Abstract
Background: Circulating saturated fatty acids (SFAs) are integrated biomarkers of diet and metabolism that may influence the pathogenesis of diabetes. In epidemiologic studies, circulating levels of palmitic acid (16:0) are associated with diabetes; however, very-long-chain SFAs (VLSFAs), with 20 or more carbons, differ from palmitic acid in their biological activities, and little is known of the association of circulating VLSFA with diabetes.
Objective: By using data from the Cardiovascular Health Study, we examined the associations of plasma phospholipid VLSFA levels measured at baseline with subsequent incident diabetes.
Design: A total of 3179 older adults, with a mean age of 75 y at study baseline (1992–1993), were followed through 2011. We used multiple proportional hazards regression to examine the associations of arachidic acid (20:0), behenic acid (22:0), and lignoceric acid (24:0) with diabetes.
Results: Baseline levels of each VLSFA were cross-sectionally associated with lower triglyceride levels and lower circulating palmitic acid. We identified 284 incident diabetes cases during follow-up. Compared with the lowest quartile, levels of arachidic acid in the highest quartile of the fatty acid distribution were associated with a 47% lower risk of diabetes (95% CI: 23%, 63%; P-trend: <0.001), after adjustment for demographics, lifestyle factors, and clinical conditions. In analogous comparisons, levels of behenic and lignoceric acid were similarly associated with 33% (95% CI: 6%, 53%; P-trend: 0.02) and 37% (95% CI: 11%, 55%; P-trend: 0.01) lower diabetes risk, respectively. Adjustment for triglycerides and palmitic acid attenuated the associations toward the null, and only the association of arachidic acid remained statistically significant (32% lower risk for fourth vs. first quartile; P-trend: 0.04).
Conclusions: These results suggest that circulating VLSFAs are associated with a lower risk of diabetes, and these associations may be mediated by lower triglycerides and palmitic acid. The study highlights the need to distinguish the effects of different SFAs and to explore determinants of circulating VLSFAs. This trial was registered at clinicaltrials.gov as NCT00005133.
Keywords: diabetes, epidemiology, fatty acids, saturated, arachidic acid, behenic acid, lignoceric acid
See corresponding editorial on page 901.
INTRODUCTION
Type 2 diabetes has reached epidemic proportions, with ∼347 million adults afflicted worldwide (1). Diabetes is a leading cause of cardiovascular disease and a major public health issue (2). Metabolic and lifestyle factors, including obesity, diet, physical activity, and smoking, influence the risk of developing diabetes, as does genetics (3–9).
Circulating SFAs are integrated biomarkers of diet and metabolism that may influence the pathogenesis of diabetes, including insulin resistance and β-cell dysfunction. The SFA palmitic acid (16:0), in particular, has been shown to impair insulin sensitivity in skeletal muscle cells (10–12) and induce lipotoxicity and apoptosis in β cells in culture (13–15). Circulating levels of 16:0 and of other fatty acids that may derive from palmitic acid during de novo lipogenesis, including palmitoleic acid (16:1n–7) and stearic acid (18:0), have been associated with insulin resistance and diabetes risk in several epidemiologic studies (16–20). However, much less is known of the associations of very-long-chain SFAs (VLSFAs), SFAs with 20 carbons or more.
VLSFAs are major components of ceramides and sphingomyelins, lipids made of a sphingosine backbone with one acylated, often saturated, fatty acid (21). Importantly, ceramides with the SFA palmitic acid may play a role in insulin resistance and the decline in β-cell function that can lead to the development of diabetes (22–24). However, ceramides with VLSFAs appear to be protective (25) and may counteract the deleterious effects of ceramides with palmitic acid. For this reason, we hypothesized that higher circulating levels of VLSFAs would be associated with a lower risk of diabetes. We used the Cardiovascular Health Study, a prospective cohort study of risk factors for cardiovascular disease among older adults (26), to examine the associations of plasma phospholipid levels of 3 circulating VLSFAs—arachidic acid (20:0), behenic acid (22:0), and lignoceric acid (24:0)—with incident diabetes.
METHODS
Study population
The Cardiovascular Health Study is a prospective, population-based cohort study of cardiovascular disease among older adults (26). Participants were recruited from 4 US communities (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Allegheny County, Pennsylvania) from a random sample generated from the Health Care Financing Administration files. Among eligible adults who were contacted, 57% agreed to participate. The cohort consists of 5201 noninstitutionalized men and women, aged ≥65 y, recruited in 1989–1990, plus an additional 687 predominantly black participants recruited in 1992–1993. Each center’s institutional review board approved the study, and all participants provided informed written consent to participate in the study.
Phospholipid fatty acids were measured on specimens drawn in 1992–1993, the baseline of our study. Among the 3941 participants with available fatty acid data, we excluded 594 participants with prevalent diabetes at the 1992–1993 examination and 168 participants with no follow-up data on diabetes. The remaining 3179 participants were included in the analyses.
Plasma phospholipid fatty acids
Blood was drawn after 12-h fasting, and plasma specimens were stored at −70°C. Plasma lipids were extracted by the method of Folch et al. (27). Phospholipids were separated from other lipids by thin-layer chromatography. Fatty acid methyl esters were prepared by direct transesterification of the phospholipid fraction (28) and separated by gas chromatography by using a fused-silica 100-m capillary column as previously described (29). Fatty acids were expressed as weight percentage of total fatty acids. Interassay CVs for the VLSFA measurements were ≤3.5%.
Ascertainment of diabetes
Participants were followed by means of annual study clinic examinations with interim phone contacts for 10 y and telephone contacts every 6 mo thereafter. Medication use was assessed annually. Fasting glucose was measured at the study baseline (1992–1993) and in 1996–1997, 1998–1999, and 2005–2006; nonfasting glucose was measured in 1994–1995. Incident and prevalent diabetes were defined by glucose ≥126 mg/dL when participants reported fasting ≥8 h before venipuncture, glucose ≥200 mg/dL when fasting was <8 h, or use of insulin or oral hypoglycemic medication. In secondary analyses, we included additional diabetes cases identified from the Centers for Medicare & Medicaid Services records. These cases were defined by having at least 2 inpatient (i.e., hospital, nursing home, or home health services) or 3 outpatient (outpatient or carrier health services) or ≥1 inpatient and ≥1 outpatient International Classification of Diseases, Ninth Revision, Clinical Modification Medicare claim codes for diabetes diagnosis over a 2-y period. All codes with the prefix 250.xx at any position within the claim were classified as diabetes.
Risk factors
Information on medical history, lifestyle, and clinical risk factors was collected at annual clinic visits as previously reported (26). BMI was calculated as body weight (kg) divided by height squared (m2). Waist circumference was measured at the umbilicus. Lipids, glucose, insulin, and inflammatory biomarkers were assessed on fasting blood samples by using enzymatic methods (26). HOMA-IR was calculated as follows: [fasting glucose (mg/dL) × fasting insulin (IU/mL)]/405. An insulin sensitivity score was calculated as follows: exp[2.63 − 0.28 × log(fasting insulin) − 0.31 × log(fasting triglycerides)] (30). Physical activity was assessed at the 1992–1993 visit by using a modified Minnesota Leisure-Time Activities questionnaire (31). Dietary habits were assessed in 1989–1990 by using a picture-sort food-frequency questionnaire validated against 6 detailed 24-h diet recall interviews spaced ∼1 mo apart. With the exception of diet, covariates measured at the same 1992–1993 visit used for blood sampling were used in the plasma phospholipid VLSFA analyses.
Statistical analysis
Baseline (unadjusted) demographic characteristics, cardiometabolic risk factors, and lifestyle habits for the study population were summarized according to quartiles of each VLSFA. Continuous variables are presented as mean (%), and categorical variables are presented as percentages. Linear and logistic regression was used to assess linear trends of characteristics across quartiles of fatty acids.
The cross-sectional associations of each VLSFA with metabolic factors (i.e., BMI, LDL cholesterol, HDL cholesterol, triglycerides, C-reactive protein, fibrinogen, fasting glucose, fasting insulin, HOMA-IR, and insulin sensitivity score) were assessed by using linear regression; the predicted probabilities (mean ± SE) of each metabolic factor were estimated in a model that held age, sex, race, clinic, education, smoking, alcohol use, physical activity, treated hypertension, ischemic heart disease, self-reported health status, BMI, and waist circumference at their mean. C-reactive protein, fasting insulin, HOMA-IR, and insulin sensitivity score were log10-transformed for these analyses because of skewed distributions.
Comparisons between quartiles of VLSFA levels were evaluated by using indicator variables, with tests for trend based on an ordinal variable. The Cox proportional hazards model was used to estimate the HR of incident diabetes associated with different quartiles of VLSFA levels. The time variable was the minimum amount of time until first diagnosis, death, or latest date of follow-up in 2011. Three models were fit to examine the association of each VLSFA and incident diabetes: 1) a model that adjusted for age and sex (minimally adjusted model); 2) a model that additionally adjusted for race, clinic, education, smoking, alcohol use, BMI, waist circumference, physical activity, treated hypertension, prevalent ischemic heart disease, and self-reported health status at baseline (primary model); and 3) a model that additionally included the potential mediators plasma phospholipid palmitic acid and triglycerides at baseline. To further assess potential mediation, we examined the ratios of HRs of the primary model and the model also adjusted for plasma phospholipid palmitic acid and triglycerides by using bootstrap percentile CIs (32).
Missing covariates (<4% for all covariates) were imputed by using data on age, sex, race, education, smoking, alcohol use, BMI, physical activity, self-reported health status, and prevalent ischemic heart disease at baseline. Results with imputed data are presented. The results were unchanged from analyses that excluded participants with missing values. In sensitivity analyses, we repeated the analyses, including the additional diabetes cases identified from the Centers for Medicare & Medicaid Services.
Modification of the association of VLSFAs with diabetes risk was evaluated for age (linear), sex, and BMI (linear), with statistical significance assessed via the Wald test for the interaction term, in models where VLSFAs were modeled linearly. Analyses used Stata 10.0 (StataCorp LP).
RESULTS
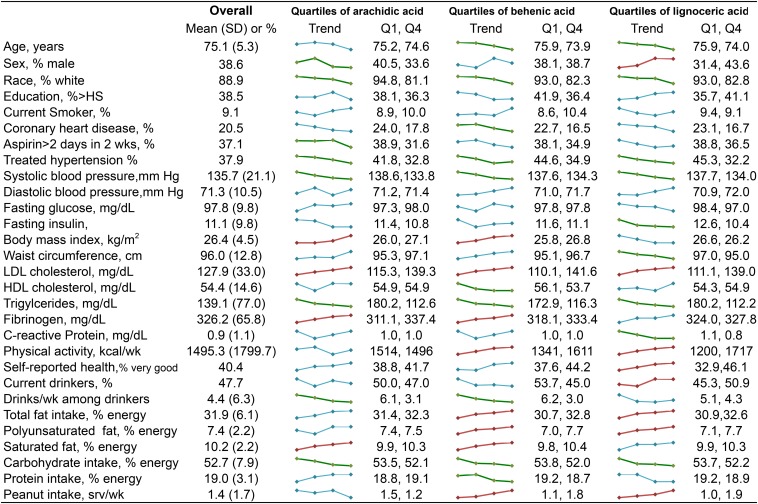
Median (25th, 75th percentile) plasma phospholipid levels of arachidic acid in the study samples were 0.50% of total fatty acids (0.45%, 0.55%). Median levels of behenic and lignoceric acid were 1.65% (1.46%, 1.85%) and 1.37% (1.21%, 1.56%) of total fatty acids, respectively. Baseline characteristics of the study participants and unadjusted trends across quartiles of VLSFAs are illustrated in Figure 1. Participants had a mean age of 75 y at baseline, 39% were male, and 20% had prevalent ischemic heart disease. Participants with higher levels of any of the 3 VLSFAs had lower systolic blood pressure, greater levels of LDL cholesterol, and lower levels of triglycerides (Figure 1). In addition, higher levels of VLSFAs were associated with a lower intake of carbohydrates; higher levels of arachidic and behenic acid were associated with lower alcohol consumption and a greater intake of saturated fat; and higher levels of behenic and lignoceric acid were associated with a greater peanut intake. Among the other plasma phospholipid fatty acids, VLSFAs were negatively correlated with palmitic and palmitoleic acids (Supplemental Table 1).
FIGURE 1.
Characteristics of study participants, overall and according to plasma phospholipid arachidic, behenic, and lignoceric acids (n = 3179). The column labeled “Overall” shows the characteristics [mean (SD) or %] in all study participants. The small colored graphics show means or percentages of each characteristic across quartiles of the fatty acids. Linear and logistic regression was used to assess linear trends of characteristics across quartiles of fatty acids. Trends that were significant at P ≤ 0.002 are colored in red (upward trends) or green (downward trends). Trends with P > 0.002 are colored in blue. The column labeled “Q1, Q4” shows mean or percentage of each characteristic for the first and fourth quartiles of the fatty acid. HS, high school; srv, serving.
We further examined the cross-sectional associations of VLSFAs with metabolic risk factors in multiple regression analyses (Table 1). After adjustment for other risk factors, higher levels of each VLSFA were associated with higher LDL cholesterol and fibrinogen but also with lower triglycerides and a greater insulin sensitivity score. In addition, lignoceric acid was associated with lower fasting insulin and HOMA-IR, lower C-reactive protein, and lower adiposity measures.
TABLE 1.
Cross-sectional associations of plasma phospholipid VLSFAs with metabolic risk factors (n = 3179)1
| Quartiles of plasma phospholipid fatty acids |
|||||
| Q1 | Q2 | Q3 | Q4 | P-trend | |
| Arachidic acid | |||||
| Percentage of total fatty acids | 0.41 (0.26–0.44)2 | 0.47 (0.45–0.50) | 0.52 (0.51–0.55) | 0.59 (0.56–0.82) | |
| Blood lipids | |||||
| LDL cholesterol, mg/dL | 115.2 ± 1.153 | 124.6 ± 1.14 | 130.8 ± 1.04 | 139.2 ± 1.20 | <0.001 |
| Triglycerides, mg/dL | 180.7 ± 3.79 | 141.2 ± 2.38 | 127.8 ± 1.92 | 111.9 ± 1.49 | <0.001 |
| HDL cholesterol, mg/dL | 54.9 ± 0.54 | 52.9 ± 0.42 | 54.8 ± 0.43 | 55.0 ± 0.41 | 0.30 |
| Inflammation | |||||
| Fibrinogen, mg/dL | 311.1 ± 2.17 | 322.5 ± 2.22 | 331.9 ± 2.22 | 337.6 ± 2.42 | <0.001 |
| Ln-CRP,4 mg/dL | 0.97 ± 0.04 | 0.81 ± 0.04 | 0.88 ± 0.04 | 1.02 ± 0.04 | 0.52 |
| Glucose-insulin homeostasis | |||||
| Ln-insulin sensitivity score,4,5 U | 3.87 ± 0.03 | 4.11 ± 0.03 | 4.21 ± 0.03 | 4.36 ± 0.02 | <0.001 |
| Ln-fasting insulin,4 μlU/mL | 2.29 ± 0.02 | 2.27 ± 0.01 | 2.27 ± 0.01 | 2.26 ± 0.01 | 0.01 |
| Ln-HOMA-IR, U | 0.85 ± 0.02 | 0.85 ± 0.02 | 0.84 ± 0.02 | 0.84 ± 0.02 | 0.05 |
| Fasting glucose, mg/dL | 97.3 ± 0.35 | 97.9 ± 0.34 | 97.9 ± 0.32 | 98.0 ± 0.32 | 0.11 |
| Adiposity | |||||
| BMI, kg/m2 | 26.0 ± 0.15 | 26.0 ± 0.15 | 26.3 ± 0.16 | 27.1 ± 0.15 | <0.001 |
| Waist circumference, cm | 95.3 ± 0.44 | 95.9 ± 0.44 | 95.7 ± 0.46 | 97.1 ± 0.44 | <0.001 |
| Behenic acid | |||||
| Percentage of total fatty acids | 1.31 (0.09–1.45) | 1.55 (1.46–1.65) | 1.74 (1.66–1.85) | 2.02 (1.86–3.36) | |
| Blood lipids | |||||
| LDL cholesterol, mg/dL | 110.3 ± 1.16 | 124.8 ± 1.08 | 132.6 ± 1.05 | 141.7 ± 1.14 | <0.001 |
| Triglycerides, mg/dL | 174.3 ± 3.98 | 138.8 ± 2.22 | 131.2 ± 1.93 | 116.2 ± 1.55 | <0.001 |
| HDL cholesterol, mg/dL | 55.8 ± 0.54 | 54.4 ± 0.45 | 53.7 ± 0.39 | 53.7 ± 0.39 | 0.04 |
| Inflammation | |||||
| Fibrinogen, mg/dL | 316.8 ± 2.52 | 325.7 ± 2.26 | 327.7 ± 2.19 | 333.7 ± 2.20 | <0.001 |
| Ln-CRP,4 mg/dL | 0.99 ± 0.04 | 0.82 ± 0.04 | 0.89 ± 0.04 | 0.99 ± 0.04 | 0.22 |
| Glucose-insulin homeostasis | |||||
| Ln-insulin sensitivity score,4,5 U | 3.95 ± 0.03 | 4.14 ± 0.03 | 4.18 ± 0.03 | 4.28 ± 0.03 | <0.001 |
| Ln-fasting insulin,4 μlU/mL | 2.27 ± 0.02 | 2.26 ± 0.01 | 2.28 ± 0.01 | 2.29 ± 0.01 | 0.07 |
| Ln-HOMA-IR,4 U | 0.84 ± 0.02 | 0.83 ± 0.02 | 0.85 ± 0.02 | 0.86 ± 0.02 | 0.21 |
| Fasting glucose, mg/dL | 97.8 ± 0.35 | 97.6 ± 0.34 | 98.0 ± 0.32 | 97.9 ± 0.32 | 0.10 |
| Adiposity | |||||
| BMI, kg/m2 | 25.8 ± 0.16 | 26.1 ± 0.15 | 26.6 ± 0.15 | 26.8 ± 0.15 | 0.007 |
| Waist circumference, cm | 95.1 ± 0.47 | 95.7 ± 0.45 | 96.4 ± 0.44 | 96.7 ± 0.42 | 0.06 |
| Lignoceric acid | |||||
| Percentage of total fatty acids | 1.08 (0.52–1.19) | 1.28 (1.20–1.36) | 1.45 (1.37–1.56) | 1.85 (1.57–3.42) | |
| Blood lipids | |||||
| LDL cholesterol, mg/dL | 111.2 ± 1.17 | 125.8 ± 1.09 | 133.0 ± 1.05 | 139.2 ± 1.15 | <0.001 |
| Triglycerides, mg/dL | 181.1 ± 3.95 | 142.3 ± 2.21 | 126.4 ± 1.88 | 112.1 ± 1.58 | <0.001 |
| HDL cholesterol, mg/dL | 54.1 ± 0.52 | 54.3 ± 0.45 | 54.2 ± 0.42 | 54.8 ± 0.40 | 0.10 |
| Inflammation | |||||
| Fibrinogen, mg/dL | 323.0 ± 2.69 | 326.4 ± 2.20 | 327.0 ± 2.23 | 327.9 ± 2.05 | 0.005 |
| Ln-CRP,4 mg/dL | 1.11 ± 0.04 | 0.92 ± 0.04 | 0.85 ± 0.04 | 0.83 ± 0.04 | <0.001 |
| Glucose-insulin homeostasis | |||||
| Ln-insulin sensitivity score,4,5 U | 3.79 ± 0.03 | 4.08 ± 0.03 | 4.26 ± 0.03 | 4.41 ± 0.03 | <0.001 |
| Ln-fasting insulin,4 μlU/mL | 2.35 ± 0.02 | 2.28 ± 0.01 | 2.24 ± 0.01 | 2.23 ± 0.01 | <0.001 |
| Ln-HOMA-IR,4 U | 0.93 ± 0.02 | 0.85 ± 0.02 | 0.82 ± 0.02 | 0.80 ± 0.02 | <0.001 |
| Fasting glucose, mg/dL | 98.3 ± 0.37 | 98.0 ± 0.33 | 97.9 ± 0.32 | 97.1 ± 0.32 | 0.93 |
| Adiposity | |||||
| BMI, kg/m2 | 26.6 ± 0.17 | 26.5 ± 0.16 | 26.2 ± 0.15 | 26.2 ± 0.15 | 0.002 |
| Waist circumference, cm | 97.1 ± 0.51 | 96.3 ± 0.45 | 95.7 ± 0.42 | 95.0 ± 0.41 | <0.001 |
P-trend values were computed by assigning indicator variables to each fatty acid quartile. CRP, C-reactive protein; Q, quartile; VLSFA, very-long-chain SFA.
Median; range in parentheses (all such values).
Adjusted mean ± SE from linear regression models (all such values). Values were adjusted for age, sex, race, clinic, education, smoking, alcohol use, physical activity, treated hypertension, ischemic heart disease, self-reported health status, BMI, and waist circumference.
Log10-transformed for the analyses due to skewed distribution.
Defined as follows: exp[2.63 − 0.28 × log(fasting insulin) − 0.31 × log(fasting triglycerides)] (30).
During 32,800 person-years of follow-up, we identified 284 cases of incident diabetes. Plasma phospholipid VLSFAs were each associated with a lower risk of diabetes after adjustment for age and sex and after adjustment for other demographic, cardiovascular, and lifestyle risk factors (Table 2). Further adjustments for levels of other fatty acids reportedly associated with diabetes (16–20, 33–37), including eicosapentaenoic acid, docosahexaenoic acid, α-linolenic acid, palmitoleic acid, and trans-palmitoleic acid, and further adjustment for dietary PUFAs did not appreciably change the results (not shown). Adjustment for levels of LDL cholesterol and fibrinogen also did not alter the results (not shown). However, adjustment for the possible mediators triglycerides and plasma phospholipid palmitic acid attenuated the associations (Table 3). CIs for ratios of the HRs comparing the primary model with the model also adjusted for plasma phospholipid palmitic acid and triglycerides, estimated from bootstrap percentile CI, all excluded one, providing statistical evidence of mediation (Supplemental Table 2).
TABLE 2.
Prospective associations of plasma phospholipid arachidic, behenic, and lignoceric acids with incident diabetes (n = 3179)1
| Quartile of plasma phospholipid fatty acid |
|||||
| Q1 | Q2 | Q3 | Q4 | P-trend | |
| Arachidic acid | |||||
| Percentage of total fatty acids | 0.41 (0.26–0.44)2 | 0.47 (0.45–0.50) | 0.52 (0.51–0.55) | 0.59 (0.56–0.82) | |
| Person-years | 7196 | 8248 | 8543 | 8813 | |
| No. of incident cases | 75 | 81 | 69 | 59 | |
| Age and sex adjusted3 | 1.00 (referent) | 0.94 (0.68, 1.28) | 0.77 (0.56, 1.07) | 0.63 (0.45, 0.89) | 0.004 |
| Multivariate adjusted4 | 1.00 (referent) | 0.87 (0.63, 1.20) | 0.72 (0.51, 1.00) | 0.53 (0.37, 0.77) | <0.001 |
| + Mediators5 | 1.00 (referent) | 0.99 (0.71, 1.38) | 0.88 (0.62, 1.27) | 0.68 (0.46, 1.00) | 0.04 |
| Behenic acid | |||||
| Percentage of total fatty acids | 1.31 (0.09–1.45) | 1.55 (1.46–1.65) | 1.74 (1.66–1.85) | 2.02 (1.86–3.36) | |
| Person-years | 7027 | 8057 | 8883 | 8833 | |
| No. of incident cases | 71 | 68 | 73 | 72 | |
| Age and sex adjusted3 | 1.00 (referent) | 0.84 (0.60, 1.17) | 0.78 (0.56, 1.08) | 0.75 (0.54, 1.04) | 0.08 |
| Multivariate adjusted4 | 1.00 (referent) | 0.80 (0.57, 1.12) | 0.73 (0.52, 1.02) | 0.67 (0.47, 0.94) | 0.02 |
| + Mediators5 | 1.00 (referent) | 0.99 (0.69, 1.43) | 0.95 (0.65, 1.37) | 0.94 (0.63, 1.40) | 0.71 |
| Lignoceric acid | |||||
| Percentage of total fatty acids | 1.08 (0.52–1.19) | 1.28 (1.20–1.36) | 1.45 (1.37–1.56) | 1.85 (1.57–3.42) | |
| Person-years | 6847 | 8113 | 8645 | 9195 | |
| No. of incident cases | 79 | 71 | 68 | 66 | |
| Age and sex adjusted3 | 1.00 (referent) | 0.74 (0.54, 1.02) | 0.64 (0.46, 0.88) | 0.56 (0.40, 0.77) | <0.0001 |
| Multivariate adjusted4 | 1.00 (referent) | 0.75 (0.54, 1.04) | 0.72 (0.51, 1.00) | 0.63 (0.45, 0.89) | 0.01 |
| + Mediators5 | 1.00 (referent) | 0.90 (0.63, 1.27) | 0.91 (0.63, 1.31) | 0.84 (0.57, 1.24) | 0.39 |
P-trend values were computed by assigning indicator variables to each fatty acid quartile. Q, quartile.
Median; range in parentheses (all such values).
All values are HRs (95% CIs) from a Cox regression model adjusted for age and sex.
All values are HRs (95% CIs) from a Cox regression model adjusted for age sex, race, clinic, education, smoking, alcohol use, BMI, waist circumference, physical activity, treated hypertension, prevalent ischemic heart disease, and self-reported health status at baseline.
All values are HRs (95% CIs) from a Cox regression model adjusted for age sex, race, clinic, education, smoking, alcohol use, BMI, waist circumference, physical activity, treated hypertension, prevalent ischemic heart disease, and self-reported health status at baseline and further adjusted for the potential mediators plasma phospholipid levels of palmitic acid and triglyceride levels.
TABLE 3.
Prospective associations of plasma phospholipid behenic and lignoceric acids with incident diabetes, stratified by categories of BMI
| BMI, kg/m2 | Total N/diabetes events | Incidence rate per 1000 person-years (95% CI) | Behenic acid1 | Lignoceric acid1 |
| <25 | 1289/59 | 4.57 (3.54, 5.90) | 0.67 (0.52, 0.86) | 0.67 (0.52, 0.86) |
| 25–30 | 1311/120 | 8.43 (7.05, 10.1) | 0.91 (0.76, 1.09) | 0.91 (0.76, 1.09) |
| >30 | 579/102 | 18.9 (15.5, 22.9) | 1.03 (0.84, 1.26) | 1.03 (0.84, 1.26) |
HRs (95% CIs) for an increase in fatty acid levels corresponding to 1 SD. HRs were obtained from Cox regression models adjusted for age, sex, race, clinic, education, smoking, alcohol use, physical activity, treated hypertension, ischemic heart disease, and self-reported health status at baseline. P values for interaction were 0.02 for continuous BMI × behenic acid and 0.005 for continuous BMI × lignoceric acid.
The association of phospholipid VLSFAs with diabetes did not vary according to age and sex. However, we found evidence for an interaction of behenic and lignoceric acid levels with BMI (P values for interaction 0.02 and 0.005, respectively). In analyses stratified by 3 categories of BMI, the association of these fatty acids with lower risk of diabetes was most pronounced among participants with normal BMI (Table 3).
We observed similar results in sensitivity analyses that included diabetes cases identified from Medicare claims (Supplemental Table 3).
DISCUSSION
In this study of older adults, we report an association of higher plasma phospholipid levels of arachidic, behenic, and lignoceric acids with a lower risk of incident diabetes. For example, compared with the lowest quartile, levels of arachidic acid corresponding to the highest quartile were associated with a 47% lower risk (95% CI: 23%, 63%), after adjustment for demographic factors, lifestyle factors, and clinical conditions. The associations were attenuated after adjustment for fasting triglycerides and plasma phospholipid levels of palmitic acid, suggesting that these 2 factors may mediate the associations of VLSFAs with diabetes risk.
Our results are in agreement with a recent report from the European Prospective Investigation into Cancer and Nutrition (EPIC)–InterAct study, published after the completion of our analysis, in which higher plasma phospholipid VLSFAs were associated with lower risk of incident diabetes (38). Our hypothesis-driven investigation, with its focus on VLSFAs, optimized VLSFA measurements, and thorough examination of multiple confounders, confirms and expands on the exploratory findings of the EPIC-InterAct study, as well as extends the findings to an older population. In addition, we provide novel evidence that associations of VLSFAs with diabetes risk are partly mediated by circulating levels of palmitic acid and triglycerides.
At baseline, each VLSFA was cross-sectionally associated with lower fasting levels of triglycerides. Fasting triglycerides are known to be associated with insulin resistance, and a score based on both insulin and triglyceride levels has been shown to be the best method to predict insulin sensitivity in individuals with normal glycemia (30). The VLSFAs were strongly positively associated with this insulin sensitivity score. Although the temporality of this association cannot be established, greater insulin sensitivity may mediate in part the association of VLSFAs with a lower risk of diabetes.
The VLSFAs were also cross-sectionally negatively correlated with levels of plasma phospholipid palmitic acid. The SFA palmitic acid is the major end product of fatty acid synthesis, and circulating levels of palmitic acid may be a biomarker of de novo lipogenesis. However, palmitoleic acid, another end product of de novo lipogenesis, was similarly negatively correlated with VLSFAs, but palmitoleic acid did not influence the associations of VLSFAs with diabetes. In addition, arachidic and behenic acids were actually associated with greater measures of adiposity, suggesting higher, not lower, lipogenesis. Although these cross-sectional findings cannot rule out the possibility that lipogenesis accounts for the association of VLSFAs with lower diabetes risk, we propose instead a hypothetical mechanism based on the specific lipids that are known carriers of VLSFAs.
The VLSFA are major components of ceramides and sphingomyelins, lipids that contain one fatty acid acylated to a sphingoid backbone (21). Ceramides have been suggested to play a role in the development of insulin resistance (22), and apoptosis due to ceramides may contribute to a decline in β cell mass and function (23), leading to diabetes. However, in experimental cell and animal studies, ceramides that contain a VLSFA have opposite biological activities from ceramides that contain the shorter SFA palmitic acid (25). For example, in CerS2 (ceramide synthase 2) null mouse liver, ceramides containing palmitic acid are increased to compensate loss of ceramides containing behenic acid and ceramides containing lignoceric acid, with no change in total ceramides, and the mice develop severe hepatopathy (39). We hypothesize that VLSFAs may lower the risk of diabetes by lowering endogenous levels of ceramides containing palmitic acid.
The VLSFAs were cross-sectionally associated with higher levels of LDL cholesterol. In cell membranes, sphingomyelins are enriched together in lipid rafts, specialized membrane domains rich in cholesterol that provide an environment for membrane traffic, signal transduction, and other membrane-level processes (24, 40). The observed association of circulating VLSFAs with LDL cholesterol may reflect the association of sphingomyelins with cholesterol in membranes rather than a pathologic mechanism.
The associations of behenic and lignoceric acids with lower diabetes risk appeared strongest in participants with a normal BMI. This observation may reflect differences in metabolism, such as dyslipidemia or excess lipogenesis in the context of excess adiposity (41), that may negate possible benefits from higher levels of VLSFAs. A chance finding is also possible, and this observation needs to be replicated.
The sources of circulating VLSFAs may be both dietary and metabolic. The VLSFAs are found in limited foods, including peanuts, macadamia nuts, and canola oil (42). We observed here that levels of behenic and lignoceric acids were associated with peanut intake measured 3 y earlier. In addition, small short-term feeding trials have shown an increase in circulating levels of arachidic and behenic acids with consumption of macadamia nuts (43) and an increase in circulating behenic and lignoceric acids with consumption of peanut butter (44). These observations suggest dietary intake may directly influence circulating levels of VLSFAs. Interestingly, peanut consumption has been associated with a lower risk of incident diabetes in the Nurses’ Health Study (45). Whether levels of VLSFAs mediated the peanut association is not known.
In addition to diet, VLSFAs may be synthesized endogenously from stearic acid by elongases. In particular, the elongase elovl1, which is ubiquitously distributed, selectively elongates SFAs with at least 18 carbons (46). In agreement with the predominant use of VLSFAs in ceramides and other sphingolipids, elovl1 appears to be coregulated with ceramide synthase 2, which produces ceramide by addition of a VLSFA to sphingosine (47). The extent to which dietary intake and endogenous metabolism contribute to circulating levels of VLSFAs is not known.
The study has several limitations and strengths. This is an observational study, and causality cannot be established. Residual confounding by unknown factors is also possible. However, results were robust to adjustment for multiple diabetes risk factors. Fatty acids were measured as a proportion of total fatty acids, and the negative correlation of the VLSFAs with palmitic acid could explain the inverse association of VLSFAs with diabetes if a lower proportion of VLSFAs directly translated into a higher proportion of palmitic acid. However, we have reported inverse associations of the VLSFAs with the risk of incident atrial fibrillation in the Cardiovascular Health Study (48) and with the risk of sudden cardiac arrest in another study (49), and adjustment for palmitic acid did not change these associations, suggesting the effect of adjusting for palmitic acid is specific to the diabetes analyses. The cross-sectional associations of VLSFAs with triglycerides and palmitic acid do not allow a distinction between mediation and confounding of the VLSFA-diabetes association. The study was conducted among older adults, and results may not be generalizable to other populations. However, similar associations were observed in the younger population of the EPIC-InterAct study. Strengths include the prospective design, population-based enrollment, use of an objective marker of diet and metabolism, and rich information on demographics, risk factors, and lifestyle habits.
In conclusion, we report an association of higher levels of plasma phospholipid VLSFAs with a lower risk of incident diabetes, which may be mediated by lower levels of triglycerides and plasma phospholipid palmitic acid. Together with our previous reports of inverse associations of VLSFAs with incident atrial fibrillation (48) and incident sudden cardiac arrest (49), the study findings should prompt further research on the effects and determinants of VLSFAs.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—RNL, AMF, DSS, and DM: designed the research; IBK and XS: conducted the research; KM, LD, DSS, JRK, and DM: provided essential materials necessary for the research; AMF, CMS, MLB, and BM: analyzed the data; RNL and AMF: wrote the manuscript; KM, LD, DSS, BM, JRK, and NS: provided critical revisions of the manuscript; RNL: had primary responsibility of the final content; and all authors: read and approved the final manuscript. DM reported ad hoc honoraria from Bunge and membership on the Unilever North America Scientific Advisory Board. Harvard University has filed a provisional patent application, which has been assigned to Harvard University, listing DM as a coinventor to the US Patent and Trademark Office for use of trans-palmitoleic acid to prevent and treat insulin resistance, type 2 diabetes, and related conditions. The other authors reported no conflicts of interest.
REFERENCES
- 1.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011;378:31–40. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Diabetes fact sheet: national estimates and general information on diabetes and pre-diabetes in the United States. Atlanta (GA): US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 3.McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med 2010;363:2339–50. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Kamineni A, Carnethon M, Djousse L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the Cardiovascular Health Study. Arch Intern Med 2009;169:798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care 2007;30:744–52. [DOI] [PubMed] [Google Scholar]
- 6.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007;298:2654–64. [DOI] [PubMed] [Google Scholar]
- 7.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010;121:2271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr 2013;98:1066–83. [DOI] [PubMed] [Google Scholar]
- 9.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34. [DOI] [PubMed] [Google Scholar]
- 10.Jové M, Planavila A, Sanchez RM, Merlos M, Laguna JC, Vazquez-Carrera M. Palmitate induces tumor necrosis factor–alpha expression in C2C12 skeletal muscle cells by a mechanism involving protein kinase C and nuclear factor-kappaB activation. Endocrinology 2006;147:552–61. [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos N, Watson M, Sakamoto K, Hundal HS. Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem J 2006;399:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jové M, Planavila A, Laguna JC, Vazquez-Carrera M. Palmitate-induced interleukin 6 production is mediated by protein kinase C and nuclear-factor kappaB activation and leads to glucose transporter 4 down-regulation in skeletal muscle cells. Endocrinology 2005;146:3087–95. [DOI] [PubMed] [Google Scholar]
- 13.Kato T, Shimano H, Yamamoto T, Ishikawa M, Kumadaki S, Matsuzaka T, Nakagawa Y, Yahagi N, Nakakuki M, Hasty AH, et al. Palmitate impairs and eicosapentaenoate restores insulin secretion through regulation of SREBP-1c in pancreatic islets. Diabetes 2008;57:2382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes 2003;52:726–33. [DOI] [PubMed] [Google Scholar]
- 15.Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, et al. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes 2002;51:1437–42. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003;78:91–8. [DOI] [PubMed] [Google Scholar]
- 17.Patel PS, Sharp SJ, Jansen E, Luben RN, Khaw KT, Wareham NJ, Forouhi NG. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr 2010;92:1214–22. [DOI] [PubMed]
- 18.Zong G, Zhu J, Sun L, Ye X, Lu L, Jin Q, Zheng H, Yu Z, Zhu Z, Li H, et al. Associations of erythrocyte fatty acids in the de novo lipogenesis pathway with risk of metabolic syndrome in a cohort study of middle-aged and older Chinese. Am J Clin Nutr 2013;98:319–26. [DOI] [PubMed] [Google Scholar]
- 19.Hodge AM, English DR, O'Dea K, Sinclair AJ, Makrides M, Gibson RA, Giles GG. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 2007;86:189–97. [DOI] [PubMed] [Google Scholar]
- 20.Kroger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Doring F, Joost HG, Boeing H, Schulze MB. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam Study. Am J Clin Nutr 2011;93:127–42. [DOI] [PubMed]
- 21.Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res 2010;51:3299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab 2012;15:585–94. [DOI] [PubMed] [Google Scholar]
- 23.Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis 2009;14:1484–95. [DOI] [PubMed] [Google Scholar]
- 24.Hanzal-Bayer MF, Hancock JF. Lipid rafts and membrane traffic. FEBS Lett 2007;581:2098–104. [DOI] [PubMed] [Google Scholar]
- 25.Grösch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res 2012;51:50–62. [DOI] [PubMed] [Google Scholar]
- 26.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–76. [DOI] [PubMed] [Google Scholar]
- 27.Folch J, Lees M, Sloane GH. A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 28.Lepage G, Roy CC. Direct transesterification of all lipids in a one-step reaction. J Lipid Res 1986;27:114–20. [PubMed] [Google Scholar]
- 29.Mozaffarian D, Lemaitre RN, King IB, Song X, Spiegelman D, Sacks FM, Rimm EB, Siscovick DS. Circulating long-chain ω-3 fatty acids and incidence of congestive heart failure in older adults: the Cardiovascular Health Study: a cohort study. Ann Intern Med 2011;155:160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, Duncan AW. Diagnosing insulin resistance in the general population. Diabetes Care 2001;24:460–4. [DOI] [PubMed] [Google Scholar]
- 31.Taylor HL, Jacobs DR Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 1978;31:741–55. [DOI] [PubMed] [Google Scholar]
- 32.Efron B, Tibshirani R. An introduction to the bootstrap. Boca Raton (FL): CRC Press; 1994. [Google Scholar]
- 33.Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, Siscovick DS, Nettleton JA. Trans-palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2013;97:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am J Clin Nutr 2010;92:1350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med 2010;153:790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Djousse L, Biggs ML, Lemaitre RN, King IB, Song X, Ix JH, Mukamal KJ, Siscovick DS, Mozaffarian D. Plasma omega-3 fatty acids and incident diabetes in older adults. Am J Clin Nutr 2011;94:527–33. [DOI] [PMC free article] [PubMed]
- 37.Wu JH, Micha R, Imamura F, Pan A, Biggs ML, Ajaz O, Djousse L, Hu FB, Mozaffarian D. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr 2012;107(Suppl 2):S214–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB, Crowe FL, Huerta JM, Guevara M, Beulens JW, et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol 2014;2:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pewzner-Jung Y, Brenner O, Braun S, Laviad EL, Ben-Dor S, Feldmesser E, Horn-Saban S, Amann-Zalcenstein D, Raanan C, Berkutzki T, et al. A critical role for ceramide synthase 2 in liver homeostasis: II. insights into molecular changes leading to hepatopathy. J Biol Chem 2010;285:10911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Insel PA, Head BP, Ostrom RS, Patel HH, Swaney JS, Tang CM, Roth DM. Caveolae and lipid rafts: G protein-coupled receptor signaling microdomains in cardiac myocytes. Ann N Y Acad Sci 2005;1047:166–72. [DOI] [PubMed] [Google Scholar]
- 41.Manteiga S, Choi K, Jayaraman A, Lee K. Systems biology of adipose tissue metabolism: regulation of growth, signaling and inflammation. Wiley Interdiscip Rev Syst Biol Med 2013;5:425–47. [DOI] [PubMed] [Google Scholar]
- 42.US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 26 [Internet]. Washington (DC): US Department of Agriculture. 2013. [cited 2014 Oct 17]. Available from: http://www.ars.usda.gov/ba/bhnrc/ndl.
- 43.Garg ML, Blake RJ, Wills RB. Macadamia nut consumption lowers plasma total and LDL cholesterol levels in hypercholesterolemic men. J Nutr 2003;133:1060–3. [DOI] [PubMed] [Google Scholar]
- 44.Lam C, Wong D, Cederbaum S, Lim B, Qu Y. Peanut consumption increases levels of plasma very long chain fatty acids in humans. Mol Genet Metab 2012;107:620–2. [DOI] [PubMed] [Google Scholar]
- 45.Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu FB. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA 2002;288:2554–60. [DOI] [PubMed] [Google Scholar]
- 46.Kihara A. Very long-chain fatty acids: elongation, physiology and related disorders. J Biochem 2012;152:387–95. [DOI] [PubMed] [Google Scholar]
- 47.Ohno Y, Suto S, Yamanaka M, Mizutani Y, Mitsutake S, Igarashi Y, Sassa T, Kihara A. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc Natl Acad Sci USA 2010;107:18439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fretts AM, Mozaffarian D, Siscovick DS, Djousse L, Heckbert SR, King IB, McKnight B, Sitlani C, Sacks FM, Song X, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc 2014;3:e000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lemaitre RN, King IB, Rice K, McKnight B, Sotoodehnia N, Rea TD, Johnson CO, Raghunathan TE, Cobb LA, Mozaffarian D, et al. Erythrocyte very long-chain saturated fatty acids associated with lower risk of incident sudden cardiac arrest. Prostaglandins Leukot Essent Fatty Acids 2014;91:149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.