Abstract
Uptake of polychlorinated biphenyls (PCBs) by fish is controlled by the bioavailability of ingested PCBs in the gut and the freely dissolved concentration in the water moving across the gills. Prediction of bioaccumulation in fish relies on models that account for these exposure routes, however, these models typically do not account for incidental ingestion of sediment by fish, which is not well studied. The literature values for the PCB assimilation efficiency (AE) in the gut have been reported for compounds in food matrices and not associated with sediment particles. It is also unclear how mitigation strategies that alter PCB bioavailability in sediments affect predictions made by the bioaccumulation models when sediment ingestion is involved. To test the bioavailability of PCBs from treated and untreated sediments, dietary AEs were measured for 16 polychlorinated biphenyl (PCB) congeners in mummichogs (Fundulus heteroclitius) that were fed four experimental diets. Diets consisted of PCB-spiked earthworms, spiked untreated sediment mixed with earthworms, spiked activated carbon (AC)-treated sediment mixed with earthworms, and spiked AC mixed with earthworms. AEs were determined by calculating the ratio of PCB mass in the fish tissue to the PCB mass in the food after a pulse feeding experiment. AEs of PCBs associated with earthworm diet were similar to the values reported in the literature. Fish that were fed the PCB-spiked untreated sediment and AC particles exhibited the highest and lowest AEs respectively over a wide KOW range, respectively. AEs of sediment-bound PCBs were significantly reduced (31 to 93% reduction for different congeners) upon amendment with AC. The present study indicates that assimilation of PCBs can be reduced by sorption to black carbon.
INTRODUCTION
Bioaccumulation models are available that can predict uptake of hydrophobic organics, such as polychlorinated biphenyls (PCBs) through water and food. These models are based on calibrated uptake rate coefficients, and site-specific values of chemical concentrations in water, sediment, and prey items [1, 2]. While these models have been generally effective in predicting uptake in large natural systems, the ability to predict changes after engineering alterations in sediments is questionable and there are key uncertainties that need to be addressed especially when bioavailability in sediments is altered. For instance, one factor that plays an important role in the PCB dietary uptake, mainly for the more hydrophobic congeners, is the efficiency with which fish assimilate chemicals from dietary sources, which can change after sediment amendment. Dietary sources not only include prey items but can also contain matrices other than food, such as sediment particles. Others in the scientific community have theorized that PCBs sorbed to activated carbon (AC) particles can be available for uptake in fish after ingestion [3]. Sediment ingestion is critically important for benthivorous fish, since they resuspend [4] and ingest considerable amounts of sediments when feeding [5]. Therefore, it is necessary to investigate changes in PCB uptake through sediment ingestion when total PCB concentration in the sediment remains constant but bioavailability is altered. Gaillard et al. [6] compared the relative bioavailability of PCB-impacted sediments containing 0.3% native black carbon with the bioavailability of PCBs carried by canola oil to carp but did not measure assimilation efficiency (AE) of sediment-bound PCBs. The findings of this study revealed that PCBs sorbed to sediment were as available as PCBs in canola oil at all three concentrations. The authors suggested that the experiment should be repeated with sediment of various ages and black carbon content to evaluate how sediment properties can affect PCB bioavailability. In our previous work [7] exposure through ingestion was calibrated (separately for the untreated and treated sediment) by accounting for the observed uptake of the dominant heptachlorobiphenyl PCB-180 that could not be explained by uptake solely from water and PCB-free food. Assuming that the fish exposed to untreated and treated sediment had the same ingestion rates, the ratio of AEs of untreated to treated sediment-bound PCBs was calculated to be 2. These findings led to exploring changes that occur in AE of PCBs after AC amendment of sediment. However, frequently used bioaccumulation models such as Arnot and Gobas [1] model allow the incidental sediment ingestion by fish to be included as part of the diet and apply the assimilation efficiency values for biotic diets to sediments. Not using characterized assimilation efficiency values for the PCBs bound to the sediment implies that these models may be less suitable for bottom-feeding fish. To address this gap, recent studies have included sediment ingestion as an exposure pathway [8, 9]. In our previous work [7] we showed that while AC amendment reduced uptake in fish, a substantial portion of the remaining uptake came from the sediment ingestion pathway (69% contribution from this pathway to the total fish PCB uptake exposed to the treated sediment). However, it should be noted that these values were obtained from fitting the model to the observed data and not from independent measures of ingestion rate and assimilation efficiency. Moermond et al. [8] modeled the PCB assimilation rate from sediment for carp based on observations that benthivorous fish ingest as much sediment as invertebrate food and estimating the lumped product of AE and ingestion rate from empirical correlations. Furthermore, their study focused on a natural lake system that did not explore changes that happen after bioavailability manipulation in sediment. Given the importance of incidental sediment ingestion as an uptake pathway, it is important to define sediment ingestion rate and AE terms independently, and as accurately as possible. Several methods such as gravimetric analysis of fish gut contents and a mass balance tracer approach have been proposed to measure sediment ingestion rate [10]. While numerous laboratory studies have measured PCB AE of contaminated food in fish [11–15] (Table 1), no study to our knowledge has assessed AE of PCBs associated with contaminated sediment in fish. Since deposit feeding invertebrates feed on sediment and detritus at high feeding rates to obtain required nutrients for energy budgets and survival [1], PCB uptake through sediment ingestion for these organisms has been widely studied. Therefore, most of the AE values for sediment ingestion that exist in the literature are for aquatic invertebrates. Researchers have reported AE values ranging from 15 to 75% for tetra- to hexachlorobiphenyl compounds in spiked sediments fed to amphipods, oligochaetes, clams, and mussels [16–18] (Table 1). McLeod et al. investigated the bioavailability to clams (macoma balthica) via PCB-spiked diets which included different carbonaceous particles [16]. Their findings are the only AE values for activated carbon reported in the literature.
Table 1.
Reported assimilation efficiency values for fish and invertebrates
| Organism | Food | Compounda | AEb % | Reference |
|---|---|---|---|---|
| Fish | ||||
| Juvenile carp | frozen bloodworm blended with cod liver oil containing PCBs | tetra, hexa, hepta-CB | 40 | (Stapleton, Letcher et al. 2004) |
| Rainbow trout | commercial fish food + PCBs dissolved in hexane and mixed with capelin fish oil (gavaged) | di to deca-CB | 75 (average) | (Niimi and Oliver 1983) |
| Juvenile rainbow trout | commercial fish food + PCBs dissolved in hexane | PCBs | 31–49 | (Fisk, Norstrom et al. 1998) |
| Pike | rainbow trout injected with PCBs dissolved in rainbow trout lipid | tri to hexa-CB | 44–71 | (Burreau, Axelman et al. 1997) |
| Goldfish | commercial fish food + PCBs dissolved in petroleum ether | tetra, hexa, octa, deca-CB | 26–53 | (Gobas, McCorquodale et al. 1993) |
| Amphipod | ||||
| Diporeia sp | sediments | HCBP | 46–58 | (Kukkonen and Landrum 1995) |
| 36–52 | (Kukkonen and Landrum 1995) | |||
| Oligochaete | ||||
| Limnodrilus hoffmeisteri | sediments | HCBP | 15–37 | (Klump, Krezoski et al. 1987) |
| Lumbriculus variegatus | sediments | PCBs | 10–36 | (Sun, Werner et al. 2009) |
| Clam | ||||
| Macoma balthica | wood particles | TCBP | 75 | (McLeod, van den Heuvel-Greve et al. 2004) |
| Macoma balthica | activated carbon | TCBP | Less than 2 | (McLeod, van den Heuvel-Greve et al. 2004) |
| Zebra mussel | ||||
| Dreissena polymorpha | sediments | HCBP | 30 | (Bruner, Fisher et al. 1994, Gossiaux, Landrum et al. 1998) |
HCBP = hexachlorobiphenyl; TCBP = tetrachlorobiphenyl.
AE = assimilation efficiency
The present study was designed to investigate the effect of sorption on fish dietary AE of PCBs in sediments. Dietary AE of PCBs in different dietary matrices, including worms, worms mixed with sediment, worms mixed with AC-treated sediment and worms mixed with AC was measured 24 hours after feeding. Understanding how the AE of particle-associated PCBs changes after AC amendment will help characterize PCB uptake by fish through the sediment ingestion pathway more accurately and quantify how uptake through this pathway changes upon addition of activated carbon to sediments.
MATERIALS AND METHODS
Food preparation
Four types of diets were used in the present study: PCB-spiked worms (W), clean worms mixed with untreated spiked sediment (U), clean worms mixed with treated spiked sediment (T), and clean worms mixed with PCB-spiked AC particles (AC). Lyophilized earthworms (Lumbricus terrestris) were used as clean food and Rhode River sediment, a fine grained silty sediment which had a measured PCB concentration below the level of detection (0.01 µg/g dry wt.), was used as the clean sediment matrix. Coal-based powdered activated carbon (≤ 44µm; Calgon Carbon) was used as an amendment to sediment. A PCB congener mixture standard from Ultra Scientific (RPC-EPA2-1), which contains 16 PCB congeners spanning a wide range of log KOW values, was used to spike the food. For PCB-spiked food, a specific volume of the PCB standard solution in acetone was added to lyophilized earthworms, completely covering them. After shaking the earthworm mixture overnight, the solvent was evaporated under nitrogen stream. For the other three diets, the spiking procedure used by McLeod et al. [16] was followed, i.e. specific volumes of the PCB standard in acetone were added to glass vials containing dichloromethane and evaporated to dryness by gentle hand swirling, leaving the compounds adhered to the inside walls of the vials. Clean Rhode River sediment was ground up in a mortar and pestle to be used as untreated sediment or mixed with AC to make the treated sediment. Untreated sediment, treated sediment (sediment +5% AC), and AC particles were added to separate vials as suspensions in 5 ml deionized water. Spike vials were sealed with Teflon septa and mixed on a rotator for 21 days. Once air-dried, 20% sediment (including AC mass for treated sediment) or AC particles and 80% clean worms by weight as well as the spiked earthworms were shaped into 2 mm pellets using carboxymethyl cellulose and water solution (additional 2.5% carboxymethyl cellulose by dry weight food) (see Figure S1 in the Supporting Information). The optimal 20 to 80% mass distribution was chosen based on test studies conducted prior to the main experiment that used pellets containing different amounts of sediment for feeding the fish. Higher percentage of sediment or AC caused the fish to avoid the pellets or the pellets to fall apart too soon. An initial mass of PCBs in the food pellets was measured before the start of the experiment.
Test organisms and feeding procedure
Mummichogs (Fundulus heteroclitius) were spawned at Delaware State University and the fertilized eggs were transported to the Institute of Marine and Environmental Technology (MD, USA) where they hatched and were raised until they reached adult size weight (~ 20 g wet). The fish used in this experiment comprised of males and females and were similar in size to minimize effects of size and age on PCB uptake. The fish were acclimated to their new diet a week prior to beginning the experiment by being fed with pellets comprised of 20% sediment or AC particles and 80% clean worms by weight. Subsequently, the fish were fed pre-weighed and equal amount of food three times during 7 hours, based on a feeding rate of 1.5% (mass dry food/mass wet fish body weight) per day, and sampled after a 17-hour fasting period. The pellets sank to the bottom of each tank and were consumed shortly after being offered to fish, with the exception of the AC pellets, for which less than 5% of the AC particles remained uneaten due to the pellets breaking apart (the 5% was determined visually). Feces at the bottom of the tanks were siphoned out frequently throughout the feeding period to avoid introducing more PCBs to the fish through ingestion of feces. It should be noted that feces were not analyzed for PCBs in the present study. This study was carried out in accordance with the guidelines of the International Animal Care and Use Committee of the University of Maryland Medical School (IACUC protocol #1015008).
Aquarium set up
Four sets of tanks (triplicate tanks for each set) were used for the four types of food (see Figure S2). Each tank contained 8 L of synthetic seawater (as grams per liter: NaCl, 21.998; MgCl, 6.89; CaCl, 1.26; KCl, 0.66; SrCl, 0.015; LiCl, 0.01; NaSO4, 2.53; MgSO4, 1.87; NaBO4, 0.04; NaMoO4, 0.00001; NaCO3, 0.06; NaHCO4, 0.23) diluted with dechlorinated tap water to a salinity of 15 psu, and one individual mummichog. There was an even distribution of male and female fish for each of the four sets of tanks (Table S1). Fish were maintained under static conditions and the chemical composition of the water (pH, conductivity, and alkalinity), ammonia and nitrite levels were monitored throughout the experiment. pH and conductivity were measured using a Horiba U-22 multiparameter probe. Alkalinity, ammonia, and nitrate were measured with water quality test kits (Hach Co.). The tanks were contained in a separate heated bath to maintain the water temperature uniformly at 28°C, the optimal temperature for the fish, in all tanks. Tanks were equipped with air stones to maintain adequate oxygen level in the water. A photoperiod of 14 hours of light and 10 hours of darkness was maintained in the laboratory.
Sampling and PCB analysis
Fish from all tanks were sampled 24 hours after feeding started, sacrificed using dry ice, and the digestive tract from the stomach to the anus, which will be referred to as the gut in this manuscript, was removed from the fish by dissection. Gut contents were purged manually and preserved for PCB analysis. The gut and the rest of the body (referred to as fish tissue in this manuscript) were each lyophilized and frozen until analysis. Separate fish tissue and gut samples were used to determine background PCB levels in the fish prior to exposure. Fish from each tank were analyzed individually. Fish tissue samples were ground with anhydrous sodium sulfate and extracted with a hexane:acetone mixture (1:1, v/v) following method SW846 3550C. Prior to extraction, surrogate recovery standards (PCB BZ#14 and 65) were added to assess processing efficiency. Percent surrogate recovery in all analyzed samples was within the criteria of 100 ± 30%. The lipids were removed by treating with concentrated sulfuric acid. Further cleanup was performed by treating the extract with activated copper and passing through a 3% deactivated florisil and acidified silica gel column. The eluate was concentrated by nitrogen evaporation and analyzed for PCB congeners as described in Fadaei et al., using PCB BZ#30 and 204 as internal standards. A quality control plan was implemented to ensure that the chemical analyses performed were accurate [7]. PCB analysis of the food pellets, gut tissue and gut content samples were similar to fish tissue except that EPA 3630C method (silica gel cleanup) was used for cleanup.
Data analysis
The assimilation efficiency (AE) was calculated from equation 1 as described in the dietary exposure test section (equation A7.7, Appendix 7) of the Organisation for Economic Co-operation and Development Guidelines for Testing of Chemicals [19]:
| (1) |
The following assumptions were made to use equation 1: (1) Losses from the fish during the experiment are insignificant, i.e. depuration and PCB loss via respiration and fecal egestion are negligible (2) biotransformation of PCBs is minimal, and (3) the sampling occurs at the linear portion of the uptake curve, i.e. days 1 to 3 in a typical exposure [19].
Since the uneaten AC particles were siphoned out and no uneaten food was observed in the case of the other three pellets, dissolution of PCBs associated with food into the water was minimized. Therefore, the ratio measured in the present study represents direct AE as this was only exposure through food and did not include aquatic exposure.
Partition constants (Kd) for three of the diets were predicted by modeling sorption to organic carbon (OC) and applied AC.
| (2) |
| (3) |
Where CW is the porewater concentration (ng/L), fOC and fAC are the fractions of OC and AC in sediments, KOC and KAC are water-sorbent distribution coefficients for OC and AC, respectively with unit of (L/kg sorbent). Total organic carbon and black carbon content in untreated Rhode River sediment were measured as 3.9±0.06% and 0.2±0.01% by dry weight (Average ± S.E.), respectively. AC content in the solids portion of the T and AC diets were 5% and 100%, respectively.
KOC (L/kg OC) values for PCB congeners were estimated using equation 4 [20]. To use this empirical correlation the octanol-water partitioning constant (KOW (−)) was obtained from Hawker and Connell [21].
| (4) |
Sorption to AC was described by a Freundlich isotherm, assuming n=1 for all PCB congeners because the n values reported by Gomez-Eyles et al. [22] were close to 1 for most of the PCB congeners. KAC values for different congeners were obtained from published isotherm study results by Gomez-Eyles et al. [22] using coal-based AC. KAC was calculated from the mean of the ratios of sediment to aqueous PCBs at different concentrations (Table S2).
Partition constant between particle and octanol (Kpo) was calculated as:
| (5) |
Where Kow is the partition constant between octanol and water as reported in Hawker and Connell [21].
RESULTS AND DISCUSSION
Fish weights and PCBs in food
Average weight of the fish in different tanks was 16±0.73 g (Average ± S.E.) (Table S1). The total PCB concentration in the different diets were 1.0 and 1.2 µg/g dry weight for W and T diets (not significantly different, p=0.09, ANOVA); 0.3 and 4.1 µg/g dry weight for U and AC diets, respectively (see Table S3 for congener specific concentrations in all the four diets). For the AC diet, AC was spiked with higher PCB levels due to anticipated reduced AE and to allow accumulation of detectable levels of PCBs in the fish. The final total PCB mass that measured in W and U diets after the spiking procedure was half of what was spiked, suggesting that a portion of the added PCBs were lost through the spiking procedure. This residual was reduced to one-fifth for the T and AC diets, likely due to lower extraction efficiency of PCBs in the presence of AC. The actual measured PCB mass in the food were used for AE calculations and may provide a conservative estimate of AE for T and AC where extraction efficiency was poor.
AE for worm diet
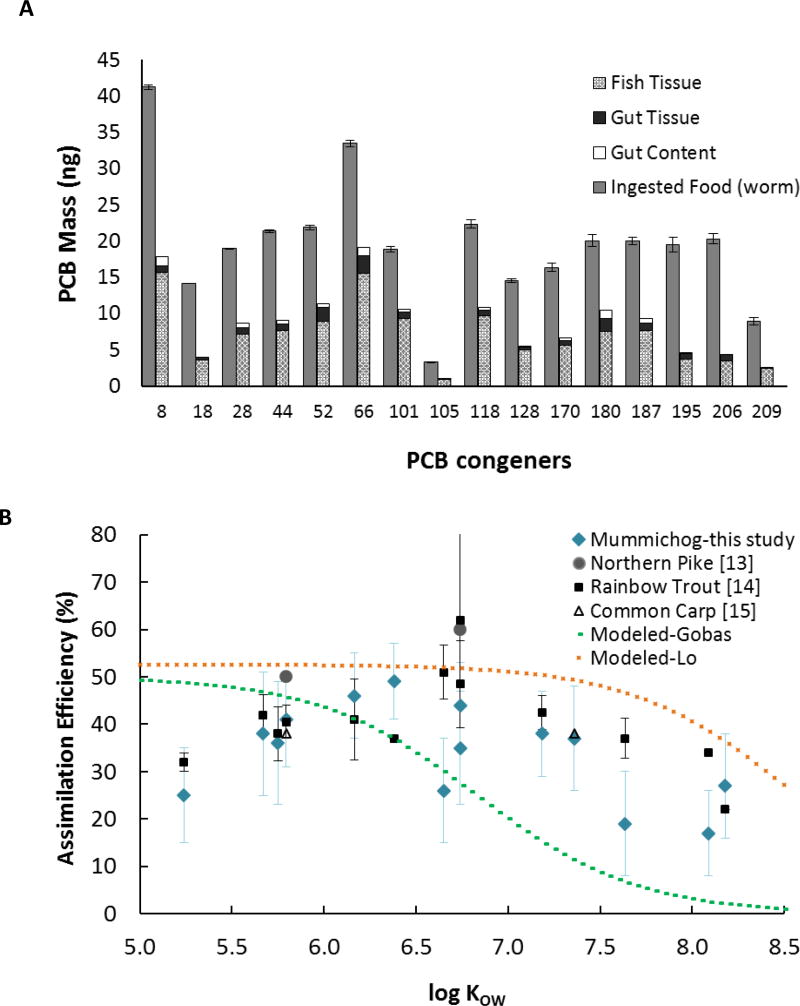
Figure 1A shows the results of PCB analyses in the food, fish tissue, gut tissue, and gut content for the PCB-spiked worms (W) diet. Reported residual PCBs in fish tissue and gut tissue were corrected for background levels. The majority of the adsorbed PCBs were assimilated in the fish tissue after 24 hours. Gut tissue PCBs contributed on average 11% of the total PCB body burden for fish that fed on W diet. The results also indicate that the post-feeding 17 hour fasting/purging period was sufficient to eliminate most of the unabsorbed PCBs through gut contents.
Figure 1.
(A) Mass of PCBs in the W (worm) diet, fish tissue, gut tissue, and gut content, and (B) assimilation efficiency of different PCBs in the W diet as a function of log KOW and their comparison with literature values. The lines in Figure 1B represent the AE-log KOW correlations developed by Gobas et al. [23] and Lo et al. [25]. Error bars represent standard error.
AE-KOW relationship
In the case of the W diet, AEs of PCBs show an initial increase (strong correlation with log KOW, p=0.007 < 0.05, Pearson’s test) up to a log KOW value of 6.5 followed by a decrease (strong correlation with log KOW, p=0.006 < 0.05, Pearson’s test) with increasing log Kow (Figure 1B). Such bell-shaped response of AE with respect to compounds Kow has been observed before such as in the data for rainbow trout [14] also shown in Figure 1B. Previous work by Gobas et al. [23, 24] reviewed empirical measurements of AE in the literature and concluded that dietary AE is initially independent of log KOW and starts decreasing at log KOW of 6 for a range of hydrophobic organic chemicals due to reduced bioavailability and steric hindrance in crossing biological membranes. The predicted values by the Gobas et al. [23] and Lo et al. models [25] were plotted in Figure 1B. In these non-linear models AE (α) is presented as α = (2 + 3 × 10−7. KOW)−1 and α = (1.9 + 5.6 × 10−9. KOW)−1, respectively. Gobas at al. model predictions were statistically different (1-R2 = 0.93) than absolute values of AE for the W diet. The predicted values by the Gobas model were derived by fitting a non-linear correlation to empirical data from various fish species which may not have the ability to predict variations in fish species, feeding rate, diet type and quality, as well as food concentration. Using the relationship derived from one species and size (rainbow trout) by Lo et al. led to improved predictions (1−R2 = 0.51) compared to the relationship derived for multiple species and sizes by Gobas et al.
Comparison of observed AE for worm diet with literature values
AE of different PCBs in the W diet was mostly similar to the values reported in the literature for fish [13–15] (Figure 1B, see also Table 1 for other literature values). Arnot and Gobas [1] reported that the empirical AE observations are highly variable in fish ranging between 0 and 90%. They attributed the variance in measured AE to the differences in composition and sorption capacity of dietary matrices (e.g., organic carbon and soot carbon content), and differences in how various species process food in the gut. The above factors explain the discrepancy between the Gobas AE model and reported values in the literature (including the results from the present study). The three cited studies in the literature used rainbow trout, commercial fish food, and frozen bloodworm as the food matrix, respectively. It should be highlighted that results from the present study reflect true AE measured at a shorter time while values reported in the cited studies are net AE values measured at longer exposure times. The observed difference is also attributed to use of fish from different species (which leads to differences in gut morphology and food digestion processes) [1], age, and size [14, 26]. In addition, different food lipid content and composition [12, 27], food digestibility [28], fish feeding rate [29], and the method used to deliver PCBs to the animal food [30] can result in variations in AE among studies. In the W diet, average AE of the 16 congeners and AE of hexachlorobiphenyl were 34 and 35%, respectively which compare well with the PCB AE values reported for members of amphipod, oligochaete, and zebra mussel group (Tables S4 and 1).
AE for sediment and AC diets
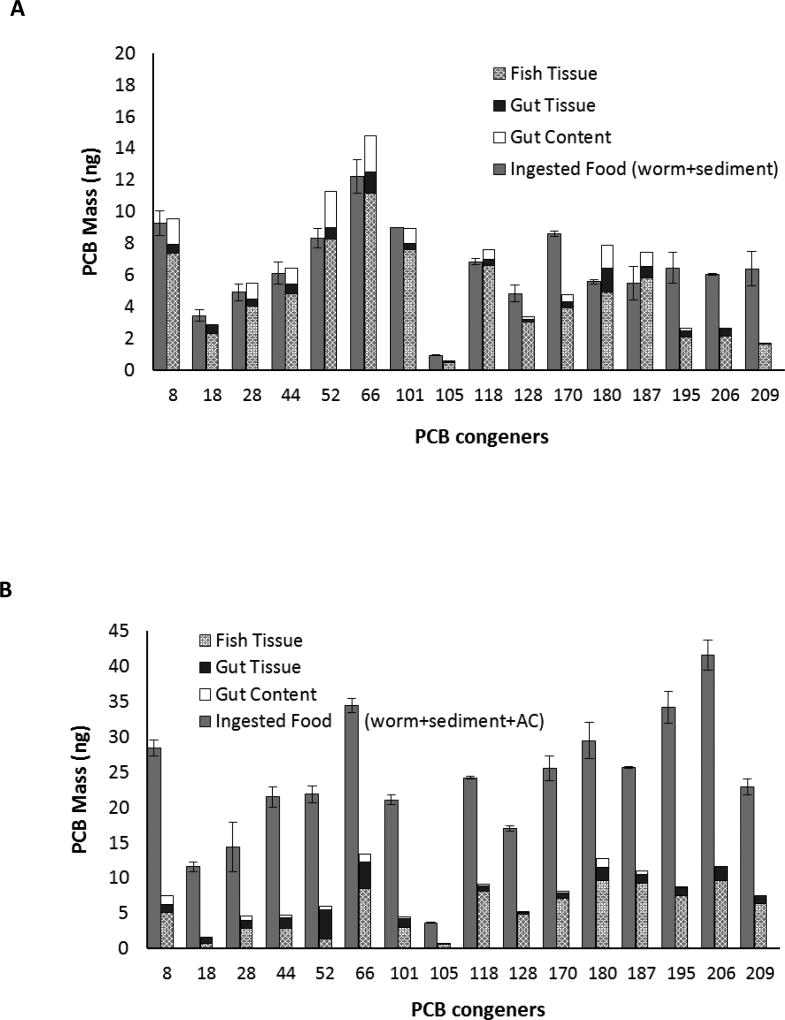
Consistency of the AE results of PCBs in food discussed in the previous section with literature values confirmed the accuracy of the experiment design and the analytical method of the present study to measure AE. This section discusses the AE of PCBs associated with the sediment and carbon phases in matrices that comprised of PCB-free worm and PCB-spiked solids. Figure 2 shows the results of PCB analyses in the food, fish tissue, gut tissue, and gut content for the three diets that contained a non-food portion: clean worms mixed with untreated spiked sediment (U), clean worms mixed with treated spiked sediment (T), and clean worms mixed with PCB-spiked AC particles (AC). Reported residual PCBs in fish tissue and gut tissue were corrected for background levels. Figures 2A through 2C indicate that the majority of the adsorbed PCBs were assimilated in the fish tissue after 24 hours with little residue in the gut tissue. Also, purging was effective in minimizing residual PCBs in the gut. Gut tissue PCBs contributed on average 10 and 13% of the total PCB body burden for fish that fed on U and AC diets, respectively and higher levels were observed with T diet (23%). Gut tissue contributed to body burden of lower chlorinated congeners more in the T and AC diets versus the U diet. As the gut tissue is the medium through which PCBs are being transported from the gut contents into the fish one would expect to see higher concentration in the gut tissue than the rest of the organism. In addition, it is possible that some of the fine AC remains stuck within the gut. It was presumed that any PCBs not measured in the tissue, gut and gut content was lost through fecal and gill elimination. In order to support the assumption that PCB loss through the gills did not interfere with the AE calculation, a kinetic bioaccumulation model [2] was used to quantitate the maximum PCB loss through the gills in the 24 hour exposure and depuration period (see Equations 2– in the Supporting Information). The loss of total PCBs through the gills was less than 1% of the total PCB ingested by the fish (see Table S5 for congener level losses through the gills).
Figure 2.
Mass of PCBs in the food, fish tissue, gut tissue, and gut content for the (A) U (worm + sediment), (B) T (worm + sediment + AC), and (C) AC (worm + AC) diets. Error bars represent standard error.
AE comparison among diets and comparison with literature values
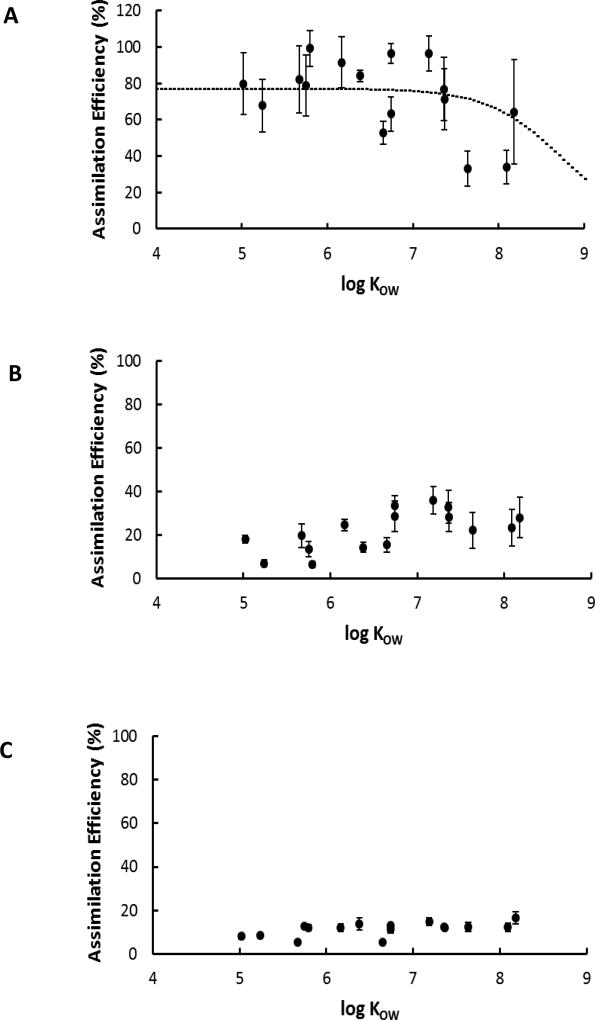
AE of sediment-bound PCBs was reduced by 31 to 93% for different congeners upon amendment of sediment with AC (Figures 3A and 3B, Table S4). The reduction in bioavailability to fish due to the addition of AC to the diet was more pronounced for the lower chlorinated PCBs, likely due to the faster mass transfer kinetics of these spiked compounds to the AC particles [31]. The ratio of directly measured AEs of untreated to treated sediment-bound PCBs was found to be 2 for the majority of the PCB congeners used in the present study (Table S4). This ratio is identical to the value calculated independently in Fadaei et. al. [7] for the ratio of the product of ingestion rate and AE for fish exposed for 90 days to untreated and treated sediment.
Figure 3.
Assimilation efficiency as a function of log KOW for the (A) U (worm + sediment), (B) T (worm + sediment + AC), and (C) AC (worm + AC) diets. Error bars represent standard error.
The sediment-bound PCBs in U diet were more bioavailable to fish than those in the W diet (Figure 3A and 1B). This suggests that PCBs bound to untreated sediment were not resistant to gut fluids. Hypothetically, this could be due to longer residence time of the PCBs in the gut in the presence of untreated sediment as time required to digest and pass the meal can differ. Further investigations may be required to better understand the relationship between food quality, gut passage time, and AE. Unfortunately feces were not quantitatively collected in the present study, preventing diet digestion efficiency calculations across the diet treatments. Gaillard et al. [6] compared differences between PCB bioavailability in sediment and food matrices. Three diets spiked with increasing masses of PCBs in canola oil and three diets contaminated via increasing masses of PCBs in sediment were fed to carp over an exposure period of 15 days. Gaillard et al. measured the relative bioavailability by comparing the slopes of the fish PCB concentration plotted against diet PCB concentration for sediment and oil-based diets. The results demonstrated that the sediment-bound PCBs were as bioavailable as those spiked into canola oil and fed to carp in standard diet (the aforementioned study also used 21% sediment by dry weight in one of their PCB contaminated diets and the sediment contained 3.4% organic carbon). DiPinto and Coull [32] observed that uptake of Aroclor 1254 by juvenile fish L. xanthurus was five times higher from contaminated sediments than contaminated prey. In a similarly designed feeding experiment, DiPinto [33] found similar response by juvenile L. xanthurus for the organophosphate pesticide APM (uptake was eight times higher from contaminated sediments than from contaminated prey).
In the case of the AC diet, the presence of AC resulted in strong absorption of PCBs to the diet matrix and hence reduced AE of all the PCB congeners including the lower chlorinated ones which had much higher AE values than the higher chlorinated PCBs in the W and U diets (Figure 3C). AC diet showed the lowest AE values and this was not surprising, given the strong sorption of PCBs to activated carbon. However, the observed uneaten food (<5%) in the tanks that received AC diet implies that the calculated AE values were likely underestimated for this diet as the fish did not ingest all the PCBs administered to the food. A comparison of AE between Figures 1B and 3C suggests that reduced bioavailability of the PCBs in the AC diet affects the extractability of the PCBs in the gut and therefore reduces the efficiency with which fish assimilate PCBs from the AC-food matrix (29 to 86% reduction in AE for different congeners when comparing AC to food only). Since AC pellets were initially spiked with 3.9 times higher sum PCBs than the W pellets (Figures 1A and 2C), concentration effects can lead to a more conservative estimate of AE for the AC diet. This is mainly because of the nonlinearity of PCB sorption to AC which can result in weaker sorption at higher concentrations [34] and therefore better extraction of the PCBs in the gut. While the calculated AE from the present study was 12±1% for PCB 52 in the AC diet (CPCB 52 in food = 0.3 µg/g), Mcleod et al. [16] reported AE of less than 2% in clam, Macoma balthica for the same congener spiked into fine-mesh activated carbon (TOG 50 × 200) (CPCB 52 in food = 800 µg/g). Mcleod et al. [16] observed lower values, likely due to the use of 100% AC in the diet as opposed to use of 20% AC by dry weight food in the present study. Furthermore, different digestion mechanisms of the fish compared to that of clam can be a contributing factor to this difference.
AE-KOW and AE-Kd relationships
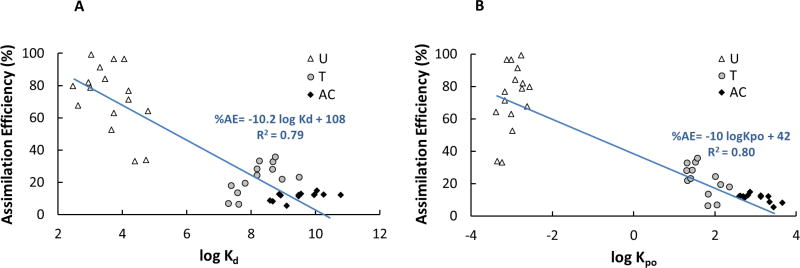
A trend similar to Figure 1B was observed for the AEs of PCBs in U diet (Figure 3A) with an inflection point around log KOW=7. Fitting the non-linear model to the data in Figure 3A resulted in: AE = (1.3 + 2.4 × 10−9. KOW)−1. For the T diet (Figure 3B), the statistical analysis did not determine a strong correlation between AE on log KOW (p=0.08 > 0.05, Pearson’s test). There was no clear relationship between PCB AE values for the AC diet and log KOW (Figure 3C). In Figure 3, assimilation efficiency values of PCBs were related only to the compounds’ hydrophobicity, while sediment geochemistry also plays some role in PCB bioavailability and hence the fish dietary uptake through sediment ingestion. Therefore, AE was plotted against estimated log Kd of U, T, and AC diets for all congeners (Figure 4) and individual congeners (Figures 5 and S3). As seen in Figure 4, there is a strong general trend of decreasing AE with increasing PCB partitioning into the ingested solids as the PCBs are becoming more associated with non-digestible carbon and are not being released through digestion. There is some scatter among the individual congeners in each solid category which is not explained by compound Kd alone (Figure 4A). Compound structure and ability to be absorbed in the gut after being released from the solid matrix is not captured by compound KOW alone. The scatter is not reduced significantly when the AE is plotted against the log Kpo (Figure 4B, Kpo is defined in Equation 5 as partitioning between particle and octanol).
Figure 4.
Assimilation efficiency of U (worm + sediment), T (worm + sediment + AC), and AC (worm + AC) diets as a function of (A) log Kd and (B) log Kpo.
Figure 5.
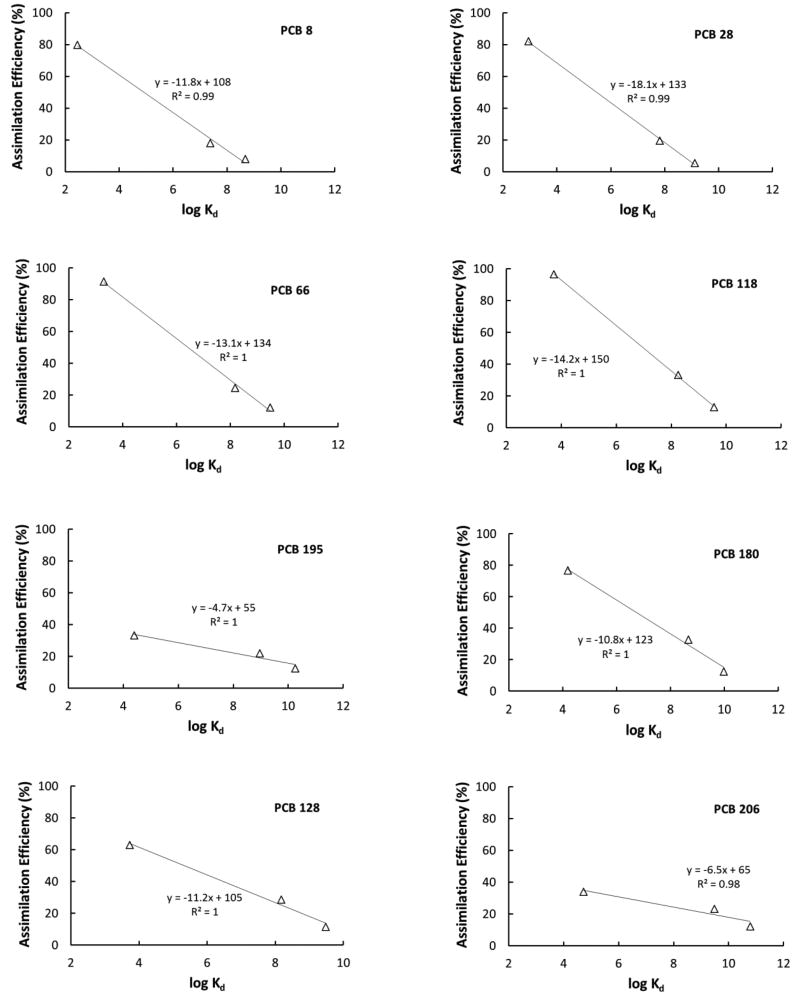
AE-log Kd plots for representative congeners from each homolog group. Partition constants (Kd) were calculated as Kd = fOC KOC for U (worm + sediment) diet and as Kd = fOC KOC + fAC KAC for T (worm + sediment + AC) and AC (worm + AC) diets. Remaining congener plots are shown in Supplemental Figure S3.
AE-log Kd plots for individual congeners (Figure 5) showed a strong relationship across different diets. R2 values improved as the degree of chlorination increased. This could be due to an experimental artifact which could lead to underestimation of the true AE values for the lower chlorinated PCBs because of their faster rate of depuration compared to the larger PCBs [14]. These relationships emphasize how the PCB bioavailability in the fish gut is impacted by the geosorbent type.
Change in PCB uptake prediction using measured AE for sediments
Three hypothetical scenarios were simulated to evaluate how the measured AE for sediments impact prediction of PCB uptake in benthivorous fish. The PCB uptake through sediment ingestion pathway was estimated based on different AE values for the different scenarios. For the three scenarios in order to predict uptake through ingestion of treated sediment, the AE for sediment treated with 5% AC was chosen to be: (1) the same as food (the AE results found for the W diet), (2) 100 % AE for all the PCB congeners, and (3) same as AE results for the T diet obtained from the present study. Predictions of PCB body burden were generated and limited to the congeners used in the present study, assuming that a 0.9 kg catfish was in contact with the sediment concentration of Cs = 1.6 µg/g d.w for 180 days. The modified version of the Connolly bioaccumulation model [2] (Equation 5 in the Supporting Information), which included gill uptake, ingestion of PCB contaminated food and sediment as the uptake pathways, was used. The model assumption was that the fish fed on benthic worms (Lumbriculus variegatus) with incidental sediment ingestion. The concentration in the food was estimated from laboratory measured porewater concentrations of treated sediment. It was assumed that catfish ingest as much sediment as invertebrate food. The simulation was also repeated for sediment ingestion rates of 50, 20 and 10% of the food ingestion rate. See Supporting Information for a more detailed discussion of the model. Table 2 summarizes the simulation results for the different scenarios and shows that using our independently measured AE for sediment PCBs as an input to the model leads to a prediction of PCB uptake in fish at 6 µg/g lipid. If we did not have access to the AE values reported from the present study and would assume that AE values for sediment-bound PCBs are either the same as PCBs in the food or 100% for all the congeners, we then would overestimate the total PCB uptake in fish at 10 and 24 µg/g lipid respectively (see row 1 in Table 2). As the sediment ingestion rate decreased, not only the uptake predictions for the fish in all the three scenarios decreased, but also the difference between predicted values from scenario 1 and 3 decreased. This is mainly due to the smaller contribution of sediment ingestion to uptake as the sediment ingestion rate is reduced.
Table 2.
Results of total PCB uptake predictions for catfish exposed to sediment for 180 days based on different choices of AE values for the sediment ingestion pathway and sediment ingestion rate. W and T represent PCB-spiked worms and clean worms mixed with treated spiked sediment, respectively.
| Choice of AE values for sediment | |||
|---|---|---|---|
| Measured AE for W diet |
100% AE for all congeners |
Measured AE for T diet |
|
| Choice of sediment ingestion rate | Total C lipid (µg/g) | ||
| 1.0 × GD | 10 | 24 | 5.5 |
| 0.5 × GD | 5.5 | 13 | 3.5 |
| 0.2 × GD | 2.8 | 5.6 | 1.9 |
| 0.1 × GD | 1.9 | 3.3 | 1.4 |
GD is the food ingestion rate
IMPLICATIONS OF THIS RESEARCH
Fish are exposed to PCBs from both particulate and dissolved phases. Few efforts have been made to modify the traditional bioaccumulation models by addition of a term for uptake through ingestion of sediment-associated PCBs. In this regard, measurement of AE of sediment-bound PCBs and estimating contaminant bioavailability in the gut is critical. The previously developed non-linear model [23] does not account for effects of geochemistry in matrices other than food and is therefore not suitable for estimating AE of PCBs associated with sediment particles. The present study took the first step towards improving the predictions made by bioaccumulation models after AC amendment of PCB-impacted sediments by looking at the differences in the uptake efficiency of PCBs in food versus sediment with and without AC amendment. Our results indicated that PCBs in the AC-amended sediment are 31 to 93% less available for uptake through the gastrointestinal tract of mummichog. Untreated sediment PCBs were assimilated more efficiently than PCBs in the worms. Reduced uptake from the T diet showed the effect of enhanced sportive properties of sediment on PCB accumulation through ingestion and addresses the issue raised by Gaillard et al. [6] that effect of black carbon contents on bioavailability of sediment-bound PCBs should be further investigated. Our simulation results for a specific case of catfish exposure showed that assuming AE for sediment and food are same, can overestimate uptake from AC-amended sediments by 31 to 82% (depending on the sediment ingestion rate chosen). The AE values reported in the present study, which are the first report of AE for sediment ingestion by fish, can support the development of bioaccumulation models that can evaluate the contribution of sediment ingestion to PCB bioaccumulation in fish in natural systems before and after application of carbonaceous material to the sediments. These modified bioaccumulation models can provide a more accurate tool for risk assessment and forecasting the success of different remedial alternatives based on the recovery of fish, the primary risk driver for PCB exposure, in an aquatic ecosystem.
Supplementary Material
Acknowledgments
We would like to thank the National Institute of Environment and Health Sciences, Superfund Research Program for financial support (Grant # R01ES020941). This is contribution #xxxx from UMCES and #xx-x for IMET. This research was funded in part by grants from OHH NIH R01ES021949-01/NSFOCE1313888 and NOAA-NOS-NCCOS-2012-2002987 to ARP.
References
- 1.Arnot JA, Gobas FAPC. A food web bioaccumulation model for organic chemicals in aquatic ecosystems. Environmental Toxicology and Chemistry. 2004;23:2343–2355. doi: 10.1897/03-438. [DOI] [PubMed] [Google Scholar]
- 2.Connolly JP. Application of a food chain model to polychlorinated biphenyl contamination of the lobster and winter flounder food chains in New Bedford Harbor. Environmental Science & Technology. 1991;25:760–770. [Google Scholar]
- 3.Weber WJ. Comment on “Addition of Carbon Sorbents to Reduce PCB and PAH Bioavailability in Marine Sediments:? Physicochemical Tests”. Environmental Science & Technology. 2005;39:1197–1198. doi: 10.1021/es048209q. [DOI] [PubMed] [Google Scholar]
- 4.Scheffer M, Portielje R, Zambrano L. Fish facilitate wave resuspension of sediment. Limnology and Oceanography. 2003;48:1920–1926. [Google Scholar]
- 5.Tolonen KT, Karjalainen J, Staff S, Leppä M. Individual and population-level food consumption by cyprinids and percids in a mesotrophic lake. Ecology of Freshwater Fish. 2000;9:153–162. [Google Scholar]
- 6.Gaillard J, Banas D, Thomas M, Fournier A, Feidt C. Bioavailability and bioaccumulation of sediment-bound polychlorinated biphenyls to carp. Environmental Toxicology and Chemistry. 2014;33:1324–1330. doi: 10.1002/etc.2561. [DOI] [PubMed] [Google Scholar]
- 7.Fadaei H, Watson A, Place A, Connolly J, Ghosh U. Effect of PCB Bioavailability Changes in Sediments on Bioaccumulation in Fish. Environmental Science & Technology. 2015;49:12405–12413. doi: 10.1021/acs.est.5b03107. [DOI] [PubMed] [Google Scholar]
- 8.Moermond CTA, Roozen FCJM, Zwolsman JJG, Koelmans AA. Uptake of Sediment-Bound Bioavailable Polychlorobiphenyls by Benthivorous Carp (Cyprinus carpio) Environmental Science & Technology. 2004;38:4503–4509. doi: 10.1021/es040029t. [DOI] [PubMed] [Google Scholar]
- 9.van Beusekom OC, Eljarrat E, Barceló D, Koelmans AA. Dynamic modeling of food-chain accumulation of brominated flame retardants in fish from the Ebro River Basin, Spain. Environmental Toxicology and Chemistry. 2006;25:2553–2560. doi: 10.1897/05-409r.1. [DOI] [PubMed] [Google Scholar]
- 10.Doyle JR, Al-Ansari AM, Gendron RL, White PA, Blais JM. A method to estimate sediment ingestion by fish. Aquatic Toxicology. 2011;103:121–127. doi: 10.1016/j.aquatox.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Niimi AJ, Oliver BG. Biological Half-lives of Polychlorinated Biphenyl (PCB) Congeners in Whole Fish and Muscle of Rainbow Trout (Salmo gairdneri) Canadian Journal of Fisheries and Aquatic Sciences. 1983;40:1388–1394. [Google Scholar]
- 12.Gobas FAPC, McCorquodale JR, Haffner GD. Intestinal absorption and biomagnification of organochlorines. Environmental Toxicology and Chemistry. 1993;12:567–576. [Google Scholar]
- 13.Burreau S, Axelman J, Broman D, Jakobsson E. Dietary uptake in pike (Esox lucius) of some polychlorinated biphenyls, polychlorinated naphthalenes and polybrominated diphenyl ethers administered in natural diet. Environmental Toxicology and Chemistry. 1997;16:2508–2513. [Google Scholar]
- 14.Fisk AT, Norstrom RJ, Cymbalisty CD, Muir DCG. Dietary accumulation and depuration of hydrophobic organochlorines: Bioaccumulation parameters and their relationship with the octanol/water partition coefficient. Environmental Toxicology and Chemistry. 1998;17:951–961. [Google Scholar]
- 15.Stapleton HM, Letcher RJ, Li J, Baker JE. Dietary accumulation and metabolism of polybrominated diphenyl ethers by juvenile carp (Cyprinus carpio) Environmental Toxicology and Chemistry. 2004;23:1939–1946. doi: 10.1897/03-462. [DOI] [PubMed] [Google Scholar]
- 16.McLeod PB, van den Heuvel-Greve MJ, Allen-King RM, Luoma SN, Luthy RG. Effects of Particulate Carbonaceous Matter on the Bioavailability of Benzo[a]pyrene and 2,2‘,5,5‘-Tetrachlorobiphenyl to the Clam, Macoma balthica. Environmental Science & Technology. 2004;38:4549–4556. doi: 10.1021/es049893b. [DOI] [PubMed] [Google Scholar]
- 17.Sun X, Werner D, Ghosh U. Modeling PCB Mass Transfer and Bioaccumulation in a Freshwater Oligochaete Before and After Amendment of Sediment with Activated Carbon. Environmental Science & Technology. 2009;43:1115–1121. doi: 10.1021/es801901q. [DOI] [PubMed] [Google Scholar]
- 18.Wang W-X, Fisher NS. Assimilation efficiencies of chemical contaminants in aquatic invertebrates: A synthesis. Environmental Toxicology and Chemistry. 1999;18:2034–2045. [Google Scholar]
- 19.OECD. OECD Guidelines for Testing of Chemicals-305. Organisation for Economic Co-operation and Development; 2011. [Google Scholar]
- 20.Werner D, Hale SE, Ghosh U, Luthy RG. Polychlorinated Biphenyl Sorption and Availability in Field-Contaminated Sediments. Environmental Science & Technology. 2010;44:2809–2815. doi: 10.1021/es902325t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawker DW, Connell DW. Octanol-water partition coefficients of polychlorinated biphenyl congeners. Environmental Science & Technology. 1988;22:382–387. doi: 10.1021/es00121a006. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-Eyles JL, Yupanqui C, Beckingham B, Riedel G, Gilmour C, Ghosh U. Evaluation of Biochars and Activated Carbons for In Situ Remediation Of Sediments Impacted With Organics, Mercury, And Methylmercury. Environmental Science & Technology. 2013;47:13721–13729. doi: 10.1021/es403712q. [DOI] [PubMed] [Google Scholar]
- 23.Gobas FAPC, Muir DCG, Mackay D. Dynamics of dietary bioaccumulation and faecal elimination of hydrophobic organic chemicals in fish. Chemosphere. 1988;17:943–962. [Google Scholar]
- 24.Arnot J, Gobas F. A food web bioaccumulation model for organic chemicals in aquatic ecosystems - SETAC Supplemental Data Archive S.3. 2004 doi: 10.1897/03-438. [DOI] [PubMed] [Google Scholar]
- 25.Lo JC, Campbell DA, Kennedy CJ, Gobas FAPC. Somatic and gastrointestinal in vivo biotransformation rates of hydrophobic chemicals in fish. Environmental Toxicology and Chemistry. 2015;34:2282–2294. doi: 10.1002/etc.3050. [DOI] [PubMed] [Google Scholar]
- 26.Barber MC. Dietary uptake models used for modeling the bioaccumulation of organic contaminants in fish. Environmental Toxicology and Chemistry. 2008;27:755–777. doi: 10.1897/07-462.1. [DOI] [PubMed] [Google Scholar]
- 27.Dabrowska H, Fisher SW, Dabrowski K, Staubus AE. Dietary uptake efficiency of 2,2ǐ,4,4ǐ,5,5ǐ-hexachlorobiphenyl in yellow perch and rainbow trout: Role of dietary and body lipids. Environmental Toxicology and Chemistry. 1999;18:938–945. [Google Scholar]
- 28.Gobas FAPC, Wilcockson JB, Russell RW, Haffner GD. Mechanism of Biomagnification in Fish under Laboratory and Field Conditions. Environmental Science & Technology. 1999;33:133–141. [Google Scholar]
- 29.Clark KE, Mackay D. Dietary uptake and biomagnification of four chlorinated hydrocarbons by guppies. Environmental Toxicology and Chemistry. 1991;10:1205–1217. [Google Scholar]
- 30.Nichols JW, Fitzsimmons PN, Whiteman FW, Kuehl DW, Butterworth BC, Jenson CT. Dietary uptake kinetics of 2,2',5,5'-tetrachlorobiphenyl in rainbow trout. Drug metabolism and disposition: the biological fate of chemicals. 2001;29:1013–1022. [PubMed] [Google Scholar]
- 31.Werner D, Ghosh U, Luthy RG. Modeling Polychlorinated Biphenyl Mass Transfer after Amendment of Contaminated Sediment with Activated Carbon. Environmental Science & Technology. 2006;40:4211–4218. doi: 10.1021/es052215k. [DOI] [PubMed] [Google Scholar]
- 32.DiPinto LM, Coull BC. Trophic transfer of sediment-associated polychlorinated biphenyls from meiobenthos to bottom-feeding fish. Environmental Toxicology and Chemistry. 1997;16:2568–2575. doi: 10.1007/BF00213396. [DOI] [PubMed] [Google Scholar]
- 33.DiPinto LM. Trophic Transfer of a Sediment-Associated Organophosphate Pesticide from Meiobenthos to Bottom Feeding Fish. Archives of environmental contamination and toxicology. 1996;30:459–466. doi: 10.1007/BF00213396. [DOI] [PubMed] [Google Scholar]
- 34.Cornelissen G, Gustafsson Ö, Bucheli TD, Jonker MTO, Koelmans AA, van Noort PCM. Extensive Sorption of Organic Compounds to Black Carbon, Coal, and Kerogen in Sediments and Soils: Mechanisms and Consequences for Distribution, Bioaccumulation, and Biodegradation. Environmental Science & Technology. 2005;39:6881–6895. doi: 10.1021/es050191b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.