د ويکيپېډيا، وړیا پوهنغونډ له خوا
Samarium, 62Sm |
|
| Pronunciation | /səˈmɛəriəm/
sə-MAIR-ee-əm |
|---|
| Appearance | silvery white |
|---|
|
|
|
| اټومي شمیره (Z) | 62 |
|---|
| گروپ, پیریود | group n/a, period 6 |
|---|
| بلاک | f-block |
|---|
| Element category | lanthanide |
|---|
| معياري اټومي وزن (Ar) | کينډۍ:Val[۱] |
|---|
| Electron configuration | [Xe] 4f6 6s2 |
|---|
Electrons per shell | 2, 8, 18, 24, 8, 2 |
|---|
|
| حالت (at STP) | جامد |
|---|
| دويلې کيدو ټکى | 1345 K (1072 °C, 1962 °F) |
|---|
| يشنا ټکی | 2173 K (1900 °C, 3452 °F) |
|---|
| Density near r.t. | 7.52 g/cm3 |
|---|
| when liquid, at m.p. | 7.16 g/cm3 |
|---|
| Heat of fusion | 8.62 kJ/mol |
|---|
| Heat of vaporization | 192 kJ/mol |
|---|
| Molar heat capacity | 29.54 J/(mol·K) |
|---|
Vapor pressure
| P (Pa)
|
1
|
10
|
100
|
1 k
|
10 k
|
100 k
|
| at T (K)
|
1001
|
1106
|
1240
|
(1421)
|
(1675)
|
(2061)
| |
|
| Oxidation states | 4, 3, 2, 1 (a mildly basic oxide) |
|---|
| Electronegativity | Pauling scale: 1.17 |
|---|
| Ionization energies | - 1st: 544.5 kJ/mol
- 2nd: 1070 kJ/mol
- 3rd: 2260 kJ/mol
-
|
|---|
| Atomic radius | empirical: 180 pm |
|---|
| Covalent radius | 198±8 pm |
|---|
|
| Crystal structure | rhombohedral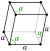 Rhombohedral crystal structure for samarium Rhombohedral crystal structure for samarium |
|---|
| Speed of sound thin rod | 2130 m/s (at 20 °C) |
|---|
| Thermal expansion | (r.t.) (α, poly) 12.7 µm/(m·K) |
|---|
| د تودوخې تېرېدنه | 13.3 W/(m·K) |
|---|
| Electrical resistivity | (r.t.) (α, poly) 0.940 µΩ·m |
|---|
| Magnetic ordering | paramagnetic[۲] |
|---|
| Magnetic susceptibility (χmol) | +1860.0·10−6 cm3/mol (291 K)[۳] |
|---|
| Young's modulus | α form: 49.7 GPa |
|---|
| Shear modulus | α form: 19.5 GPa |
|---|
| Bulk modulus | α form: 37.8 GPa |
|---|
| Poisson ratio | α form: 0.274 |
|---|
| Vickers hardness | 410–440 MPa |
|---|
| Brinell hardness | 440–600 MPa |
|---|
| CAS Number | 7440-19-9 |
|---|
|
| Naming | after the mineral samarskite (itself named after Vasili Samarsky-Bykhovets) |
|---|
| Discovery and first isolation | Lecoq de Boisbaudran (1879) |
|---|
|
|
|
| | references | in Wikidata |
ساماريوم (په انگرېزي: Samarium) له کیمیاوي عناصرو څخه یو دی چې په تناوبي جدول کې د Sm سمبول او ۶۲ اتمي شمېرې په وسیله ښودل شوی دی.
- ↑ Meija, J.; Coplen, T. B. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure Appl. Chem. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
- ↑ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ↑ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
|
 دا ليکنه نابشپړه ده، تاسې ددې ليکنې په بشپړولو کې د ويکيپېډيا سره مرسته کولای شئ. دا ليکنه نابشپړه ده، تاسې ددې ليکنې په بشپړولو کې د ويکيپېډيا سره مرسته کولای شئ.
|

