Key Points
Erk1/2 are required for the maintenance of hematopoietic stem cells and immature progenitors in vivo.
Abstract
Extracellular signal-regulated kinase 1 (Erk1) and Erk2 play crucial roles in cell survival, proliferation, cell adhesion, migration, and differentiation in many tissues. Here, we report that the absence of Erk1 and Erk2 in murine hematopoietic cells leads to bone marrow aplasia, leukopenia, anemia, and early lethality. Mice doubly-deficient in Erk1 and Erk2 show rapid attrition of hematopoietic stem cells and immature progenitors in a cell-autonomous manner. Reconstitution studies show that Erk1 and Erk2 play redundant and kinase-dependent functions in hematopoietic progenitor cells. Moreover, in cells transformed by the oncogenic KRasG12D allele, the presence of either Erk1 or Erk2 with intact kinase activity is sufficient to promote cytokine-independent proliferation.
Introduction
Extracellular signal-regulated kinase 1 (Erk1) and Erk2 (Erk/12) encode serine/threonine kinases belonging to the mitogen-activating protein kinase family, and play critical roles in transmitting signals from a plethora of cytokines and growth factors.1 ERK1 and ERK2 are >80% identical, and both phosphorylate a myriad of substrates, many of which are cell line- and agonist-specific.1-3 Erk1−/− mice are viable and fertile,4,5 whereas Erk2−/− mice are embryonic lethal due to placental defects.6-9
ERK1/2 activation appears to be required for the survival and differentiation of many types of hematopoietic cells,2,3,10 but most studies of ERK1/2 function in hematopoiesis have used pharmacologic blockade of mitogen-activated protein kinase kinase 1 (MEK1) and MEK2, the kinases that activate ERK1/2. Genetic experiments argued that Erk1 is required for T-cell development,4 although this observation is controversial.5,9,11 T-cell–specific deletion of Erk2 also leads to defective T-lymphocyte maturation, suggesting that in some cellular contexts, ERK1 cannot compensate for ERK2.9 By contrast, either Erk1 or Erk2 is sufficient for normal B-cell development,12 although this process is markedly impaired in Erk1/2 doubly-deficient B cells.12 Together, these observations suggest that ability of Erk1 and Erk2 to compensate for one another might be cell-type specific. To date, the role of Erk1/2 in hematopoietic stem cell (HSC) function remains to be characterized. Here, we show that Erk1/2 are required in a cell-autonomous manner for the maintenance of HSCs.
Methods
Erk1−/−5 and Erk2flox/flox9 mice were crossed to Mx1-Cre mice (The Jackson Laboratory, Bar Harbor, ME), as indicated. All mice were maintained in accord with guidelines approved by the animal welfare committee of the University Health Network.
Results and discussion
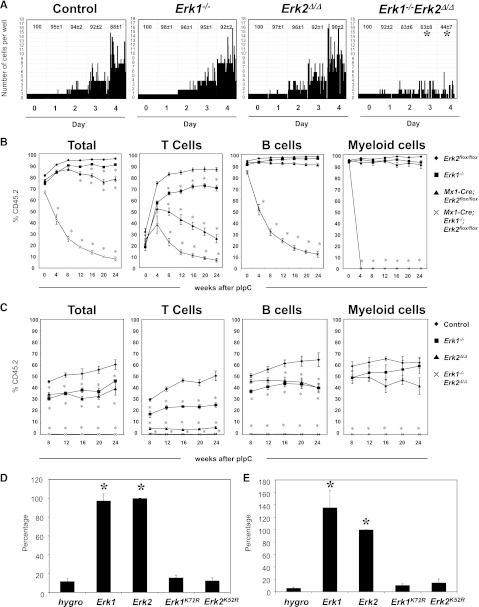
To assess ERK1 and ERK2 function in hematopoiesis, we generated Mx1-Cre;Erk2flox/flox and Mx1-Cre Erk1−/−;Erk2flox/flox mice in a C57BL/6J background and induced Cre recombinase expression by administration of polyinosinic-polycytidylic acid (pIpC). Hematopoiesis in untreated or pIpC-treated Erk2flox/flox (hereafter, “control”) mice was indistinguishable from that in wild-type C57BL/6J animals (data not shown). Most induced Mx1-Cre Erk1−/−;Erk2flox/flox (hereafter, Erk1−/−Erk2Δ/Δ) mice became moribund or died 30 to 40 days after pIpC injection (Figure 1A), whereas Erk1−/− and induced Mx1-Cre;Erk2flox/flox (hereafter, Erk2Δ/Δ) mice were indistinguishable from controls. Erk1−/−Erk2Δ/Δ mice were leukopenic, severely anemic, and had markedly reduced numbers of bone marrow (BM) cells (Figure 1B-D and supplemental Figure 1A, available on the Blood website). There also was a trend toward decreased numbers of mature myeloid, B cells, and erythroid cells in the BM of Erk1−/−Erk2Δ/Δ mice, although this difference did not reach statistical significance (supplemental Figure 1B).
Figure 1.
Deletion of Erk1 and Erk2 leads to fatal BM failure and loss of HSC. (A) Kaplan-Meier survival analysis of a cohort of Erk1−/− (n = 23), Mx1-Cre;Erk flox/flox (n = 21), Mx1-Cre Erk1−/−;Erk2 flox/flox (n = 22), and littermate control (n = 18) mice following pIpC treatment. Erk1−/−Erk2Δ/Δ mice have a median lifespan of 30 days; control mice remain healthy for >120 days. *P < .05, log-rank test. (B) Hematoxylin and eosin–stained sections from humeri of control, Erk1−/−, Erk2Δ/Δ, and Erk1−/−Erk2Δ/Δ animals 30 days after receiving their last dose of pIpC. (C-D) White blood cell count (WBC) and hematocrit (HCT) of control, Erk1−/−, Erk2Δ/Δ, and Erk1−/−Erk2Δ/Δ mice (*P < .05, ANOVA). (E) Absolute number (mean ± SEM, *P < .05, ANOVA) of LSK, CMP (Lin−Sca1−cKit+CD34+FcγRlo), GMP (Lin−Sca1−cKit+CD34+FcγRhi), MEP (Lin−Sca1−cKit+CD34−FcγR−/lo) of control (n = 6), Erk1−/− (n = 5), Erk2Δ/Δ (n = 5), and Erk1−/−Erk2Δ/Δ mice (n = 6). *P < .05, ANOVA. (F) Absolute number (mean ± SEM, * P < .05, Student t test) of signaling lymphocyte activation molecule (SLAM)-HSC from control (n = 6), Erk1−/− (n = 5), Erk2Δ/Δ (n = 5), and Erk1−/−Erk2Δ/Δ mice (n = 6). *P < .05, ANOVA.
Next, we examined the HSC-enriched Lin−Sca1+c-Kit+ (LSK) compartment and early progenitors in control and Erk-deficient BM. Erk1−/− and Erk2Δ/Δ mice showed normal numbers of LSK cells, common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs), and megakaryocte-erythrocyte progenitors (MEPs), but all of these progenitors were reduced markedly in Erk1−/−Erk2Δ/Δ BM (Figure 1E). Analysis of a more highly enriched HSC compartment (LSKCD150+CD48−, SLAM-HSC) also revealed a marked reduction in the frequency and the absolute cell number of these cells in Erk1−/−Erk2Δ/Δ mice (Figure 1F and data not shown), indicating a defect at the earliest stage of adult hematopoiesis. Thus, either Erk1 or Erk2 is sufficient to maintain hematopoiesis, but deletion of both Erk1 and Erk2 results in rapid hematopoietic failure and depletion of HSCs and progenitors.
Next, we investigated the ability of Erk-deficient HSCs to respond to cytokines that promote HSC maintenance. SLAM-HSCs were isolated from control and Erk-mutant mice 10 days after pIpC treatment, and placed at 1 cell per well in a medium that preserves multipotency in vitro.13,14 Mutant cells harvested at this time point all showed complete ablation of the appropriate Erk messenger RNA (supplemental Figure 2A-B). After 4 days of culture, >85% of cells from control, Erk1−/−, Erk2Δ/Δ mice were viable and had shown substantial proliferation. By contrast, Erk1−/−Erk2Δ/Δ cells underwent at most 1 or 2 cell divisions, and only ∼40% remained at the end of the experiment (Figure 2A). These data indicate that compound loss of Erk1 and Erk2 is incompatible with the proliferation and survival of HSCs in vitro.
Figure 2.
Requirement of Erk1 and Erk2 in HSCs and immature progenitors. (A) Single SLAM-HSC from of control, Erk1−/−, Erk2Δ/Δ, and Erk1−/−Erk2Δ/Δ mice were sorted into 60-well plates (1 cell per well) and cultured in media containing SCF, Tpo, Flt3l, and IL-11. Proliferation of each clone was evaluated microscopically over 4 days. Representative data from 1 of 4 experiments with similar results are shown. The average percentages (±SEM) of surviving clones from the 4 experiments are shown at the top of each panel. *P < .05, ANOVA. (B) Lethally irradiated CD45.1+ recipients (6 mice per group) were transplanted with 106 BM cells from control, Erk1−/−, Erk2Δ/Δ, and Erk1−/−Erk2Δ/Δ mice, along with 105 wild-type CD45.1+ cells. After 5 weeks of engraftment, chimeric mice were treated with 5 doses of pIpC, and the percentage of donor-derived peripheral blood cells (Total), CD3+ (T cells), B220+ (B cells), and Gr1+ (myeloid cells) were determined flow cytometry. *P < .05, ANOVA. (C) Control, Erk1−/−, Erk2−/−, and Mx1-Cre Erk1−/−Erk2flox/flox mice were treated with pIpC for 14 days before their BM cells (5 × 105) were harvested and mixed with equal numbers of wild-type CD45.1+ cells and intrafemorally injected into lethally irradiated CD45.1+ recipients (5 mice per group). After 8 weeks of engraftment, the percentage of donor-derived peripheral blood cells (Total), CD3+ (T cells), B220+ (B cells), and Gr1+ (myeloid cells) were determined flow cytometry. *P < .05, ANOVA. (D) Primary lin− BM cells from Erk1−/−;Erk2 flox/flox mice were transduced with the indicated retroviruses, and FACS-purified GFP+ cells were cultured in Iscove modified Dulbecco medium containing IL-3, IL-6, and SCF. MIG-Cre vectors coexpress the hygromycin resistance gene (hygro), Erk1, Erk2, Erk1KR, and Erk2KR. Relative cell proliferation in each experiment is normalized to that of Erk2. Results are shown as mean ± SEM of 4 independent experiments. *P < .05, ANOVA. (E) Lin− BM cells from Erk1−/−;Erk2 flox/flox;LSL-KrasG12D mice were transduced with retroviruses as described in panel D, and GFP+ cells were cultured in IMDM medium containing no cytokines. Cells were harvested and proliferation was determined. Cell proliferation in each experiment is normalized to that of Erk2 and results are shown as mean ± SEM of 4 independent experiments. *P < .05, ANOVA.
To test whether the requirement for Erk1 and Erk2 in hematopoiesis is cell-autonomous, we transferred uninduced Erk2flox/flox, Erk1−/−, Mx1-Cre;Erk2flox/flox, and Mx1-Cre Erk1−/−;Erk2flox/flox BM cells, together with wild-type congenic CD45.1-expressing carrier BM, into lethally irradiated CD45.1 animals. Recipients were maintained for 5 weeks to allow establishment of steady-state hematopoiesis. These mice were then injected with pIpC, and the ratio of CD45.1+ vs CD45.2+ cells in the peripheral blood was monitored. All mice showed comparable levels of CD45.2 engraftment before pIpC treatment (Figure 2B). Moreover, the percentage of donor chimerism of the B-cell and myeloid lineages in recipients of control, Erk1−/−, and Erk2Δ/Δ BM cells did not differ significantly. By contrast, there was a significant reduction in the percentage of donor-derived T cells in recipients of Erk1−/− or Erk2Δ/Δ BM cells, with Erk2Δ/Δ BM cells recipients showing a more pronounced defect. Finally, mice that had received Mx1-Cre Erk1−/−;Erk2flox/flox BM cells showed profound attrition of donor-derived T, B, and myeloid cells following pIpC treatment, indicating that the defect in HSC maintenance in the absence of ERK1 and ERK2 is cell-autonomous.
To further assess the effects of Erk deficiency on the functional competence of HSCs, we performed competitive reconstitution studies. In these experiments, control or Erk-deficient BM cells were mixed with CD45.1+ BM cells at 1:1 ratio before they were injected to lethally irradiated CD45.1+ recipients. Beginning 8 weeks after transplantation, CD45.2-derived cells in the peripheral blood of recipient mice were quantified. Recipients of Erk1−/− and Erk2Δ/Δ BM cells showed a reduced proportion of donor-derived T and B cells (Figure 2C). However, the frequencies of donor-derived myeloid cells in recipients of control, Erk1−/−, and Erk2Δ/Δ BM cells were similar. By contrast, animals that received Erk1−/−Erk2Δ/Δ BM cells had almost no contribution of donor-derived cells from any lineage in their peripheral blood. Thus, single Erk knockout HSCs and myeloid progenitors are not disadvantaged under competitive conditions, but deletion of both Erk isoforms leads to gross attrition of all hematopoietic lineages.
Although Erk2 is known to play a crucial role in T-cell development and activation,9,11 the role of Erk1 in T-lineage cells remains controversial.4,5,9,11 Importantly, the development and maintenance of Erk1-deficient T cells in an Erk1-sufficient background has never been examined. Our data suggest that both Erk1 and Erk2 are necessary for normal T-cell development/maintenance in vivo. Furthermore, our data indicate that the development and maintenance of Erk1- and Erk2-deficient B cells are diminished in the presence of wild-type B cells, indicating that B lymphocytes lacking a single ERK isoform have a subtle functional impairment not revealed in the noncompetitive setting.
Isoform-specific functions of Erk1 and Erk2 remain controversial.2,3 Moreover, ERK1 and ERK2 are known to have kinase-independent functions.15 To ask whether Erk1 and Erk2 have unique functions in hematopoietic cells and whether catalytic activity is required for hematopoietic progenitor function, we constructed retroviral vectors coexpressing a green fluorescent protein (GFP)–Cre fusion protein and either the hygromycin resistance gene (hygro), Erk1, Erk2, Erk1K72R, or Erk2K52R. Erk1K72R and Erk2K52R encode kinase-defective mutants of ERK1 and ERK2.16 Lineage-negative (lin−) BM cells from Erk1−/−Erk2flox/flox mice were infected with these viruses, and GFP+ cells were purified by fluorescence-activated cell sorter (FACS) and plated in liquid medium containing IL-3, IL-6, and stem cell factor (SCF) (Figure 2D). In infected cells, GFP-Cre excises the endogenous Erk2 alleles, generating Erk1−/−Erk2null cells, while concomitantly, different Erk isoforms are expressed under the control of the same viral promoter. We observed nearly complete loss of cell proliferation in Erk1−/−Erk2flox/flox cells coexpressing GFP-Cre and hygro. Coexpression of wild-type Erk1 or Erk2 with GFP-Cre restored cell proliferation to a similar extent. In sharp contrast, cells coexpressing kinase-defective Erk1 or Erk2 showed minimal proliferation. Thus, Erk1 and Erk2 have redundant, kinase-dependent functions in hematopoietic progenitors.
Pharmacologic blockade of MEK attenuates KRasG12D-evoked myeloproliferative disease in mice.17 However, the specific role(s) of Erk1 and Erk2 in KRasG12D-transformed hematopoietic cells remained be to be addressed. Notably, Erk2 has allele-specific effects on Ras-induced epithelial-to-mesenchymal transformation,18 raising the possibility that a single ERK isoform might be important for KRAS-evoked transformation of hematopoietic cells. To test this possibility, we infected lin− BM cells from Erk1−/−Erk2flox/floxLSL-KRasG12D mice with the retroviral vectors described in the previous paragraph. In infected cells, Cre expression promotes simultaneous deletion of Erk2 and expression of KRasG12D under the control of its endogenous promoter. GFP+ cells were purified by FACS and plated in media containing no cytokines to assess KRasG12D-evoked cytokine-independent proliferation. Comparable cell proliferation was observed in cells coexpressing either wild-type Erk1 or Erk2 (Figure 2E). By contrast, cells expressing catalytically defective ERK1 or ERK2 showed a complete loss of KRasG12D-evoked cytokine-independent proliferation. Thus, myeloid cell transformation by oncogenic KRAS requires catalytically active ERK1 or ERK2.
In summary, we found that deletion of both Erk genes results in the rapid loss of HSCs and progenitors, leading to leukopenia, anemia, and early lethality in mice. Similar data were reported recently by Staser et al.19 Together, these results are consistent with previous findings reported by us and others that hematopoietic-specific loss of Ptpn11 (encoding Src homology 2 domain containing phosphatase 2 [SHP2]), a positive regulator of RAS/ERK signaling, also leads to attrition of HSCs and progenitors.14,20
Supplementary Material
Acknowledgments
The authors thank Tharsan Velauthapillai for expert assistance with flow cytometry.
This work was supported by National Institutes of Health grants RO1 CA114945 and R37CA49132 (B.G.N.) and was also funded in part by the Ontario Ministry of Health and Long Term Care (OMOHLTC) and the Princess Margaret Hospital Foundation.
B.G.N. is a Canada Research Chair, Tier 1.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: G.C. designed research, performed experiments, analyzed data, and wrote the paper; S.G. performed experiments and analyzed data; and B.G.N. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gordon Chan, Ontario Cancer Institute, 101 College St, TMDT Room 8-301, Toronto, ON M5G 1L7, Canada; e-mail: gordon.chan@uhnresearch.ca
References
- 1.Roskoski R., Jr ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66(2):105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Geest CR, Coffer PJ. MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol. 2009;86(2):237–250. doi: 10.1189/jlb.0209097. [DOI] [PubMed] [Google Scholar]
- 3.Chung E, Kondo M. Role of Ras/Raf/MEK/ERK signaling in physiological hematopoiesis and leukemia development. Immunol Res. 2011;49(1-3):248–268. doi: 10.1007/s12026-010-8187-5. [DOI] [PubMed] [Google Scholar]
- 4.Pagès G, Guérin S, Grall D, et al. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286(5443):1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 5.Nekrasova T, Shive C, Gao Y, et al. ERK1-deficient mice show normal T cell effector function and are highly susceptible to experimental autoimmune encephalomyelitis. J Immunol. 2005;175(4):2374–2380. doi: 10.4049/jimmunol.175.4.2374. [DOI] [PubMed] [Google Scholar]
- 6.Hatano N, Mori Y, Oh-hora M, et al. Essential role for ERK2 mitogen-activated protein kinase in placental development. Genes Cells. 2003;8(11):847–856. doi: 10.1046/j.1365-2443.2003.00680.x. [DOI] [PubMed] [Google Scholar]
- 7.Saba-El-Leil MK, Vella FD, Vernay B, et al. An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep. 2003;4(10):964–968. doi: 10.1038/sj.embor.embor939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao Y, Li W, Wu J, et al. Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc Natl Acad Sci U S A. 2003;100(22):12759–12764. doi: 10.1073/pnas.2134254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer AM, Katayama CD, Pagès G, Pouysségur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23(4):431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DE. Src family kinases and the MEK/ERK pathway in the regulation of myeloid differentiation and myeloid leukemogenesis. Adv Enzyme Regul. 2008;48:98–112. doi: 10.1016/j.advenzreg.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Souza WN, Chang CF, Fischer AM, Li M, Hedrick SM. The Erk2 MAPK regulates CD8 T cell proliferation and survival. J Immunol. 2008;181(11):7617–7629. doi: 10.4049/jimmunol.181.11.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasuda T, Sanjo H, Pagès G, et al. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity. 2008;28(4):499–508. doi: 10.1016/j.immuni.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Benveniste P, Cantin C, Hyam D, Iscove NN. Hematopoietic stem cells engraft in mice with absolute efficiency. Nat Immunol. 2003;4(7):708–713. doi: 10.1038/ni940. [DOI] [PubMed] [Google Scholar]
- 14.Chan G, Cheung LS, Yang W, et al. Essential role for Ptpn11 in survival of hematopoietic stem and progenitor cells. Blood. 2011;117(16):4253–4261. doi: 10.1182/blood-2010-11-319517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez J, Crespo P. Working without kinase activity: phosphotransfer-independent functions of extracellular signal-regulated kinases. Sci Signal. 2011;4(196):re3. doi: 10.1126/scisignal.2002324. [DOI] [PubMed] [Google Scholar]
- 16.Robinson MJ, Harkins PC, Zhang J, et al. Mutation of position 52 in ERK2 creates a nonproductive binding mode for adenosine 5′-triphosphate. Biochemistry. 1996;35(18):5641–5646. doi: 10.1021/bi952723e. [DOI] [PubMed] [Google Scholar]
- 17.Lyubynska N, Gorman MF, Lauchle JO, et al. A MEK inhibitor abrogates myeloproliferative disease in Kras mutant mice. Sci Transl Med. 2011;3(76):76ra27. doi: 10.1126/scitranslmed.3001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin S, Dimitri CA, Yoon SO, Dowdle W, Blenis J. ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. Mol Cell. 2010;38(1):114–127. doi: 10.1016/j.molcel.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staser K, Park SJ, Rhodes SD, et al. Normal hematopoiesis and neurofibromin-deficient myeloproliferative disease require Erk. J Clin Invest. 2013;123(1):329–334. doi: 10.1172/JCI66167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu HH, Ji K, Alderson N, et al. Kit-Shp2-Kit signaling acts to maintain a functional hematopoietic stem and progenitor cell pool. Blood. 2011;117(20):5350–5361. doi: 10.1182/blood-2011-01-333476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.