye Q, Arteaga CL, et al. Inhibition of Hsp90 down-regulates mutant epidermal growth factor receptor (EGFR) expression and sensitizes EGFR mutant tumors to paclitaxel. Cancer Res. 2008;68:589–96. doi: 10.1158/0008-5472.CAN-07-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hostein I, Robertson D, DiStefano F, Workman P, Clarke PA. Inhibition of signal transduction by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosis. Cancer Res. 2001;61:4003–9. [PubMed] [Google Scholar]
- 36.Guo W, Reigan P, Siegel D, Zirrolli J, Gustafson D, Ross D. Formation of 17-allylamino-demethoxygeldanamycin (17-AAG) hydroquinone by NAD(P)H:quinone oxidoreductase 1: role of 17-AAG hydroquinone in heat shock protein 90 inhibition. Cancer Res. 2005;65:10006–15. doi: 10.1158/0008-5472.CAN-05-2029. [DOI] [PubMed] [Google Scholar]
- 37.Gaspar N, Sharp SY, Pacey S, Jones C, Walton M, Vassal G, et al. Acquired resistance to 17-allylamino-17-demethoxygeldanamycin (17-AAG, tanespimycin) in glioblastoma cells. Cancer Res. 2009;69:1966–75. doi: 10.1158/0008-5472.CAN-08-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pacey S, Wilson RH, Walton M, Eatock MM, Hardcastle A, Zetterlund A, et al. A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clin Cancer Res. 2011;17:1561–70. doi: 10.1158/1078-0432.CCR-10-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janin YL. ATPase inhibitors of heat-shock protein 90, second season. Drug Discov Today. 2010;15:342–53. doi: 10.1016/j.drudis.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Sequist LV, Gettinger S, Senzer NN, Martins RG, Janne PA, Lilenbaum R, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol. 2010;28:4953–60. doi: 10.1200/JCO.2010.30.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo W, Reigan P, Siegel D, Ross D. Enzymatic reduction and glutathione conjugation of benzoquinone ansamycin heat shock protein 90 inhibitors: relevance for toxicity and mechanism of action. Drug Metab Dispos. 2008;36:2050–7. doi: 10.1124/dmd.108.022004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samuni Y, Ishii H, Hyodo F, Samuni U, Krishna MC, Goldstein S, et al. Reactive oxygen species mediate hepatotoxicity induced by the Hsp90 inhibitor geldanamycin and its analogs. Free Radic Biol Med. 2010;48:1559–63. doi: 10.1016/j.freeradbiomed.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biamonte MA, Van de Water R, Arndt JW, Scannevin RH, Perret D, Lee WC. Heat shock protein 90: inhibitors in clinical trials. J Med Chem. 2010;53:3–17. doi: 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]
- 44.Chiosis G, Timaul MN, Lucas B, Munster PN, Zheng FF, Sepp-Lorenzino L, et al. A small molecule designed to bind to the adenine nucleotide pocket of Hsp90 causes Her2 degradation and the growth arrest and differentiation of breast cancer cells. Chem Biol. 2001;8:289–99. doi: 10.1016/s1074-5521(01)00015-1. [DOI] [PubMed] [Google Scholar]
- 45.Wright L, Barril X, Dymock B, Sheridan L, Surgenor A, Beswick M, et al. Structure-activity relationships in purine-based inhibitor binding to HSP90 isoforms. Chem Biol. 2004;11:775–85. doi: 10.1016/j.chembiol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 46.Caldas-Lopes E, Cerchietti L, Ahn JH, Clement CC, Robles AI, Rodina A, et al. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc Natl Acad Sci U S A. 2009;106:8368–73. doi: 10.1073/pnas.0903392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheung KM, Matthews TP, James K, Rowlands MG, Boxall KJ, Sharp SY, et al. The identification, synthesis, protein crystal structure and in vitro biochemical evaluation of a new 3,4-diarylpyrazole class of Hsp90 inhibitors. Bioorg Med Chem Lett. 2005;15:3338–43. doi: 10.1016/j.bmcl.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 48.Eccles SA, Massey A, Raynaud FI, Sharp SY, Box G, Valenti M, et al. NVP-AUY922: a novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res. 2008;68:2850–60. doi: 10.1158/0008-5472.CAN-07-5256. [DOI] [PubMed] [Google Scholar]
- 49.Woodhead AJ, Angove H, Carr MG, Chessari G, Congreve M, Coyle JE, et al. Discovery of (2,4-dihydroxy-5-isopropylphenyl)-[5-(4-methylpiperazin-1-ylmethyl)-1,3-di hydroisoindol-2-yl]methanone (AT13387), a novel inhibitor of the molecular chaperone Hsp90 by fragment based drug design. J Med Chem. 2010;53:5956–69. doi: 10.1021/jm100060b. [DOI] [PubMed] [Google Scholar]
- 50.Nakashima T, Ishii T, Tagaya H, Seike T, Nakagawa H, Kanda Y, et al. New molecular and biological mechanism of antitumor activities of KW-2478, a novel nonansamycin heat shock protein 90 inhibitor, in multiple myeloma cells. Clin Cancer Res. 2010;16:2792–802. doi: 10.1158/1078-0432.CCR-09-3112. [DOI] [PubMed] [Google Scholar]
- 51.Messaoudi S, Peyrat J-F, Brion D-J, Alami M. Heat shock protein 90 inhibitors as antitumor agents: A survey of the literature from 2005 to 2010. Expert Opin Therapeutic Patents. 2011;21:1501–42. doi: 10.1517/13543776.2011.594041. [DOI] [PubMed] [Google Scholar]
- 52.Fadden P, Huang KH, Veal JM, Steed PM, Barabasz AF, Foley B, et al. Application of chemoproteomics to drug discovery: identification of a clinical candidate targeting hsp90. Chem Biol. 2010;17:686–94. doi: 10.1016/j.chembiol.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 53.Brough PA, Barril X, Borgognoni J, Chene P, Davies NG, Davis B, et al. Combining hit identification strategies: fragment-based and in silico approaches to orally active 2-aminothieno[2,3-d]pyrimidine inhibitors of the Hsp90 molecular chaperone. J Med Chem. 2009;52:4794–809. doi: 10.1021/jm900357y. [DOI] [PubMed] [Google Scholar]
- 54.Bao R, Lai CJ, Qu H, Wang D, Yin L, Zifcak B, et al. CUDC-305, a novel synthetic HSP90 inhibitor with unique pharmacologic properties for cancer therapy. Clin Cancer Res. 2009;15:4046–57. doi: 10.1158/1078-0432.CCR-09-0152. [DOI] [PubMed] [Google Scholar]
- 55.Lyman SK, Crawley SC, Gong R, Adamkewicz JI, McGrath G, Chew JY, et al. High-content, high-throughput analysis of cell cycle perturbations induced by the HSP90 inhibitor XL888. PLoS One. 2011;6:e17692. doi: 10.1371/journal.pone.0017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCleese JK, Bear MD, Fossey SL, Mihalek RM, Foley KP, Ying W, et al. The novel HSP90 inhibitor STA-1474 exhibits biologic activity against osteosarcoma cell lines. Int J Cancer. 2009;125:2792–801. doi: 10.1002/ijc.24660. [DOI] [PubMed] [Google Scholar]
- 57.Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–50. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 58.Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 59.Modi S, Ismail-Khan R, Munster PN, Lucas M, Galluppi GR, Tangri S, et al. Phase I dose escalation study of the heat shock protein 90 inhibitor BIIB021 with trastuzumab in HER2+ metastatic breast cancer. San Antonio Breast Cancer Symposium; 2010. abstract. [Google Scholar]
- 60.Goldman JW, Raju RN, Gordon GA, Vukovic VM, Bradley R, Rosen LS. A phase I dose escalation study of the Hsp90 inhibitor STA-9090 administered once weekly in patients with solid tumors. J Clin Oncol. 2010;28 abstract 2529. [Google Scholar]
- 61.Cleary JM, Heath EI, Kwak EL, Dezube BJ, Gandhi L, Zack C, et al. A phase I dose escalation study of the Hsp90 inhibitor STA-9090 adminstered twice weekly in patients with solid tumors. J Clin Oncol. 2010;28 abstract 3083. [Google Scholar]
- 62.Samuel TA, Sessa C, Britten C, Milligan KS, Mita MM, Banerji U, et al. AUY922, a novel HSP90 inhibitor: Final results of a first-in-human study in patients with advanced solid malignancies. J Clin Oncol. 2010;28 abstract 2528. [Google Scholar]
- 63.Bryson JC, Infante JR, Ramanathan RK, Jones SF, Von Hoff DD, Burris HA. A phase I dose escalation study of the safety and pharmacokinetics (PK) of the oral Hsp90 inhibitor SNX-5422. J Clin Oncol. 2008;26 abstract 14613. [Google Scholar]
- 64.Lancet JE, Gojo I, Burton M, Quinn M, Tighe SM, Kersey K, et al. Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia. 2010;24:699–705. doi: 10.1038/leu.2009.292. [DOI] [PubMed] [Google Scholar]
- 65.Wong K, Koczywas M, Goldman JW, Paschold EH, Horn L, Lufkin JM, et al. An open-label phase II study of the Hsp90 inhibitor ganetespib (STA-9090) as monotherapy in patients with advanced non-small cell lung cancer (NSCLC) ASCO Meeting Abstracts. 2011:7500. [Google Scholar]
- 66.Fionda C, Soriani A, Malgarini G, Iannitto ML, Santoni A, Cippitelli M. Heat shock protein-90 inhibitors increase MHC class I-related chain A and B ligand expression on multiple myeloma cells and their ability to trigger NK cell degranulation. J Immunol. 2009;183:4385–94. doi: 10.4049/jimmunol.0901797. [DOI] [PubMed] [Google Scholar]
- 67.Annamalai B, Liu X, Gopal U, Isaacs JS. Hsp90 is an essential regulator of EphA2 receptor stability and signaling: implications for cancer cell migration and metastasis. Mol Cancer Res. 2009;7:1021–32. doi: 10.1158/1541-7786.MCR-08-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kawabe M, Mandic M, Taylor JL, Vasquez CA, Wesa AK, Neckers LM, et al. Heat shock protein 90 inhibitor 17-dimethylaminoethylamino-17-demethoxygeldanamycin enhances EphA2+ tumor cell recognition by specific CD8+ T cells. Cancer Res. 2009;69:6995–7003. doi: 10.1158/0008-5472.CAN-08-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang YC, Williams BR, Siegel JJ, Amon A. Identification of aneuploidy-selective antiproliferation compounds. Cell. 2011;144:499–512. doi: 10.1016/j.cell.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robles AI, Wright MH, Gandhi B, Feis SS, Hanigan CL, Wiestner A, et al. Schedule-dependent synergy between the heat shock protein 90 inhibitor 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin and doxorubicin restores apoptosis to p53-mutant lymphoma cell lines. Clin Cancer Res. 2006;12:6547–56. doi: 10.1158/1078-0432.CCR-06-1178. [DOI] [PubMed] [Google Scholar]
- 71.Richardson PG, Badros AZ, Jagannath S, Tarantolo S, Wolf JL, Albitar M, et al. Tanespimycin with bortezomib: activity in relapsed/refractory patients with multiple myeloma. Br J Haematol. 2010;150:428–37. doi: 10.1111/j.1365-2141.2010.08264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richardson PG, Chanan-Khan AA, Alsina M, Albitar M, Berman D, Messina M, et al. Tanespimycin monotherapy in relapsed multiple myeloma: results of a phase 1 dose-escalation study. Br J Haematol. 2010;150:438–45. doi: 10.1111/j.1365-2141.2010.08265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Usmani SZ, Bona RD, Chiosis G, Li Z. The anti-myeloma activity of a novel purine scaffold HSP90 inhibitor PU-H71 is via inhibition of both HSP90A and HSP90B1. J Hematol Oncol. 2010;3:40. doi: 10.1186/1756-8722-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ri M, Iida S, Nakashima T, Miyazaki H, Mori F, Ito A, et al. Bortezomib-resistant myeloma cell lines: a role for mutated PSMB5 in preventing the accumulation of unfolded proteins and fatal ER stress. Leukemia. 2010;24:1506–12. doi: 10.1038/leu.2010.137. [DOI] [PubMed] [Google Scholar]
- 75.Chavany C, Mimnaugh E, Miller P, Bitton R, Nguyen P, Trepel J, et al. p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2. J Biol Chem. 1996;271:4974–7. doi: 10.1074/jbc.271.9.4974. [DOI] [PubMed] [Google Scholar]
- 76.Murphy CG, Modi S. HER2 breast cancer therapies: a review. Biologics. 2009;3:289–301. [PMC free article] [PubMed] [Google Scholar]
- 77.Normant E, Paez G, West KA, Lim AR, Slocum KL, Tunkey C, et al. The Hsp90 inhibitor IPI-504 rapidly lowers EML4-ALK levels and induces tumor regression in ALK-driven NSCLC models. Oncogene. 2011;30:2581–6. doi: 10.1038/onc.2010.625. [DOI] [PubMed] [Google Scholar]
- 78.Montagut C, Sharma SV, Shioda T, McDermott U, Ulman M, Ulkus LE, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 2008;68:4853–61. doi: 10.1158/0008-5472.CAN-07-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pashtan I, Tsutsumi S, Wang S, Xu W, Neckers L. Targeting Hsp90 prevents escape of breast cancer cells from tyrosine kinase inhibition. Cell Cycle. 2008;7:2936–41. doi: 10.4161/cc.7.18.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shimamura T, Li D, Ji H, Haringsma HJ, Liniker E, Borgman CL, et al. Hsp90 inhibition suppresses mutant EGFR-T790M signaling and overcomes kinase inhibitor resistance. Cancer Res. 2008;68:5827–38. doi: 10.1158/0008-5472.CAN-07-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang S, Pashtan I, Tsutsumi S, Xu W, Neckers L. Cancer cells harboring MET gene amplification activate alternative signaling pathways to escape MET inhibition but remain sensitive to Hsp90 inhibitors. Cell Cycle. 2009;8:2050–6. doi: 10.4161/cc.8.13.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Katayama R, Khan TM, Benes C, Lifshits E, Ebi H, Rivera VM, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci U S A. 2011;108:7535–40. doi: 10.1073/pnas.1019559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peng C, Brain J, Hu Y, Goodrich A, Kong L, Grayzel D, et al. Inhibition of heat shock protein 90 prolongs survival of mice with BCR-ABL-T315I-induced leukemia and suppresses leukemic stem cells. Blood. 2007;110:678–85. doi: 10.1182/blood-2006-10-054098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruiz MI Gallegos, Floor K, Roepman P, Rodriguez JA, Meijer GA, Mooi WJ, et al. Integration of gene dosage and gene expression in non-small cell lung cancer, identification of HSP90 as potential target. PLoS One. 2008;3:e0001722. doi: 10.1371/journal.pone.0001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Forafonov F, Toogun OA, Grad I, Suslova E, Freeman BC, Picard D. p23/Sba1p protects against Hsp90 inhibitors independently of its intrinsic chaperone activity. Mol Cell Biol. 2008;28:3446–56. doi: 10.1128/MCB.02246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McDowell CL, Sutton R Bryan, Obermann WM. Expression of Hsp90 chaperone [corrected] proteins in human tumor tissue. Int J Biol Macromol. 2009;45:310–4. doi: 10.1016/j.ijbiomac.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 87.Smith JR, Clarke PA, de Billy E, Workman P. Silencing the cochaperone CDC37 destabilizes kinase clients and sensitizes cancer cells to HSP90 inhibitors. Oncogene. 2008 doi: 10.1038/onc.2008.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith JR, Workman P. Targeting CDC37: an alternative, kinase-directed strategy for disruption of oncogenic chaperoning. Cell Cycle. 2009;8:362–72. doi: 10.4161/cc.8.3.7531. [DOI] [PubMed] [Google Scholar]
- 89.Gray PJ, Jr., Stevenson MA, Calderwood SK. Targeting Cdc37 inhibits multiple signaling pathways and induces growth arrest in prostate cancer cells. Cancer Res. 2007;67:11942–50. doi: 10.1158/0008-5472.CAN-07-3162. [DOI] [PubMed] [Google Scholar]
- 90.Holmes JL, Sharp SY, Hobbs S, Workman P. Silencing of HSP90 cochaperone AHA1 expression decreases client protein activation and increases cellular sensitivity to the HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 2008;68:1188–97. doi: 10.1158/0008-5472.CAN-07-3268. [DOI] [PubMed] [Google Scholar]
- 91.Zhang T, Li Y, Yu Y, Zou P, Jiang Y, Sun D. Characterization of celastrol to inhibit hsp90 and cdc37 interaction. J Biol Chem. 2009;284:35381–9. doi: 10.1074/jbc.M109.051532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bagatell R, Paine-Murrieta GD, Taylor CW, Pulcini EJ, Akinaga S, Benjamin IJ, et al. Induction of a heat shock factor 1-dependent stress response alters the cytotoxic activity of hsp90-binding agents. Clin Cancer Res. 2000;6:3312–8. [PubMed] [Google Scholar]
- 93.McCollum AK, Teneyck CJ, Sauer BM, Toft DO, Erlichman C. Up-regulation of heat shock protein 27 induces resistance to 17-allylamino-demethoxygeldanamycin through a glutathione-mediated mechanism. Cancer Res. 2006;66:10967–75. doi: 10.1158/0008-5472.CAN-06-1629. [DOI] [PubMed] [Google Scholar]
- 94.Powers MV, Clarke PA, Workman P. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell. 2008;14:250–62. doi: 10.1016/j.ccr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 95.Powers MV, Workman P. Inhibitors of the heat shock response: biology and pharmacology. FEBS Lett. 2007;581:3758–69. doi: 10.1016/j.febslet.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 96.Davenport EL, Zeisig A, Aronson LI, Moore HE, Hockley S, Gonzalez D, et al. Targeting heat shock protein 72 enhances Hsp90 inhibitor-induced apoptosis in myeloma. Leukemia. 2010;24:1804–7. doi: 10.1038/leu.2010.168. [DOI] [PubMed] [Google Scholar]
- 97.Powers MV, Jones K, Barillari C, Westwood I, Montfort RL, Workman P. Targeting HSP70: The second potentially druggable heat shock protein and molecular chaperone? Cell Cycle. 2010;9:1542–50. doi: 10.4161/cc.9.8.11204. [DOI] [PubMed] [Google Scholar]
- 98.Evans CG, Chang L, Gestwicki JE. Heat shock protein 70 (hsp70) as an emerging drug target. J Med Chem. 2010;53:4585–602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hadchity E, Aloy MT, Paulin C, Armandy E, Watkin E, Rousson R, et al. Heat shock protein 27 as a new therapeutic target for radiation sensitization of head and neck squamous cell carcinoma. Mol Ther. 2009;17:1387–94. doi: 10.1038/mt.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mollapour M, Tsutsumi S, Donnelly AC, Beebe K, Tokita MJ, Lee MJ, et al. Swe1Wee1-dependent tyrosine phosphorylation of Hsp90 regulates distinct facets of chaperone function. Mol Cell. 2010;37:333–43. doi: 10.1016/j.molcel.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mollapour M, Neckers L. Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbamcr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J Biol Chem. 2000;275:37181–6. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 103.Marcu MG, Schulte TW, Neckers L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J Natl Cancer Inst. 2000;92:242–8. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 104.Donnelly A, Blagg BS. Novobiocin and additional inhibitors of the Hsp90 C-terminal nucleotide-binding pocket. Curr Med Chem. 2008;15:2702–17. doi: 10.2174/092986708786242895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao H, Donnelly AC, Kusuma BR, Brandt GE, Brown D, Rajewski RA, et al. Engineering an Antibiotic to Fight Cancer: Optimization of the Novobiocin Scaffold to Produce Anti-proliferative Agents. J Med Chem. 2011;54:3839–53. doi: 10.1021/jm200148p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Conde R, Belak ZR, Nair M, O’Carroll RF, Ovsenek N. Modulation of Hsf1 activity by novobiocin and geldanamycin. Biochem Cell Biol. 2009;87:845–51. doi: 10.1139/o09-049. [DOI] [PubMed] [Google Scholar]
- 107.Gangaraju VK, Yin H, Weiner MM, Wang J, Huang XA, Lin H. Drosophila Piwi functions in Hsp90-mediated suppression of phenotypic variation. Nat Genet. 2011;43:153–8. doi: 10.1038/ng.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Specchia V, Piacentini L, Tritto P, Fanti L, D’Alessandro R, Palumbo G, et al. Hsp90 prevents phenotypic variation by suppressing the mutagenic activity of transposons. Nature. 2010 doi: 10.1038/nature08739. [DOI] [PubMed] [Google Scholar]
- 109.Maloney A, Clarke PA, Naaby-Hansen S, Stein R, Koopman JO, Akpan A, et al. Gene and protein expression profiling of human ovarian cancer cells treated with the heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 2007;67:3239–53. doi: 10.1158/0008-5472.CAN-06-2968. [DOI] [PubMed] [Google Scholar]
- 110.Choo A, Palladinetti P, Passioura T, Shen S, Lock R, Symonds G, et al. The role of IRF1 and IRF2 transcription factors in leukaemogenesis. Curr Gene Ther. 2006;6:543–50. doi: 10.2174/156652306778520683. [DOI] [PubMed] [Google Scholar]
- 111.Narayan V, Eckert M, Zylicz A, Zylicz M, Ball KL. Cooperative regulation of the interferon regulatory factor-1 tumor suppressor protein by core components of the molecular chaperone machinery. J Biol Chem. 2009;284:25889–99. doi: 10.1074/jbc.M109.019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huntoon CJ, Nye MD, Geng L, Peterson KL, Flatten KS, Haluska P, et al. Heat shock protein 90 inhibition depletes LATS1 and LATS2, two regulators of the mammalian hippo tumor suppressor pathway. Cancer Res. 2010;70:8642–50. doi: 10.1158/0008-5472.CAN-10-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.St John MA, Tao W, Fei X, Fukumoto R, Carcangiu ML, Brownstein DG, et al. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat Genet. 1999;21:182–6. doi: 10.1038/5965. [DOI] [PubMed] [Google Scholar]
- 114.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–60. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 115.Park Y, Kubo A, Komiya T, Coxon A, Beebe K, Neckers L, et al. Low-penetrant RB allele in small-cell cancer shows geldanamycin instability and discordant expression with mutant ras. Cell Cycle. 2008;7:2384–91. doi: 10.4161/cc.6363. [DOI] [PMC free article] [PubMed] [Google Scholar]