Abstract
Introduction
Heat shock proteins (Hsps) are proteins with important functions in regulating disease phenotypes. Historically, Hsp90 has first received recognition as a target in cancer, with consequent efforts extending its potential role to other diseases. Hsp70 has also attracted interest as a therapeutic target for its role as a co-chaperone to Hsp90 as well as its own anti-apoptotic roles.
Areas covered
Herein, patents from 2008 to 2013 are reviewed to identify those that disclose composition of matter claimed to inhibit Hsp90 or Hsp70.
Expert opinion
For Hsp90, there has been considerable creativity in the discovery of novel pharmacophores that fall outside the three initially discovered scaffolds (i.e., ansamycins, resorcinols and purines). Nonetheless, much of the patent literature appears to build on previously reported structure activity relationship through slight modifications of Hsp90 inhibitor space by finding weaknesses in existing patents. The major goal of future development of Hsp90 inhibitors is not necessarily identifying better molecules but rather understanding how to rationally use these agents in the clinic. The development of Hsp70 inhibitors has lagged behind. It will require a more concerted effort from the drug discovery community in order to begin to realize the potential of this target.
Keywords: ATPase, cancer, chaperone, heat shock protein 70, heat shock protein 90, targeted therapy
1. Introduction
Heat shock proteins (Hsps) are required for proper folding and activity of proteins in the cell [1,2] and are characterized by their ability to become overexpressed under conditions of stress (i.e., heat shock, oxidative stress, malignant transformation). Hsps are classified according to their approximate molecular weight and include the small Hsps (i.e., Hsp27), Hsp40, Hsp60, Hsp70, Hsp90 and Hsp110. Their relevance in disease is currently a hot topic in medicine and attempts to modulate their activity is a highly pursued and active area of research. Currently, the Hsps receiving most attention from a drug discovery perspective are Hsp90 and Hsp70. Both have been shown to be overexpressed in cancer [3-6] and to act together in cancer-promoting multi-chaperone complexes [7,8].
Many of Hsp90's client proteins play a role in the development and progression of cancer (i.e., EGFR, human epidermal growth factor receptor 2 [HER2], breakpoint cluster region, abelson, c-Kit, mitogen-activated protein kinase/ERK kinase, VEGFR, Fms-like tyrosine kinase 3, androgen receptor, BRAF), and these proteins are especially dependent on Hsp90 function for their activity. Concordantly, inhibition of Hsp90 has been demonstrated in numerous preclinical models of cancer to result in antitumor activity. Similarly, Hsp70 is essential for the survival of cancer cells as knockout of both Hsp70 and Hsc70 function results in selective cancer cell death [9]. In addition to its role as a co-chaperone of Hsp90, Hsp70 is also a powerful pro-survival protein through its inhibition of apoptosis at numerous points within the intrinsic and extrinsic cell death pathways [10]. Although historically Hsps were first recognized for their implication in cancer, understanding has been rapidly building over the past decade recognizing a role for these proteins in neurodegenerative disease [11,12], autoimmune disease [13,14] and infections caused by bacteria, fungi, viruses and other parasites [15,16].
Both Hsp90 and Hsp70 are ATPases that utilize repetitive cycles of ATP binding and hydrolysis in a complex cycle required for protein folding [17]. Both undergo extensive conformational changes that are regulated by binding of nucleotides, substrates and co-chaperones. The function of Hsp90 is modulated by a number of co-chaperones such as Hsp70, HIP, HOP, p50, AHA1 and p23. Hsp70 acts with two types of co-chaperones in protein folding, J-domain proteins such as Hsp40, which serve both to deliver substrate proteins to Hsp70 as well as to stimulate their ATPase activity, and nucleotide exchange factors such as Hsp110, which is essential for nucleotide exchange. The complex structural movements undertaken by Hsp90 and Hsp70 during their substrate regulation provide a structural basis for their vulnerability to inhibition. Indeed, small molecules that either interfere directly with nucleotide binding or allosterically alter the conformational flexibility of the Hsps diminish protein activity [18-21].
Driven by both a strong biological rationale for Hsps as targets and a good structural understanding for inhibitor discovery, much effort has been subsequently devoted toward the development of Hsp inhibitors as a potential anticancer strategy [20-23]. This review will focus on patents that have been disclosed from approximately 2008 to 2013 and which describe novel composition of matter claimed to inhibit either Hsp90 or Hsp70. There are many excellent reviews available describing structures disclosed before 2008 and the readers should refer to these [21,24-27].
Patents related to Hsp90 predominate as it has been more thoroughly validated as an anticancer strategy and also more effectively pursued. Aside from this, it is also clear for reasons discussed below, that it has been a target far more druggable than Hsp70. Efforts to develop Hsp70 inhibitors have been less fruitful as can be seen by the rather limited number of patents disclosing such composition of matter. As a further indication to this point, there are currently about 17 clinical trials ongoing that examine the use of Hsp90 inhibitors in cancer, whereas there are none yet investigating Hsp70 inhibitors.
2. Hsp90 inhibitors
Hsp90 contains a Bergerat fold specific to the GHKL family of ATPases [28] and thus binds ATP in a rather unique bent conformation. These features have enabled the development of highly selective inhibitors of Hsp90. In fact, all of the compounds that have been advanced to clinic thus far function by competing with ATP for binding to this unique nucleotide-binding site [23]. Other means for inhibiting Hsp90 function have been reported and include molecules that target the C terminus and those which inhibit interaction between Hsp90 with either its co-chaperones or client proteins. This review has been limited to ATP-competitive inhibitors that target the nucleotide-binding pocket and a discussion on molecules which inhibit Hsp90 by alternative mechanisms is beyond the scope of this review. Readers interested in learning about these alternative modes of Hsp90 inhibition are referred to earlier review articles on this topic [18,29,30].
Many compounds were claimed as Hsp90 inhibitors in earlier patents (c. 2008) [25,26,31,32] and will not be described here. A number of these have been developed into compounds which are currently being evaluated in the clinic for cancer and include those from the ansamycin class (17-allyl-17-desmethoxygeldanamycin [17-AAG] [1], 17-desmethoxy-17-N,N-dimethylaminoethylaminogeldanamycin [17-DMAG] [2] and IPI-504 [3]), purine-scaffold class (BIIB021 [4], PU-H71 [5], MPC-3100 [6]), resorcinols (NVP-AUY922 [7], AT-13387 [8], KW-2478 [9]), dihydroindazolones (SNX-5422 [10]) and others (XL-888 [11], NVP-HSP990 [12]) (Figure 1) [23,25,33,34]. The discovery and development of Hsp90 inhibitors continues to be an active area of research and this review will outline efforts in this area from 2008 to 2013, as described below.
Figure 1.
Hsp90 inhibitors disclosed before 2008 that have advanced to clinic.
2.1 Ansamycin derivatives
The ansamycins have a significant historical relevance in the field of Hsp90 inhibitors. Geldanamycin (GM; 13) was the first Hsp90 inhibitor discovered, but its displayed hepatotoxicity prevented its evaluation in humans (Figure 2) [23]. Other derivatives such as 17-AAG (1) and 17-DMAG (2), showed improved toxicity profile and in fact 17-AAG was the first Hsp90 inhibitor to be evaluated in humans (Figure 1) [23]. However, its development was halted for a variety of reasons, one being its dose- and schedule-dependent propensity for liver toxicity. This stems from the presence of a benzoquinone moiety on the GM-type scaffold, a ring that is reactive toward nucleophiles such as glutathione [35]. It is, thus, of no surprise that ensuing synthetic efforts in this class focused on modifying the GMs to limit glutathione conjugation, while maximizing their reduction to stable Hsp90 inhibitory hydroquinones. This was deemed as a strategy for optimizing the therapeutic index.
Figure 2.
The ansamycin class of Hsp90 inhibitors. Hsp: Heat shock protein.
Along these lines, Discovery Partner International claimed oxime derivatives of GM, 17-DMAG and herbimycin 14 – 16, respectively (Figure 2) [36]. Compounds 14 – 16 retained affinity for Hsp90 comparable to the parent compounds, while exhibiting enhanced water solubility and oral bioavailability. These oximes were unreactive toward nucleophiles and, therefore, claimed to be devoid of hepatotoxicity.
The University of Colorado disclosed novel ansamycin analogs substituted at the 19-position of the molecule. Compounds exemplified in the patent were of general formula 17 (Figure 2), in which X = -OCH3, -NH-(CH2)2N(CH3)2 or -NHCH2-CH=CH2 and R = alkyl, allyl or 5- or 6-membered aryl or heteroaryl [37]. The rationale behind the synthesis of these derivatives was to block potential thiol conjugation at the C19 position with endogenous nucleophiles such as glutathione and thereby decrease the potential for such reaction to occur upon in vivo administration. Although these modifications reduced the glutathione reactivity of these molecules, they came at a significant cost to Hsp90-binding affinity. For example, 19-aryl ansamycins which were the most active compounds of this series, had a low micromolar activity, which compared to the parent unmodified benzoquinone was a 1- to 2-log drop in activity (e.g., 19-Phe-DMAG [18]; IC50 = 3 μM against MiaPaCa-2 pancreatic cancer cell lines compared to 0.13 μM for 17-DMAG) (Figure 2) [38].
2.2 Purine-scaffold-derived
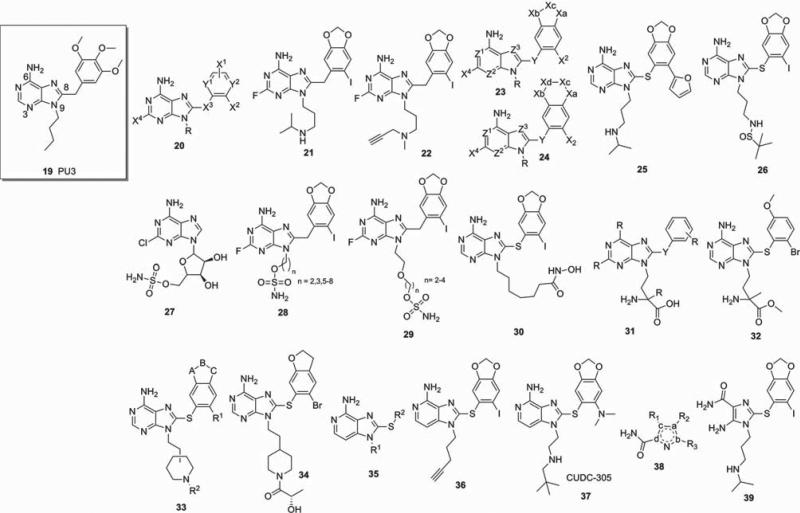
Purine-derived Hsp90 inhibitors are one of the most patented classes of small molecule inhibitors and trace their origins to the first reported synthetic Hsp90 inhibitor, PU3 (19; Figure 3) [39]. This compound along with several other Hsp90-binding pharmacophores was claimed by Memorial Sloan Kettering Cancer Center (MSKCC) [40]. Subsequently, several groups have adopted this scaffold as a lead for optimization and as a result numerous patents are available which claim particular compounds, several of which have already entered the clinic. Efforts by MSKCC to optimize compound 19 resulted in compound PU-H71 (Figure 1) [41-45], which is currently being evaluated in clinical trials for cancer. Similar efforts by other groups have resulted in the clinical compounds BIIB021 [46-49] by Conforma Therapeutics (acquired by Biogen Idec) and MPC-3100 by Myrexis (Figure 1) [50]. As a result of the success of this class, compounds continue to be patented and many of the claims are based on derivatization of the purine-scaffold at N3-, N9- or C8-position of the purine ring (Figure 3).
Figure 3.
Purine and purine-like scaffold inhibitors of Hsp90. Hsp: Heat shock protein.
Within the 8-aryl-substituted purine class, the N9-substituent has proven to be highly amenable to structural modification. In these molecules, this position is oriented toward the solvent exposed region of the protein and as a result numerous compounds have been reported that modify this part of the molecule [51]. A number of patents have claimed molecules with such structural alterations, as is detailed below.
In 2008, MSKCC claimed purine-derived Hsp90 inhibitors of general structure 20 for the treatment of neurodegenerative diseases [52]. Unlike the previous derivatives destined for cancer applications, these compounds were designed with blood–brain barrier (BBB) permeability in mind. The patent discloses both composition of matter and a testing strategy for the discovery of Hsp90 inhibitors for neurodegenerative diseases. Appropriately substituted analogs such as compounds 21 and 22 were able to cross the BBB and favorably modulate several hallmarks associated with tauopathies, such as Alzheimer's disease and frontotemporal dementias (Figure 3).
In 2011 and 2012, MSKCC extended their claims on substituted purine derivatives and related compounds of the formulas 23 and 24 (Figure 3) [53]. These wide-ranging patents encompass numerous compounds. A major focus in one patent application was placed upon replacement of the X2 moiety with aromatic, heteroaromatic as well as alkyne groups. These compounds, such as compound 25, were obtained through palladium catalyzed cross-coupling reactions (i.e., Suzuki, Stille, Sonagashira) of compounds such as PU-H71, which are substituted with an iodine at X2. Derivatives with R group at N9 being amide, sulfonamide or sulfinamide such as representative example 26 (Figure 3) had the unexpected benefit of limited to no activity when tested for potential human ether-à-go-go-related gene (hERG) liability, while retaining potent Hsp90-mediated biological activity (EC50 = 12 nM Hsp90 binding; > 100 μM hERG binding).
In 2008, Wyeth claimed compounds containing sulfamoyl moieties attached to the purine scaffold at the N9 position as well as to the corresponding triazolo-, pyrazolo- and pyrrolopyrimidines [54]. This series of inhibitors is claimed to be inspired by the naturally occurring antibiotic 2-chloro-5′-sulfamoyl adenosine (CSA; 27) [55], which was also found to inhibit Hsp90 (Figure 3). Compound 27 was toxic to mice as a result of its protein synthesis-inhibiting properties through inhibition of aminoacyl tRNA synthetase [56]; thus, efforts reported in this patent were aimed at increasing potency and safety. Important examples are purine-scaffold derivatives modified at N9 position with a sulfamoyl moiety and consist of one of two types. In the first type, an all-carbon alkyl chain of variable length is followed by the sulfamoyl group (28) and in the second type, an oxygen-containing carbon chain of variable length is followed by a sulfamoyl chain (29) (Figure 3). The activity of these compounds was evaluated by a battery of assays including: i) a competition binding assay; ii) a luciferase refolding assay; iii) a client protein degradation assay; and iv) a cell growth inhibition assay. Representative compounds such as compounds 28 and 29 were claimed to have activity in these assays, which is not surprising as they closely mimic PU-H71 (Figure 1), the MSKCC developed inhibitor and not CSA.
In 2008, Curis claimed a series of chimeric Hsp90 inhibitors, which were designed to simultaneously act as histone deacetylase (HDAC) inhibitors as well (30, Figure 3). Combined inhibition of both these targets was hypothesized to potentially provide advantageous results in the treatment of cancer [57]. In addition to containing an Hsp90 binding moiety, these compounds also contained an N-hydroxyalkylamide group to act as HDAC or matrix metalloproteinase inhibitors by virtue of their ability to bind zinc ions. The benefit of such chimeric molecules may be that they may have enhanced activity when compared to a combination of separate molecules and, further, may be liable to decreased toxicity since only one molecule is administered. The activities of the compounds were determined in separate in vitro inhibition assays of Hsp90 chaperone activity and HDAC enzymatic activity, but no in vivo assessment was provided to prove that such dual inhibitors do indeed provide an advantage over individual Hsp90 and HDAC inhibitors. The purine part of compound 30 is similar to the potent Hsp90 inhibitor PU-H71 claimed earlier by MSKCC.
In 2009, Chroma claimed purine-derived amino acids (31) and the corresponding esters for the treatment of proliferative diseases which are mediated by inappropriate Hsp90 activity as well as inflammatory and immune disorders or for the protection of normal cells against cytotoxic agents (Figure 3) [58]. However, biological activity data were provided only for the amino acid methyl ester (32; Figure 3). Its activity in the binding assay was rather ambiguous (IC50 = < 1 μM) and was not potent in the cytotoxicity assay (IC50 = > 5 μM against U937 and HUT78 cancer cells).
Myrexis described a series of N-substituted ethylpiperidines attached to the purine ring via the N9 or N3 position (33; Figure 3) [59] as an extension of an earlier related patent in which MPC-3100 (Figure 1) was disclosed [60]. As mentioned previously, MPC-3100 had entered clinical trials and completed a Phase I clinical evaluation in refractory, or recurrent, cancer patients. The compounds described in this patent maintained the ethylpiperidine moiety at the N9 or N3 position of the purine, while varying the right side aryl component (Figure 3; compound 34). As was the case for MPC-3100, the presence of bromine at the R1 position on these structures rendered these compounds of lower Hsp90 inhibitory activity when compared to the corresponding iodo-compounds [61].
2.3 Purine-like: imidazopyridine
In 2008, Curis disclosed a series of imidazopyridine inhibitors (35) for the treatment of Hsp90-related diseases, including cancer, autoimmune and neurodegenerative diseases (Figure 3) [62]. These compounds are similar to the purine-scaffold compounds, however, the nitrogen at 3-position is replaced by a carbon. These compounds are generally good Hsp90 inhibitors, although it appears that replacement of N with C diminishes activity [63]. Some examples (compounds 36 and 37) are shown in Figure 3. One compound from this series, CUDC-305 (or Debio093; 37), is currently in clinical evaluation. It shows favorable affinity for Hsp90α/β (IC50 ~ 100 nM) and the Hsp90 complex derived from various cancer cells (average IC50 = 48.8 nM) and displays anti-proliferative activity against a broad range of cancer cell lines (average IC50 = 220 nM). CUDC-305 crosses the BBB to reach therapeutic levels in brain tissue [64]. In 2010, Curis extended their claims for the use of these compounds for the treatment of malignancies associated with brain and lung [65].
2.4 Purine-like: imidazole derivatives
Crystax Pharmaceuticals described a series of derivatives of generic formula 38, where one of the a-d atoms is N and the remaining are C and R3 is substituted thioaryl or benzyl (Figure 3) [66]. Most of the compounds described in the patent have imidazole as the core and are exemplified by compound 39 (Figure 3). These compounds also bear similarity to PUH71, but have diminished Hsp90-mediated activity (IC50 = 0.88 μM against Hsp90 in a fluorescence polarization [FP] assay using PU-H71-Bodipy as a tracer and in cell activity of 15.9 μM in MCF7 cells).
2.5 Pyrrolopyridine/pyrrolopyrimidine/ pyrazolopyrimidine derivatives
In 2008, Vernalis described a series of pyrrolopyridine derivatives of generic formula 40, which they narrowed to sub-genus compound 41, in which R1 and S1 = H, alkyloxy or alkylthio groups of varying length, branch and heteroatoms; R3 and R4 = halogen, cyano, alkyl groups (Figure 4) [67]. This patent did not provide any further biological data for these compounds other than modest Hsp90 inhibitory activity determined by FP assay. The following year, Vernalis disclosed a pyrrolopyrimidine class of compounds as Hsp90 inhibitors [68]. These compounds were of the generic structure 42, in which R = methoxy or cyano; R1 and R2 = H, alkyl, haloalkyl, cycloalkyl, saturated heterocyclic ring when N is considered as part of the ring; R3 and R4 = H, alkyl up to 3 carbon long, 3 – 6 membered cycloalkyl ring when C is considered as part of the ring (Figure 4). Only 22 compounds were described in the patent, including compound 43, which had an IC50 of 24 nM in a FP competition assay. These compounds are similar to a previously disclosed compound, NVP-BEP800 (44), which has a pyrimidothiophene core instead of pyrrolopyrimidine (Figure 4) [69]. NVP-BEP800 (44) is a potent Hsp90 inhibitor that has demonstrated significant anticancer effect in a BT474 human breast cancer model [70,71], however, it has not yet entered into clinical trials.
Figure 4.
Pyrrolopyridine/pyrrolopyrimidine/pyrazolopyrimidines Hsp90 inhibitors. Hsp: Heat shock protein.
In 2010, Chroma Therapeutics claimed a series of pyrrolopyrimidines as inhibitors of Hsp90 [72]. They reported on 7-(pyridin-2-ylmethyl)-pyrrolopyrimidines with an amino acid function connected to an alkoxy-4-(prop-1-yn-1-yl)benzene linker at position-5 of the pyrrolopyrimidine scaffold. The most potent disclosed compound was 45 (Figure 4). The susceptibility of compound 45 to hydrolysis suggests that the acid may be the active form in cells. These compounds appear to be similar to a series of pyrrolopyrimidines previously claimed in a patent by Conforma Therapeutics in 2006, which contained EC144 (46), an orally available compound that induced HER2 degradation (i.e., a functional read-out of Hsp90 inhibition in cancer cells) with an EC50 = 14 nM (Figure 4) [73,74].
Pfizer claimed related 2-aminopyrimidines as Hsp90 inhibitors of general structure 47 (Figure 4) [75]. Representative example (48) showed a Ki = 9 nM in a scintillation proximity assay (Figure 5). In a subsequent patent, related pyrazole-containing derivatives such as compound 49 were claimed [76]. Compound 49 is an optimized compound which shows a Ki = 6 nM in the abovementioned assay and cellular activity of IC50 = 36 nM for degradation of AKT kinase in NCI-H1299 cells (i.e., a functional readout of Hsp90 inhibition in cancer cells). Good stability in human liver microsomes and oral bioavailability in rats (15 – 20%) and dogs (50 – 60%) were also claimed for this compound. When administered to mice bearing A2058 melanoma xeno-grafts, compound 49 displayed 75 and 96% inhibition of tumor growth when dosed orally at 10 or 25 mg/kg/day, respectively, for over 14 days.
Figure 5.
Quinazoline and tetrahydropteridine Hsp90 inhibitors. Hsp: Heat shock protein.
Daiichi Sankyo claimed cyclized pyrazolopyrimidines of general structure 50 (Figure 4) [77,78]. A compound derived from these series, compound 51, inhibited the ATPase activity of Hsp90 with an IC50 = 0.61 μM (Figure 4). In a subsequent patent, compound 52 is reported to have an IC50 = 13 and 26 nM in inhibiting the growth of SKBr3 and NCI-H460 cancer cell lines, respectively (Figure 4) [79,80]. These compounds in large appear to closely mimic the structure of the Conforma Therapeutics-derived compound BIIB012 (Figure 1).
Poniard Pharmaceuticals describe compounds of general formula 53, in which derivatives such as compound 54 were claimed to have growth inhibitory IC50s of < 70 nM against MCF-7, SKBr3, BT-474 and HT29 cancer cells (Figure 4) [81]. Additionally, compound 54 had an IC50 = 90 nM in an assay to measure HER2 degradation in SKBr3 cancer cells.
2.6 Quinazoline derivatives
Scientists at DAC-SRL, an oncology division of the Genextra group, described compounds related to 2-amino-7,8-dihydro-6H-quinazolin-5-one oximes (55) as Hsp90 inhibitors for the treatment of cancers and other proliferative diseases (Figure 5) [82]. Compounds described in the patent are of generic formula 55, where R1 = H, halogen, alkyl, OH; R2, R3 = H, alkyl, substituted or unsubstituted aryl; R6 = H, alkyl. Optimization led to derivatives of type 56, which showed IC50 < 1 μM for Hsp90 inhibition in a FP assay (Figure 5). In a subsequent patent, the same group disclosed several optimized compounds of generic formula 57, where R = H, up to 5 carbon alkyl, alkylene, non-aromatic heterocyclyl with at least one heteroatom (Figure 5) [83]. An example from this series is compound 58, which had an IC50 of 24 nM for Hsp90 inhibition in the FP assay and ~ 100 nM in inhibiting select cancer cells (Figure 5). Interestingly, Takeda Pharmaceuticals have claimed a similar series of oxime derivatives in addition to oxime derivatives of related d-lactam analogs similar to NVP-HSP990 (Figure 1) [84].
In 2009, Merck disclosed quinazolinamide derivatives of general structure 59 to treat diseases by the inhibition, regulation or modulation of Hsp90 (Figure 5) [85]. The evaluation of these new inhibitors was performed by a radioligand binding filtration assay, wherein their ability to replace radiolabeled 17-AAG from recombinant human Hsp90α was determined. Structure activity relationship (SAR) in this class of inhibitors indicated electron withdrawing or donating groups at the 2′-position of R′ (59, Figure 5) of the phenyl ring to be well tolerated for Hsp90 inhibitory activity. Compound 60 is a representative example from the series (Figure 5). In a subsequent patent, structures of type 61 were disclosed, which are similar to compound 60 (Figure 5). Most of the compounds described in the patent, exemplified by derivative 62, showed IC50 values from 10 nm to 1 μM (Figure 5) [86]. In two subsequent patents [87,88], scientists from Merck described optimized structures with modification at the 6-position of the quinazoline ring such as compounds 63 and 64 (Figure 5).
2.7 Tetrahydropteridine and derivatives
In 2008, Astra Zeneca published a patent describing 5,6,7,8-tetrahydropteridine derivatives of general formula 65 (Figure 5). Derivatives belonging to subgroup 66 showed most potent Hsp90 inhibition activity in a FP assay (Figure 5; IC50 of 67 = 49 nM) [89]. Poniard Pharmaceuticals also disclosed aminopteridine derivatives as Hsp90 inhibitors with generic formula 68 [81], similar in structure to those described previously by Astra Zeneca (Figure 5). Compound 69 is a representative example of this series that had activity in cancer cells at ~ 100 nM.
2.8 Resorcinol derivatives
Radicicol (RD; 70) (Figure 6), a macrocyclic antibiotic isolated from Monosporium bonorden, was the first resorcinol-containing molecule found to bind to the N-terminal domain of Hsp90 and to exert in cancer cells Hsp90-mediated biological activity [90,91]. Since then many pharmaceutical companies have been actively pursuing the development of small-molecule Hsp90 inhibitors based on the resorcinol core of RD. This is epitomized by the filing of various patents having diverse chemical structure that incorporate the resorcinol core and the advancement of these agents to clinic (e.g., NVP-AUY922, AT13387, KW-2478; Figure 1).
Figure 6.
Resorcinol derivatives as Hsp90 inhibitors. Hsp: Heat shock protein.
Synta Pharmaceuticals disclosed a series of compounds of generic formula 71 containing 1,2,4-triazole ring as Hsp90 inhibitors, in which ring A = substituted aryl or heteroaryl; R1 = -OH, -SH, -NHR7, and the like; R5 = substituted or unsubstituted, monocyclic or bicyclic and aryl or heteroaryl ring (Figure 6) [92]. In this patent, compound 72 was shown to be more potent than 17-AAG in inhibiting Hsp90 ATPase activity (Figure 6). This patent also describes the synthesis of STA-9090 (73; Ganetespib), which is currently undergoing clinical evaluation for the treatment of various solid tumors (Figure 6). Ganetespib killed cancer cells with BRAF mutation at low nanomolar concentrations with IC50s in the range of 4 – 40 nM. These effects were greater in combination with the BRAF inhibitor vemurafenib, which led to a patent filing of ganetespib in combination with a BRAF inhibitor for the treatment of cancers with BRAF mutation V600E [93].
In a subsequent patent, the oxygen at the 4-position of the resorcinol was incorporated into a five- or six-member ring. These derivatives, however, represented by the prototype compound 74, demonstrated modest activity (Figure 6) [94]. Derivatives of general formula 75, where R1 = R2 = OH; R5 and R6 = branched or unbranched alkyl groups and R7 = alkyl chain, heteroalkyl and heteroaryl ring, were also claimed (Figure 6) [95]. Compound 76 is an example from this series (Figure 6). In yet another patent from Synta, the resorcinol moiety was replaced by 6-hydroxyindazole that resulted in compounds such as compound 77, which in contrast to earlier agents from Synta is claimed to be orally bioavailable in rats [96]. In further series of inhibitors, the triazole was replaced with pyrrole or an open hydrazonamide [97,98]. These were not favored modifications, as compound 78, an example from the pyrrole series, and compound 79 from the hydrazonamide series both had modest activity.
Arqule, Inc. has patented a series of resorcinol-containing tetrazoles of generic formula 80 as Hsp90 inhibitors (Figure 6) [99]. In these compounds, R4 = cyclic or linear, branched or unbranched alkyl or substituted aryl, and compounds such as 81 and 82 were determined to be effective Hsp90 inhibitors in a fluorescent ELISA assay with IC50 < 1 μM (Figure 6). These, however, showed only modest activity in cancer cells.
Sigma-Tau Research group also patented resorcinol type derivatives of generic structure 83 as Hsp90 inhibitors (Figure 6) [100]. The compounds described in the patent have isoxazole 3-carboxamide as a core with substituted or unsubstituted amino or amido group at the 4-position of isoxazole and a chlorine or isopropyl group as X-substituent in the resorcinol moiety. A representative compound is 84. In another patent, Sigma-Tau Research group described 1,2,3-triazole derivatives of general structure 85 (Figure 6) [101]. Compound 86 is a representative example of this series [101]. These derivatives show similarity to NVP-AUY922 (Figure 1), a clinical compound developed by Novartis/Vernalis that is currently in clinical evaluation.
A series of resorcinol compounds of generic formula 87 was described as Hsp90 inhibitors by Merck GMBH, where dihydroisoindole was attached at position-5 of the resorcinol core (Figure 6) [102]. These compounds, such as derivative 88, bear structural resemblance to the clinical compound AT13387 (Figure 1) developed by Astex Pharmaceuticals and are substituted with a carboxamide at position-1 of the resorcinol ring instead of the isopropyl group seen in AT13387.
Nerviano Medical Sciences claimed a series of bicyclic pyrazole and isoxazole derivatives of general structure 89 as Hsp90 inhibitors (Figure 6) [103]. The most potent compound reported was compound 90 which, however, had a modest, micromolar range activity (Figure 6). Nerviano also patented a series of resorcinols of general structure 91 (Figure 6) [104]. In this patent, numerous heterocycles attached to the resorcinol ring were claimed, including isoxazole and pyrazoles. Compound 92 (NMS-E973) is a representative example (Figure 6). It induced HER2 degradation (IC50 = 0.11 μM) and inhibited the proliferation of A2780 ovarian cancer cells (IC50 = 0.06 μM). Compound 92 showed favorable solubility in water at pH 7 (179 μM) [105]. When administered intravenously at 30 and 60 mg/kg to mice bearing A2780 tumors for 10 consecutive days, compound 92 resulted in a tumor growth inhibition of 53 and 74%, respectively.
3. Hsp70 inhibitors
For the reasons described above, Hsp70 has attracted considerable interest as an anticancer target. However, it has proven to be more of a challenge to drug than Hsp90 and a number of reasons can be attributed to this [106]. Unlike the case with Hsp90, drug-like natural product inhibitors with specific binding mode and, moreover, captured by a crystal structure to potentially guide structure-based drug design are unavailable for Hsp70. Another reason is related to the nature of the nucleotide-binding pocket of Hsp70. This pocket is considerably more hydrophilic compared to that of Hsp90 and adopts an actin-like fold whereby ATP binds in a more extended conformation and makes important polar contacts deep within the pocket through its β- and γ-phosphate groups.
In the first reported rational design approach to develop ATP-competitive Hsp70 inhibitors, nucleotide mimetics such as the dibenzyl-8-aminoadenosine analog VER-155008 (93) were developed by scientists at Vernalis to bind into the N-terminal ATP pocket of Hsp70 (Figure 7) [107]. Despite this compound binding Hsp70 with reasonably high affinity (Kd = 0.3 μM), it exhibited low cellular potency in a cytotoxicity assay against HCT116 colon cancer cells (GI50 = 5 μM). Although a poor permeability profile cannot be excluded as a major factor toward this compound's limited cellular activity, other factors also contribute to this effect. Mainly, Hsp70 has a high affinity for ADP (0.11 – 0.5 μM) and combined with the high intracellular concentrations of ATP (1 – 6 mM), it makes it difficult to obtain reversible competitive inhibitors, altogether underscoring the difficulty in targeting the ATP pocket of Hsp70 with reversible competitive inhibitors.
Figure 7.
Structures of some known Hsp70 inhibitors. Hsp: Heat shock protein.
Other efforts took advantage of the highly dynamic nature and the conformational flexibility of Hsp70. These compounds, reported before 2008, were identified from library screens designed to identify inhibitors of either the ATPase activity or the folding capacity of yeast or bacterial Hsp70. Follow-up biochemical studies indicated that they altered Hsp70 activity through a variety of distinct interaction modes [20,21,108].
MAL3-101 (94) and MAL3-39 (95) are dihydropyrimi-dines [109] that inhibit J-domain-stimulated ATPase activity of yeast Hsp70 without effecting endogenous ATPase activity (i.e., non-stimulated) (Figure 7). These compounds were identified from a library of 31 compounds with structural similarity to NSC 630668-R/1 [110] and 15-deoxyspergualin (DSG) [111,112] – two compounds previously known to modulate the function of Hsp70. NSC 630668-R/1 is an inhibitor of endogenous and J-domain-stimulated ATPase activity, whereas DSG is a stimulator of Hsp70 ATPase activity. Methylene blue (96), azure c (97) and myricetin (98) were identified as inhibitors of the ATPase activity of Hsp70 from a high-throughput screening of a 2800-member library of bioactive compounds (Figure 7) [113]. MKT-077 (99) was initially discovered following optimization efforts [114] that had previously identified such rhodacyanine dyes as possessing anticancer activity (Figure 7) [115]. Only after its initiation into clinical trials was it found to bind with Hsp70 (actually mortalin, the mitochondrial Hsp70 member) [116]. As no crystal structure of inhibitor bound to Hsp70 is available, the precise mode of binding for these compounds is not known. However, myricetin [117] and MKT-077 [118] by NMR studies are proposed to interact with allosteric sites outside the nucleotide-binding domain (NBD) of Hsp70.
Although these compounds inhibit Hsp70 function, they are generally believed to have a pleiotropic mechanism of action and likely bind to multiple targets within the cell.
These molecules have also been hindered by a largely non-tractable SAR and due to their little drug-like character it is unclear whether they will become useful drugs. Therefore, there remains clearly a strong need for Hsp70 inhibitors based on pharmacophores that enable substantial medicinal chemistry for the discovery of Hsp70 drug candidates. The efforts listed below reveal some of the most recent patents claiming certain molecules as Hsp70 inhibitors.
3.1 Imidazole derivatives
In 2008, Yonsei University claimed a number of imidazole compounds that were reported to induce apoptosis [119]. Using a cell-based screen of compounds capable of inducing apoptosis, researchers identified compounds 100 – 103 from an imidazole library of compounds (Figure 8). Apoptozole (100) induced significant cell death through apoptosis in SK-OV-3 (ovarian cancer), HCT-15 (colon cancer cells), A549 (lung cancer) with IC50 of 0.22, 0.25 and 0.13 μM, respectively [120]. Using an apoptozole-conjugated agarose matrix, a single heavy band at about 70 kDa was identified by affinity purification from P19 embryonic carcinoma cells following SDS–PAGE. This was identified as Hsc70 by nanoLC-MS/MS and confirmed by western blot analysis, which showed that binding of both recombinant Hsc70 and Hsp70 could be competed off the apoptozole matrix with soluble apoptozole. Apoptozole inhibited the ATPase activity of Hsp70 (20% at 50 μM and 55% at 200 μM) and was determined to bind to the NBD [121]. A biotinylated-apoptozole bound Hsc70 and Hsp70 with Kd = 0.21 and 0.14 μM, respectively [120].
Figure 8.
Structures of Hsp70 inhibitors. Hsp: Heat shock protein.
3.2 Aptamers/polypeptides
In 2009, the Institut National de la Sante et de la Recherche Medicale have claimed certain peptide aptamers of < 15 amino acids that bind selectively to Hsp70 [122,123]. These aptamers were identified through an optimized yeast two-hybrid procedure for their ability to selectively bind to human Hsp70 [124]. Two of the aptamers, A8 (104) and A17 (105), demonstrated a strong interaction with Hsp70 and it was determined that A8 bound to the substrate binding domain (SBD) and A17 bound to the NBD (Figure 8). Although none of the aptamers exhibited toxicity when administered alone, both A8 and A17 strongly sensitized cancer cells to apoptotic cell death following treatment with cisplatin as well as with fluorouracil or etoposide. When B16F10 melanoma cells transfected with either A8 or A17 aptamer expression vectors were grown in non-immunodeficient C57/BL6 mice, both resulted in smaller tumors as compared to control. Furthermore, both A8 and A17 expressed mice showed significant antitumor effect in combination with cisplatin with most exhibiting complete response. However, in immunocompromised athymic nude (nu/nu) mice, there was no observed anticancer activity, suggesting involvement of the immune system. Further study revealed that A8 and A17 induced an increase in the number of tumor infiltrating CD8+ T cells and macrophages. When the 13-aa peptide P17 was directly administered, it exhibited all of the observed effects of the aptamer A17 in vitro and in vivo.
3.3 Derivatives of pyrrhocoricin
Pyrrhocoricin (106) is a 20-amino acid glycopeptide identified from a subset of insect-derived peptides possessing antibacterial activity (Figure 8) [125]. It was shown to inhibit the ATPase activity of the bacterial chaperone protein DnaK, although its precise site of binding is not known. Early research had shown that aa's 1 – 9 had activity comparable to the full-length peptide [125]. In humans, it is highly susceptible to degradation and, furthermore, is toxic at higher doses. As a result, Chaperone Technologies, Inc. initiated efforts to modify pyrrhocoricin and in 2010 claimed a series of small-molecule peptide inhibitors [126]. The researchers optimized the minimal peptide sequence of pyrrhocoricin, Tyr-Leu-Pro (aa 6 – 8), required for binding to DnaK and extensive derivatization was carried out. Among all the derivatives reported, compound 107 was found to be the most active inhibitor of DnaK-induced refolding of denatured firefly luciferase with an IC50 = 3 μM (pyrrhocoricin IC50 = 29 μM) (Figure 8). Another derivative, compound 108, was also active in this assay (IC50 = 14 μM), and furthermore demonstrated antibacterial activity in strain 109 of H. influenza (MIC = 2 μg/ml) (Figure 8). The potential use for these compounds in cancer against human Hsp70 has not been explored.
3.4 Sulfonamides
In 2011, the University of Pennsylvania disclosed a series of sulfonamides of general structure 109 that selectively inhibit Hsp70 and Hsc70 (Figure 8) [127,128]. Two compounds of significance are described herein, 2-phenylethynesulfonamide (PES; 110) as well as its more active chloro-substituted analog PES-Cl (111) (Figure 8). PES was originally identified from a screen of molecules designed to evaluate ability to impair the mitochondrial localization of p53 [129] and further evaluation showed it to function by inhibiting Hsp70 and disrupting association with some of its co-chaperones (i.e., CHIP, BAG-1, Hsp40) and substrate proteins [130,131]. When exposed to cancer cells, these compounds were found to induce cell death by impairing autophagy through inhibition of Hsp70-dependent lysosomal function and reduced proteasome function, thereby affecting the two major pathways of protein degradation. PES-induced cell death is not dependent on caspase activation or p53 function nor was it inhibited by overexpression of BCL-xL. Treatment of tumor cells with PES resulted in cytoplasmic vacuolization, accumulation of misfolded and aggregated proteins and induction of autophagy. Furthermore, PES altered the expression of Hsp70/Hsp90 client proteins [132]. Deletion-based analysis suggested that PES may interact with the C-terminal SBD of Hsp70. Further, in silico docking and site-directed mutagenesis suggests that PES binds to the α-helical ‘lid’ of the SBD with N548, I607 and Y611 each making significant contacts with the ligand.
3.5 Pyrimidine derivatives
In 2011, MSKCC claimed a series of pyrimidine-based small molecule inhibitors that were discovered using a structure-based design strategy targeting a novel allosteric pocket located in a cleft region outside of the ATP/ADP-binding pocket and flanked by subregions Ib and IIb (Figure 8) [133]. A homology model of human Hsp70 was developed and computational analyses used to reveal this unanticipated allosteric binding site. In addition, Cys267 was observed to be present as one of the binding site amino acids and was used as an advantage in the design of covalent modifiers targeting this site. Structure-based drug design coupled with phenotypic assays designed to measure the effect on Hsp70 within a cancer cell were used to identify a number of inhibitors, including the 2,5′-thiodipyrimidine series exemplified by compound 112, which was one of the most active compounds claimed (Figure 8). Through the use of a biotinylated analog of compound 112, it was shown to bind selectively to Hsp70 and Hsc70 present in cancer cells and to form a covalent bond with Cys267 residue through Michael addition with its acrylamide moiety [134]. Compound 112 inhibited the refolding of heat-denatured luciferase by purified Hsc70 and DJA2 and, at low micromolar concentrations, induced degradation of several Hsp90–Hsp70 complex onco-client proteins without feedback induction of Hsp70. Compound 112 also induced apoptosis as indicated by substantial poly (ADP-ribose) polymerase cleavage and altered the formation of the oncogenic Hsp90–HOP–Hsp70 complexes at similar concentrations.
4. Conclusion
Hsp90 and Hsp70 are important targets in a number of diseases. Both have received significant attention as targets for the treatment of cancer, especially Hsp90, which has been one of the most highly pursued cancer targets during the past 10 years. Although no Hsp90 inhibitor has yet been approved by the FDA, these efforts have resulted in 17 different Hsp90 inhibitors to enter the clinic. Interestingly, each of these molecules targets the nucleotide-binding pocket and competes with ATP for binding. GM was the first Hsp90 inhibitor to be discovered and served as a template for a number of agents to have entered clinical evaluation, including 17-AAG, 17-DMAG, IPI-493 and IPI-504. Patents based on the ansamycin class of inhibitor continue to be published and these describe derivatives claimed to possess decreased toxicity. However, it appears that the clinical utility of these agents is limited and no degree of derivatization can overcome the inherent hepatotoxicity associated with this class. Another major class is the resorcinol series which is reminiscent of the core moiety found in the natural product inhibitor RD. Although all representative compounds of this series contain a resorcinol moiety, these synthetic compounds show considerable diversity compared to RD and exhibit improved properties. Inhibitors of this class to have reached the clinic include NVP-AUY922, AT13387, KW-2478 and STA-9090. Compounds from this class are steadily advancing in the clinic with STA-9090 being furthest along. Many more novel resorcinol derivatives have been claimed in patents, a trend that is likely to continue owing to the relative success of this class. The third major class of inhibitor is the purine-scaffold which originated with PU3 and was the first synthetic inhibitor described. Since then, it has been elaborated into a number of compounds currently being evaluated in the clinic, including PU-H71, BIIB021, MPC-3100 and CUDC-305. These compounds are also steadily advancing through clinic and numerous patents describing purine analogs have been reported and it is expected that they will continue to be in the future. In addition to these, numerous other classes of inhibitors have been reported. Although unrelated, the clinical agents SNX-5422 and XL888 both contain benzamide functionality and another clinical agent, NVP-HSP990, is an aminopyrimidine. Other classes of compounds have been patented, which in many ways represent modifications to the purine and aminopyrimidine classes and include scaffolds such as pyrrolopyridine, pyrrolopyrimidine, pyrazolopyrimi-dine, pyrimidothiophene and tetrahydropteridine.
A potential drawback of Hsp90 inhibition is the induction of Hsps that can potentially limit the anticancer activity of these agents in the clinic. This is mediated through activation of heat shock factor-1, a feedback mechanism not associated with some Hsp70 inhibitors. There are relatively few reports describing Hsp70 inhibitors and of these only two classes have been rationally designed. Vernalis has described a series of aden-osine inhibitors such as VER155008 that target the nucleotide-binding pocket. These compounds directly compete with ATP, and despite potent binding, these compounds have limited cellular activity. Researchers at MSKCC have claimed a series of pyrimidines which were designed to target an allosteric site outside the ATP-binding pocket. Other compounds have been claimed, including sulfonamides, imidazoles and peptides. Most of the inhibitors reported to date bind to Hsp70 by an alternative mechanism to direct ATP-competition and reveal a general trend toward the development of compounds that function through an allosteric mechanism. In contrast to Hsp90, it does not appear that direct targeting of the ATP-pocket with small-molecule inhibitors will be a viable anticancer strategy for Hsp70. Rational-based strategies like that used in discovering compound 112 may be useful in advancing small-molecule Hsp70 inhibitors as a viable anticancer strategy. Although there are only a limited number of patents claiming compounds as Hsp70 inhibitors, interest in this class of molecules in the future is predicted to be high.
5. Expert opinion
Hsp90 and Hsp70 are targets of significant interest to the drug discovery community. These proteins are chaperones with a multitude of functions in the cell and as such put a wrinkle in the one drug-one target paradigm that has driven cancer drug discovery since the inception of the age of targeted therapies. Unlike conventional targeted therapies which aim to effect a single cancer-related pathway (i.e., kinases, receptors), Hsp90 is a nodal protein interacting with multiple pathways, and whose function is co-opted by cancer cells for their own survival. Many of Hsp90s client proteins are involved in aberrant signaling which have been implicated in the development and progression of cancer. Therefore, inhibition of Hsp90 with a single small molecule can be considered akin to inhibiting a multitude of proteins which would otherwise require numerous different molecules. Similarly, Hsp70 is recognized for its role as a co-chaperone to Hsp90 as well as its powerful role in cell survival through inhibition of both intrinsic and extrinsic apoptotic pathways. Taken together, chaperones such as Hsp90 and Hsp70 represent a potential solution to the primary issue related to current targeted therapies, namely the heterogeneous nature of tumors that is a cause of resistance that frequently leads to treatment failure.
Despite the immense interest and potential for inhibitors of Hsp90 or Hsp70, no such agents are yet approved by the FDA. However, it is clear at this point that efforts to drug Hsp90 are far more advanced compared to Hsp70. To date, 17 different Hsp90 inhibitors have been or are being evaluated in man whereas no designated Hsp70 inhibitor has entered clinical trial, a fact borne out by the difference in the number of patents described here for each. We have earlier elaborated on the relative difficulty in ‘drugging’ Hsp70, but suffice it to say that much more intense efforts have been devoted towards Hsp90. It is a reality that Hsp70 inhibitors represent higher hanging fruit that to pick would at the very least require efforts from the drug discovery community on a scale that has been devoted to Hsp90.
Currently, the discovery of Hsp70 inhibitors is largely an academic endeavor, similar to the state of affairs in the early days of Hsp90 inhibitor development, which was largely performed by researchers at academic institutions and smaller biotech companies. It was only after publication of the landmark paper by Kamal et al. [8], which for the first time gave a sound rational for the observed therapeutic index of Hsp90 inhibitors, that large pharmaceutical companies began to take notice and devote their enormous resources to this. A similar potential exists for Hsp70 because it remains an extremely interesting target.
Much of the efforts surrounding Hsp90 inhibitor development continue to be focused on the elaboration around three initially discovered scaffolds, the natural products ansamycins and RD (i.e., resorcinol) as well as the purine-scaffold, with the goal of optimizing pharmaceutical properties. Although there has been considerable creativity in the discovery of novel chemotypes which fall outside the three initially discovered scaffolds, much of the patent literature appears to build on previously reported SAR through slight modifications of Hsp90 inhibitor space by finding weakness in existing patents. Some of the efforts were not necessarily meant to improve affinity, which was already good, but rather sought to address potential liabilities related to absorption, distribution, metabolism, excretion, toxicity properties. In this context, some of these efforts have been successful but not so for others. For example, attempts to modify the ansamycins have been plagued by numerous liabilities that cannot be overcome by chemical modification, despite intense efforts to do so.
Essentially we already have an array of excellent agents in the clinic. In preclinical species, these agents show desirable pharmacokinetic properties, whereby they are selectively retained in tumor tissues at therapeutic concentrations for a prolonged period of time. Therefore, the major goal for future development of Hsp90 inhibitors is not necessarily identifying better molecules but rather it will be to better understand how to best use these agents in the clinic. Important questions that will need to be answered in this regard include: What is the ideal dosing schedule? Who are the patients to most likely benefit from therapy and how can they be identified? How can an Hsp90 inhibitor be combined with other agents to effect maximal anticancer responses? Additionally, there has been progress in the development of Hsp90 paralog selective inhibitors [135]. Such isoform selective molecules may increase the safety/tolerability/efficacy that is observed with the pan-Hsp90 inhibitors currently in the clinic.
Given the real promise for the potential of these molecules to affect cancer and the fact that no drug has yet been approved it is highly likely that patents describing Hsp90 inhibitors will continue to be published at high rates. It is likely that the future will be as bountiful for patents describing Hsp70 inhibitors as well.
Article highlights.
Heat shock protein (Hsp)90 and Hsp70 are two important chaperones often overexpressed in cancer and whose function in the development and progression of cancer have made them important targets in drug discovery.
Intense efforts have been directed toward the discovery and development of Hsp90 inhibitors resulting in a large number of patent applications and 17 different molecules to enter clinical trials evaluating their effectiveness in cancer.
Efforts to develop Hsp70 inhibitors have not yet proven as fruitful as those directed toward Hsp90, as evidenced by the limited number of patent applications filed and the lack of a clinical candidate.
Whereas development of ATP-competitive inhibitors has proven a good strategy for Hsp90, structure-based design targeting allosteric sites may yield Hsp70 inhibitors with potent in vivo anticancer activity.
Future development of Hsp90 inhibitors will focus on how best to use these compounds in the clinic.
Due to increasing interest in chaperone biology as it relates to cancer and other diseases, interest in inhibitors of Hsp90 and Hsp70 will remain high for the foreseeable future.
This box summarizes key points contained in the article.
Acknowledgments
Memorial Sloan-Kettering Cancer Center holds the intellectual rights to the Hsp90 and Hsp70 portfolio. Samus Therapeutics, of which G Chiosis has partial ownership, has licensed the Hsp90 cancer portfolio. This work was supported in part by Jane H Gordon Breast Cancer Research Fund, the Hirshberg Foundation for Pancreatic Cancer, the Byrne Fund, Susan G Komen for the Cure, Leukemia and Lymphoma Society #6330-11, R01 CA172546, R01 CA155226, U01 AG032969 and R21 CA158609. G Chiosis is a director at Samus Therapeutics.
Footnotes
Declaration of interest
The authors declare no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Kim YE, Hipp MS, Bracher A, et al. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–55. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- 2••.Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Cancer. 2013;13:630–42. doi: 10.1038/nrm3658. [Ref [1,2] are comprehensive and up-to-date reviews on the structure and function of molecular chaperones.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santarosa M, Favaro D, Quaia M, et al. Expression of heat shock protein 72 in renal cell carcinoma: possible role and prognostic implications in cancer patients. Eur J Cancer. 1997;33:873–7. doi: 10.1016/s0959-8049(97)00002-6. [DOI] [PubMed] [Google Scholar]
- 4.Nanbu K, Konishi I, Mandai M, et al. Prognostic significance of heat shock proteins HSP70 and HSP90 in endometrial carcinomas. Cancer Detect Prev. 1998;22:549–55. doi: 10.1046/j.1525-1500.1998.00069.x. [DOI] [PubMed] [Google Scholar]
- 5.Trieb K, Lechleitner T, Lang S, et al. Heat shock protein 72 expression in osteosarcomas correlates with good response to neoadjuvant chemotherapy. Hum Pathol. 1998;29:1050–5. doi: 10.1016/s0046-8177(98)90412-9. [DOI] [PubMed] [Google Scholar]
- 6.Uozaki H, Ishida T, Kakiuchi C, et al. Expression of heat shock proteins in osteosarcoma and its relationship to prognosis. Pathol Res Pract. 2000;196:665–73. doi: 10.1016/S0344-0338(00)80118-1. [DOI] [PubMed] [Google Scholar]
- 7••.Moulick K, Ahn JH, Zong H, et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol. 2011;7:818–26. doi: 10.1038/nchembio.670. [This paper shows that cancer cells contain distinct pools of Hsp90 which can be selectively targeted by certain Hsp90 inhibitors.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Kamal A, Thao L, Sensintaffar J, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–10. doi: 10.1038/nature01913. [This paper shows that Hsp90 in cancer cells are predominantly bound in high affinity chaperone complexes and describes the origins for the increased sensitivity of cancer cells to Hsp90 inhibitors.] [DOI] [PubMed] [Google Scholar]
- 9••.Powers MV, Clarke PA, Workman P. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell. 2008;14:250–62. doi: 10.1016/j.ccr.2008.08.002. [This paper shows that knockdown of both Hsc70 and Hsp70 in cancer cells is required in order to elicit significant anticancer effects. Furthermore, dual knockdown of Hsc70 and Hsp70 in non-tumor cell lines does not result in comparable cytotoxic effects, thus indicating a potential therapeutic window.] [DOI] [PubMed] [Google Scholar]
- 10.Powers MV, Clarke PA, Workman P. Death by chaperone: HSP90, HSP70 or both? Cell Cycle. 2009;8:518–26. doi: 10.4161/cc.8.4.7583. [DOI] [PubMed] [Google Scholar]
- 11.Luo W, Sun W, Taldone T, et al. Heat shock protein 90 in neurodegenerative diseases. Mol Neurodegener. 2010;5:24. doi: 10.1186/1750-1326-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo W, Dou F, Rodina A, et al. Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc Natl Acad Sci USA. 2007;104:9511–16. doi: 10.1073/pnas.0701055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsan MF, Gao B. Heat shock protein and innate immunity. Cell Mol Immunol. 2004;1:274–9. [PubMed] [Google Scholar]
- 14.Yun TJ, Harning EK, Giza K, et al. EC144, a synthetic inhibitor of heat shock protein 90, blocks innate and adaptive immune responses in models of inflammation and autoimmunity. J Immunol. 2011;186:563–75. doi: 10.4049/jimmunol.1000222. [DOI] [PubMed] [Google Scholar]
- 15.Rochani AK, Singh M, Tatu U. Heat shock protein 90 inhibitors as broad spectrum anti-infectives. Curr Pharm Des. 2013;19:377–86. doi: 10.2174/138161213804143608. [DOI] [PubMed] [Google Scholar]
- 16.Csermely P, Schnaider T, Soti C, et al. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–68. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 17•.Mayer MP. Gymnastics of molecular chaperones. Mol Cell. 2010;39:321–31. doi: 10.1016/j.molcel.2010.07.012. [A review article which discusses the conformational dynamics of Hsp70 and Hsp90 as well as other ATP-dependent chaperones such as Hsp60 and Hsp100.] [DOI] [PubMed] [Google Scholar]
- 18.Trepel J, Mollapour M, Giaccone G, et al. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–49. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–28. doi: 10.1038/nrm2918. [Ref [18,19] are excellent review articles concerned with Hsp90 biology and implications for therapeutic intervention.] [DOI] [PubMed] [Google Scholar]
- 20.Evans CG, Chang L, Gestwicki JE. Heat shock protein 70 (hsp70) as an emerging drug target. J Med Chem. 2010;53:4585–602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Brodsky JL, Chiosis G. Hsp70 molecular chaperones: emerging roles in human disease and identification of small molecule modulators. Curr Top Med Chem. 2006;6:1215–25. doi: 10.2174/156802606777811997. [Ref [20,21] are interesting review articles on Hsp70.] [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Carbonero R, Carnero A, Paz-Ares L. Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet Oncol. 2013;14:e358–e69. doi: 10.1016/S1470-2045(13)70169-4. [DOI] [PubMed] [Google Scholar]
- 23•.Jhaveri K, Taldone T, Modi S, et al. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta. 2012;1823:742–55. doi: 10.1016/j.bbamcr.2011.10.008. [Ref [22,23] are review articles which discusses the current state of Hsp90 inhibitor development in the clinic.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drysdale MJ, Brough PA. Medicinal chemistry of Hsp90 inhibitors. Curr Top Med Chem. 2008;8:859–68. doi: 10.2174/156802608784911644. [DOI] [PubMed] [Google Scholar]
- 25•.Janin YL. ATPase inhibitors of heat-shock protein 90, second season. Drug Discov Today. 2010;15:342–53. doi: 10.1016/j.drudis.2010.03.002. [A comprehensive survey of Hsp90 inhibitors reported in the literature from around 2005 to 2010.] [DOI] [PubMed] [Google Scholar]
- 26.Janin YL. Heat shock protein 90 inhibitors. A text book example of medicinal chemistry? J Med Chem. 2005;48:7503–12. doi: 10.1021/jm050759r. [DOI] [PubMed] [Google Scholar]
- 27.Taldone T, Sun W, Chiosis G. Discovery and development of heat shock protein 90 inhibitors. Bioorg Med Chem. 2009;17:2225–35. doi: 10.1016/j.bmc.2008.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Chène P. ATPases as drug targets: learning from their structure. Nat Rev Drug Discov. 2002;1:665–73. doi: 10.1038/nrd894. [A review article which describes the structural features of the nucleotide-binding site of a number of ATPases, including Hsp70 and Hsp90, and defines the conformational differences adopted by ADP/ATP when protein-bound.] [DOI] [PubMed] [Google Scholar]
- 29.Brandt GEL, Blagg BSJ. Alternate strategies of Hsp90 modulation for the treatment of cancer and other diseases. Curr Top Med Chem. 2009;9:1447–61. doi: 10.2174/156802609789895683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel HJ, Modi S, Chiosis G, et al. Advances in the discovery and development of heat-shock protein 90 inhibitors for cancer treatment. Expert Opin Drug Discov. 2011;6:559–87. doi: 10.1517/17460441.2011.563296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messaoudi S, Peyrat JF, Brion JD, et al. Heat-shock protein 90 inhibitors as antitumor agents: a survey of the literature from 2005 to 2010. Expert Opin Ther Pat. 2011;21:1501–42. doi: 10.1517/13543776.2011.594041. [DOI] [PubMed] [Google Scholar]
- 32.Dymock BW, Drysdale MJ, McDonald E, et al. Inhibitors of HSP90 and other chaperones for the treatment of cancer. Expert Opin Ther Pat. 2004;14:837–47. [Google Scholar]
- 33.Biamonte MA, Van de Water R, Arndt JW, et al. Heat shock protein 90: inhibitors in clinical trials. J Med Chem. 2010;53:3–17. doi: 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]
- 34.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo W, Reigan P, Siegel D, et al. Enzymatic reduction and glutathione conjugation of benzoquinone ansamycin heat shock protein 90 inhibitors: relevance for toxicity and mechanism of action. Drug Metab Dispos. 2008;36:2050–7. doi: 10.1124/dmd.108.022004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansske FG, Werner S, Eckard P, et al. Synthesis of ansamycin derivatives. 2008 WO2008034895.
- 37.Ross D, Siegel D, Guo W, et al. 19-Substituted geldanamycin derivative Hsp90 inhibitors with modified toxicity, and use in the treatment of cancers and other proliferative disorders. 2009 WO2009026548A1.
- 38.Ross D, Siegel D, Moody CJ, et al. HSP90 inhibitors with modified toxicity. 2013 WO2013074695.
- 39•.Chiosis G, Timaul MN, Lucas B, et al. A small molecule designed to bind to the adenine nucleotide pocket of Hsp90 causes Her2 degradation and the growth arrest and differentiation of breast cancer cells. Chem Biol. 2001;8:289–99. doi: 10.1016/s1074-5521(01)00015-1. [This paper describes the design and validation of the first reported synthetic Hsp90 inhibitor, PU3.] [DOI] [PubMed] [Google Scholar]
- 40.Chiosis G, Rosen N. Small molecule compositions for binding to HSP90. 2002 WO2002036075.
- 41•.He H, Zatorska D, Kim J, et al. Identification of potent water soluble purine-scaffold inhibitors of the heat shock protein 90. J Med Chem. 2006;49:381–90. doi: 10.1021/jm0508078. [This paper describes the design and synthesis of PU-H71 and SAR of related purine-scaffold compounds.] [DOI] [PubMed] [Google Scholar]
- 42.Rodina A, Vilenchik M, Moulick K, et al. Selective compounds define Hsp90 as a major inhibitor of apoptosis in small-cell lung cancer. Nat Chem Biol. 2007;3:498–507. doi: 10.1038/nchembio.2007.10. [DOI] [PubMed] [Google Scholar]
- 43.Caldas-Lopes E, Cerchietti L, Ahn JH, et al. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc Natl Acad Sci USA. 2009;106:8368–73. doi: 10.1073/pnas.0903392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cerchietti LC, Lopes EC, Yang SN, et al. A purine scaffold Hsp90 inhibitor destabilizes BCL-6 and has specific antitumor activity in BCL-6-dependent B cell lymphomas. Nat Med. 2009;15:1369–76. doi: 10.1038/nm.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marubayashi S, Koppikar P, Taldone T, et al. HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans. J Clin Invest. 2010;120:3578–93. doi: 10.1172/JCI42442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Kasibhatla SR, Hong K, Biamonte MA, et al. Rationally designed high-affinity 2-amino-6-halopurine heat shock protein 90 inhibitors that exhibit potent antitumor activity. J Med Chem. 2007;50:2767–78. doi: 10.1021/jm050752+. [This paper describes the design and synthesis of BIIB021 and SAR of related purine-scaffold compounds.] [DOI] [PubMed] [Google Scholar]
- 47.Lundgren K, Zhang H, Brekken J, et al. BIIB021, an orally available, fully synthetic small-molecule inhibitor of the heat shock protein Hsp90. Mol Cancer Ther. 2009;8:921–9. doi: 10.1158/1535-7163.MCT-08-0758. [DOI] [PubMed] [Google Scholar]
- 48.Yin X, Zhang H, Lundgren K, et al. BIIB021, a novel Hsp90 inhibitor, sensitizes head and neck squamous cell carcinoma to radiotherapy. Int J Cancer. 2010;126:1216–25. doi: 10.1002/ijc.24815. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Neely L, Lundgren K, et al. BIIB021, a synthetic Hsp90 inhibitor, has broad application against tumors with acquired multidrug resistance. Int J Cancer. 2010;126:1226–34. doi: 10.1002/ijc.24825. [DOI] [PubMed] [Google Scholar]
- 50•.Kim S-H, Bajji A, Tangallapally R, et al. Discovery of (2S)-1-[4-(2-{6-Amino-8-[(6-bromo-1,3-benzodioxol-5-yl) sulfanyl]-9H-purin-9-yl}ethyl)piperidin-1-yl]-2-hydroxypropan-1-one (MPC-3100), a Purine-Based Hsp90 Inhibitor. J Med Chem. 2012;55:7480–501. doi: 10.1021/jm3004619. [This paper describes the design and synthesis of MPC-3100 and SAR of related purine-scaffold compounds.] [DOI] [PubMed] [Google Scholar]
- 51.Immormino RM, Kang Y, Chiosis G, et al. Structural and quantum chemical studies of 8-aryl-sulfanyl adenine class Hsp90 inhibitors. J Med Chem. 2006;49:4953–60. doi: 10.1021/jm060297x. [DOI] [PubMed] [Google Scholar]
- 52.Chiosis G, Greengard P, Dou F, et al. Treatment of neurodegenerative diseases through inhibition of hsp90. 2008 WO2008005937.
- 53.Chiosis G, Taldone T, Sun W. Purine derivatives useful as Hsp90 inhibitors. 2011 WO2011044394.
- 54.Chen J, Sperl G, Gullo V, et al. Preparation of sulfamoyl-containing heterocycles as anticancer agents. 2008 WO2008049105.
- 55.Takahashi E, Beppu T. A new nucleosidic antibiotic AT-265. J Antibiot. 1982;35:939–47. doi: 10.7164/antibiotics.35.939. [DOI] [PubMed] [Google Scholar]
- 56.Beautement K, Chrystal EJT, Howard J, et al. N-(alpha-aminoacyl)-5’-O-sulfamoyladenosines: natural product based inhibitors of amino acyl tRNA synthetases. Spec Publ R Soc Chem. 2000;257:288–94. [Google Scholar]
- 57.Qian C, Cai X, Gould S, et al. Preparation of benzodioxolyl purine derivatives as HSP90 inhibitors containing a zinc binding moiety. 2008 WO2008115262.
- 58.Moffat DCF, Baker KWJ, Donald ADG, et al. Preparation of purine amino acid derivatives for the treatment of cancer, autoimmune and inflammatory diseases. 2009 WO2009136144.
- 59.Bajji AC, Kim S-H, Tangallapally R, et al. Preparation of arylthiopurinamine derivatives for use as antitumor agents. 2009 WO2009065035.
- 60.Bajji AC, Kim S-H, Markovitz B, et al. Preparation of substituted purinamines as antitumor agents. 2007 WO2007134298.
- 61.Llauger L, He H, Kim J, et al. Evaluation of 8-arylsulfanyl, 8-arylsulfoxyl, and 8-arylsulfonyl adenine derivatives as inhibitors of the heat shock protein 90. J Med Chem. 2005;48:2892–905. doi: 10.1021/jm049012b. [DOI] [PubMed] [Google Scholar]
- 62.Cai X, Qian C, Zhai H. Preparation of imidazo[4,5-c]pyridine derivatives as HSP90 inhibitors. 2008 WO2008115719.
- 63.Taldone T, Patel PD, Patel M, et al. Experimental and structural testing module to analyze paralogue-specificity and affinity in the Hsp90 inhibitors series. J Med Chem. 2013:6803–18. doi: 10.1021/jm400619b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.Bao R, Lai C-J, Qu H, et al. CUDC-305, a novel synthetic HSP90 inhibitor with unique pharmacologic properties for cancer therapy. Clin Cancer Res. 2009;15:4046–57. doi: 10.1158/1078-0432.CCR-09-0152. [This paper describes the pharmacological properties of the imidazopyridine CUDC-305.] [DOI] [PubMed] [Google Scholar]
- 65.Cai X, Qian C. Fused amino pyridines for the treatment of brain tumors. 2010 WO2010083403.
- 66.Martinell PM, Navarro MI, Soler LM, et al. Preparation of 1H-imidazole-4-carboxamide derivatives as Hsp90 inhibitors. 2009 WO2009007399.
- 67.Brough P, Drysdale M. Preparation of aryl-1H-pyrrolo[2,3-b]pyridine derivatives for use as HSP90 inhibitors. 2008 WO2008025947.
- 68.Brough P, Drysdale M, Davis N. Preparation of pyrrolopyrimidine derivatives having HSP90 inhibitory activity. 2009 WO2009030871.
- 69.Brough PA, Barril-Alonso X, Drysdale MJ. Preparation of pyrimidothiophene derivatives for use as HSP90 inhibitors. 2006 WO2006090094.
- 70•.Brough PA, Barril X, Borgognoni J, et al. Combining hit identification strategies: fragment-based and in silico approaches to orally active 2-aminothieno [2,3-d]pyrimidine inhibitors of the Hsp90 molecular chaperone. J Med Chem. 2009;52:4794–809. doi: 10.1021/jm900357y. [This paper describes the design and synthesis of NVP-BEP800 and SAR of related compounds.] [DOI] [PubMed] [Google Scholar]
- 71.Massey AJ, Schoepfer J, Brough PA, et al. Preclinical antitumor activity of the orally available heat shock protein 90 inhibitor NVP-BEP800. Mol Cancer Ther. 2010;9:906–19. doi: 10.1158/1535-7163.MCT-10-0055. [DOI] [PubMed] [Google Scholar]
- 72.Day FA, Launay DFM, Charlton MH, et al. Pyrrolo[2,3-d]pyrimidine derivatives as HSP90 inhibitors and their preparation, pharmaceutical compositions and use in the treatment of diseases. 2010 WO2010043867.
- 73.Kasibhatla SR, Biamonte MA, Shi J, et al. Alkynylpyrrolo[2,3-d]pyrimidines as HSP90 inhibitors, their preparation, pharmaceutical compositions, and use in therapy. 2006 WO2006105372.
- 74•.Shi J, Van de Water R, Hong K, et al. EC144 is a potent inhibitor of the heat shock protein 90. J Med Chem. 2012;55:7786–95. doi: 10.1021/jm300810x. [This paper describes the design and synthesis of EC144 and SAR of related pyrrolopyrimidines.] [DOI] [PubMed] [Google Scholar]
- 75.Kung P-P, Meng JJ. Preparation of 2-aminopyrimidine derivatives as HSP-90 inhibitors patent. 2008 WO2008059368.
- 76.Kung P-P, Meng JJ. Preparation of pyrazolylethoxyphenyl pyrroloyrimidinamines as heat shock protein-90 (HSP-90) inhibitors. 2010 WO2010018481.
- 77.Ohsuki S, Tengeiji A, Ikeda M, et al. Preparation of pyrazolopyrimidine derivatives as inhibitors of heat shock protein 90 (HSP 90). 2008 WO2008035629.
- 78.Ousu S, Tengeiji A, Ikeda M, et al. Preparation of pyrazolopyrimidine derivatives as inhibitors of heat shock protein 90 (HSP 90). 2009 JP2009256323.
- 79.Ohki H, Okayama T, Ikeda M, et al. Preparation of tricyclic pyrazolopyrimidine derivatives as Hsp90 inhibitors. 2010 WO2010098344.
- 80.Oki H, Okayama T, Ikeda M, et al. Preparation of tricyclic pyrazolopyrimidine derivatives as Hsp90 inhibitors. 2012 JP2012067087.
- 81.Sun CL, Li X, Zhu Y. Preparation of aminopteridinone derivatives and analogs for use as HSP90 inhibitors. 2009 WO2009139834.
- 82.Courtney SM, Whittaker M, Mather OC, et al. Preparation of 2-amino-7,8-dihydro-6H-quinazolin-5-one oximes having HSP90 inhibitory activity. 2008 WO2008142720.
- 83.Amici R, Colombo A, Courtney SM, et al. Quinazoline derivatives with Hsp90 inhibitory activity. 2013 WO2013064919.
- 84.Chen YK, Co EW, Guntupalli P, et al. Oxime derivatives as HSP90 inhibitors and their preparation, pharmaceutical compositions and use in the treatment of diseases. 2009 WO2009097578.
- 85.Eggenweiler H-M, Sirrenberg C, Buchstaller H-P. Preparation of quinazoline amides as HSP90 modulators. 2009 WO2009010139.
- 86.Eggenweiler H-M, Sirrenberg C, Buchstaller H-P. Preparation of quinazoline amides as HSP90 inhibitors. 2010 WO2010066324.
- 87.Eggenweiler H-M, Sirrenberg C, Buchstaller H-P. Quinazoline derivatives as HSP90 inhibitors and their preparation and use in the treatment of diseases. 2011 WO2011060873A1.
- 88.Eggenweiler H-M, Sirrenberg C, Buchstaller H-P. Phenylquinazoline derivatives as HSP90 inhibitors and their preparation. 2012 WO2012041435. [Google Scholar]
- 89.Nowak T. 5,6,7,8-Tetrahydropteridine derivatives as Hsp90 inhibitors. 2008 WO2008093075.
- 90.Schulte TW, Akinaga S, Soga S, et al. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones. 1998;3:100–8. doi: 10.1379/1466-1268(1998)003<0100:arbttn>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kwon HJ, Yoshida M, Fukui Y, et al. Potent and specific inhibition of p60v-src protein kinase both in vivo and in vitro by radicicol. Cancer Res. 1992;52:6926–30. [PubMed] [Google Scholar]
- 92.Ying W. Preparation of triazoles and related compounds as Hsp90 inhibitors. 2008 WO2008051416.
- 93.Proia D, Acquaviva J. Combination therapy of Hsp90 inhibitors with BRAF inhibitors. 2013 WO2013074594.
- 94.Ying W, Chimmanamada DU, Burlison JA, et al. Preparation of triazole compounds that modulate Hsp90 activity. 2010 WO2010017479.
- 95.Ying W, Chimmanamada DU, Burlison JA, et al. Preparation of substituted triazoles, particularly 4,5-diphenyl-4H-1,2,4-triazole-3-carboxamides, that modulate Hsp90 activity. 2010 WO2010017545.
- 96.Chimmanamada D, Demko Z, Ying W. Triazole derivatives as hsp90 inhibitors. 2013 WO2013148857. [Google Scholar]
- 97.Burlison JA, Chimmanamada DU, Ying W, et al. Preparation of phenyl hydrazonamide derivatives as modulators of Hsp90 activity. 2009 WO2009158026.
- 98.Chimmanamada DU, Ying W. Pyrrole compounds that modulate HSP90 activity. 2009 WO2009148599.
- 99.Yang R-Y, Ali SM, Ashwell MA, et al. Preparation of substituted tetrazole compounds as HSP90 inhibitors for treating cell proliferative disorder. 2009 WO2009049305.
- 100.Giannini G, Cabri W, Simoni D, et al. Preparation of 5-phenylisoxazole-3-carboxamides as Hsp90 modulators with antitumor activity. 2010 WO2010000748. [Google Scholar]
- 101.Giannini G, Cabri W, Vesci L, et al. Preparation of aryl triazoles as Hsp90 inhibitors for treating cancer. 2012 WO2012084602. [Google Scholar]
- 102.Eggenweiler H-M, Sirrenberg C, Buchstaller H-P. 1,3-Dihydroisoindole derivatives as HSP90 inhibitors, their preparation, pharmaceutical compositions, and use in therapy. 2009 DE102007041116.
- 103.Mantegani S, Brasca MG, Casuscelli F, et al. Preparation of bicyclic pyrazole and isoxazole derivatives as antitumor and antineurodegenerative agents and as agents for treating other diseases mediated by HSP90 protein. 2010 WO2010060854.
- 104.Brasca MG, Casale E, Ferguson R, et al. Preparation of resorcinol derivatives as HSP90 inhibitors. 2010 WO2010121963.
- 105.Brasca MG, Mantegani S, Amboldi N, et al. Discovery of NMS-E973 as novel, selective and potent inhibitor of heat shock protein 90 (Hsp90). Bioorg Med Chem. 2013;21:7047–63. doi: 10.1016/j.bmc.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 106••.Massey AJ. ATPases as drug targets: insights from heat shock proteins 70 and 90. J Med Chem. 2010;53:7280–6. doi: 10.1021/jm100342z. [This paper offers a valuable perspective into targeting the ATP pockets of Hsp70 and Hsp90 and describes the difficulties in this approach against Hsp70.] [DOI] [PubMed] [Google Scholar]
- 107•.Williamson DS, Borgognoni J, Clay A, et al. Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. J Med Chem. 2009;52:1510–13. doi: 10.1021/jm801627a. [This paper describes the first efforts of a rational approach toward inhibitors of Hsp70 by designing ATP-competitive adenosine-based inhibitors.] [DOI] [PubMed] [Google Scholar]
- 108.Patury S, Miyata Y, Gestwicki JE. Pharmacological targeting of the Hsp70 chaperone. Curr Top Med Chem. 2009;9:1337–51. doi: 10.2174/156802609789895674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fewell SW, Smith CM, Lyon MA, et al. Small molecule modulators of endogenous and co-chaperone-stimulated Hsp70 ATPase activity. J Biol Chem. 2004;279:51131–40. doi: 10.1074/jbc.M404857200. [DOI] [PubMed] [Google Scholar]
- 110.Fewell SW, Day BW, Brodsky JL. Identification of an inhibitor of hsc70-mediated protein translocation and ATP hydrolysis. J Biol Chem. 2001;276:910–14. doi: 10.1074/jbc.M008535200. [DOI] [PubMed] [Google Scholar]
- 111.Nadler SG, Eversole AC, Tepper MA, et al. Elucidating the mechanism of action of the immunosuppressant 15-deoxyspergualin. Ther Drug Monit. 1995;17:700–3. doi: 10.1097/00007691-199512000-00026. [DOI] [PubMed] [Google Scholar]
- 112.Brodsky JL. Selectivity of the molecular chaperone-specific immunosuppressive agent 15-deoxyspergualin: modulation of Hsc70 ATPase activity without compromising DnaJ chaperone interactions. Biochem Pharmacol. 1999;57:877–80. doi: 10.1016/s0006-2952(98)00376-1. [DOI] [PubMed] [Google Scholar]
- 113.Jinwal UK, Miyata Y, Koren J, III, et al. Chemical manipulation of hsp70 ATPase activity regulates tau stability. J Neurosci. 2009;29:12079–88. doi: 10.1523/JNEUROSCI.3345-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kawakami M, Koya K, Ukai T, et al. Structure-activity of novel rhodacyanine dyes as antitumor agents. J Med Chem. 1998;41:130–42. doi: 10.1021/jm970590k. [DOI] [PubMed] [Google Scholar]
- 115.Kawakami M, Koya K, Ukai T, et al. Synthesis and evaluation of novel rhodacyanine dyes that exhibit antitumor activity. J Med Chem. 1997;40:3151–60. doi: 10.1021/jm9702692. [DOI] [PubMed] [Google Scholar]
- 116.Wadhwa R, Sugihara T, Yoshida A, et al. Selective toxicity of MKT-077 to cancer cells is mediated by its binding to the hsp70 family protein mot-2 and reactivation of p53 function. Cancer Res. 2000;60:6818–21. [PubMed] [Google Scholar]
- 117.Chang L, Miyata Y, Ung PM, et al. Chemical screens against a reconstituted multiprotein complex: myricetin blocks DnaJ regulation of DnaK through an allosteric mechanism. Chem Biol. 2011;18:210–21. doi: 10.1016/j.chembiol.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118•.Rousaki A, Miyata Y, Jinwal UK, et al. Allosteric drugs: the interaction of antitumor compound MKT-077 with human Hsp70 chaperones. J Mol Biol. 2011;411:614–32. doi: 10.1016/j.jmb.2011.06.003. [This paper shows that MKT-077 inhibits Hsp70 through an allosteric mechanism.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shin I-J, Lee M-R, Williams D. Preparation of imidazoles (apoptazoles) as inducers of apoptosis. 2008 WO2008105565.
- 120.Williams DR, Ko SK, Park S, et al. An apoptosis-inducing small molecule that binds to heat shock protein 70. Angew Chem Int Ed Engl. 2008;47:7466–9. doi: 10.1002/anie.200802801. [DOI] [PubMed] [Google Scholar]
- 121.Cho HJ, Gee HY, Baek KH, et al. A small molecule that binds to an ATPase domain of Hsc70 promotes membrane trafficking of mutant cystic fibrosis transmembrane conductance regulator. J Am Chem Soc. 2011;133:20267–76. doi: 10.1021/ja206762p. [DOI] [PubMed] [Google Scholar]
- 122.Garrido C, Colas P, Bickle M, et al. New polypeptides binding to HSP70 ATPase domain and uses in cancer therapy. 2009 WO2009043942.
- 123.Peptide inhibitors of heat shock protein 70 as inducers of apoptosis in cancer therapy. 2009 EP2050758.
- 124.Rérole AL, Gobbo J, De Thonel A, et al. Peptides and aptamers targeting HSP70: a novel approach for anticancer chemotherapy. Cancer Res. 2011;71:484–95. doi: 10.1158/0008-5472.CAN-10-1443. [DOI] [PubMed] [Google Scholar]
- 125.Cociancich S, Dupont A, Hegy G, et al. Novel inducible antibacterial peptides from a hemipteran insect, the sapsucking bug Pyrrhocoris apterus. Biochem J. 1994;300:567–75. doi: 10.1042/bj3000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sturgess MA, Kotch F. Preparation of small molecule and peptide DnaK inhibitors for treating bacterial infections. 2010 US20100087466.
- 127.George DL, Leu JI-J, Murphy M. Screening methods for identifying HSP70/DNAK modulators and uses thereof in treatment of cancer. 2011 US20110189125.
- 128.George DL, Leu JI-J, Murphy M. Modulators of HSP70/DnaK function, particularly 2-phenylethynesulfonamide (PES) derivatives, for use in potentiating the anticancer and antibacterial effects. 2010 WO2010033771.
- 129.Strom E, Sathe S, Komarov PG, et al. Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nat Chem Biol. 2006;2:474–9. doi: 10.1038/nchembio809. [DOI] [PubMed] [Google Scholar]
- 130.Leu JI, Pimkina J, Frank A, et al. A small molecule inhibitor of inducible heat shock protein 70. Mol Cell. 2009;36:15–27. doi: 10.1016/j.molcel.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leu JI, Pimkina J, Pandey P, et al. HSP70 inhibition by the small-molecule 2-phenylethynesulfonamide impairs protein clearance pathways in tumor cells. Mol Cancer Res. 2011;9:936–47. doi: 10.1158/1541-7786.MCR-11-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132•.Balaburski GM, Leu JI, Beeharry N, et al. A modified HSP70 inhibitor shows broad activity as an anticancer agent. Mol Cancer Res. 2013;11:219–29. doi: 10.1158/1541-7786.MCR-12-0547-T. [Ref [130-132] describe the anticancer activities of PES and PES-Cl, Hsp70 inhibitors of the sulfonamide class that are believed to bind in the C terminus.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chiosis G, Taldone T, Rodina A, et al. Pyrimidine-based heat shock protein binding compounds, compositions, and methods for making and using same. 2011 WO2011022440.
- 134•.Rodina A, Patel PD, Kang Y, et al. Identification of an allosteric pocket on human hsp70 reveals a mode of inhibition of this therapeutically important protein. Chem Biol. 2013;20:1469–80. doi: 10.1016/j.chembiol.2013.10.008. [This paper describes a rational approach to targeting a novel allosteric pocket on Hsp70 through irreversible interaction with a native cysteine.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135•.Patel PD, Yan P, Seidler PM, et al. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat Chem Biol. 2013;9:677–84. doi: 10.1038/nchembio.1335. [This paper describes the design of a Grp94-selective inhibitor and offers valuable insights into their application in HER2-overexpressing breast cancers.] [DOI] [PMC free article] [PubMed] [Google Scholar]