Abstract
Resident memory T cells are non-recirculating memory T cells that persist long term in epithelial barrier tissues, including the gastrointestinal tract, lung, skin and reproductive tract. Resident memory T cells persist in the absence of antigens, have impressive effector functions and provide rapid on-site immune protection against known pathogens in peripheral tissues. A fundamentally distinct gene expression program differentiates resident memory T cells from circulating T cells. Although these cells likely evolved to provide rapid immune protection against pathogens, autoreactive, aberrantly activated and malignant resident memory cells contribute to numerous human inflammatory diseases including mycosis fungoides and psoriasis. This review will discuss both the science and medicine of resident memory T cells, exploring how these cells contribute to healthy immune function and discussing what is known about how these cells contribute to human inflammatory and autoimmune diseases.
Introduction
Resident memory T cells (TRM) are a recently described subset of memory T cells that persist long-term in peripheral tissues. TRM undergo a distinct differentiation program that discriminates them from circulating T cells; this program likely evolved to populate epithelial barrier tissues - skin, gut, lung and reproductive tracts - with highly protective T cells specific for the pathogens most commonly encountered through each tissue (1, 2). In this way, the immune system distributes memory T cells to the tissue sites where they will likely to be needed in the future. However, dysregulation of TRM can give contribute to human autoimmune and inflammatory diseases. TRM differentiate and accumulate in tissues after pathogen infection but evidence suggests they also develop after sensitization to otherwise harmless environmental or self antigens. Pathogenic autoreactive TRM induce the fixed, recurrent skin lesions of psoriasis and TRM specific for environmental allergens likely underlie development and worsening of allergic asthma and contact dermatitis. Moreover, the TRM differentiation program is likely also engaged when T cells are activated in non-barrier tissues such as the kidney, brain and joints. As a result, pathogenic TRM likely contribute to chronic inflammatory diseases in non-barrier tissues as well. This review will discuss both the basic biology and human diseases associated with TRM, exploring how these cells support healthy immune function and contribute to human inflammatory disease.
The discovery of resident memory T cells
In prior decades, it was thought that tissue tropic T cells remained in the circulation until recruited to sites of inflammation and that non-inflamed peripheral tissues contained very few T cells. T cells recruited to tissues during infections were thought to either exit tissues or undergo apoptosis following clearance of the infection. However, CD8+ T cells that remained long term in mouse lung following influenza virus infection were observed as early as 2001 (3, 4) and antigen-specific CD8+ T cells were found to migrate to non-lymphoid tissues and remain as long-lived memory cells following infections with Listeria and vesicular stomatitis virus (5). In 2004, it was observed that skin grafts of normal appearing, non-lesional skin from psoriatic patients gave rise to active psoriatic skin lesions after transplantation onto immunodeficient mice, demonstrating that a population of resident pathogenic T cells existed in the non-lesional skin of patients with psoriasis (6, 7). Subsequently, it was discovered that the healthy skin surface of an adult human being contained nearly 20 billion T cells, approximately twice as many as are present in the entire blood volume (8). Human skin T cells were all CD45RO+ memory T cells, co-expressed the skin homing addressins CLA and CCR4, had potent effector functions and a diverse T cell repertoire (8–11). Studies designed to determine the location of skin tropic memory T cells found that 98% were located in human skin under non-inflamed conditions and only 2% were in the circulation. (8) These findings challenged the idea that T cells must be recruited from peripheral blood during infectious challenges and suggested that at least some subsets of tissue tropic T cells spend the majority of their time in peripheral tissues. Subsequent studies demonstrated large numbers of antigenically diverse TRM in human lung, gastrointestinal tract, peritoneum, reproductive tract and bone marrow (12–19).
The Mission of Resident Memory T cells: To Persist, Protect & stay Put
The large numbers of memory T cells in human epithelial barrier tissues is consistent with the idea that some T cells may reside long-term in peripheral tissues but a series of elegant mouse models were required to clarify how these cells are generated, distributed and maintained. Mice kept in clean, pathogen-free barrier facilities had few skin TRM, likely because these cells are generated by the infections barrier facilities are designed to prevent. However, experimentally administered skin infections with HSV and vaccinia virus both generated populations of CD8+ T cells that remained in skin long after resolution of the acute infection and provided rapid viral clearance after skin reinfection (20–24). Dendritic cells (DC) were capable of presenting antigen to local populations of HSV-specific TRM, leading to a local recall immune response entirely within the skin (25). TRM generated by prior vaccinia skin infection were more protective than circulating T cells and rapidly cleared virus from the skin after reinfection in the complete absence of both antibodies and circulating T cells (21, 23, 24). Moreover, local skin infection with vaccinia virus led to seeding of the entire cutaneous surface with long lived, highly protective TRM, although the highest concentration of these cells occurred at the site of infection (21) (Figure 1). Subsequent re-infections at different skin locations led to progressive increases in the number of protective TRM throughout the skin; in this way local skin infections led to a global protection of the entire skin by long lived, highly protective TRM (21). Interestingly, preferential deposition of TRM at inflammatory sites following HSV infection was found to be antigen independent (22). TRM progenitor cells generated by recent skin infection migrated in greatest numbers into areas of inflamed skin, regardless of whether the inflammation was caused by local HSV infection or by irritation of the skin by the contact allergen 2,4-dinitrofluorobenzene (DNFB) (22).
Figure 1. Local skin infection leads to seeding of the entire skin surface with protective TRM.
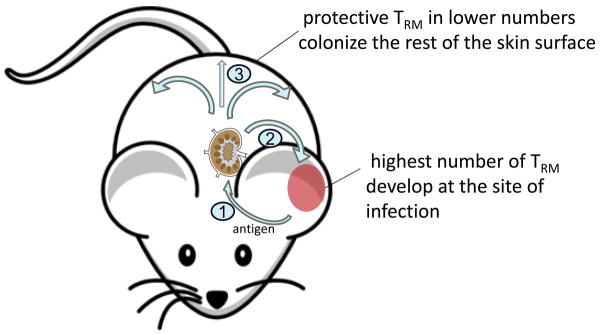
After a localized skin infection with vaccinia virus in mice, highly protective virus-specific TRM were generated that remained long term in the skin and provided local protection against reinfection (21). TRM were most numerous at the site of initial infection but these cells were also found in lower numbers in never infected skin. Note: Draft figures are shown. For professionally drawn figures, please see the published manuscript in Science Translational Medicine.
The best possible outcome of vaccination is the generation of highly protective TRM in the epithelial barrier tissue most likely to be infected. Skin scarification with live vaccinia virus, associated with local keratinocyte infection, generated long-lived CD8+ T cell-mediated immunity that was 100,000 times more effective than subcutaneous, intradermal and intramuscular vaccination in protecting against viral reinfection of the skin (24). This protection was mediated entirely by skin TRM and did not require antibodies or recruitment of circulating T cells from blood (21, 24). These studies call into question the current vaccination strategy of injecting vaccines into muscle. Muscle lacks a resident population of antigen presenting cells and intramuscular vaccination is unlikely to effectively focus immune responses to the relevant peripheral tissues.
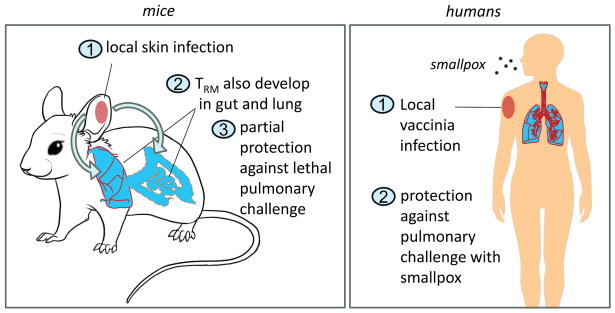
A surprising additional benefit of local skin infection with vaccinia was the generation of a smaller but functionally important population of lung TRM that could, in the absence of antibody and circulating T cells, partially protect mice from an otherwise lethal pulmonary challenge with vaccinia virus (23, 24). This spillover of immune protection occurred as a result of early release from skin draining lymph nodes of a subset of T cells proliferating in response to antigen following skin infection. These released T cells traveled to distant lymph nodes draining other peripheral tissues where they continued to proliferate in the absence of antigen and developed tissue tropism for these distinct, noncutaneous tissues (23) (Figure 2, left panel). In other words, local infection of one barrier tissue led to at least some immune TRM-mediated protection of other epithelial barrier tissues. A similar mechanism clearly exists in humans, where smallpox vaccination, delivered by skin scarification, has historically provided effective protection against smallpox, which is primarily transmitted as a respiratory infection (Figure 2, right panel).
Figure 2. Local skin infection leads to protective TRM in other epithelial barrier tissues.
After local skin infection with vaccinia virus in mice, protective TRM developed not only in the skin but also in lower numbers in the lungs and gastrointestinal tract (23, 24). Lung resident TRM induced by skin infection provided partial protection against an otherwise lethal pulmonary challenge with vaccinia. This protection was found to be mediated by early release of viral specific T cells proliferating in the skin draining lymph nodes after primary cutaneous infection. These T cells migrated to lymph nodes draining other tissues, proliferated in the absence of antigen and were imprinted with tropism for these distant tissues (23). Historical evidence suggests a similar biology exists in human beings. Smallpox vaccination, carried out by skin scarification with vaccinia virus, leads to protection against smallpox, an infection that is acquired via the respiratory route.
The immune protection provided by TRM is more than skin deep; infection models in mouse gut, lung and reproductive tract confirm that TRM persist, protect and do not recirculate. Gut infection with lymphocytic choriomeningitis virus, listeria, and other pathogens generated long-lived intraepithelial T cells with potent protective capacity (26). CD4+ TRM in the lung generated by influenza infection persisted, did not recirculate (14) and provided potent in situ protection against an otherwise lethal influenza challenge (27, 28). Both intranasal and intraperitoneal infection with influenza induced viral specific effector memory T cells but only nasal infection generated T cells that mediated protection against an otherwise lethal intranasal influenza challenge (29). In the genitourinary tract, intravaginal infection with HSV induced the accumulation of highly protective TRM (30). Moreover, topical intravaginal chemokine application “pulled” circulating effector T cells generated by subcutaneous HSV infection into the vaginal mucosa where they differentiated into protective TRM. Likewise, intravaginal inflammation induced by topical nonoxynol-9 application pulled circulating HSV-specific effector cells into the vaginal mucosa where they differentiated into protective TRM (22).
TRM generation in peripheral tissues: A distinct differentiation program
Studies in both mice and humans have demonstrated that TRM from peripheral tissues express CD69 and a subset also express CD103 (8, 16, 20, 21, 28, 31–33). In mice, epithelial resident CD8+CD103+ T have been most fully characterized although CD4+ TRM have also been observed and are in fact the dominant population of TRM in human skin (34, 35). After HSV infection in mice, sessile CD8+ TRM took up residence in the epidermis whereas CD4+ TRM remained in the dermis and were locally more mobile (34). Interestingly, CD8+ TRM that took up residence in the epidermis after HSV infection in mice progressively displaced existing populations of dendritic epithelial T cells (DETC) from their epidermal niches (36). In contrast to CD8+ TRM, which take up residence in epidermis only after skin infection, γδ DETC are present in mouse epidermis prior to infections, although they are not observed in humans. γδ DETC were fixed in place within the epidermis whereas CD8+ TRM moved laterally between keratinocytes, frequently interacting with Langerhans cells, but remained within a limited territory of migration (36). Lastly, decreased expression of the KLF2 transcription factor leading to sphingosine 1 phosphate receptor 1 (S1P1) down-regulation was also necessary for retention of CD8+ TRM in skin (37). CD69 suppresses the activity of S1P1, suggesting CD69 expression by TRM may assist in retaining these cells in peripheral tissues (38).
Gene profiling of CD103+ CD8+ TRM isolated from mouse skin, lung, gut and brain has demonstrated that TRM are the product of a distinct differentiation pathway that renders TRM extracted from different peripheral tissues more similar to each other than they are to circulating T cells (1, 2). The TRM differentiation program is engaged only after migration of recently activated T cells into peripheral tissues such as the skin (1) (Figure 3). The exact precursor cell type for TRM remains unidentified but in mice they are derived from the KLRG1− T cell population (1). Localization of HSV specific CD8+ TRM in the epidermis was dependent on a chemokine mediated process that could be inhibited by pertussis toxin. Maintenance of epidermal TRM cells required IL-15 and CD103 expression on these cells was induced in a TGF-β dependent manner after entry into the epidermis (1). A second study confirmed that TGF-β was important for establishing epithelial TRM residence and demonstrated additional requirements for IL-33 and TNFα (37).
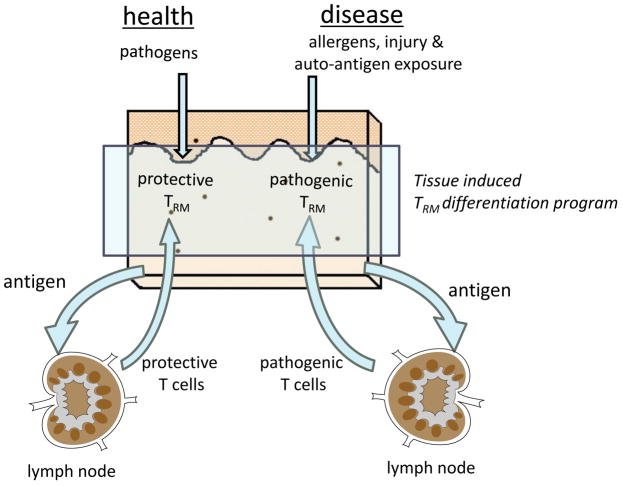
Figure 3. TRM are generated via a distinct, tissue induced differentiation program.
Under conditions of both health and disease, antigens are transferred from the skin to the draining lymph nodes, where responding T cells are generated. A subset of these T cells home back to the skin and undergo a distinct TRM differentiation program that is induced only after entry into the peripheral tissues (1, 2). In the case of infectious pathogens, TRM provide rapid local immune protection against re-infection. However, the TRM program can also be engaged after exposure to allergens and auto-antigens and in these situations pathogenic TRM can be generated in peripheral tissues. In the skin, pathogenic TRM have been demonstrated in psoriasis and fixed drug eruption (60–62).
Peripheral tissues also contain populations of recirculating T cells and one common feature of these T cells is their expression of CCR7. CCR7 expression is required for the exit of T cells from peripheral tissues via the afferent lymphatics (39–41). Studies in Kaede mice demonstrated that skin contained both non-recirculating CD4+CCR7− TRM and recirculating CD4+ CD69−CCR7+L-selectinlo T cells that exited skin in a CCR7 dependent mechanism (40). Human skin contains a recirculating population of L-selectin+CCR7+ central memory T cells that co-express the skin addressins CLA and CCR4. (35)
Resident Memory T Cells in Humans
Mouse model systems have provided invaluable insights into many biologic processes. However, there are significant differences in the immune systems of humans and mice and these differences remain incompletely characterized. Some mouse models, such as the C3H model for alopecia areata, closely resemble the human disease but others, including proposed mouse models of psoriasis, only partially recapitulate the human disease (42–44). It is therefore critical that observations made in mouse models be confirmed in humans.
Evidence has accumulated that the three critical characteristics of mouse TRM - that these cells persist, protect and do not recirculate - also hold true in humans. The presence of 20 billion T cells in human skin and the observation that T cell numbers in skin remain constant even in patients in their 90s is circumstantial evidence that these cells persist long-term (8, 45). However, the persistence and protective nature of human TRM has now has been elegantly and directly demonstrated in studies of patients with genital HSV (46, 47). HSV specific CD8αα T cells were found to persist at the dermo-epidermal junction long term after resolution of acute HSV outbreaks (47). During subsequent episodes of subclinical viral reactivation, asymptomatic viral shedding was noted accompanied by HSV protein production by infected keratinocytes. TRM were observed to cluster around the infected keratinocytes and produce perforin. Suppression of viral reactivation was observed when TRM were present in high density whereas low numbers of HSV specific TRM were associated with full viral reactivation and progression to clinically apparent lesions (47). These elegant studies, utilizing multicolor immunofluorescence and high throughput TCR sequencing, are illustrative of the elegant work that can now be done to characterize TRM in human tissues.
Additional evidence that TRM in the skin persist, protect and do not recirculate comes from studies of patients with leukemic cutaneous T-cell lymphoma (L-CTCL). Alemtuzumab, an anti-CD52 antibody, used in the treatment of CLL and CTCL, depletes via ADCC requiring accessory cells that are most frequent in the circulation (35). Low dose alemtuzumab treatment depleted all circulating T cells and purged the skin of both malignant and benign L-selectin+/CCR7+ central memory T cells (TCM), a skin tropic subpopulation of highly migratory T cells (35, 48). Despite complete depletion of all circulating and recirculating T cells, low dose alemtuzumab was not associated in this patient population with an increased risk of infection. Biopsy of the skin demonstrated that large numbers of TRM with marked effector functions and diverse antigen specificities remained in the skin of treated patients. These studies demonstrated that human skin is colonized by a diverse, highly protective and non-recirculating population of TRM. In addition, these studies demonstrated that a subset of L-selectin+/CCR7+ TCM, a subset of T cells thought to primarily recirculate between the blood and lymph nodes, also express skin homing addressins in humans and can enter and recirculate through the skin (35).
Large numbers of diverse CD69+ TRM with marked effector functions and enriched for influenza specificity have also been detected in histologically noninflamed human lung (14, 19). CD8+ T cells in human lung expressing the TRM marker CD103 were found to be the subset that were specific for influenza, compared to non-resident T cell populations from the same patients (14, 49). More recent studies in other tissues have demonstrated the presence of TRM in human stomach, colon, small intestine and cervix (12, 16, 18). CD8+ T cells were excluded from high-grade cervical dysplastic lesions (18) but patients vaccinated against oncogenic papilloma virus proteins had robust T cell infiltration of high-grade cervical intraepithelial neoplasias (50).
Findings in both human and mice suggest that the TRM populations in each epithelial barrier tissue may be enriched for the pathogens commonly encountered through those sites. Although the antigen specificity of TRM in human peripheral tissues has been difficult to interrogate directly, a recent study evaluated the antigen specificity of circulating effector T cells tropic for the skin (CLA+), gut (α4β7 integrin+) and other sites (including lung) (51). T cells tropic for skin were enriched for specificity against skin associated pathogens and commensals (S. epidermidis, C. albicans, HSV-1), gut-tropic T cells were enriched for specificity against gut associated pathogens (S. Typhi, Rotavirus) and remaining T cells (including lung tropic T cells) were enriched for pathogens encountered through the lung (Influenza A and Myocplasma).
TRM in human disease
The TRM genetic differentiation program evolved to progressively populate epithelial barrier tissues with nonrecirculating memory T cells with potent effector functions that are specific for the pathogens encountered through those sites. Under conditions of health, these cells provide extremely rapid on-site immune protection against known pathogens. However, when T cells specific for allergens or auto-antigens enter peripheral tissues and differentiate into TRM or when TRM themselves become malignant, TRM cease to be the solution and instead become part of the problem (Figures 3–5).
Figure 5. TRM in human autoimmune, inflammatory and allergic disorders.
Pathologic TRM have been directly demonstrated in psoriasis, mycosis fungoides and fixed drug eruption (shown in bold) but the clinical characteristics of many other human inflammatory diseases suggest a role for TRM. Clinical characteristics of TRM-mediated diseases include recurrent inflammation in the same anatomic locations, discrete, well demarcated inflammatory lesions, rapid onset of inflammation within 12 to 24 hours and progressively worsening disease with subsequent exposures.
The clinical characteristics of TRM mediated human diseases reflect TRM biology. Because TRM do not recirculate, inflammatory lesions caused by TRM tend to be well demarcated, with distinct margins and an abrupt cutoff with normal tissue (Figure 4). TRM mediated lesions tend to persist long-term in a particular location and, although they may appear to resolve with anti-inflammatory therapy, will often recur in the same location and grow to the previously observed size after therapy is discontinued if the inciting agent is still present. Because TRM are already on site and can respond to antigen presented by local tissue resident dendritic cells (25), TRM mediated tissue inflammation is extremely rapid and can occur within hours of antigen exposure. In general, onset of inflammation within 24 to 48 hours after antigen exposure is suspicious for TRM involvement. Lastly, because of the tendency of TRM to progressively accumulate in tissues with repeated antigen exposures, TRM mediated inflammatory diseases tend to worsen over time, with an increasingly rapid onset of inflammation and increasing inflammatory severity with each exposure.
Figure 4. TRM and skin-tropic TCM give rise to distinct inflammatory lesions in the skin.
CTCL are a heterogeneous collection of lymphomas derived from skin tropic T cells. (A) In mycosis fungoides, a skin limited variant of CTCL, malignant T cells have the phenotype of TRM, do not recirculate and induce well demarcated stable inflammatory skin lesions that appear to resolve with topical therapies but often recur after treatment is discontinued (35, 56). (B) In contrast, patients with leukemic CTCL (L-CTCL) have malignant T cells that co-express both TCM markers (L-selectin, CCR7) and skin homing addressins (CLA, CCR4) (56). These T cells recirculate between the blood and skin (35), likely also enter lymph nodes and give rise to diffuse erythema of the skin.
Two prototypic TRM skin diseases: Mycosis fungoides and psoriasis
Mycosis fungoides
Cutaneous T cell lymphomas (CTCL) are a heterogeneous collection of non-Hodgkin’s lymphomas derived from T cells that traffic to the skin. The common feature of all CTCL variants is the formation of inflammatory skin lesions caused by malignant T cells. However CTCL patients can have remarkably different clinical presentations and prognoses. Patients with mycosis fungoides (MF), a relatively benign skin limited variant of CTCL, have malignant T cells that are found only in well demarcated fixed inflammatory skin lesions (Figure 4A). These inflammatory lesions often persist for decades and, although they may appear to resolve with topical steroid therapy, usually recur in the same locations once therapy is discontinued. Approximately 80% of patients with MF will have stable disease and a normal life expectancy (52). Patients who progress often develop tumors and worsening skin disease but rarely develop circulating malignant T cells. In contrast, patients with leukemic CTCL (L-CTCL), including Sézary syndrome, have malignant T cells that accumulate in the skin, blood, lymph nodes and sometimes other organs. These patients tend to present with patchy, ill-defined inflammatory skin lesions or with diffusely erythematous skin (erythroderma, Figure 4B). Patients with L-CTCL have an average life expectancy of 3 to 4 years, definitive cure requires stem cell transplantation and patients die most commonly from infections (53).
MF and L-CTCL were previously thought to represent stages in a disease continuum but most patients present with either one subtype or the other (52). More recently, profiling and phenotypic studies of malignant T cells have suggested that these variants actually represent lymphomas derived from distinct T-cell subsets (54–56). Malignant T cells in MF lacked L-selectin and CCR7 expression and phenotypically resembled TRM, consistent with their tendency to form fixed inflammatory skin lesions (56). In contrast, malignant T cells in L-CTCL, whether they were isolated from the blood or inflamed skin, co-expressed L-selectin and CCR7 along with the skin homing addressin CLA and CCR4 (35, 56). These skin tropic central memory cells are observed in healthy human skin as well and would be expected to recirculate between the blood lymph nodes and skin, exactly the tissues involved in patients with L-CTCL (35).
The most effective treatment modalities for these disorders reflect the biology of the T cells that cause them. MF responds, at least temporarily, to skin directed therapy with topical steroids, retinoids, nitrogen mustard, phototherapy, and low dose irradiation whereas L-CTCL patients require systemic therapies and eventually, stem cell transplantation (53). We found that alemtuzumab, a humanized monoclonal antibody against CD52, depletes circulating and recirculating T cells in L-CTCL but spares TRM in skin. This medication is remarkably effective in L-CTCL and completely ineffective in MF (35).
Psoriasis
Psoriasis is a prototypic TRM mediated autoimmune inflammatory skin disease and a uniquely human problem. Mouse models recapitulate some of the histologic disease features but mice do not spontaneously develop psoriasis. As a result, most insights into the biology of psoriasis have resulted from observing patient responses to clinical therapeutics. The findings that T cell immunosuppressive medications, such as cyclosporine, suppress psoriatic symptoms pointed to a T cell mediated etiology for this disease, as opposed to a primary keratinocyte abnormality (57). Most recently, remarkably complete responses of patients to the IL-17A inhibitor secukinumab have demonstrated that psoriasis is, as its core, an IL-17 mediated disease (58). One of the chief frustrations of physicians and sufferers of psoriasis is the fact that psoriatic skin lesions occur to completely resolve with therapy but often recur in the same locations, regrowing to their prior size, once therapy is discontinued.
The first clue that psoriasis may be a TRM mediated disease came from the surprising clinical observation that blockade of E-selectin, which blocks the migration of T cells from the blood into the skin, was completely ineffective in the treatment of psoriasis (59). Next came a groundbreaking set of experiments in which non-lesional, normal appearing skin grafts derived from patients with psoriasis developed full-blown psoriatic lesions when transferred onto immunodeficient mice (6). These lesions resulted from activation and proliferation of a presumably autoreactive T cell population transferred with the normal appearing skin graft. These experiments demonstrated that autoreactive pathogenic T cells were present in even the normal healthy appearing skin of patients with psoriasis and that psoriatic skin lesions can develop in the complete absence of any recruitment of cells from blood. Gene profiling studies of active psoriatic lesions and previously lesional, normal appearing skin after treatment with TNFα blockade demonstrated persistently abnormal expression of a subset of genes, including CD8 (60). Histologic studies demonstrated that there was a residual resident population of CD8+ T cells in previously lesional psoriatic skin (60). More recent studies demonstrated that CD4+ cells making IL-22 and CD8+ cells making IL-17 remained resident in previously lesional psoriatic skin after clinical resolution with a number of effective psoriatic therapies (61). These studies definitively demonstrate that TRM persist in clinically resolved psoriatic lesions and produce the cytokines known to cause the primary pathology of this disease.
Other human TRM mediated diseases
In the skin, histologic studies also support TRM as the cause of fixed drug eruption, a cutaneous eruption consisting of a single or few inflammatory skin lesions that occur after taking a particular medication. Once the medication is discontinued, these lesions appear to completely resolve, only to recur in exactly the same places years or even decades later when the medication is taken again. Histologic stains demonstrated the presence of a resident population of CD8+ T cells producing IFNγ in sites of resolved fixed drug eruption lesions, supporting a role for TRM in this process (62). Other fixed, rapidly recurrent and progressively worsening cutaneous eruptions including vitiligo, contact dermatitis and chronic lesions of eczematous dermatitis are likely to involve TRM but this has not been directly demonstrated (Figure 5).
Inflammatory diseases in other barrier tissues, including the gut, lung and genitourinary tracts, have clinical characteristics that suggest TRM participation. Asthma, other allergic airway diseases, food allergies and celiac sprue show a rapid onset of inflammation, at least some of it T cell mediated, after antigen exposure (63). Crohn’s disease produces areas of discrete gut inflammation separated by normally appearing mucosa, known as “skip lesions”, which are morphologically similar to the well demarcated skin lesions seen in TRM mediated skin disease. However, TRM presence, generation and pathogenicity have yet to be directly demonstrated in these disorders.
Although the TRM genetic differentiation program likely evolved to populate epithelial barrier tissues with protective T cells, T cells responding to inflammation in other tissue sites can also give rise to TRM and corresponding pathology at the sites (Figure 5). CD103+ TRM with transcriptional profiles distinct from effector and central memory T cells were generated and remained resident within the brains of mice after infection with vesicular stomatitis virus (2). In humans, TRM have been postulated to contribute to brain injury in multiple sclerosis and schizophrenia (64, 65). In mouse models of spondyloarthropathy, enthesial-resident Th17 TRM were essential for disease progression (66) and recurrence of arthritis in particular joints is a hallmark of human rheumatoid arthritis. Malaria infection led to the generation of TRM in the livers of infected mice (67). In humans, T cells infiltrate the liver in patients with chronic hepatitis C but these cells have not been definitively identified as TRM (68). In patients with lupus erythematosus and nephritis, individual T cell clones in renal inflammatory infiltrates were found to persist for years in repeat biopsy samples from individual patients, suggesting that TRM may participate in the progressive, devastating kidney damage observed in patients with lupus (69).
Conclusions and perspectives
In the years to come, it will be critical to determine how long TRM remain in peripheral tissues, how often they turn over, what environmental niches within the peripheral tissue support their continued long-term survival and what if anything can be done to mobilize these cells from peripheral tissues if their presence is detrimental. It will be critical to identify which human diseases have a TRM mediated component and to evaluate the therapies for their impacts on TRM. Many treatment modalities in current clinical use, including total body irradiation, skin brachytherapy, phototherapy and a variety of systemic chemotherapy drugs are completely uncharacterized with respect to their effects on TRM. For example, alemtuzumab, a medication previously thought to deplete all T and B cells in the body, depletes only circulating and recirculating T cells but leaves at least skin TRM intact (35). A better understanding of how and where these drugs act on memory T cells could lead to more selective therapies that preferentially target the pathogenic T cells while sparing nonpathogenic T cell subsets.
The discovery and characterization of TRM has proved once again that nature and biology are smarter than we are. A system that distributes memory T cells specific for the pathogens likely to be encountered through those sites directly to the tissues at risk is a remarkably elegant way to focus potentially dangerous effector T cells only to the places they are most likely to be needed. Work remains to be done to identify which vaccination strategies generate the greatest number of TRM in the target tissues and similar approaches are needed to induce the formation of TRM a tumor sites, where they can locally protect against recurrence. However, the strengths of TRM become a problem when these cells are maladaptive and cause inappropriate inflammation. The long lived, non-recirculating nature of the cells combined with their potent effector functions results in inflammatory diseases that are remarkably intransigent and tend to recur in the same anatomic locations. Further studies of the basic biology of these cells may identify an Achilles’ heel in TRM biology that will allow their depletion and local control within tissues.
Literature cited
- 1.Mackay LK, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nature immunology. 2013 Dec;14:1294. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 2.Wakim LM, et al. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol. 2012 Oct 1;189:3462. doi: 10.4049/jimmunol.1201305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogan RJ, et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 2001 Feb 1;166:1813. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- 4.Wei CH, et al. Tissue-resident memory CD8+ T cells can be deleted by soluble, but not cross-presented antigen. J Immunol. 2005 Nov 15;175:6615. doi: 10.4049/jimmunol.175.10.6615. [DOI] [PubMed] [Google Scholar]
- 5.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001 Mar 23;291:2413. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 6.Boyman O, et al. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. The Journal of experimental medicine. 2004 Mar 1;199:731. doi: 10.1084/jem.20031482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyman O, Conrad C, Tonel G, Gilliet M, Nestle FO. The pathogenic role of tissue-resident immune cells in psoriasis. Trends in immunology. 2007 Feb;28:51. doi: 10.1016/j.it.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Clark RA, et al. The vast majority of CLA+ T cells are resident in normal skin. The Journal of Immunology. 2006 Apr 1;176:4431. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 9.Clark RA, et al. A novel method for the isolation of skin resident T cells from normal and diseased human skin. Journal of Investigative Dermatology. 2006 May;126:1059. doi: 10.1038/sj.jid.5700199. [DOI] [PubMed] [Google Scholar]
- 10.Clark RA. Skin resident T cells: the ups and downs of on site immunity. Journal of Investigative Dermatology. 2010 Feb;130:362. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 2007 Jan 1;109:194. doi: 10.1182/blood-2006-02-002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Booth JS, et al. Characterization and functional properties of gastric tissue-resident memory T cells from children, adults, and the elderly. Frontiers in immunology. 2014;5:294. doi: 10.3389/fimmu.2014.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okhrimenko A, et al. Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proceedings of the National Academy of Sciences of the United States of America. 2014 Jun 24;111:9229. doi: 10.1073/pnas.1318731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner DL, et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal immunology. 2014 May;7:501. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts GW, et al. Functional effector memory T cells enrich the peritoneal cavity of patients treated with peritoneal dialysis. J Am Soc Nephrol. 2009 Sep;20:1895. doi: 10.1681/ASN.2008101127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sathaliyawala T, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013 Jan 24;38:187. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKinnon LR, et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011 Dec 1;187:6032. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 18.Trimble CL, et al. Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. The Journal of Immunology. 2010 Dec 1;185:7107. doi: 10.4049/jimmunol.1002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purwar R, et al. Resident Memory T Cells (T(RM)) Are Abundant in Human Lung: Diversity, Function, and Antigen Specificity. PloS one. 2011;6:e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature immunology. 2009;10:524. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 21.Jiang X, et al. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012 Mar 8;483:227. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackay LK, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proceedings of the National Academy of Sciences of the United States of America. 2012 May 1;109:7037. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity. 2006 Sep;25:511. doi: 10.1016/j.immuni.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, et al. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nature medicine. 2010 Feb;16:224. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008 Jan 11;319:198. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 26.Klonowski KD, et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004 May;20:551. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 27.Teijaro J, Ndjembi MP, Lorenzo L, Chandran S, Farber D. 2007;178:S36-d–37. [Google Scholar]
- 28.Teijaro JR, et al. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011 Dec 1;187:5510. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu T, et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol. 2014 Feb;95:215. doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012 Nov 15;491:463. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bos JD, et al. The skin immune system (SIS): distribution and immunophenotype of lymphocyte subpopulations in normal human skin. The Journal of investigative dermatology. 1987 May;88:569. doi: 10.1111/1523-1747.ep12470172. [DOI] [PubMed] [Google Scholar]
- 32.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. The Journal of experimental medicine. 2010 Mar 15;207:553. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunkel EJ, et al. Expression of the Chemokine Receptors CCR4, CCR5, and CXCR3 by Human Tissue-Infiltrating Lymphocytes. The American journal of pathology. 2002 Jan 1;160:347. doi: 10.1016/S0002-9440(10)64378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gebhardt T, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011 Sep 8;477:216. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 35.Clark RA, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Science Translational Medicine. 2012 Jan 18;4:117ra7. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaid A, et al. Persistence of skin-resident memory T cells within an epidermal niche. Proceedings of the National Academy of Sciences of the United States of America. 2014 Apr 8;111:5307. doi: 10.1073/pnas.1322292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skon CN, et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nature immunology. 2013 Dec;14:1285. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiow LR, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006 Mar 23;440:540. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 39.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nature immunology. 2005 Sep;6:895. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 40.Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol. 2013 Feb 1;190:970. doi: 10.4049/jimmunol.1202805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Debes GF, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nature immunology. 2005 Sep;6:889. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sundberg JP, Cordy WR, King LE., Jr Alopecia areata in aging C3H/HeJ mice. The Journal of investigative dermatology. 1994 Jun;102:847. doi: 10.1111/1523-1747.ep12382416. [DOI] [PubMed] [Google Scholar]
- 43.McElwee KJ, Boggess D, King LE, Jr, Sundberg JP. Experimental induction of alopecia areata-like hair loss in C3H/HeJ mice using full-thickness skin grafts. The Journal of investigative dermatology. 1998 Nov;111:797. doi: 10.1046/j.1523-1747.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- 44.Swindell WR, et al. Genome-wide expression profiling of five mouse models identifies similarities and differences with human psoriasis. PloS one. 2011;6:e18266. doi: 10.1371/journal.pone.0018266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neuber K, Schmidt S, Mensch A. Telomere length measurement and determination of immunosenescence-related markers (CD28, CD45RO, CD45RA, interferon-gamma and interleukin-4) in skin-homing T cells expressing the cutaneous lymphocyte antigen: indication of a non-ageing T-cell subset. Immunology. 2003 May;109:24. doi: 10.1046/j.1365-2567.2003.01640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu J, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. The Journal of experimental medicine. 2007 Mar 19;204:595. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu J, et al. Immune surveillance by CD8alphaalpha+ skin-resident T cells in human herpes virus infection. Nature. 2013 May 23;497:494. doi: 10.1038/nature12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999 Oct 14;401:708. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 49.Piet B, et al. CD8(+) T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. The Journal of clinical investigation. 2011 Jun;121:2254. doi: 10.1172/JCI44675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maldonado L, et al. Intramuscular Therapeutic Vaccination Targeting HPV16 Induces T Cell Responses That Localize in Mucosal Lesions. Science Translational Medicine. 2014 Jan 29;6:221ra13. doi: 10.1126/scitranslmed.3007323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlapbach C, et al. Human TH9 Cells Are Skin-Tropic and Have Autocrine and Paracrine Proinflammatory Capacity. Science Translational Medicine. 2014 Jan 15;6:219ra8. doi: 10.1126/scitranslmed.3007828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Archives of dermatology. 2003 Jul;139:857. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- 53.(© 2009 National Comprehensive Cancer Network, Inc., 2009), vol. 2009, pp. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org.
- 54.Shin J, et al. Lesional gene expression profiling in cutaneous T-cell lymphoma reveals natural clusters associated with disease outcome. Blood. 2007 Oct 15;110:3015. doi: 10.1182/blood-2006-12-061507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Doorn R, et al. Oncogenomic analysis of mycosis fungoides reveals major differences with Sezary syndrome. Blood. 2009 Jan 1;113:127. doi: 10.1182/blood-2008-04-153031. [DOI] [PubMed] [Google Scholar]
- 56.Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010 Aug 5;116:767. doi: 10.1182/blood-2009-11-251926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mueller W, Herrmann B. Cyclosporin A for psoriasis. The New England journal of medicine. 1979 Sep 6;301:555. doi: 10.1056/NEJM197909063011016. [DOI] [PubMed] [Google Scholar]
- 58.Langley RG, et al. Secukinumab in Plaque Psoriasis - Results of Two Phase 3 Trials. The New England journal of medicine. 2014 Jul 9; doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 59.Bhushan M, et al. Anti-E-selectin is ineffective in the treatment of psoriasis: a randomized trial. British Journal of Dermatology. 2002 May;146:824. doi: 10.1046/j.1365-2133.2002.04743.x. [DOI] [PubMed] [Google Scholar]
- 60.Suarez-Farinas M, Fuentes-Duculan J, Lowes MA, Krueger JG. Resolved psoriasis lesions retain expression of a subset of disease-related genes. Journal of Investigative Dermatology. 2011 Feb;131:391. doi: 10.1038/jid.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheuk S, et al. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J Immunol. 2014 Apr 1;192:3111. doi: 10.4049/jimmunol.1302313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teraki Y, Shiohara T. IFN-gamma-producing effector CD8+ T cells and IL-10-producing regulatory CD4+ T cells in fixed drug eruption. Journal of Allergy and Clinical Immunology. 2003 Sep;112:609. doi: 10.1016/s0091-6749(03)01624-5. [DOI] [PubMed] [Google Scholar]
- 63.Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nature medicine. 2012 May;18:705. doi: 10.1038/nm.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sasaki K, et al. Relapsing-remitting central nervous system autoimmunity mediated by GFAP-specific CD8 T cells. J Immunol. 2014 Apr 1;192:3029. doi: 10.4049/jimmunol.1302911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Debnath M, Berk M. Th17 Pathway-Mediated Immunopathogenesis of Schizophrenia: Mechanisms and Implications. Schizophrenia bulletin. 2014 Apr 7; doi: 10.1093/schbul/sbu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sherlock JP, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4−CD8− entheseal resident T cells. Nature medicine. 2012 Jul;18:1069. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 67.Tse SW, Cockburn IA, Zhang H, Scott AL, Zavala F. Unique transcriptional profile of liver-resident memory CD8+ T cells induced by immunization with malaria sporozoites. Genes and immunity. 2013 Jul-Aug;14:302. doi: 10.1038/gene.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yanagisawa K, et al. Ex vivo analysis of resident hepatic pro-inflammatory CD1d-reactive T cells and hepatocyte surface CD1d expression in hepatitis C. Journal of viral hepatitis. 2013 Aug;20:556. doi: 10.1111/jvh.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winchester R, et al. Immunologic characteristics of intrarenal T cells: trafficking of expanded CD8+ T cell beta-chain clonotypes in progressive lupus nephritis. Arthritis Rheum. 2012 May;64:1589. doi: 10.1002/art.33488. [DOI] [PMC free article] [PubMed] [Google Scholar]