Abstract
In this review, we examine how experiences in social confrontations alter gene expression in mesocorticolimbic cells. The focus is on the target of attack and threat due to the prominent role of social defeat stress in the study of coping mechanisms and victimization. The initial operational definition of the socially defeated mouse by Ginsburg and Allee (1942) enabled the characterization of key endocrine, cardiovascular, and metabolic events during the initial response to an aggressive opponent and during the ensuing adaptations. Brief episodes of social defeat stress induce an augmented response to stimulant challenge as reflected by increased locomotion and increased extracellular dopamine (DA) in the nucleus accumbens (NAC). Cells in the ventral tegmental area (VTA) that project to the NAC were more active as indicated by increased expression of c-fos and Fos-immunoreactivity and BDNF. Intermittent episodes of social defeat stress result in increased mRNA for MOR in brainstem and limbic structures. These behavioral and neurobiological indices of sensitization persist for several months after the stress experience. The episodically defeated rats also self-administered intravenous cocaine during continuous access for 24 h (“binge”). By contrast, continuous social stress, particularly in the form of social subordination stress, leads to reduced appetite, compromised endocrine activities, and cardiovascular and metabolic abnormalities, and prefer sweets less as index of anhedonia. Cocaine challenges in subordinate rats result in a blunted psychomotor stimulant response and a reduced DA release in NAC. Subordinate rats self-administer cocaine less during continuous access conditions. These contrasting patterns of social stress result from continuous vs. intermittent exposure to social stress, suggesting divergent neuroadaptations for increased vulnerability to cocaine self-administration vs. deteriorated reward mechanisms characteristic of depressive-like profiles.
Keywords: Social stress, Defeat, Ventral tegmental area, Dorsal raphe, Amphetamine, Cocaine, Sensitization, Tolerance, Anhedonia
Introduction
Studies in molecular biology have begun to provide tools for studying how experiences in aggressive confrontations alter the activity of corticolimbic cells, via both genomic and non-genomic action (e.g., Caspi et al. 2002; Craig and Halton 2009; Day et al. 2011; Lesch and Merschdorf 2000; Taylor and Kim-Cohen 2007; Tecott and Barondes 1996). The link between a specific social experience and altered gene expression in the cells of discrete mesocorticolimbic pathways involves a cascade of events that ultimately leads to changes in translation and transcription (Joppa et al. 1995; Kollack-Walker et al. 1997; Martinez et al. 1998b, 2002; Nikulina et al. 1998; Young and Wang 2004). The study of social experience-dependent gene expression complements the traditional approaches of behavior genetics, namely to trace the genetic basis of a particular behavioral phenotype. While classic strategies have relied on comparisons of closely related animals such as strains of mice selectively bred for a specific phenotype such as high levels of aggressive behavior (“top-down genetics”; Ginsburg 1967), more recent efforts devoted themselves to single or double gene mutations of mice that engage in very high levels of aggressive behavior (“bottom-up genetics”). Given the polygenic basis of aggressive behavior patterns, it is not surprising that the latter approach has failed to find “mean genes” or to identify specific candidate genes (Ginsburg 1967; Hen 1996; Meyer-Lindenberg et al. 2006; Miczek et al. 2001).
Salient social experiences can readily alter gene expression. Attachment early in life, particularly in animal species with strong bonds such as the montane vole, is a prominent example of a social experience that—when disrupted by maternal separation distress—results in activation of transcriptional factors in discrete diencephalic cells (Pitkow et al. 2001; Winslow et al. 1993; Young et al. 1997). Some of these genes encode for the synthesis of opioid peptides, and manipulations of opioid receptors shortly after their discovery demonstrated the importance of these neuropeptides in social attachment in both avian and mammalian species (Panksepp et al. 1978). Similarly, sexual experiences are of remarkable salience and readily engender transcriptional activation in discrete brain areas such as the extended amygdala (Baum and Everitt 1992; Joppa et al. 1995; Kollack-Walker and Newman 1995; Ogawa et al. 1997; Parfitt and Newman 1998; Potegal et al. 1996). In the following account, the focus is on behavior during social conflict, particularly on the individual who is the target of attack and threat behavior by aggressive opponents. Social defeat stress in animals appears particularly relevant for the study of strategies for coping with stress and victimization in human and veterinary medicine (Björkqvist 2001; Koolhaas et al. 1999).
Social defeat stress during aggressive confrontations
One of the early experimental analyses of the socially defeated mouse was performed by Ginsburg and Allee (1942), who were also the first to describe the behavioral details: “The chased mouse rears on its hind legs, draws one fore-leg close to the body, extends the other stiffly, remains motionless, and squeals when touched by the other mouse.” Even in a pugnacious species such as mice (Mus musculus), intense aggressive episodes are relatively rare, but when they do occur, the physiological and behavioral consequences endure for weeks and months. From an evolutionary perspective, aggressive behavior is found in all phyla, subserving adaptive functions in the cohesion and dispersal of individuals, and this class of behaviors cannot be considered a behavioral aberration (Scott 1958). In socially cohesive species such as rats (Rattus norvegicus) that live in colonies, attacks and threats by dominant individuals are responded to by defensive and evasive behavior, including defensive attacks, defensive upright posture, and full flight (Barnett 1975; Blanchard and Blanchard 1977; Lore et al. 1984; Lucion et al. 1996; Miczek and de Boer 2005). Rats emit loud, frequent and long ultrasonic vocalizations in the 20–25 kHz range in response to the attacks and threats of an aggressive opponent (Thomas et al. 1982; van der Poel and Miczek 1991). When escape is not possible, the reaction to aggressive behavior consists primarily of crouch, defensive and submissive supine postures which comprise a passive coping strategy (Fig. 1; Koolhaas et al. 1998). These postures signal submission and decrease the probability of being attacked further.
Fig. 1.

The characteristic defeat posture by an intruder mouse that has been attacked by a resident. The submissive-supine posture by an intruder rat as displayed in reaction to an aggressive posture by a resident aggressive rat. (Mouse picture from Miczek et al. 1982)
When resources are sparse, the social organization of mice differs from that of rats, and consequently the pattern and function of aggressive behavior vary significantly (Berdoy and Drickamer 2007). Behaviorally, the most obvious difference is that resident mice patrol and mark the boundaries of a territory with limited resources (Crowcroft and Rowe 1963), whereas rats are a colonial species with a repertoire of threat and submission signals (vide infra). In both species, the initial reaction to aggressive behavior comprises retaliatory bites, eventually followed by retreat and escapes. Mice rarely display the supine posture, but when cornered, they assume the upright defeat posture with the head angled upward, ears retracted, frontpaws limp, and they fail to orient toward the approaching attacking opponent (Fig. 1; Ginsburg and Allee 1942; Miczek et al. 1982).
Stress physiology has established that responses to stressors are determined by critical parameters such as the frequency, intensity, and duration of the stressor. The characteristic inverted U-shaped curve for relating the stress response to the stress stimulus emerges (Miczek et al. 2008). The ascending limb of the curve depicts how mild, short and infrequent stress activates and mobilizes, whereas the descending limb of the curve represents how intense, long, frequent stress impairs and immobilizes (Sapolsky 1996). In addition to the critical parameters of intensity, frequency and duration, psychological work has highlighted predictability and controllability of stress as being of paramount significance (Maier and Seligman 1976). Even infrequent and low intensity stress can have significant ill effects, when it is unpredictable and uncontrollable (Weiss et al. 1976).
Subordination stress
Traditionally, experimental studies of social stress have focused on continuous subordination stress which in many animal species engenders a morbid course, particularly under laboratory conditions with limited opportunities for escape (Martinez et al. 1998a; Von Holst 1998). For example, even visual exposure to a dominant male treeshrew (Tupaia belangerii) is sufficient to precipitate severe physiological impairment in an intruding male leading to a deterioration of endocrine regulation and weight loss and eventually to death (Von Holst 1985). If breeding colonies of laboratory rats live in open spaces with localized food sources, it is necessary to rescue subordinate members in order to ensure their survival (Blanchard et al. 1985; McKittrick et al. 1995). A third of longhaired rats that were introduced into a small colony of Rattus villosissimus died as a result of the residents’ attacks, even though no major wounds were visible (Barnett et al. 1975). In colonies of mice with clumped food and water supplies, subordinate animals that have access to survival resources only opportunistically show increased adrenal epinephrine content and essential hypertension (Ely 1981; Henry and Stephens 1977; Hucklebridge et al. 1981). These subordinate animals do not die of their injuries; rather they are physiologically compromised to a degree that renders them incapable of adjusting to even moderate stresses and strains. Continuously subordinate animals are characterized by a severely constrained behavioral repertoire; they exhibit lower reproductive success, disturbed circadian physiological rhythms with prominent ultradian components, persistent endocrine activation that results in gonadal atrophy and adrenal hypertrophy, and suppressed immune function (Barnett et al. 1975; Brain 1972; Bronson 1973; Miczek et al. 1991; Raab et al. 1986; Sgoifo et al. 1996). Active and passive coping styles differentiate subordinate animals on a morbid course from those that do adapt successfully (Koolhaas et al. 1999).
Regulation of monoaminergic and peptidergic receptors has been studied in the brains of chronically subordinate rodents and primates (Albeck et al. 1997; Dhingra et al. 1997; Grant et al. 1998; McKittrick et al. 1995; Watanabe et al. 1995). Hippocampal regions of subordinate rats show significant reductions in the mRNA for mineralocorticoid and glucocorticoid receptors and lower 5-HT1A receptor density in comparison to non-stressed control rats. PET imaging studies indicate that in the basal ganglia of subordinate cynomologous monkeys the binding potential of a selective dopamine D2 ligand was reduced relative to that of dominants, suggesting a downregulation of D2 receptors (Grant et al. 1998). These subordination stress-induced long-term changes in peptidergic and aminergic receptors promise to serve as markers for individuals that differ in terms of vulnerability and resilience to behavioral and physiological impairments.
Intermittent episodes of social defeat stress
A different type of social stress is defined by the characteristic defeat response to attacks and threats during a brief aggressive confrontation. Under captive conditions such as in a laboratory setting where dispersal is only feasible in a limited way, the study of intermittent brief episodes of social defeat stress offers the opportunity to dissect the immediate responses to attacks and threats, the recovery period after the confrontation and also the enduring neuroadaptations that persist long after the brief social defeat episode has terminated without risk of morbidity.
The physiological costs of being defeated in a brief encounter consist of an initial massive adrenocortical and sympathetic activation that is followed by a parasympathetic rebound (e.g., Adams et al. 1969). The tachycardic, hypertensive, hyperthermic responses during the initial phase of an aggressive encounter are seen in both the resident attacker and the defeated intruder. However, these large deviations from their homeostatic set points are much longer lasting in defeated animals than in dominant animals. For example, normal heart rate and core temperature is established in a defeated intruder more than 3–4 h after the end of a 10-min confrontation with an attacking and threatening resident rat (Fig. 2; Tornatzky and Miczek 1993). Similarly, plasma corticosterone levels rise in both the resident attacker and the intruding defeated rat, but the elevated corticosterone levels take nearly 24 h to return to normal values in defeated rats, whereas dominant rats recover within a few hours (Covington III and Miczek 2005; Schuurman 1980). After brief episodes of social defeat, intruder rats show evidence of reduced antibody production, indicating immunosuppression (Fleshner et al. 1989; Stefanski 2001).
Fig. 2.
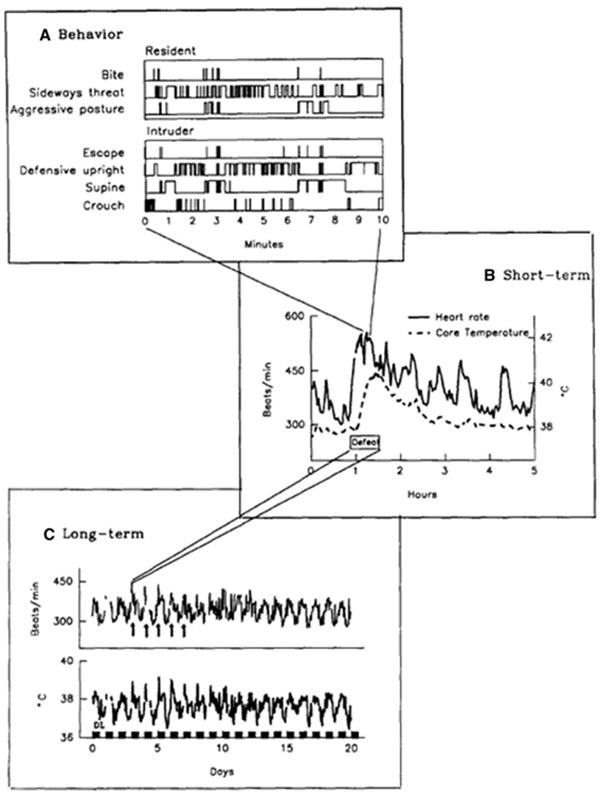
The time course of social behavior in resident and intruder rats during a confrontation, and the subsequent effect of a confrontation on an intruder’s circadian heart rate and core body temperature over subsequent days. a Agonistic behavior during the confrontation of a resident rat threatening and attacking an intruder. Top: Event records of bites, sideways threats and aggressive postures by a resident rat. Bottom: Event records of escapes, defensive upright postures, supine postures, and crouch by the intrude, identified as upward deflections from the time line (in minutes). b Short-term effects of being attacked and threatened on the intruder’s heart-rate and core body temperature before, during and after defeat (h) as obtained by telemetry. Defeat consisted of three phases: PRE = intruder in resident’s home cage, separated by an opaque divider for 10 min; DEF = physical interactions leading to defeat, maximally for 10 min or until the intruder has been attacked 20 times; POST = 10 min of intruder activity in the resident’s home cage after the defeat with the resident removed; thereafter, the intruder was placed back into his own home cage. Sampling period for heart rate and core body temperature was 20 s during the resident-intruder confrontation and 5 min in the intruder’s home cage. c Long-term effects of five consecutive defeats on the magnitude and rhythmicity of heart rate and core temperature as mean values per hour (sampling period: 5 min). Black horizontal bars denote dark periods; arrows point to the defeats (Adapted from Tornatzky and Miczek 1993)
A most remarkable feature of episodic social defeat stress is its persistent, long-lasting nature as evidenced by the lack of behavioral and physiological habituation upon repeated exposure (Covington III and Miczek 2005). Studies in intruder rats that confronted an aggressive resident opponent every 3–4 days over the course of several months show that telemetrically assessed tachycardic, hypertensive, and hyperthermic responses to social defeat stress remained consistent after many repeated social defeats. Actually, the hyperthermia, tachycardia and antinociception become readily conditioned so that the imminent demands of an aggressive confrontation are anticipated by rises in core temperature, heart rate and pain blockade prior to the actual confrontation (Meehan et al. 1995; Meerlo et al. 1996; Tornatzky and Miczek 1993; Tornatzky and Miczek 1994).
Neuroadaptations to social stress
Opioid tolerance
One of the immediate physiological adaptations during social defeat stress as a result of attacks by an aggressive opponent is an antinociceptive response (Miczek et al. 1982). When the defeated mouse shows evidence for a blockade of pain perception by not responding to nociceptive thermal or chemical stimuli, but remains reactive to other tactile stimuli, functionally selective centrifugal inhibition of nociception is evident. From an ethological perspective, the attacks by the aggressive opponent constitute the releasers that activate the passive coping response of defeat and narrow the sensory filters for nociceptive stimuli. From a pharmacological perspective, tolerance develops to the initial defeat-induced analgesia, and the duration and magnitude of this tolerance are systematically related to the amount of defeat (Miczek 1991; Miczek et al. 1986, 1999a, 2004). A second type of adaptation, termed behavioral and neural sensitization, develops to the psychomotor stimulant-activation after intermittent social defeat stress (Covington III et al. 2005; Covington III and Miczek 2001; Miczek et al. 1999b; Nikulina et al. 2004, 2008).
The discovery of opioid-mediated antinociception in defeated mice (Miczek et al. 1982) was confirmed in several strains of mice under a range of conditions by several laboratories (Rodgers and Hendrie 1983; Siegfried et al. 1984; Teskey et al. 1984). Initial pharmacological data implicated endogenous opioid peptides and their receptors in the mediation of the antinociceptive response in mice experiencing social defeat stress. Opioid receptor antagonists such as naloxone, naltrexone and β-chlornaltrexamine blocked the defeat stress-induced analgesia (Frischknecht and Siegfried 1989; Külling et al. 1988; Miczek et al. 1982, 1985; Rodgers and Randall 1985; Siegfried and Frischknecht 1989; Teskey et al. 1984). The quaternary forms of naloxone and naltrexone that do not readily penetrate the blood brain barrier failed to antagonize the defeat stress-induced analgesia, pointing to a site of action in the central nervous system (Miczek et al. 1982; Rodgers and Randall 1985). Microinjection studies demonstrated that naloxone acting on receptors in the periaqueductal grey area and in the region of the arcuate nucleus prevented the defeat stress-induced analgesia (Fig. 3; Miczek et al. 1985). In addition to the naloxone-reversible defeat stress-induced analgesia, a modest level of antinociception results from exposure to mild and short attacks by an opponent, and this type of antinociceptive response does not appear to be opioid-mediated since naloxone does not reverse this effect (Rodgers and Randall 1988).
Fig. 3.

Tail-flick latencies in response to a heat stimulus as a function of the number of attack bites received by intruder mice, and the specificity of this tolerance to analgesia by opiate receptors in the periaqueductal grey and arcuate nucleus. Left: The effect of saline or the opiate receptor antagonist naloxone (10 μg) injected into the periaquiductal grey, at a volume of 0.5 μl, on the latency to flick the tail from a heat stimulus. Center: The effect of saline or naloxone (10 μg in 0.5 μl) injected into the arcuate nucleus on the tail flick latency. Right: The effect of saline or naloxone (1 or 10 μg in 0.25 μl) injected into the periaqueductal grey. Asterisks indicate significant differences (p < 0.05) from vehicle injections (Adapted from Miczek et al. 1985)
Subsequent pharmacological evidence identified μ and δ opioid receptors as critical sites that were changed by the defeat stress experience. In acutely defeated male and female rats, morphine and selective μ and δ opioid receptor agonists such as DAMGO and DPDPE produced analgesic effects at much lower doses than in non-defeated control animals (Fig. 4; Haney and Miczek 1995; Vivian and Miczek 1998). When an intruder rat is defeated in a brief confrontation and then protected behind a protective screen while still being threatened by the aggressive opponent, the concurrent determination of the nociceptive response reveals that the dose–effect curves for the analgesic effects of μ and δ opioid receptor agonists are shifted to the left. This leftward shift indicates a potentiation of opioid analgesia during the actual exposure to the social stress, which can be interpreted to result from the combined action of the injected peptides in conjunction with the defeat stress-activated endogenous opioid peptides. The role of the μ opioid receptors in the action of this effect of defeat stress is further shown by the selective antagonism by naltrexone, but not by naltrindole, of stress-potentiated DAMGO analgesia (Vivian and Miczek 1998). After more frequent attacks, β-endorphin-like immunoreactivity decreased in the periaqueductal region of intruder mice (Külling et al. 1988).
Fig. 4.

The effects of morphine (top), DAMGO (middle), and DPDPE (bottom) on tail flick latencies in socially inexperienced (open circles) and socially defeated (filled circles) rats. Lines indicate first order regression equations for % M.P.E. for increasing doses of each drug. Asterisks indicate significant differences (p < 0.05) from vehicle injections (Adapted from Vivian and Miczek 1998)
One to seven days after the experience of a single defeat in rats, the morphine dose–effect curve for antinociceptive effects is shifted to the right, indicating the development of tolerance (Miczek et al. 1991). This shift to the right in the morphine analgesia dose–effect curve is larger in animals that have experienced several episodes of defeat experiences than after a single defeat. For example, starting 7 days after the last of five daily defeats, eight times more morphine is required to produce equivalent levels of antinociceptive effects than in non-defeated controls (Fig. 5; Miczek and Winslow 1987). Moreover, tolerance to opiate analgesia after multiple defeat experiences persists for weeks and months. The long-lasting tolerance after social defeat stress points to profound neural adaptations that may be based on genomic and non-genomic changes in the CNS and that promote synthesis and release of enkephalin and induce regulatory changes in the synthesis of receptor proteins. The available pharmacological data support both mechanisms, and direct measurements of the transcriptional and translational events for enkephalin and for the μ opioid receptor provide evidence how defeat experiences trigger gene expression.
Fig. 5.

The effect of morphine on tail flick latencies in mice, before (open circles) and after (solid circles) a single (left) or five (right) defeat experiences (Adapted from Miczek and Winslow 1987)
A single episode of social defeat stress induced a rapid and significant expression of μ-opioid receptor-encoding mRNA in the VTA, as evident by the larger number of labeled cells and the elevated amount of mRNA per cell starting 30 min after the social defeat stress (Nikulina et al. 1999). This increase in μ opioid receptor-encoding mRNA contrasted with the decrease in the expression of preproenkephalin mRNA in the periaqueductal gray matter (Fig. 6a, b; Nikulina et al. 1999). It is inviting to link the changes in the message for the μ opioid receptor and the precursor of enkephalin in the VTA and PAG after a single defeat in an aggressive confrontation to the ensuing tolerance in morphine analgesia (Miczek et al. 1991). Moreover, repeated exposure to social defeat stress upregulates μ-opioid receptor mRNA in the VTA for weeks after the last stress exposure (Fig. 7; Nikulina et al. 2005, 2008). μ-Opioid receptors within the VTA are mainly localized in non-dopamine interneurons containing GABA (Garzon and Pickel 2001; Johnson and North 1992), many of which make synaptic contact with dopamine neurons. Stress-induced enhancement of μ-opioid activity in the VTA also affects dopaminergic neurotransmission indirectly and the local stimulation of VTA μ-opioid receptors by DAMGO causes greater locomotor activity after social defeat stress exposure than after handling that suggest this elevated expression of μ-opioid receptor mRNA is translated into functional receptor proteins in the VTA (Nikulina et al. 2005). This selective regional alteration confers a molecular substrate that may underlie the impact of social defeat stress on the development of drug sensitization or other mesolimbic dopamine-related behaviors.
Fig. 6.

The number of Fos-Li positive cells in the VTA, PAG, dorsal raphe (DR) and locus coeruleus (LC) in mice, 1 h after an injection of saline or an experience of social defeat. Asterisks indicate significant differences (p < 0.01) from saline injection (Adapted from Nikulina et al. 1998)
Fig. 7.

The amount of preproenkephalin (a) and μ-opiate receptor (b) mRNA in the PAG as a function of time (hours) after a social defeat experience. Asterisks indicate significant differences (*p < 0.05, **p < 0.01) from control rats with no defeat experience
Brief social defeat stress, behavioral and neural sensitization associated with immediate early gene expression
During recent decades numerous studies have demonstrated how distinctive behavioral experiences engender the expression of immediate early genes. The examples begin early in development when differential maternal care leads to the expression of hippocampal glucocorticoid receptors in adulthood (Liu et al. 1997). Mating and aggressive experiences engender c-fos expression in discrete limbic and mesencephalic regions in female hamsters (Joppa et al. 1995). By now, considerable evidence documents how social defeat stress modulates the expression of immediate early genes in the core of the neuroaxis, most frequently studied in voles, mice, rats and hamsters (Cooper et al. 2009; Day et al. 2011; Fekete et al. 2009; Kirkpatrick et al. 1994; Kollack-Walker et al. 1999; Kollack-Walker and Newman 1995; Martinez et al. 2002; Nikulina et al. 1998; Vivian and Miczek 1998).
Compared to other types of stressors, such as restraint or immobilization, which on repeated exposure produce rapid habituation of neuronal activation measured by Fos induction (Chen and Herbert 1995; Melia et al. 1994; Umemoto et al. 1994), social defeat stress does not appear to produce habituation of Fos expression in many autonomic and monoaminergic brain structures. Based on group comparison data in rats and hamsters those experiencing social defeat stress once acutely versus those that are exposed repeatedly indicate that c-fos expression is attenuated in the region of the hippocampus and amygdala, particularly in the central amygdala, lateral septum, preoptic area, lateral hypothalamus, arcuate nucleus, supra-chiasmatic nucleus and the nucleus of the solitary tract (Martinez et al. 2002). By contrast, in repeatedly defeated mice c-fos expression persisted and was increased in forebrain and brainstem areas (Matsuda et al. 1996). Even in rats and hamsters, cellular activation as indicated by c-fos expression remains elevated after repeated social defeat stress in brain areas of the medial amygdala, anterior and ventromedial hypothalamus, the central grey area and the raphe nuclei.
Behavioral and neural sensitization to psychomotor stimulants
Sensitization is induced by repeated and intermittent exposure to a drug injection and is expressed by an augmented behavioral or neural response to a challenge by the same drug, often given at a lower dose (Robinson 2010). A most frequent example is the augmented locomotor response to an amphetamine challenge after intermittent treatments with amphetamine injections (Segal and Mandell 1974). The term “cross-sensitization” refers to an augmented behavioral response to a drug challenge produced by intermittent exposure to an agent other than the challenge drug. For example, repeated exposure to social defeat stress can induce an increased behavioral and neural response that continues to be expressed to a challenge with amphetamine or cocaine, and therefore, social defeat stress can be considered to induce cross-sensitization (Covington III and Miczek 2001; Miczek et al. 1999b). A single brief episode of social defeat stress during an aggressive confrontation is sufficient to sensitize a mouse (Miczek et al. 1999b; Nikulina et al. 1998) or a rat (de Jong et al. 2005) to the psychomotor activating effects when subsequently challenged with a psychomotor stimulant drug, but these effects are as enduring as the sensitizing effects of multiple defeats. The progressively augmented locomotor response to psychomotor stimulant drugs is long-lasting and is based on altered changes in corticolimbic circuits, involving dopamine cells (e.g., Kalivas and Stewart 1991; Vezina et al. 2002). A similar behavioral and neural augmentation of response to stimulant challenge is seen in animals that have experienced an environmental stressor such as tail pinch, electric foot shock or immobilization, and these represent examples of “cross-sensitization” (e.g., Antelman et al. 1980; Deroche et al. 1992; Herman et al. 1984; Sorg and Kalivas 1991).
A single episode of social defeat stress increases c-fos expression, an index of neural activity, in several brainstem nuclei as much as a high cocaine dose (Fig. 8; Nikulina et al. 1998). Most significantly, 1 week after the social defeat stress, evidence for cross-sensitization is apparent when the defeat-stressed animals are challenged with cocaine, both in terms of augmented hyperactivity and in terms of increased c-fos expression in the periaqueductal grey area, the locus coeruleus, and the raphe nuclei. However, social defeat stress prevents cocaine-mediated Fos induction in the raphe, PAG and LC, when both occur concurrently. As time elapses after the social defeat stress, cocaine begins to activate c-fos expression again, peaking at 1 week after the stress, coinciding with the peak of behavioral sensitization (Nikulina et al. 1998).
Fig. 8.

The effect of an acute cocaine (40 mg/kg) challenge in the absence of social stress (white bars) or immediately after social defeat (gray bars), on the number of Fos-Li positive cells in the ventral tegmental area, periaqueductal grey, dorsal raphe and locus coeruleus 1 h after injection (Adapted from Nikulina et al. 1998)
In fact, the time courses of sensitization to cocaine and cross-sensitization to social defeat stress parallel each other closely. Immediately, after an intruder mouse experiences a single social defeat stress, the characteristic psychomotor stimulating effects of cocaine are inhibited. However, when challenged 3 days later, cocaine engenders a typical hyperactivity, and by the fifth and seventh days after the social defeat stress, an augmented hyperactivity emerges (Fig. 9; Miczek et al. 1999b). This latter response pattern constitutes evidence for behavioral sensitization and shows some parallels to the cellular activation in the brainstem nuclei of the monoaminergic systems.
Fig. 9.

Left: The effects of 40 mg/kg cocaine (Coc) or saline (Sal) on motor activity in non-defeated mice. Right: the effects of cocaine on motor activity in separate groups of mice that were socially defeated either during the 20 min injection-test interval, or 3, 5, 7 or 9 days earlier (n = 15/group, filled circle). In an additional group of mice, the effects of saline, administered before the social defeat stress, on motor activity are shown (open circle). The data are portrayed as medians; the vertical lines depict the interquartile ranges. The behavioral measurements consisted of (a) walking frequency based on direct behavioral observation of video records, (b) distance traveled measured by a video tracking system, and (c) total counts recorded by a photocell apparatus. *p < 0.05 for the comparisons that are indicated by the brackets, using non-parametric randomization tests. A regression line was fitted in order to aid in visualizing the time course of the effects of a cocaine challenge at different times (Adapted from Miczek et al. 1999b)
A most significant facet of sensitization and cross-sensitization is its enduring nature. In rats, when episodes of social defeat stress occur repeatedly the physiological, behavioral and neural indices of sensitization in response to stimulant challenge persist for months after the conclusion of the actual stress experiences. Such a long-lasting impact of salient stress exposures is characteristic of experiences during a critical developmental period in the postnatal infant (Liu et al. 1997), but is rarely seen in mature animals. When rats are exposed to social defeat stress on four occasions over the course of 10 days, their hyperactive response to a challenge with cocaine or amphetamine remains at a sensitized level for at least 2 months (Fig. 10; Covington III et al. 2005). This persistent cross-sensitization between repeated social defeat stress and stimulant hyperactivity appears to be based on activity in a neural circuit that involves the ventral tegmental area, the nucleus accumbens and nuclei within the amygdaloid complex as well as prefrontal cortical areas (Miczek et al. 2011).
Fig. 10.

The effect of stimulant challenge on horizontal locomotor activity for defeat stressed rats (gray bars) and unstressed controls (open bars). Baseline measurements of locomotor activity were obtained after a saline injection before the first stress exposure. Stressed rats experienced episodes of social defeat once every 72 h over 10 days (1, 4, 7, and 10). One cohort was challenged with cocaine (10.0 mg/kg) on day 20 (top) for the expression of sensitization. Two separate cohorts were challenged with amphetamine (1.0 mg/kg) on days 20, 40, 60 and 70 (middle), or only on day 70 (bottom). Bars (±SEM) represent average walking frequencies over 5–10 and 25–30 min post-injection for each group. *Indicate that groups of stressed and corresponding unstressed control rats were significantly different from each other for that day (p < 0.05) (Adapted from Covington III et al. 2005)
Using immunohistochemistry for Fos-like proteins, representing the Fos-related antigens, 10 days after the last of four defeat stress episodes significantly more Fos-like immunoreactive cells were labeled in the prelimbic and infralimbic cortical regions, the shell and core of the nucleus accumbens septi, the medial, central and basolateral amygdala, and the ventral tegmental area in intruder rats (Nikulina et al. 2004). Increased stress-induced Fos-like immunoreactive labeling in the medial amygdala was seen after challenge with a moderate amphetamine dose, providing evidence for neural sensitization. Even 70 days later, an amphetamine challenge produced sensitized Fos-like immunoreactive labeling in the ventral tegmental area and central amygdala (Fig. 11). Using zif268 immediate early gene expression as an indicator of functional activation (Covington III et al. 2005), the cells in the central amygdala were significantly activated in rats that had experienced social defeat stress 2 months earlier. By contrast, zif268 expression in the prefrontal cortex was suppressed in response to amphetamine challenge 2 months after social defeat stress. Animals showed a sensitized hyperactivity in response to amphetamine challenge that coincided with the cellular changes in the central amygdala and prefrontal cortex. Moreover, in a parallel group of animals that had experienced repeated social defeat stress, prolonged and more intense intravenous cocaine self-administration persisted during continuous access for 24 h (Fig. 12). The enduring changes in cellular activity in the central amygdala may in fact be critical for such intense cocaine self-administration in animals that are sensitized by social defeat stress.
Fig. 11.

The effect of social defeat stress on Fos-LI labeling in the ventral tegmental area (a) and the central amygdala (b), 2 h after a saline or amphetamine (1 mg/kg) challenge, 17 and 70 days after initiating social defeat stress exposures. Bars indicate the mean ± SEM. *indicate significant differences (p < 0.05) (Adapted from (Nikulina et al. 2004)
Fig. 12.

Top: Cocaine intake expressed as mg/kg/h for stress-sensitized and unstressed control rats. Stress-sensitized rats (filled circles) self-administered cocaine for 24 h effectively abolishing the circadian pattern of intake revealed by control rats (open circles) (p < 0.01). Motor activity for each hour of the binge is indicated by the distance (cm) traveled (middle), and the frequency of focused stereotypies (bottom). Data for each curve were fitted with a Weibull equation (Adapted from Covington III et al. 2005)
Behavioral and neural sensitization after intermittent repeated social defeat stress, particularly in mesocorticolimbic structures, is relevant to several stress disorders, most prominently in the pathogenesis of psychosis, affective disorders and drug abuse (e.g., Kreek and Koob 1998; Post et al. 1987). Vulnerability and resilience to salient stress experiences such as defeat in an aggressive confrontation may be based on translational and transcriptional changes in the projection neurons from parts of the amygdala to the prefrontal cortex, which in turn modulates accumbal dopamine neurons.
Morphine
A striking feature of repeated exposure to social defeat stress is the concurrent development of two divergent types of neuroadaptation in endogenous opioid peptides and their receptors, namely tolerance and sensitization. As discussed above, repeated defeat experiences result in an incrementally reduced antinociceptive response suggesting the development of tolerance. Social defeat stress-induced activation of μ-opioid receptor function in the ventral tegmental area –nucleus accumbens pathway could be a substrate for cross-sensitization to psychostimulant drugs following repeated social defeat stress exposure. It was shown that prolonged amphetamine-induced behavioral sensitization is accompanied by elevated μ-opioid, but not δ-opioid receptor mRNA expression in the VTA (Magendzo and Bustos 2003) This observation is consistent with our suggestion that increased VTA μ-opioid receptor mRNA expression predisposes animals to a sensitized response to a psychostimulant challenge (Covington III and Miczek 2001; Nikulina et al. 1999). Social defeat stress effectively induced conditioned place preference reinstatement to morphine after reliable extinction in mice which suggests an essential role of defeat stress-induced relapse to opiates (Ribeiro Do Couto et al. 2006).
Aggressive experiences and gene expression
The acute experience of offensive aggressive behavior may also modify gene expression, similar to the more frequently studied defensive, submissive and defeat experiences. The transient state changes resulting from aggressive experiences differ from the long-known trait characteristics of aggressive animals, and it is useful to consider these state changes to be superimposed on the aggressive traits. Classic genetic strategies provide evidence for genetic influences on aggressive traits, chiefly based on selective breeding studies, strain comparisons, and quantitative trait locus (QTL) analysis (e.g., Brodkin et al. 2002; Dow et al. 2011; Maxson 1996; Miczek et al. 2001; Nehrenberg et al. 2010; Roubertoux et al. 2005).
Immediate early gene expression in brain tissue from intruder animals can be precisely related to the specific time and magnitude of the defeat stress. By contrast, the first appearance of intense aggressive behavior in the developmental trajectory of an individual remains uncertain, even under controlled laboratory conditions. One strategy is to study the cellular activation in rodents with a specific background of high or low aggressive behavior, and then examine c-fos expression after an aggressive encounter. In resident hamsters that attack an intruder in their home cage more Fos-immunoreactive neurons were found in the dorsal periaqueductal grey area, dorsal central grey, the anterior hypothalamus, medial periventricular nucleus, the bed nucleus of the stria terminalis and the medial amygdala (Delville et al. 2000; Kollack-Walker et al. 1997; Kollack-Walker and Newman 1995). Activation of cells in these nuclei after the display of attack behavior contrasts with the neural activation after mating bouts (Kollack-Walker and Newman 1995). When the hamster has been instigated by a brief confrontation with an opponent (i.e. “attack-priming”), it engages in very rapid and intense fighting during a subsequent encounter and shows an increased number of Fos-immunoreactive neurons in the corticomedial amygdala (Potegal et al. 1996). Similarly, in resident singly housed Wistar rats that have attacked an intruder, large increases in Fos labeling were seen in the medial amygdala, the lateral hypothalamus and the periaqueductual grey area, and this response pattern was intensified by glucocorticoid hypofunction (Halasz et al. 2002). After having initiated intense aggressive behavior rapidly and executed aggressive acts frequently, serotonergic neurons in the dorsal raphe of highly aggressive ferally derived rats were more active than in low aggressive or control rats (Van Der Vegt et al. 2003). Defeated mice show greater Fos protein levels in the DRN and periaqueductal grey area after a single social defeat when compared to control animals (Nikulina et al. 1998).
These initial studies with immediate early gene expression after the display of offensive aggressive behavior in rodents complement earlier information with electrical and chemical lesion and stimulation techniques to delineate critical elements of neural circuits. These studies provide evidence for a circuit that consists of medial and central amygdala—stria terminalis—bed nucleus of the stria terminalis and lateral septum—diagonal band of Broca—anterior and lateral hypothalamus—periaqueductal grey and raphe nuclei (Fig. 13). A further important microcircuit for neural activity mediating offensive aggressive behavior consists of the ascending monoaminergic neurons from tegmental and raphe nuclei to corticolimbic terminals, as illustrated by in vivo microdialysis studies (Ferrari et al. 2003; Takahashi et al. 2010; Van Erp and Miczek 2000). As with repeated defeat experiences, it is likely that repeated episodes of offensive aggressive behavior may engender enduring changes in cellular activity, possibly even changes in neural morphology (Amorim and Almada 2005; Coates and Herbert 2008; Fuxjager et al. 2010; Hsu et al. 2006; Oliveira et al. 2009; Oyegbile and Marler 2005).
Fig. 13.

Circuit diagram of neural structures and connecting pathways, based on cellular activation after social defeat stress (From Miczek et al. 2008)
Among the unresolved questions is the extensive anatomical overlap between patterns of c-fos expression in cells that subserve offensive aggressive and defeat experiences. An important task will be to characterize the cells that express c-fos neurochemically more adequately, such as by labeling additional gene products (Van Der Vegt et al. 2003). A further strategy will be to go beyond Fos labeling and track entire arrays of genes that may have been induced or suppressed by aggressive and defeat experiences.
Acknowledgments
The authors would like to thank Mr. J. Thomas Sopko for his exceptional technical assistance. Preparation of this review and the original research from our own laboratory were supported by USPHS research grants AA05122, DA02632, DA026451 (EMN) and grants from the Alcoholic Beverage Medical Research Foundation (KAM, PI).
Footnotes
The paper originated in a festschrift, Nurturing the Genome, to honor Benson E. Ginsburg on June 2, 2002 and is part of a special issue of Behavior Genetics based on that festschrift.
Contributor Information
Klaus A. Miczek, Email: klaus.miczek@tufts.edu, Department of Psychology, Tufts University, 530 Boston Ave. (Bacon Hall), Medford, MA 02155, USA; Department of Psychiatry, Tufts University School of Medicine, Boston, MA, USA; Departments of Pharmacology and Neuroscience, Tufts University, Boston, MA, USA.
Ella M. Nikulina, Department of Psychiatry, Tufts University School of Medicine, Boston, MA, USA Department of Basic Medical Sciences, University of Arizona College of Medicine, Phoenix, AZ, USA.
Aki Takahashi, Mouse Genomics Resource Laboratory, National Institute of Genetics, Mishima, Shizuoka, Japan.
Herbert E. Covington, III, Departments of Psychology and Neuroscience, Duke University, Durham, NC, USA.
Jasmine J. Yap, Center for Neuroscience, University of Colorado, Boulder, CO, USA
Christopher O. Boyson, Department of Psychology, Tufts University, 530 Boston Ave. (Bacon Hall), Medford, MA 02155, USA
Akiko Shimamoto, Department of Psychology, Tufts University, 530 Boston Ave. (Bacon Hall), Medford, MA 02155, USA.
Rosa M. M. de Almeida, Instituto de Psicologia, UFRGS, Porto Alegre, RS, Brazil
References
- Adams DB, Baccelli G, Mancia G, Zanchetti A. Cardiovascular changes during naturally elicited fighting behavior in the cat. Am J Physiol. 1969;216:1226–1235. doi: 10.1152/ajplegacy.1969.216.5.1226. [DOI] [PubMed] [Google Scholar]
- Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, Sakai RR. Chronic Social Stress Alters Levels of Corticotropin-Releasing Factor and Arginine Vasopressin mRNA in Rat Brain. J Neurosci. 1997;17:4895–4903. doi: 10.1523/JNEUROSCI.17-12-04895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim MC, Almada VC. The outcome of male-male encounters affects subsequent sound production during courtship in the cichlid fish Oreochromis mossambicus. Anim Behav. 2005;69:595–601. [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Barnett SA. A Study in Behavior. University of Chicago Press; Chicago: 1975. The Rat. [Google Scholar]
- Barnett SA, Hocking WE, Munro KMH, Walker KZ. Socially induced renal pathology of captive wild rats. Aggress Behav. 1975;1:123–133. [Google Scholar]
- Baum MJ, Everitt BJ. Increased expression of C-Fos in the medial preoptic area after Mating in male-rats—role of afferent inputs from the Medial Amygdala and Midbrain Central Tegmental Field. Neuroscience. 1992;50:627–646. doi: 10.1016/0306-4522(92)90452-8. [DOI] [PubMed] [Google Scholar]
- Berdoy M, Drickamer LC. Comparative social organization and life history of Rattus and Mus. In: Wolff JO, Sherman PW, editors. Rodent societies: an ecological and evolutionary perspective. University of Chicago Press; Chciago: 2007. pp. 380–392. [Google Scholar]
- Björkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard CD. Aggressive behavior in the rat. Behav Biol. 1977;21:197–224. doi: 10.1016/s0091-6773(77)90308-x. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Flannelly KJ. Social stress, mortality and aggression in colonies and burrowing habitats. Behav Process. 1985;11:209–213. doi: 10.1016/0376-6357(85)90062-2. [DOI] [PubMed] [Google Scholar]
- Brain PF. Endocrine and behavioral differences between dominant and subordinate male house mice housed in pairs. Psychonom Sci. 1972;28:260–262. [Google Scholar]
- Brodkin ES, Goforth SA, Keene AH, Fossella JA, Silver LM. Identification of quantitative trait Loci that affect aggressive behavior in mice. J Neurosci. 2002;22:1165–1170. doi: 10.1523/JNEUROSCI.22-03-01165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson FH. Establishment of social rank among grouped male mice: Relative effects on circulating FSH, LH, and corticosterone. Physiol Behav. 1973;10:947–951. doi: 10.1016/0031-9384(73)90065-6. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Chen X, Herbert J. Regional changes in c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlation with cardiovascular, hypothermic and endocrine responses. Neuroscience. 1995;64:675–685. doi: 10.1016/0306-4522(94)00532-a. [DOI] [PubMed] [Google Scholar]
- Coates JM, Herbert J. Endogenous steroids and financial risk taking on a London trading floor. Proc Natl Acad Sci USA. 2008;105:6167–6172. doi: 10.1073/pnas.0704025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Grober MS, Nicholas CR, Huhman KL. Aggressive encounters alter the activation of serotonergic neurons and the expression of 5-HT1A mRNA in the hamster dorsal raphe nucleus. Neuroscience. 2009;161:680–690. doi: 10.1016/j.neuroscience.2009.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology. 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology. 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Craig IW, Halton KE. Genetics of human aggressive behaviour. Hum Genet. 2009;126:101–113. doi: 10.1007/s00439-009-0695-9. [DOI] [PubMed] [Google Scholar]
- Crowcroft P, Rowe FP. Social organization and territorial behaviour in the wild house mouse. Proc Zool Soc London. 1963;140:517–531. [Google Scholar]
- Day DE, Cooper MA, Markham CM, Huhman KL. NR2B subunit of the NMDA receptor in the basolateral amygdala is necessary for the acquisition of conditioned defeat in Syrian hamsters. Behav Brain Res. 2011;217:55–59. doi: 10.1016/j.bbr.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JG, Wasilewski M, Van Der Vegt BJ, Buwalda B, Koolhaas JM. A single social defeat induces short-lasting behavioral sensitization to amphetamine. Physiol Behav. 2005;83:805–811. doi: 10.1016/j.physbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evolution. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Maccari S, Le Moal M, Simon H. Stress-induced sensitization to amphetamine and morphine psychomotor effects depend on stress-induced corticosterone secretion. Brain Res. 1992;598:343–348. doi: 10.1016/0006-8993(92)90205-n. [DOI] [PubMed] [Google Scholar]
- Dhingra NK, Raju TR, Meti BL. Selective reduction of monoamine oxidase A and B in the frontal cortex of subordinate rats. Brain Res. 1997;758:237–240. doi: 10.1016/s0006-8993(96)01477-1. [DOI] [PubMed] [Google Scholar]
- Dow HC, Kreibich AS, Kaercher KA, Sankoorikal GM, Pauley ED, Lohoff FW, Ferraro TN, Li H, Brodkin ES. Genetic dissection of intermale aggressive behavior in BALB/cJ and A/J mice. Genes Brain Behav. 2011;10:57–68. doi: 10.1111/j.1601-183X.2010.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely DL. Hypertension, social rank, and aortic arteriosclerosis in CBA/J mice. Physiol Behav. 1981;26:655–661. doi: 10.1016/0031-9384(81)90140-2. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zhao Y, Li C, Sabino V, Vale WW, Zorrilla EP. Social defeat stress activates medial amygdala cells that express type 2 corticotropin-releasing factor receptor mRNA. Neuroscience. 2009;162:5–13. doi: 10.1016/j.neuroscience.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Van Erp AMM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Laudenslager ML, Simons L, Maier SF. Reduced serum antibodies associated with social defeat in rats. Physiol Behav. 1989;45:1183–1187. doi: 10.1016/0031-9384(89)90107-8. [DOI] [PubMed] [Google Scholar]
- Frischknecht HR, Siegfried B. Relationship between behavioral and nociceptive changes in attacked mice: effects of opiate antagonists. Psychopharmacology. 1989;97:160–162. doi: 10.1007/BF00442241. [DOI] [PubMed] [Google Scholar]
- Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP, Marler CA. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc Natl Acad Sci USA. 2010;107:12393–12398. doi: 10.1073/pnas.1001394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon M, Pickel VM. Plasmalemmal μ-opioid receptor distribution mainly in nondopaminergic neurons in the rat ventral tegmental area. Synapse. 2001;41:311–328. doi: 10.1002/syn.1088. [DOI] [PubMed] [Google Scholar]
- Ginsburg BE. Genetic parameters in behavioral research. In: Hirsch J, editor. Behavior-genetic analysis. McGraw-Hill; New York: 1967. pp. 135–153. [Google Scholar]
- Ginsburg B, Allee WC. Some effects of conditioning on social dominance and subordination in inbred strains of mice. Physiol Zool. 1942;15:485–506. [Google Scholar]
- Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29:80–83. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Halasz J, Liposits Z, Kruk MR, Haller J. Neural background of glucocorticoid dysfunction-induced abnormal aggression in rats: involvement of fear- and stress- related structures. Eur J Neurosci. 2002;15:561–569. doi: 10.1046/j.0953-816x.2001.01883.x. [DOI] [PubMed] [Google Scholar]
- Haney M, Miczek KA. Delta opioid receptors: reflexive, defensive and vocal affective responses in female rats. Psychopharmacology. 1995;121:204–212. doi: 10.1007/BF02245631. [DOI] [PubMed] [Google Scholar]
- Hen R. Mean genes. Neuron. 1996;16:17–21. doi: 10.1016/s0896-6273(00)80019-7. [DOI] [PubMed] [Google Scholar]
- Henry JP, Stephens PM. The social environment and essential hypertension in mice: possible role of inervation of the adrenal cortex. In: de Jong W, Provoost AP, Shapiro AP, editors. Progress in brain research: hypertension and brain mechanisms. Vol. 47. Elsevier; New York: 1977. pp. 263–273. [DOI] [PubMed] [Google Scholar]
- Herman JP, Stinus L, Le Moal M. Repeated stress increases locomotor response to amphetamine. Psychopharmacology. 1984;84:431–435. doi: 10.1007/BF00555227. [DOI] [PubMed] [Google Scholar]
- Hsu Y, Earley RL, Wolf LL. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol Rev Camb Philos Soc. 2006;81:33–74. doi: 10.1017/S146479310500686X. [DOI] [PubMed] [Google Scholar]
- Hucklebridge FH, Gamal-El-Din L, Brain PF. Social status and the adrenal medulla in the house mouse (Mus musculus, L.) Behav Neural Biol. 1981;33:345–363. [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joppa MA, Meisel RL, Garber MA. c-Fos expression in female hamster brain following sexual and aggressive behaviors. Neuroscience. 1995;68:783–792. doi: 10.1016/0306-4522(95)00179-m. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Kim JW, Insel TR. Limbic system fos expression associated with paternal behavior. Brain Res. 1994;658:112–118. doi: 10.1016/s0006-8993(09)90016-6. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: Defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollack-Walker S, Don C, Watson SJ, Akil H. Differential expression of c-fos mRNA within neurocircuits of male hamsters exposed to acute or chronic defeat. J Neuroendocrinol. 1999;11:547–559. doi: 10.1046/j.1365-2826.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Everts H, de Ruiter AJH, de Boer SF, Bohus B. Coping with stress in rats and mice: Differential peptidergic modulation of the amygdala-lateral septum complex. Prog Brain Res. 1998;119:437–448. doi: 10.1016/s0079-6123(08)61586-1. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, de Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: Stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Külling P, Frischknecht HR, Pasi A, Waser PG, Siegfried B. Social conflict-induced changes in nociception and beta-endorphin-like immunoreactivity in pituitary and discrete brain areas of C57BL/6 and DBA/2 mice. Brain Res. 1988;450:237–246. doi: 10.1016/0006-8993(88)91563-6. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Merschdorf U. Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behav Sci Law. 2000;18:581–604. doi: 10.1002/1099-0798(200010)18:5<581::aid-bsl411>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lore R, Nikoletseas M, Takahashi L. Colony aggression in laboratory rats: a review and some recommendations. Aggress Behav. 1984;10:59–71. [Google Scholar]
- Lucion AB, de Almeida RMM, DaSilva RSM. Territorial aggression, body weight, carbohydrate metabolism and testosterone levels of wild rats maintained in laboratory colonies. Braz J Med Biol Res. 1996;29:1657–1662. [PubMed] [Google Scholar]
- Magendzo K, Bustos G. Expression of amphetamine-induced behavioral sensitization after short- and long-term withdrawal periods: participation of mu- and delta-opioid receptors. Neuropsychopharmacology. 2003;28:468–477. doi: 10.1038/sj.npp.1300063. [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman MEP. Learned helplessness—theory and evidence. J Exp Psychol Gen. 1976;105:3–46. [Google Scholar]
- Martinez M, Calvo-Torrent A, Pico-Alfonso MA. Social defeat and subordination as models of social stress in laboratory rodents: A review. Aggress Behav. 1998a;24:241–256. [Google Scholar]
- Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 1998b;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress. 2002;5:3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Peng H, Yoshimura H, Wen TC, Fukuda T, Sakanaka M. Persistent c-fos expression in the brains of mice with chronic social stress. Neurosci Res. 1996;26:157–170. [PubMed] [Google Scholar]
- Maxson SC. Searching for candidate genes with effects on an agonistic behavior, offense, in mice. Behav Genet. 1996;26:471–475. doi: 10.1007/BF02359751. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biol Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- Meehan WP, Tornatzky W, Miczek KA. Blood pressure via telemetry during social confrontations in rats: Effects of clonidine. Physiol Behav. 1995;58:81–88. doi: 10.1016/0031-9384(95)00036-i. [DOI] [PubMed] [Google Scholar]
- Meerlo P, de Boer SF, Koolhaas JM, Daan S, van den Hoofdakker RH. Changes in daily rhythms of body temperature and activity after a single social defeat in rats. Physiol Behav. 1996;59:735–739. doi: 10.1016/0031-9384(95)02182-5. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 1994;14:5929–5938. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Nat Acad Sci U S A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA. Tolerance to the analgesic, but not discriminative stimulus effects of morphine after brief social defeat in rats. Psychopharmacology. 1991;104:181–186. doi: 10.1007/BF02244176. [DOI] [PubMed] [Google Scholar]
- Miczek KA, de Boer SF. Aggressive, defensive, and submissive behavior. In: Whishaw IQ, Kolb B, editors. The Behavior of the Laboratory Rat: A Handbook with Tests. Oxford University Press; New York: 2005. pp. 344–352. [Google Scholar]
- Miczek KA, Winslow JT. Psychopharmacological research on aggressive behavior. In: Greenshaw AJ, Dourish CT, editors. Experimental psychopharmacology: concepts and methods. Humana Press; Clifton: 1987. pp. 27–113. [Google Scholar]
- Miczek KA, Thompson ML, Shuster L. Opioid-like analgesia in defeated mice. Science. 1982;215:1520–1522. doi: 10.1126/science.7199758. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Shuster L. Naloxone injections into periaqueductal grey area and arcuate nucleus block analgesia in defeated mice. Psychopharmacology. 1985;87:39–42. doi: 10.1007/BF00431775. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Shuster L. Analgesia following defeat in an aggressive encounter: development of tolerance and changes in opioid receptors. In: Kelly DD, editor. Ann N Y Acad Sci. New York: 1986. pp. 14–29. Stress-induced Analgesia. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Tornatzky W. Subordinate animals: behavioral and physiological adaptations and opioid tolerance. In: Brown MR, editor. Stress: neurobiology and neuroendocrinology. Marcel Dekker; New York: 1991. pp. 323–357. [Google Scholar]
- Miczek KA, Mutschler NH, Van Erp AMM, Blank AD, McInerney SC. d-Amphetamine “cue” generalizes to social defeat stress: sensitization and role of accumbens dopamine. Psychopharmacology. 1999a;147:190–199. doi: 10.1007/s002130051160. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina E, Kream RM, Carter G, Espejo EF. Behavioral sensitization to cocaine after a brief social defeat stress: c-fos expression in the PAG. Psychopharmacology. 1999b;141:225–234. doi: 10.1007/s002130050829. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Covington HE, Nikulina EM, Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., III Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE., III Escalated or suppressed cocaine reward, tegmental BDNF and accumbal dopamine due to episodic vs. continuous social stress in rats. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.0637-11.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehrenberg DL, Wang S, Buus RJ, Perkins J, de Villena FP, Pomp D. Genomic mapping of social behavior traits in a F2 cross derived from mice selectively bred for high aggression. BMC Genet. 2010;11:113. doi: 10.1186/1471-2156-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina EM, Marchand JE, Kream RM, Miczek KA. Behavioral sensitization to cocaine after a brief social stress is accompanied by changes in fos expression in the murine brainstem. Brain Res. 1998;810:200–210. doi: 10.1016/s0006-8993(98)00925-1. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Hammer RP, Jr, Miczek KA, Kream RM. Social defeat stress increases expression of mu-opioid receptor mRNA in rat ventral tegmental area. NeuroReport. 1999;10:3015–3019. doi: 10.1097/00001756-199909290-00026. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, III, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Miczek KA, Hammer RP., Jr Prolonged effects of repeated social defeat stress on mRNA expression and function of mu-opioid receptors in the ventral tegmental area of rats. Neuropsychopharmacology. 2005;30:1096–1103. doi: 10.1038/sj.npp.1300658. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Arrillaga-Romany I, Miczek KA, Hammer RP., Jr Long-lasting alteration in mesocorticolimbic structures after repeated social defeat stress in rats: time course of Δ-opioid receptor mRNA and FosB/ΔFosB immunoreactivity. Eur J Neurosci. 2008;27:2272–2284. doi: 10.1111/j.1460-9568.2008.06176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lubahn DB, Korach KS, Pfaff EW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RF, Silva A, Canario AV. Why do winners keep winning? Androgen mediation of winner but not loser effects in cichlid fish. Proc Biol Sci. 2009;276:2249–2256. doi: 10.1098/rspb.2009.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyegbile TO, Marler CA. Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm Behav. 2005;48:259–267. doi: 10.1016/j.yhbeh.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman B, Conner R, Bishop P, Scott JP. The biology of social attachments: opiates alleviate separation distress. Biol Psychiatry. 1978;13:607–618. [PubMed] [Google Scholar]
- Parfitt DB, Newman SW. Fos-immunoreactivity within the extended amygdala is correlated with the onset of sexual satiety. Horm Behav. 1998;34:17–29. doi: 10.1006/hbeh.1998.1459. [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM, Weiss SR, Pert A, Uhde TW. Chronic cocaine administration: sensitization and kindling effects. In: Fisher S, Raskin A, Uhlenhuth EH, editors. Cocaine: Clinical and Biobehavioral Aspects. Oxford University Press; New York: 1987. pp. 109–173. [Google Scholar]
- Potegal M, Ferris CF, Hebert M, Meyerhoff J, Skaredoff L. Attack priming in female Syrian golden hamsters is associated with a c-fos-coupled process within the corticomedial amygdala. Neuroscience. 1996;75:869–880. doi: 10.1016/0306-4522(96)00236-9. [DOI] [PubMed] [Google Scholar]
- Raab A, Dantzer R, MIchaud B, Mormede P, Taghzouti K, Simon H, Lemoal M. Behavioural, physiological and immunological consequences of social status and aggression in chronically coexisting resident-intruder dyads of male rats. Physiol Behav. 1986;36:223–228. doi: 10.1016/0031-9384(86)90007-7. [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodríguez-Arias M, Armario A, Miñarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology. 2006;185:459–470. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- Robinson TE. Sensitization to drugs. In: Stolerman IP, editor. Encyclopedia of psychopharmacology. Springer; Heidelberg: 2010. [Google Scholar]
- Rodgers RJ, Hendrie CA. Social conflict activates status-dependent endogenous analgesic or hyperalgesic mechanisms in male mice: effects of naloxone on nociception and behaviour. Physiol Behav. 1983;30:775–780. doi: 10.1016/0031-9384(83)90177-4. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Randall JI. Social conflict analgesia: Studies on naloxone antagonism and morphine cross-tolerance in male DBA/2 mice. Pharmacol Biochem Behav. 1985;23:883–887. doi: 10.1016/0091-3057(85)90087-5. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Randall JI. Blockade of non-opioid analgesia in intruder mice by selective neuronal and non-neuronal benzodiazepine recognition site ligands. Psychopharmacology. 1988;96:45–54. doi: 10.1007/BF02431532. [DOI] [PubMed] [Google Scholar]
- Roubertoux PL, Guillot PV, Mortaud S, Pratte M, Jamon M, Cohen-Salmon C, Tordjman S. Attack behaviors in mice: from factorial structure to quantitative trait loci mapping. Eur J Pharmacol. 2005;526:172–185. doi: 10.1016/j.ejphar.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, glucorticoids, and damage to the nervous system: the current state of confusion. Stress. 1996;1:1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- Schuurman T. Hormonal correlates of agonistic behavior in adult male rats. In: McConnel PS, Boer GJ, Romijn HJ, Van de Poll NE, Corner MA, editors. Progress in brain research, vol 53: adaptive capabilities of the nervous system. Elsevier Biomedical Press; Amsterdam: 1980. pp. 415–420. [DOI] [PubMed] [Google Scholar]
- Scott JP. Aggression. The University of Chicago Press; Chicago: 1958. [Google Scholar]
- Segal DS, Mandell AJ. Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav. 1974;2:249–255. doi: 10.1016/0091-3057(74)90060-4. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, deBoer SF, Haller J, Koolhaas JM. Individual differences in plasma catecholamine and corticosterone stress responses of wild-type rats: relationship with aggression. Physiol Behav. 1996;60(6):1403–1407. doi: 10.1016/s0031-9384(96)00229-6. [DOI] [PubMed] [Google Scholar]
- Siegfried B, Frischknecht HR. Place avoidance learning and stress-induced analgesia in the attacked mouse: role of endogenous opioids. Behav Neural Biol. 1989;52:95–107. doi: 10.1016/s0163-1047(89)90206-9. [DOI] [PubMed] [Google Scholar]
- Siegfried B, Frischknecht HR, Waser PG. Defeat, learned submissiveness, and analgesia in mice: effect of genotype. Behav Neural Biol. 1984;42:91–97. doi: 10.1016/s0163-1047(84)90484-9. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991;559:29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- Stefanski V. Social stress in laboratory rats. Behavior, immune function, and tumor metastasis. Physiol Behav. 2001;73:385–391. doi: 10.1016/s0031-9384(01)00495-4. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA. GABA(B) receptor modulation of serotonin neurons in the dorsal raphe nucleus and escalation of aggression in mice. J Neurosci. 2010;30:11771–11780. doi: 10.1523/JNEUROSCI.1814-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Kim-Cohen J. Meta-analysis of gene–environment interactions in developmental psychopathology. Dev Psychopathol. 2007;19:1029–1037. doi: 10.1017/S095457940700051X. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Barondes SH. Genes and aggressiveness. Behavioral genetics. Curr Biol. 1996;6:238–240. doi: 10.1016/s0960-9822(02)00466-9. [DOI] [PubMed] [Google Scholar]
- Teskey GC, Kavaliers M, Hirst M. Social conflict activates opioid analgesic and ingestive behaviors in male mice. Life Sci. 1984;35:303–315. doi: 10.1016/0024-3205(84)90114-0. [DOI] [PubMed] [Google Scholar]
- Thomas DA, Howard SB, Barfield RJ. Male-produced postejaculatory 22-kHz vocalizations and the mating behavior of estrous female rats. Behav Neural Biol. 1982;36:403–410. doi: 10.1016/s0163-1047(82)90802-0. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Behavioral and autonomic responses to intermittent social stress: Differential effects of clonidine and metoprolol. Psychopharmacology. 1994;116:346–356. doi: 10.1007/BF02245339. [DOI] [PubMed] [Google Scholar]
- Umemoto S, Noguchi K, Kawai Y, Senba E. Repeated stress reduces the subsequent stress-induced expression of Fos in rat brain. Neurosci Lett. 1994;167:101–104. doi: 10.1016/0304-3940(94)91037-5. [DOI] [PubMed] [Google Scholar]
- van der Poel AM, Miczek KA. Long ultrasonic calls in male rats following mating, defeat and aversive stimulation: frequency modulation and bout structure. Behaviour. 1991;119:127–142. [Google Scholar]
- Van Der Vegt BJ, Lieuwes N, van de Wall EHEM, Kato K, Moya-Albiol L, Martinez-Sanchis S, de Boer SF, Koolhaas JM. Activation of serotonergic neurotransmission during the performance of aggressive behaviour in rats. Behav Neurosci. 2003;117:667–674. doi: 10.1037/0735-7044.117.4.667. [DOI] [PubMed] [Google Scholar]
- Van Erp AMM, Miczek KA. Aggressive behavior, increased accumbal dopamine and decreased cortical serotonin in rats. J Neurosci. 2000;15:9320–9325. doi: 10.1523/JNEUROSCI.20-24-09320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Miczek KA. Effects of mu and delta opioid agonists and antagonists on affective vocal and reflexive pain responses during social stress in rats. Psychopharmacology. 1998;139:364–375. doi: 10.1007/s002130050727. [DOI] [PubMed] [Google Scholar]
- Von Holst D. Coping behaviour and stress physiology in male tree shrews (Tupaia belangeri) In: Hölldobler B, Lindberg I, editors. Experimental behavioral ecology and sociobiology. Sinauer Associates; Sunderland: 1985. pp. 461–470. [Google Scholar]
- Von Holst D. The concept of stress and its relevance for animal behavior. In: Moller AP, Milinski M, Slater PJB, editors. Advances in the study of behavior, vol 27: stress and behavior. Academic Press; New York: 1998. pp. 1–131. [Google Scholar]
- Watanabe Y, McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Effects of chronic social stress on tyrosine hydroxylase mRNA and protein levels. Mol Brain Res. 1995;32:176–180. doi: 10.1016/0169-328x(95)00081-3. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Pohorecky LA, Salman S, Gruenthal M. Attenuation of gastric lesions by psychological aspects of aggression in rats. J Comp Physiol Psychol. 1976;90:252–259. doi: 10.1037/h0088038. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Young LJ, Winslow JT, Wang Z, Gingrich B, Guo Q, Matzuk MM, Insel TR. Gene targeting approaches to neuroendocrinology: oxytocin, maternal behavior, and affiliation. Horm Behav. 1997;31:221–231. doi: 10.1006/hbeh.1997.1377. [DOI] [PubMed] [Google Scholar]
