Abstract
Rationale
Exposure to intermittent episodes of social defeat stress can increase drug seeking and leads to intense drug taking in rats.
Objectives
This study investigated the consequences of repeated, intermittent social defeat stress on patterns of drug self-administration in rats with access to heroin, cocaine, or a heroin-cocaine combination (“speedball”).
Methods
Male Long-Evans rats were either handled (controls) or subjected to 25 min social defeat stress episodes on days 1, 4, 7 and 10 during confrontations with an aggressive resident. Ten days following the last defeat, rats were assessed for locomotor cross-sensitization in response to heroin or cocaine. Animals were then prepared with intrajugular catheters for drug self-administration. Separate groups of controls and defeated rats were examined for self-administration of heroin (Experiment 1), a heroin-cocaine combination (Experiment 2), or cocaine (Experiment 3). Drug self-administration patterns were evaluated using fixed or progressive ratio schedules (FR, PR respectively) of reinforcement during limited access sessions or a 24-h unlimited access binge.
Results
Rats with a history of intermittent social defeat stress showed sensitized locomotor behavior when challenged with heroin or cocaine relative to controls. During the 24-h binge session, defeated rats escalated cocaine taking behavior (ca. 110 mg/kg vs. 66 mg/kg in controls), persisted in self-administering cocaine or the heroin-cocaine mixture for more hours, and showed a tendency for increased heroin-cocaine intake, but no effects on heroin taking.
Conclusions
A history of social defeat stress seems to preferentially promote escalated intake of cocaine but not heroin, unless a heroin-cocaine combination is available.
Keywords: addiction, intravenous self-administration, opiate, psychostimulant, locomotor sensitization, stress
INTRODUCTION
Exposure to stressful events can render individuals more prone to abuse addictive substances (Koob and Kreek 2007; Miczek et al. 2008; Sinha 2001). Several studies show a positive association between stress and increased drug intake and/or relapse to drug use (Brown et al. 1995; Dembo et al. 1988; Harrison et al. 1997; Jacobsen et al. 2001; Kosten et al. 1986; Sinha 2001). In experimental models, a history of exposure to such stressors as, for example, foot shock, food restriction or social defeat stress can promote and escalate drug self-administration (Covington, III and Miczek 2001; Covington, III and Miczek 2005; Goeders and Guerin 1994; Haney et al. 1995; Kosten et al. 2000; Miczek and Mutschler 1996; Piazza et al. 1990; Ramsey and van Ree 1993; Shaham and Stewart 1994). Social stress, most often studied in the form of social defeat, subordination stress, maternal separation stress and social isolation, may represent types of stressors with ecological and ethological validity that influences the initiation, maintenance and relapse to drug-taking and -seeking behaviors (Miczek et al. 2008).
The capacity of social and other stressors to promote increased sensitivity to drug’s stimulant effects and escalation of drug intake, has been associated with stress-induced neuroadaptations in brain reward pathways (for reviews, see (Miczek et al. 2008; Shalev et al. 2002)). Exposure to a brief, intermittent episode of social defeat stress (and other stressors), just like most drugs of abuse, activates mesolimbic dopamine pathways that project from the ventral tegmental area (VTA) to the nucleus accumbens and medial prefrontal cortex (Anstrom et al. 2009; DiChiara and Imperato 1988; Tidey and Miczek 1997). Single or repeated exposure to stress or drugs can induce neurochemical sensitization as demonstrated by augmented drug-induced dopamine responses in the nucleus accumbens (del Rosario et al. 2002; Grimm et al. 2003; Miczek et al. 1999a; Miczek et al. submitted; Rouge-Pont et al. 1995), often accompanied by augmented drug-induced psychomotor responses (del Rosario et al. 2002; Deroche et al. 1992; Deroche et al. 1995; Leyton and Stewart 1990; Nikulina et al. 2005; Stohr et al. 1999).
Rodents with a history of exposure to intermittent or brief episodes of social defeat stress show augmented sensitivity to psychostimulant-induced hyperactivity (locomotor “cross-sensitization”); facilitated acquisition of cocaine self-administration; increased motivation to obtain cocaine according to a progressive ratio (PR) schedule of reinforcement; and escalated drug intake during a 24-h “binge” session (Covington, III et al. 2008; Covington, III and Miczek 2001; Covington, III and Miczek 2005; Haney et al. 1995; Kabbaj et al. 2001; Nikulina et al. 1998; Tidey and Miczek 1997). Despite strong evidence suggesting that social defeat promotes escalated cocaine self-administration, it remains to be determined whether or not the consequences of social defeat will generalize to escalated patterns of intake for other drugs of abuse besides cocaine. In particular, the role of neuroadaptations in μ-opioid receptor function in the VTA as a neural mechanism underlying social defeat stress (Nikulina et al. 1999; Nikulina et al. 2005), might suggest that the rewarding effects of opiates (e.g., heroin or morphine) may be potentiated. To test this hypothesis, the first aim of this study was to study the consequences of repeated social defeat stress on patterns of heroin self-administration.
In humans, opiates and psychostimulants are often abused in combination (Darke and Hall 1995; Hartel et al. 1995; Hasin et al. 1988; Kosten et al. 1986; Leri et al. 2004). It has been suggested that the combination of cocaine and heroin (“speedball”) can be even more rewarding than each drug alone (Foltin and Fischman 1992; Hemby et al. 1999). For instance, using a progressive ratio schedule of drug reinforcement, it was demonstrated that rats tolerate progressively higher response demands when being reinforced with a opioid/psychostimulant combination relative to these drugs alone, i.e. achieve a higher “breakpoint” (Duvauchelle et al. 1998; Ranaldi and Wise 2000). The increased rewarding effects of “speedball” can be associated with an augmentation of dopamine concentrations in the nucleus accumbens when the two drugs are self-administered in combination, as compared to either cocaine or heroin alone (Smith et al. 2006). Thus, the reinforcing effects of cocaine may be enhanced by the addition of heroin and vice versa. The second aim of the present study was to determine how the behaviorally sensitizing effects of social defeat stress will affect the combined self-administration of heroin and cocaine (“speedball”). A third comparative study confirmed the effects of social defeat stress on cocaine self-administration.
MATERIALS AND METHODS
Subjects and housing
Male Long-Evans rats (n= 73; Charles River Laboratories, Wilmington, MA), weighing 225–250 g at arrival, were individually housed in custom-built clear acrylic chambers (30 × 30.5 × 24.5 cm), with cellulose pellet bedding (CelluDri™, Shepherd Specialty Papers, Kalamazoo, MI). Animals had free access to food (Purina Laboratory Chow) and water throughout all phases of each experiment. Rats were continuously maintained on a reversed 12 h light cycle (lights on 20:00 to 08:00) with controlled temperature (21°C) and humidity (35–40%). The chamber walls were fitted with removable stainless wire mesh panels. Rats were habituated to saline injections (i.p. and/or s.c.) before expression tests (see below).
Additional Long-Evans rats (n=10), weighing 500–600 g (Charles River Laboratories), served as aggressive stimulus rats (residents). Each resident was housed with a female in a large stainless steel cage (45.7 × 71.1 × 45.7 cm). Residents were selected by demonstrating consistent aggressive behavior toward an intruder rat during regularly scheduled confrontations (Miczek 1979). All experimental procedures were approved by the Tufts University Institutional Animal Care and Use Committee, following the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Drugs
Heroin
For s.c. administration, 0.25 mg/ml heroin (diacetylmorphine HCl, NIDA Drug Supply Program, Bethesda MD) was injected in a volume of 1 ml/kg (0.25 mg/kg dose). For intravenous self-administration, 0.2 mg/ml heroin was infused in a volume of 0.15 ml/kg (0.03 mg/kg/infusion) over 3–4 sec, depending on body weight. During the 24-h binge, the heroin concentration was doubled (0.4 mg/ml) and the infusion volume halved (0.075 ml/kg).
Cocaine
For i.p. administration, 10 mg/ml cocaine HCl (NIDA Drug Supply Program, Bethesda MD) was injected in a volume of 1 ml/kg (10 mg/kg dose). For intravenous self-administration, cocaine was prepared in concentrations of 5 mg/ml or 2 mg/ml and infused in a volume of 0.15 ml/kg (0.75 or 0.3 mg/kg/infusion, respectively) over 3–4 sec. During the 24-h binge, the cocaine concentration was doubled (4 mg/ml) and the infusion volume halved (0.075 ml/kg).
The heroin-cocaine mixture contained 0.08 mg/ml heroin plus 1.67 mg/ml cocaine or 0.17 mg/ml heroin plus 1.67 mg/ml cocaine, with an infusion volume of 0.15 mg/ml (0.012 or 0.025 mg/kg/infusion heroin respectively plus 0.25 mg/kg/infusion cocaine) over 3–4 sec. During the 24-h binge, the concentrations were doubled (0.08 mg/ml heroin plus 0.84 mg/ml cocaine) and the infusion volume halved (0.075 ml/kg).
All drugs were dissolved in sterile saline. The doses were selected on the basis of preliminary experiments and previous studies (Covington and Miczek 2005, Hemby et al. 1995, 1999; Quadros and Miczek 2009, Shaham and Stewart 1994).
Experimental Design
The following experiments assessed whether rats with a history of intermittent, brief exposures to repeated social defeat stress would show locomotor cross-sensitization to a heroin or cocaine challenge injection. Furthermore, it assessed whether socially-defeated rats show increased self-administration of different drugs of abuse (heroin, cocaine, and the mixture of heroin and cocaine, “speedball”), as assessed using fixed ratio (FR) and PR schedules of reinforcement, or during a 24-h unlimited “binge” self-administration session.
Social defeat stress procedure
Experimental rats were exposed to four brief episodes of intermittent social defeat stress on days 1, 4, 7 and 10, as previously described (Covington, III and Miczek 2001). Each defeat episode consisted of three separate phases. First, the home cage of the experimental rat (“intruder”) was placed within the aggressive resident's cage for 10 min to allow the resident to display species-typical threats (Tornatzky and Miczek 1993). Subsequently, the intruder was removed from the protective home cage, and the resident-intruder confrontation occurred until the attacks by a resident resulted in defeat. To reduce variability of stress intensity, each physical confrontation was standardized by establishing two criteria for defeat: the display of 6 s of supine posture by the intruder or a maximum duration of 5 min. Finally, the intruder was placed back in its home cage, and was again exposed for 10 min to the resident's threats while protected. During the entire 10-day procedure, control and stressed rats were weighed and handled daily. Two separate groups of animals were used to assess the consequences of social defeat stress on heroin or heroin-cocaine (speedball) self-administration, and a third group of rats was used for comparison purposes to replicate and further extend previous findings on cocaine self-administration.
Expression test: cross-sensitization to heroin or cocaine
The expression of locomotor cross-sensitization to heroin or cocaine was assessed in stressed and control rats on experimental day 20 (10 days after the last defeat), in two separate experiments. All expression tests were recorded in the home cage after the i.p. administration of saline (for baseline levels of motor activity), followed 15 min later by an injection of heroin (0.25 mg/kg s.c., Experiment 1) or cocaine (10 mg/kg i.p., Experiments 2 and 3). Five-min video samples were collected 5–10 min after the saline injection, and 5–10 and 25–30 min after the drug challenge. A trained observer using a custom keyboard and commercial software (The Observer Video-Pro v. 4.0, Noldus, Netherlands) analyzed each video record. Frequency and duration of walking, rearing and grooming were measured during each 5-min sample.
Intravenous drug self-administration
Surgery
One to four days after the expression test, stressed and control rats were implanted with permanently indwelling catheters (Silastic™ silicon tubing, ID 0.63 mm, OD 1.17 mm) into the right jugular vein under a combination of ketamine (100 mg/kg) and xylazine (6 mg/kg) anesthesia. The catheter was passed subcutaneously to the rat's back where it exited through a small incision and was affixed to a plastic pedestal (Plastics One, Roanoke, VA) mounted inside a harness system (Instech Laboratories Inc., Plymouth Meeting, PA). Rats were allowed to recover from surgery for 5 days.
After recovering from surgery, each rat while housed in its home cage was permanently relocated in a procedure room that was equipped for i.v. self-administration (for more details see Miczek and Mutschler 1996). A tether (Plastics One, Roanoke, VI) attached to a single channel counterbalanced swivel (Lomir Biomedical Inc., Malone NY) connected the catheter to a syringe that was activated by a syringe pump (Med Associates Inc., St. Albans, VT). One wall of the home cage was replaced with a panel equipped with two retractable levers (Med Associates Inc.). During self-administration sessions, a red cue light above the active lever signaled drug delivery and a green session light in the middle of the panel signaled drug availability. Before each daily session, the catheter was flushed with heparinized saline (20 IU/ml), and whenever drug was not available, 0.17 ml pulses of saline were delivered every 30 min.
General procedures for drug self-administration
Three different experiments were carried out, each of which had a group of socially defeated rats, and a non-defeated control group. Each experiment assessed the consequences of social defeat stress on patterns of self-administration of: heroin (Experiment 1); a combination of heroin and cocaine (“speedball”; Experiment 2); or cocaine alone (Experiment 3). For clarity, the general procedures for drug self-administration are presented together. Details of drugs, doses and schedules of reinforcement for each experiment are presented in Table 1.
Table 1.
Experimental design, timeline and drugs used in each experiment.
| Experimental Days |
Experiment 1 Heroin Self- Administration |
Experiment 2 “Speedball” Self- Administration |
Experiment 3 Cocaine Self- Administration |
|
|---|---|---|---|---|
| Intermittent social defeat stress | Days 1, 4, 7 and 10 | Defeat Stress (N=13); Controls (N=10) | Defeat Stress (N=13); Controls (N=11) | Defeat Stress (N=12); Controls (N=11) |
| Drug challenge (locomotor sensitization) | Day 21 | Heroin (0.25 mg/kg, s.c.) | Cocaine (10 mg/kg, i.p.) | Cocaine (10 mg/kg, i.p.) |
| Intravenous catheter implantation | Day 22 | |||
| Acquisition of self-administration (drugs and doses, mg/kg/infusion) | Days 27–35 | Heroin 0.03 | Heroin 0.025 + Cocaine 0.25 | Cocaine 0.75 |
| Fixed Ratio (FR) schedule for maintenance sessions | Days 36–40 | FR 3 | FR 5 | FR 5 |
| Progressive Ratio (drugs and doses, mg/kg/infusion; 3 trials/dose) | Days 41–45 | Heroin 0.03 | Heroin 0.013 + Cocaine 0.25 | Cocaine 0.3 |
| Days 46–51 | ↓ | Heroin 0.025 + Cocaine 0.25 | ↓ | |
| 24-h unlimited access “binge” (drugs and doses, mg/kg/infusion) | Days 47–48 | Heroin 0.03 | ↓ | Cocaine 0.3 |
| Days 53–54 | Heroin 0.025 + Cocaine 0.25 |
Acquisition and Maintenance
All rats were initially given limited access to drug self-administration, with each lever press being reinforced with an i.v. drug infusion (Fixed Ratio 1 (FR1) schedule of reinforcement), followed by a 30-s time out. During the time out period, the green cue light was extinguished and lever presses were recorded but had no consequences. Each daily session was terminated after 15 drug infusions or 5 h of access, whichever occurred first. Behavioral shaping was used to facilitate lever pressing in rats (approximately 30%) that did not begin responding for drug within the first two daily sessions. After completing 15 drug infusions within 5 h over two consecutive days, the FR schedule was progressively increased to every third response (FR 3 for heroin) or every fifth response being reinforced (FR 5, for cocaine and speedball), over four additional days. The FR 5 schedule was implemented in Experiments 2 and 3, since cocaine and the heroin-cocaine combination maintained responding much more effectively than heroin alone. Rats were maintained for at least four additional days on a limited access FR schedule before being examined during a progressive ratio schedule of reinforcement.
Progressive Ratio Schedule of Drug Reinforcement
After the acquisition and maintenance phase, self-administration according to a progressive ratio (PR) schedule of drug reinforcement was verified. The progression of response requirements followed the algorithm developed by Richardson and Roberts (1996): 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178... and the PR session was terminated when the rat failed to obtain an infusion during 60 min. The final infusion delivered was defined as the break point. The average number of total infusions obtained across the three PR trials for each individual rat was calculated. PR sessions were alternated with limited access FR self-administration maintenance sessions. When PR tests were carried out using more than one dose of the drug (as in Experiment 2), each dose was tested on a different day, in a counterbalanced manner and with alternating FR sessions (see Table 1).
24-h Unlimited Access Drug “Binge”
After the final PR session, each rat was allowed one additional day of limited drug access (maintenance dose, FR schedule, total of 15 infusions). The very next day, a 24-h binge protocol was implemented starting at approximately 10:00 a.m. (i.e., 2 h after the start of the dark phase). Each rat was allowed continuous access to drug infusions (see doses on Table 1) during the entire 24-h binge. The amounts of drug self-administered as well as the pattern of responding were recorded.
Statistical analysis
Locomotor sensitization was determined by comparing frequency of walking behavior between groups (stress vs. control) across 3 repeated observations (5–10 min after saline injection and 5–10 and 25–30 min after drug injection), using mixed-model Analysis of Variance. When appropriate, post-hoc mean comparisons to control were performed using the Holm-Sidak method.
All subsequent self-administration data in each separate experiment were analyzed using Student’s t-tests comparing social defeat stress and control groups. In all analyses, significant effects were detected when p<0.05.
RESULTS
Experiment 1 – Social Defeat Stress and Heroin
Locomotor Cross-Sensitization to Heroin
As shown in Table 2, analysis of walking frequency in response to a 0.25 mg/kg heroin administration (s.c.) showed a significant main effect for Observation Time (F2,44=1.90, p=0.034) and the main effect for Stress approached statistical significance (F1,22=4.09, p=0.055). Post-hoc mean comparisons revealed that the locomotor response to heroin was significantly enhanced in socially-defeat stressed rats compared to non-defeated controls in the interval from 5 to 10 minutes after heroin injection (Table 2), while no significant differences were found between previously defeated rats and controls following saline administration or 25–30 min after heroin injection (data not shown).
Table 2.
Data summary for the cross-sensitization challenge, acquisition of self-administration, FR response rate, responding on the non-reinforced lever and PR break points for each separate experiment.
| Experiment 1: Heroin Self-Administration | ||||||
| Walking after heroin challenge (frequency, 5–10 min post-inj.) |
Time to acquire FR1(hours) |
FR3 Rate during maintenance (resp/min) |
Responding on non-reinforced lever during FR3 (resp/min) |
PR break point (infusions obtained) |
||
| Controls (n=10) | 18.3±3.7 | 11.0±3.8 | 0.33±0.07 | 0.08±0.03 | 12.9±1.4 | |
| Social Defeat (14) | 37.4±6.9** | 11.1±2.1 | 0.39±0.10 | 0.02±0.01 | 9.9±1.3 | |
| Experiment 2: Heroin+Cocaine Self-Administration | ||||||
| Walking after cocaine challenge (frequency, 5–10 min post-inj.) |
Time to acquire FR1(hours) |
FR5 Rate during maintenance (resp/min) |
Responding on non-reinforced lever during FR5 (resp/min) |
PR break point (infusions obtained) | ||
| 0.025 mg/kg/inf Heroin + 0.25 Cocaine |
0.013 mg/kg/inf Heroin + 0.25 Cocaine |
|||||
| Controls (11) | 40.7±6.2 | 10.9±1.5 | 0.69±0.08 | 0.11±0.05 | 14.4±1.2 | 14.2±0.9 |
| Social Defeat (13) | 64.1±7.1* | 9.6±1.3 | 0.70±0.09 | 0.06±0.02 | 14.1±0.9 | 14.0±0.9 |
| Experiment 3: Cocaine Self-Administration | ||||||
| Walking after cocaine challenge (frequency, 5–10 min post-inj.) |
Time to acquire FR1(hours) |
FR5 Rate during maintenance (resp/min) |
Responding on non-reinforced lever during FR5 (resp/min) |
PR break point (infusions obtained) |
||
| Controls (11) | 37.3±6.3 | 19.1±4.4 | 1.62±0.75 | 0.04±0.02 | 10.8±0.6 | |
| Social Defeat (12) | 68.9±4.6** | 17.9±2.5 | 1.03±0.09 | 0.02±0.01 | 11.0±0.5 | |
Asterisks and bold type indicate significant group differences (*p<0.05, **p<0.01).
Heroin Self-Administration
Acquisition and maintenance of heroin self-administration
It took rats an average of 11.1 ± 2.0 hours across 2.9 ± 0.3 days to acquire lever-pressing behavior that was reinforced with heroin infusions (0.03 mg/kg/infusion), with no significant difference between stressed animals and controls (see Table 2). The schedule of reinforcement was moved up to FR 3, and rats were maintained on this schedule for 4 consecutive daily sessions, in which self-administration was limited to 15 infusions per session. There were no group differences in the rate of heroin-reinforced lever-pressing behavior during the maintenance phase or in the rate of responding on the non-reinforced lever (see Table 2).
Responding for heroin on a progressive ratio schedule of reinforcement
There were no group differences in the number of heroin (0.03 mg/kg/infusion) reinforcements obtained during the PR sessions (Table 2).
Heroin self-administration during a 24-h access “binge”
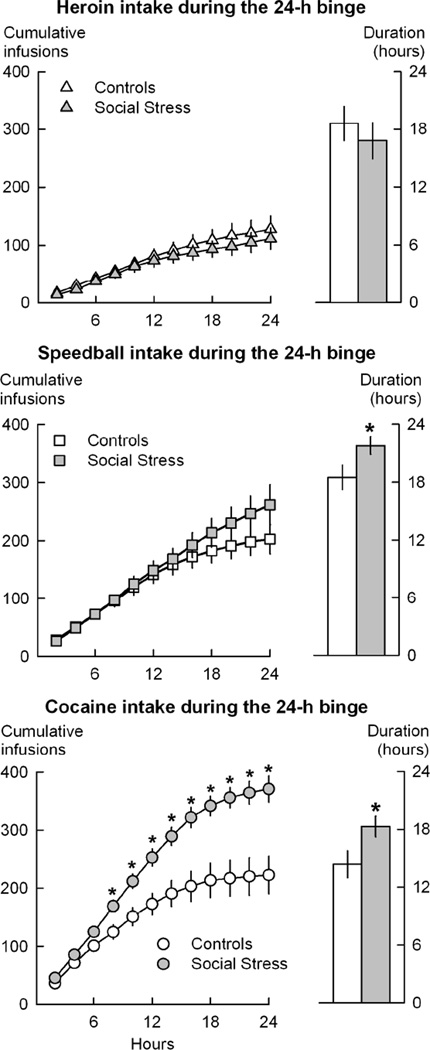
As shown in Figure 1, there were no group differences on the pattern of heroin self-administration during the 24-h access session. Rats with a history of defeat stress accumulated similar amounts of heroin as controls, and were actively responding for heroin for a similar duration of time during the 24-h binge (Figure 1 top). Rats were considered to be “actively responding” for the drug as long as the inter-infusion interval did not exceed 60 min; when this occurred, the time of the last infusion before the greater than 60-min interval was considered the “duration of active responding”.
Figure 1.
Patterns of drug self-administration during a 24-hour unlimited access “binge” session, in rats with a history of social defeat stress and non-stressed controls. Top Left: Cumulative heroin infusions (0.03 mg/kg/infusion) portrayed in 2-hour bins. Top Right: Duration of active responding for heroin during the binge session. Middle Left: Cumulative heroin-cocaine (“speedball”, 0.025 mg/kg/infusion of heroin plus 0.25 mg/kg/infusion of cocaine) infusions in 2-hour bins. Middle Right: Duration of active responding for speedball during the binge session. Bottom Left: Cumulative cocaine infusions (0.30 mg/kg/infusion) in 2-hour bins. Bottom Right: Period of active responding for cocaine during the binge session. All values are means ± SEM; * = p < 0.05.
Experiment 2 – Social defeat stress and heroin-cocaine mixture (speedball)
Locomotor Cross-Sensitization to Cocaine
Analysis of walking behavior in response to a 10 mg/kg cocaine i.p. administration showed a significant main effect for Observation Time (F2,46=8.17, p<0.001) and a significant Stress × Observation Time interaction (F2,46=5.96, p=0.005). Post-hoc mean comparisons revealed that the locomotor response to cocaine was significantly enhanced in socially-defeat stressed rats compared to non-defeated controls in the interval from 5 to 10 minutes after cocaine injection (Table 2), while no significant differences were found between previously defeated rats and controls following saline administration or 25–30 min after cocaine injection (data not shown).
Heroin-Cocaine (Speedball) Self-Administration
Acquisition and maintenance of heroin-cocaine self-administration
It took rats an average of 10.2±1.0 hours across 2.9±0.2 days to acquire lever-pressing behavior that was reinforced with heroin-cocaine infusions, with no significant difference between stressed animals and controls (Table 2). The schedule of reinforcement was moved up to FR5, and rats were maintained on this schedule for 3–4 consecutive daily sessions, in which self-administration was limited to 15 infusions per session. There were no group differences in the rate of lever-pressing for heroin-cocaine or in the rate of responding on the non-reinforced lever during the maintenance phase (Table 2).
Progressive ratio responding for heroin-cocaine
There were no group differences in the number of heroin-cocaine reinforcements obtained during the PR sessions (Table 2). Furthermore, the average number of infusions obtained during PR sessions was similar for the different dose combinations of heroin and cocaine (0.013 mg/kg/infusion heroin + 0.25 mg/kg/infusion cocaine vs. the training dose of 0.025 mg/kg/infusion heroin + 0.25 mg/kg/infusion cocaine).
Heroin-cocaine self-administration during a 24-h access “binge”
As shown in Figure 1, rats with a history of defeat stress showed increased persistence in self-administration behavior during the 24-h binge, as revealed by the longer time spent actively responding for the combination of heroin-cocaine [t(19)=2.16, p<0.05]. Socially defeated rats also showed trends for escalated intake of heroin-cocaine relative to controls during the final 12-h of the 24-h binge [t(19)=2.9; p<0.06], as shown in Figure 1. Three rats failed to run the 24-h binge session due to catheter failure (2 animals) or sickness (1 rat), resulting in 10 controls and 11 stressed animals for these comparisons.
Experiment 3 – Social defeat stress and cocaine
Locomotor Cross-Sensitization to Cocaine
Analysis of walking behavior in response to a 10 mg/kg cocaine i.p. administration showed a significant main effect for Stress (F1,18=18.96, p<0.001) and a significant Stress × Observation Time interaction (F2,36=3.42, p=0.044), while the main effect for Observation Time was just short of significant (F2,36=3.09, p=0.058). Post-hoc mean comparisons revealed that the locomotor response to cocaine was significantly enhanced in socially-defeat stressed rats compared to non-defeated controls in the intervals from 5–10 (Table 2) and 25–30 min after cocaine injection, while no significant differences were found between previously defeated rats and controls following saline administration (data not shown).
Cocaine Self-Administration
Acquisition and maintenance of cocaine self-administration
It took rats an average of 18.6 ± 2.4 hours across 4.6 ± 0.4 days to acquire lever pressing for cocaine infusions, with no significant difference between stressed animals and controls. Rats were maintained on an FR 5 schedule for 3–4 consecutive daily sessions. There were no group differences between stressed and non-stressed controls in the rate of lever-pressing for cocaine or in responding on the non-reinforced lever during the maintenance phase (see Table 2).
Progressive ratio responding for cocaine
In this group of rats, no effects of social defeat stress were detected on the number of cocaine reinforcements obtained during the PR sessions (Table 2).
Cocaine self-administration during a 24-h access “binge”
As shown in Figure 1, socially-defeated rats accumulated higher amounts of cocaine during the 24-h binge relative to controls [t(21)=3.83, p=0.001], in confirmation of previous reports (Covington, III et al. 2008; Covington, III and Miczek 2005). Furthermore, stressed rats actively persisted in cocaine taking behavior for longer period of time relative to controls [t(21)=2.23, p=0.037], and showed increased rates of responding during the binge ([t(21)=3.10, p=0.005], calculated by dividing the number of responses by the time spent actively self-administering cocaine: 1.7±0.08 resp/min vs. 1.3±0.1 resp/min in controls).
DISCUSSION
In confirmation of earlier reports, the current data demonstrate that repeated, brief episodes of social defeat can promote long-lasting behavioral and neurobiological consequences that promote increased sensitivity to drug-induced psychostimulant effects as well as escalated drug self-administration (Covington, III et al. 2005; Covington, III et al. 2008; Covington, III and Miczek 2001; Kabbaj et al. 2001; Miczek et al. submitted; Quadros and Miczek 2009; Tornatzky and Miczek 1993; Yap et al. 2006). Social defeat stress promoted locomotor cross-sensitization to heroin and cocaine, while failing to affect patterns of heroin self-administration when assessed on fixed or progressive ratio schedules of reinforcement, including a 24-h unlimited access session. Conversely, when rats with a history of defeat were self-administering the heroin-cocaine cocktail (speedball), they showed increased persistence in responding and a tendency for escalated drug intake during the 24-h binge. When rats self-administered cocaine alone, escalated intake of cocaine during the unlimited access binge was replicated in socially-defeated rats. Thus, a history of social defeat stress seems to more selectively promote escalated drug intake for psychostimulants (i.e. cocaine), with little effects on heroin self-administration when heroin is not offered in combination with cocaine.
Social defeat and heroin self-administration
Social defeat stress promoted cross-sensitization to heroin, without affecting heroin-taking behavior. One difficulty concerning the study of heroin self-administration is that it can be confounded by other effects of heroin, including sedation, long half-life and satiety (see (Arnold and Roberts 1997)for a review). Furthermore, the current PR schedule may not be the most sensitive to assess behavior maintained by opiate agonists (Arnold and Roberts 1997; Hubner and Koob 1990; Mello et al. 1988; Richardson and Roberts 1996; Roberts and Bennett 1993; Rowlett and Woolverton 1997). Nonetheless, Shaham and Stewart (Shaham and Stewart 1994) found augmented PR break points for heroin in rats receiving footshock stress, despite failing to observe differences in FR responding. In that case, the exposure to footshock stress preceded each self-administration test session (Shaham and Stewart 1994). Factors associated with stressor specificity (footshock vs. social defeat) and with the temporal relationship between stress exposure and drug access could contribute for the different outcomes found in our study and that of Shaham and Stewart (1994; see Miczek et al. 2008 for a review).
The sensitized locomotor response to a heroin injection is in accord with previous studies showing enhanced locomotor response to moderate doses of cocaine, amphetamine and morphine after repeated social defeat episodes (Covington, III et al. 2005; Covington, III and Miczek 2001; Miczek et al. 1999b; Nikulina et al. 1998; Yap and Miczek 2007). Locomotor cross-sensitization with heroin would be consistent with an up-regulation of μ-opioid receptors in the VTA of rats with a history of social defeat (Nikulina et al. 2004). In the VTA, increased mRNA for μ-opioid receptors after defeat stress are likely associated with an increased functional and behavioral response to a μ-opiod agonist microinfused into the VTA (Nikulina et al. 2005). Thus, it is possible that the cross-sensitization after the systemic heroin challenge can be attributed to heroin’s action on upregulated μ-opioid receptors in the VTA.
In contrast, the increased sensitivity to a systemic heroin challenge was not associated with escalated heroin self-administration in the present study, suggesting that different mechanisms mediate heroin’s stimulant and rewarding effects. Additionally, it is possible that daily exposure to heroin during the self-administration sessions could further regulate and affect the μ-opiod receptor function in the VTA and other brain areas (Nikulina et al. 2004, 2005).
Social defeat and speedball self-administration
Rats previously exposed to four brief episodes of social defeat tended to accumulate more drug during a 24-h binge session with access to a heroin-cocaine mixture, particularly during the final 12-h period. The major impact of social defeat was on the persistence of drug-taking behavior. Stressed rats spent most of the 24-h session actively self-administering speedball, whereas controls stopped taking drug after 18-h into the session. Speedball seems to maintain active self-administration behavior for a longer period of time when compared to cocaine alone, especially in non-defeated controls (14h of active cocaine self-administration vs. 18h of speedball self-administration; see also Quadros and Miczek 2009). However, there was no indication that defeat stress increased other parameters of speedball self-administration, including responding on a PR schedule of reinforcement.
Contradictory findings have been reported on whether the cocaine-heroin mixture can be more rewarding than either drug alone in non-human primates and rats, as assessed with PR break points (Duvauchelle et al. 1998; Mello et al. 1995; Ranaldi and Munn 1998; Rowlett et al. 1998; Rowlett and Woolverton 1997; Smith et al. 2006; Ward et al. 2005). Using several combinations of doses, Ward et al. (2005) failed to observe additive effects of the heroin-cocaine mixture on PR responding in rats. However, during a choice procedure, rats showed preference for the mixture of heroin-cocaine relative to cocaine alone, suggesting increased rewarding effect for speedball (Ward et al. 2005). The present study was not designed to compare the rewarding effects of each particular drug, dose or combination of them, but was rather focused on the effects on social defeat stress in promoting escalated taking of the different drugs.
It seems remarkable that social defeat stress promotes cross-sensitization to both psychostimulants and opiates, but seems to promote preferentially escalation of psychostimulants self-administration. The most prominent consequence of defeat stress is the escalated cocaine taking behavior during a 24-h binge, which is accompanied by increased persistence in lever pressing and increased rate of self-administration (Covington, III et al. 2005; Covington, III and Miczek 2001; Quadros and Miczek 2009). None of these parameters were changed when stressed rats were offered the opportunity to self-administer heroin in this and other doses tested during pilot studies (0.015 and 0.0075 mg/kg/infusion). When the combination of heroin-cocaine was self-administered, an intermediate profile of effects emerged, with more modest effects of defeat stress on the persistence in self-administration behavior during the 24-h binge. This observation is not without precedents. Other environmental manipulations (for example, when rats self-administer in a distinct environment relative to the home cage) that promote increased psychostimulant self-administration have also been shown to produce opposite effects on heroin self-administration (Caprioli et al. 2007, 2008; Celentano et al. 2009).
It is possible that such differential effects are due to the recruitment of distinct brain reward mechanisms by cocaine, heroin, and the cocaine-heroin mixture. While the self-administration of cocaine and psychostimulants are classically associated with increased dopamine release in the nucleus accumbens, the effects of opiates and speedball self-administration on accumbal dopamine are not as clear (Hemby et al. 1995, 1999; Smith et al. 2006; Wise et al. 1995). A history of exposure to brief episodes of defeat stress, similar to the current stress protocol, promotes a sensitized dopamine response after a cocaine challenge (Miczek et al. submitted). Thus, it is possible that the effects of social defeat stress are more specifically associated with a sensitized dopamine response, which in turn may be more relevant for escalated cocaine self-administration, but not necessarily for heroin, and only partially important for heroin-cocaine reward. Further studies will investigate which DA cells in the VTA-accumbens pathway are recruited by social defeat stress and subsequently engender cross-sensitization to both heroin and cocaine challenges, but preferentially escalate cocaine self-administration.
REFERENCES
- Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JM, Roberts DCS. A critique of fixed and progressive ratio schedules used to examine the neural subsrtrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Celentano M, Paolone G, Badiani A. Modeling the role of environment in addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1639–1653. doi: 10.1016/j.pnpbp.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Celentano M, Paolone G, Lucantonio F, Bari A, Nencini P, Badiani A. Opposite environmental regulation of heroin and amphetamine self-administration in the rat. Psychopharmacology. 2008;198:395–404. doi: 10.1007/s00213-008-1154-3. [DOI] [PubMed] [Google Scholar]
- Celentano M, Caprioli D, Dipasquale P, Cardillo V, Nencini P, Gaetani S, Badiani A. Drug context differently regulates cocaine versus heroin self-administration and cocaine- versus heroin-induced Fos mRNA expression in the rat. Psychopharmacology. 2009;204:349–360. doi: 10.1007/s00213-009-1467-x. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Repeated social-defeat stress, cocaine or morphine Effects on locomotor sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology. 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, Miczek KA., III Ïntense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology. 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA. NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology. 2008;197:203–216. doi: 10.1007/s00213-007-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Hall W. Levels and correlates of polydrug use among heroin users and regular amphetamine users. Drug Alcohol Depend. 1995;39:231–235. doi: 10.1016/0376-8716(95)01171-9. [DOI] [PubMed] [Google Scholar]
- del Rosario CN, Pacchioni AM, Cancela LM. Influence of acute or repeated restraint stress on morphine-induced locomotion: involvement of dopamine, opioid and glutamate receptors. Behav Brain Res. 2002;134:229–238. doi: 10.1016/s0166-4328(02)00038-4. [DOI] [PubMed] [Google Scholar]
- Dembo R, Williams L, Berry E, Getreu A, Washburn M, Wish ED, Schmeidler J. The relationship between physical and sexual abuse and illicit drug use: a replication among a new sample of youths entering a juvenile detention center. Int J Addict. 1988;23:1101–1123. doi: 10.3109/10826088809056189. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Maccari S, Lemoal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids .1. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7181–7188. doi: 10.1523/JNEUROSCI.15-11-07181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Maccari S, Le Moal M, Simon H. Stress-induced sensitization to amphetamine and morphine psychomotor effects depend on stress-induced corticosterone secretion. Brain Res. 1992;598:343–348. doi: 10.1016/0006-8993(92)90205-n. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvauchelle CL, Sapoznik T, Kornetsky C. The synergistic effects of combining cocaine and heroin (“speedball”) using a progressive-ratio schedule of drug reinforcement. Pharmacol Biochem Behav. 1998;61:297–302. doi: 10.1016/s0091-3057(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. The cardiovascular and subjective effects of intravenous cocaine and morphine combinations in humans. J Pharmacol Exp Ther. 1992;261:623–632. [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Non-contingent electric footshock facilitates the acquisition of intravenous cocain self-administration in rats. Psychopharmacology. 1994;114:63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Harrison PA, Fulkerson JA, Beebe TJ. Multiple substance use among adolescent physical and sexual abuse victims. Child Abuse Negl. 1997;21:529–539. doi: 10.1016/s0145-2134(97)00013-6. [DOI] [PubMed] [Google Scholar]
- Hartel DM, Schoenbaum EE, Selwyn PA, Kline J, Davenny K, Klein RS, Friedland GH. Heroin use during methadone maintenance treatment: the importance of methadone dose and cocaine use. Am J Public Health. 1995;85:83–88. doi: 10.2105/ajph.85.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Grant BF, Endicott J, Harford TC. Cocaine and heroin dependence compared in poly-drug abusers. Am J Public Health. 1988;78:567–569. doi: 10.2105/ajph.78.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Co C, Dworkin SI, Smith JE. Synergistic elevations in nucleus accumbens extracellular dopamine concentrations during self-administration of cocaine/heroin combinations (Speedball) in rats. J Pharmacol Exp Ther. 1999;288:274–280. [PubMed] [Google Scholar]
- Hemby SE, Martin TJ, Co C, Dworkin SI, Smith JE. The effects of intravenous heroin administration on extracellular nucleus accumbens dopamine concentrations as determined by in vivo microdialysis. J Pharmacol Exp Ther. 1995;273:591–598. [PubMed] [Google Scholar]
- Hubner CB, Koob GF. Bromocriptine produces decreases in cocaine self-administration in the rat. Neuropsychopharmacology. 1990;3:101–108. [PubMed] [Google Scholar]
- Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: A review of the literature. Am J Psychiatry. 2001;158:1184–1190. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H. Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology. 2001;158:382–387. doi: 10.1007/s002130100918. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Gawin FH, Rounsaville BJ, Kleber HD. Cocaine abuse among opioid addicts: demographic and diagnostic factors in treatment. Am J Drug Alcohol Abuse. 1986;12:1–16. doi: 10.3109/00952998609083739. [DOI] [PubMed] [Google Scholar]
- Leri F, Stewart J, Tremblay A, Bruneau J. Heroin and cocaine co-use in a group of injection drug users in Montreal. J Psychiatry Neurosci. 2004;29:40–47. [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Stewart J. Preexposure to foot-shock sensitizes the locomotor response to subsequent systemic morphine and intra-nucleus accumbens amphetamine. Pharmacol Biochem Behav. 1990;37:303–310. doi: 10.1016/0091-3057(90)90339-j. [DOI] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Bree MP, Mendelson JH. Progressive ratio performance maintained by buprenorphine, heroin and methadone in Macaque monkeys. Drug Alcohol Depend. 1988;21:81–97. doi: 10.1016/0376-8716(88)90053-1. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS, Lukas SE, Mendelson JH, Sholar JW, Drieze J. A primate model of polydrug abuse: Cocaine and heroin combinations. J Pharmacol Exp Ther. 1995;274:1325–1337. [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: Differential effects of d-amphetamine and cocaine. Psychopharmacology. 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology. 1996;128:256–264. doi: 10.1007/s002130050133. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH, Van Erp AMM, Blank AD, McInerney SC. d-Amphetamine “cue” generalizes to social defeat stress: sensitization and role of accumbens dopamine. Psychopharmacology. 1999a;147:190–199. doi: 10.1007/s002130051160. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina E, Kream RM, Carter G, Espejo EF. Behavioral sensitization to cocaine after a brief social defeat stress: c- fos expression in the PAG. Psychopharmacology. 1999b;141:225–234. doi: 10.1007/s002130050829. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., III Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington DC: National Academy Press; 1996. [Google Scholar]
- Nikulina EM, Covington HE, III, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Hammer RP, Jr, Miczek KA, Kream RM. Social defeat stress increases expression of mu-opioid receptor mRNA in rat ventral tegmental area. NeuroReport. 1999;10:3015–3019. doi: 10.1097/00001756-199909290-00026. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Marchand JE, Kream RM, Miczek KA. Behavioral sensitization to cocaine after a brief social stress is accompanied by changes in fos expression in the murine brainstem. Brain Res. 1998;810:200–210. doi: 10.1016/s0006-8993(98)00925-1. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Miczek KA, Hammer RP., Jr Prolonged effects of repeated social defeat stress on mRNA expression and function of mu-opioid receptors in the ventral tegmental area of rats. Neuropsychopharmacology. 2005;30:1096–1103. doi: 10.1038/sj.npp.1300658. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Stress- and pharmacologically- induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res. 1990;514:22–26. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- Quadros IM, Miczek KA. Two modes of intense cocaine bingeing: increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacology. 2009;206:109–121. doi: 10.1007/s00213-009-1584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey NF, van Ree JM. Emotional but not physical stress enhances intravenous cocaine self- administration in drug-naive rats. Brain Res. 1993;608:216–222. doi: 10.1016/0006-8993(93)91461-z. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Munn E. Polydrug self-administration in rats: Cocaine-heroin is more rewarding than cocaine alone. NeuroReport. 1998;9:2463–2466. doi: 10.1097/00001756-199808030-00007. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Wise RA. Intravenous self-administration of methamphetamine-heroin (speedball) combinations under a progressive-ratio schedule of reinforcement in rats. NeuroReport. 2000;11:2621–2623. doi: 10.1097/00001756-200008210-00003. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA. Heroin self-administration in rats under a progressive ratio schedule of reinforcement. Psychopharmacology. 1993;111:215–218. doi: 10.1007/BF02245526. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Marinelli M, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids .2. Sensitization of the increase in extracellular dopamine induced by cocaine depends on stress- induced corticosterone secretion. J Neurosci. 1995;15:7189–7195. doi: 10.1523/JNEUROSCI.15-11-07189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Wilcox KM, Woolverton WL. Self-administration of cocaine-heroin combinations by rhesus monkeys: antagonism by naltrexone. J Pharmacol Exp Ther. 1998;286:61–69. [PubMed] [Google Scholar]
- Rowlett JK, Woolverton WL. Self-administration of cocaine and heroin combinations by rhesus monkeys responding under a progressive-ratio schedule. Psychopharmacology. 1997;133:363–371. doi: 10.1007/s002130050415. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Exposure to mild stress enhances the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology. 1994;114:523–527. doi: 10.1007/BF02249346. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Smith JE, Co C, Coller MD, Hemby SE, Martin TJ. Self-administered heroin and cocaine combinations in the rat: additive reinforcing effects-supra-additive effects on nucleus accumbens extracellular dopamine. Neuropsychopharmacology. 2006;31:139–150. doi: 10.1038/sj.npp.1300786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohr T, Almeida OFX, Landgraf R, Shippenberg TS, Holsboer F, Spanagel R. Stress- and corticosteroid-induced modulation of the locomotor response to morphine in rats. Behav Brain Res. 1999;103:85–93. doi: 10.1016/s0166-4328(99)00027-3. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology. 1997;130:203–212. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Morgan D, Roberts DC. Comparison of the reinforcing effects of cocaine and cocaine/heroin combinations under progressive ratio and choice schedules in rats. Neuropsychopharmacology. 2005;30:286–295. doi: 10.1038/sj.npp.1300560. [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology. 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA. Social defeat stress, sensitization, and intravenous cocaine self-administration in mice. Psychopharmacology. 2007;192:261–273. doi: 10.1007/s00213-007-0712-4. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Takase LF, Kochman LJ, Fornal CA, Miczek KA, Jacobs BL. Repeated brief social defeat episodes in mice: Effects on cell proliferation in the dentate gyrus. Behav Brain Res. 2006;172:344–350. doi: 10.1016/j.bbr.2006.05.027. [DOI] [PubMed] [Google Scholar]