2022-24 Mpox (Monkeypox)
Outbreak:
Global Trends
World Health Organization
Produced on 08 January 2025
Key figures
Weekly trends in Africa
Data as updated weekly; from 01 January 2024 to 05 January 2025. Note that data shown here includes laboratory confirmed cases only. The most recent weeks presented in the epidemic curves should be interpreted with caution, as there are delays associated with reporting.
In the Democratic Republic of the Congo, confirmed and suspected cases reported at the national level are not updated as of the 17th November 2024. Efforts are ongoing to update this data.

Totals for key countries
Democratic Republic of the Congo: totals from 2024
As of 17 November 2024
In the Democratic Republic of the Congo, confirmed and suspected cases reported at the national level are not updated as of the 17th November 2024. Efforts are ongoing to update this data.
Case numbers in the Democratic Republic of the Congo have been revised. As of 20 November 2024, suspected mpox cases that tested negative for MPXV have been removed from overall numbers. Overall numbers of suspected cases have been reduced in DRC for this reason. This only applies to numbers at the national level.
Cases*
43 862
Lab confirmed cases
9 513
Deaths*
1 138
Lab confirmed deaths
43
Burundi: totals from 2024
As of 05 January 2025
Lab confirmed cases
3 035
Lab confirmed deaths
1
*Note that cases here refers to all cases, including suspected and laboratory confirmed cases. Laboratory confirmed cases are a subset of all cases. The same applies to deaths and laboratory confirmed deaths. In instances where suspected cases are tested and the laboratory results are negative, these cases are now retrospectively removed from the total case numbers.
Totals for Africa
Data as updated weekly; from 01 January 2022 to 05 January 2025. Note that data shown here refers to laboratory confirmed cases only, and are collected from the continent of Africa, across the WHO African and Eastern Mediterranean regions.
Total lab confirmed cases from 2024
14 700
Total lab confirmed deaths from 2024
66
Countries reporting cases from 2024
20
Total lab confirmed cases
17 104
Total lab confirmed deaths
89
Countries reporting cases
24
Global totals
Data as updated monthly; from 01 January 2022 to 30 November 2024
Total lab confirmed cases in November 2024
2 726
Total lab confirmed deaths in November 2024
4
Countries reporting cases inNovember 2024
42
Total lab confirmed cases from 2024
22 441
Total lab confirmed deaths from 2024
78
Countries reporting cases from 2024
82
Total lab confirmed cases
117 663
Total lab confirmed deaths
263
Countries reporting cases
127
Clades detected globally
Known clade distribution as of 05 January 2025. Clade detection includes cases associated with local transmission and imported cases. Data is based on a combination of sequencing databanks, published literature, epidemiological links, and communication to WHO since 2022.
Map can be clicked to view on a larger scale

1 Overview
This report provides an overview of the mpox1 epidemiological situation in Africa, on a weekly basis (as of 05 January 2025), as well as the global epidemiological situation on a monthly basis (as of November 2024).
Data in this report are based on global surveillance data collected from 01 January 2022, initiated due to the unprecedented human-to-human spread of monkeypox virus (MPXV) globally occurring in the same year.
On 14 August 2024, under the International Health Regulations (2005), the WHO Director General declared that the increase in mpox cases in the Democratic Republic of the Congo and its expansion to neighboring countries constitutes a Public Health Emergency of International Concern (PHEIC). This spread presents a public health risk to other Member States and requires a coordinated international response.
Based on currently available information, the spread of mpox cases in the Democratic Republic of the Congo is attributed to two main outbreaks - spread of MPXV clade Ia in Equateur and other previously affected provinces of the country, and the spread of clade Ib MPXV in the provinces of North and South Kivu, as well as several clade Ib cases detecrted in Kinshasa. Current sequencing in the country is limited and clade distribution might be broader than what is currently known.
WHO conducted the latest global mpox rapid risk assessment in November 2024. Based on the available information, the risk is assessed as follows:
- Clade Ib MPXV: Predominantly affecting non-endemic
areas for mpox in the Democratic Republic of the Congo and neighboring
countries — High
- Clade Ia MPXV: Primarily affecting endemic areas
for mpox within the Democratic Republic of the Congo —
High
- Clade II MPXV: Observed in Nigeria and other
endemic countries in West and Central Africa —
Moderate
- Clade IIb MPXV: Associated with the global mpox epidemic — Moderate
Please note that regardless of geographic area, epidemiological context, biological sex, gender identity or sexual behaviour, individual-level risk is largely dependent on individual factors such as exposure risk and immune status.
This report mainly focuses on laboratory confirmed case and deaths2 as defined by WHO’s working case definition published in the Surveillance, case investigation and contact tracing for monkeypox interim guidance. In Africa, laboratory confirmed and suspected cases are both shown where possible. Note that countries3 may use their own case definitions separate from those outlined in the above document.
On of 28 November 2022, WHO recommended using the name mpox as a new name for monkeypox. The words were used synonymously for one year as the term monkeypox was phased out. The virus causing mpox is named monkeypox virus (MPXV).
For the WHO European region, both confirmed and probable cases are included within confirmed case counts and detailed case data.
Throughout this document, any use of the word country should be considered shorthand for a country, area, or territory
2 Situation in Africa
This section of the report is jointly authored by the WHO Regional Office for Africa, the WHO Regional Office for the Eastern Mediterranean4 and WHO Headquarters.
Since 1 January 2022, cases of mpox have been reported to WHO from 24 Member States across Africa. As of 05 January 2025 , a total of 17 104 laboratory confirmed cases, including 89 deaths, have been reported to WHO.
From 2024, as of 05 January 2025, 20 countries have reported 14 700 confirmed cases, including 66 deaths. The three countries with the majority of the cases from 2024 are Democratic Republic of the Congo, (n = 9 513), Burundi, (n = 3 035), and Uganda, (n = 1 552).
A significant number of suspected mpox cases, that are clinically compatible with mpox remain untested due to limited diagnostic capacity in some African countries and thus never get confirmed. For this reason, we include suspected cases in this section of the report. As of 20 November 2024, the all cases indicator no longer includes test-negative cases at a national level.
This indicator should be interpreted with caution, as suspected mpox cases are recorded according to varying national case definitions. Moreover, not all countries have robust surveillance systems for mpox, meaning reported case counts are likely underestimate the extent of community transmission.
Case definitions for some countries can be seen in the case definitions subsection.
- On the African continent there are 47 Member States in the WHO African Region and seven in the Eastern Mediterranean Region.
2.1 Outbreak status and MPXV clade distribution
The distribution of clades reported in Africa, and the outbreak status of the continent is shown in the maps below. Countries with active mpox transmission are classified as those that have reported cases to WHO within the past 42 days. If a country does not share information on mpox cases or provide regular zero-case reporting to WHO or any other open-access resources, its outbreak status may not accurately reflect its current situation on the map. The distribution of reported mpox clades in Africa is also shown below. It should be noted that in many cases, sequencing may not capture all circulating clades, leading to under-representation of where clades are circulating.
Maps can be clicked to view on a larger scale.
2.1.1 Outbreak status

2.1.2 Clade Ia, Ib, and II (a and/or b) presence

2.1.3 Confirmed cases in the past six weeks
Note that the six week period used here is relative to the date cases were last reported in each country.

2.2 Epidemic curves
Epidemic curve shown by week for cases reported up to 05 Jan 2025. Note that for the purposes of these epidemic curves, countries with more than one clade present are presented in multiple epidemic curves. The most recent weeks presented in the epidemic curves should be interpreted with caution, as there are delays associated with reporting.
2.2.1 Confirmed cases in all countries in Africa
In the Democratic Republic of the Congo, confirmed and suspected cases reported at the national level are not updated as of the 17th November 2024. Efforts are ongoing to update this data.

2.2.2 Confirmed cases in countries with only clade Ib
Cases in the DRC are excluded from this figure due to cocirculation of clades Ia and Ib.

2.2.3 Confirmed cases in countries with only clade Ia
Cases in the DRC are excluded from this figure due to cocirculation of clades Ia and Ib.

2.2.4 Confirmed cases in the DRC

2.2.5 Confirmed cases in countries with Clade II (a and/or b)

2.2.6 All cases in the Democratic Republic of the Congo
All cases, including suspected and lab confirmed cases are shown from 2024. We exceptionally highlight the Democratic Republic of the Congo due to the testing shortage in country, where over half of suspected cases do not go on to be tested.

2.3 Maps
Maps can be clicked to view on a larger scale. Note that data are only shown for Africa - data from elsewhere are reflected in the global sections of the report.
2.3.1 Cumulative confirmed cases

2.3.2 Cumulative confirmed cases from 2024

2.3.3 Cumulative confirmed deaths

2.3.4 Cumulative confirmed deaths from 2024

2.4 Data by country
2.4.1 Laboratory confirmed cases
2.4.2 All cases
Note that as of 20 November 2024, cases that test negative are no longer included in all cases - for this reason, a number of countries have shown decreases in numbers of all cases.
2.5 Epidemic curves by country
Epidemic curve shown by week for cases confirmed reported up to 05 Jan 2025.
Angola

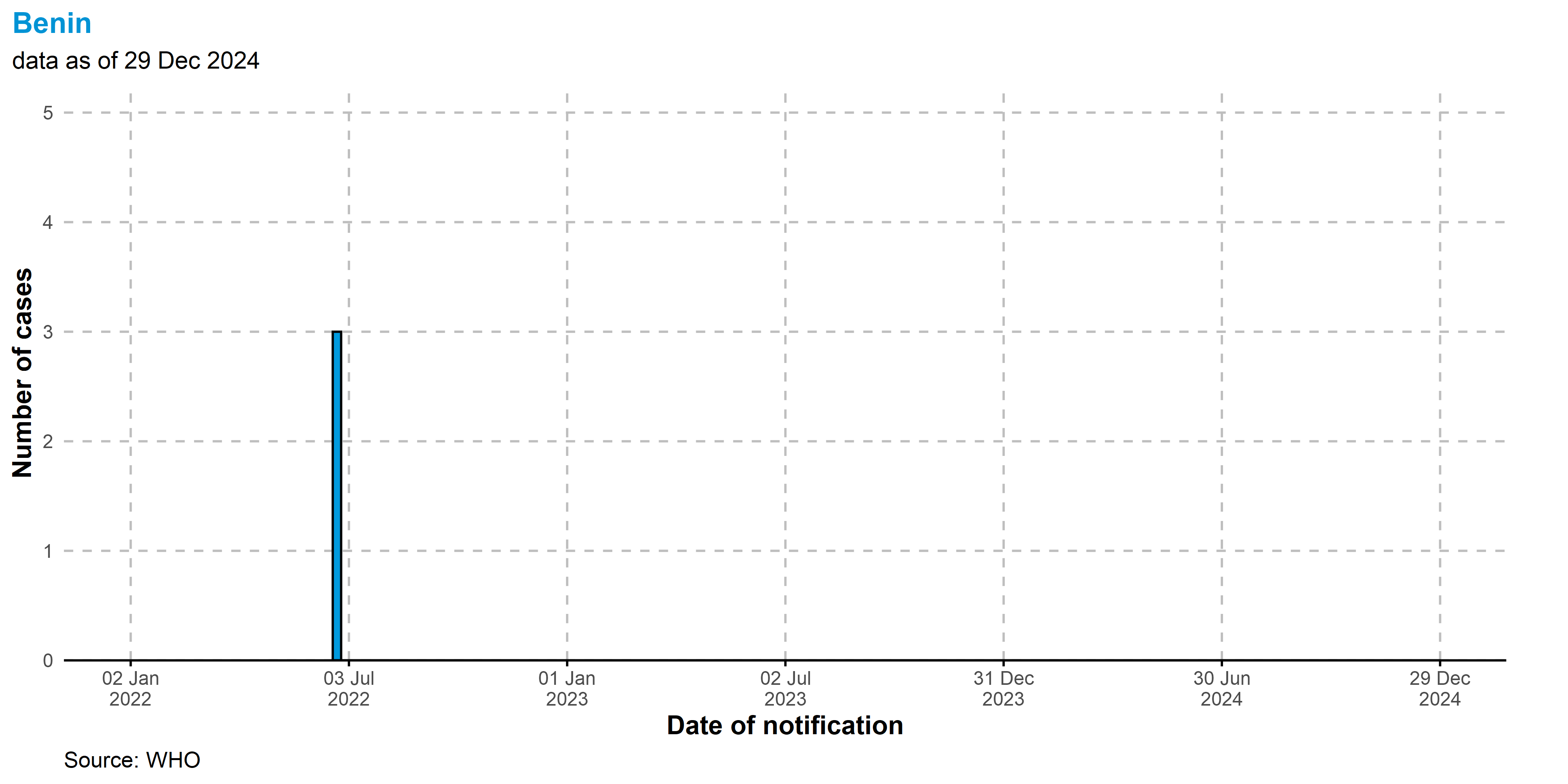
Benin
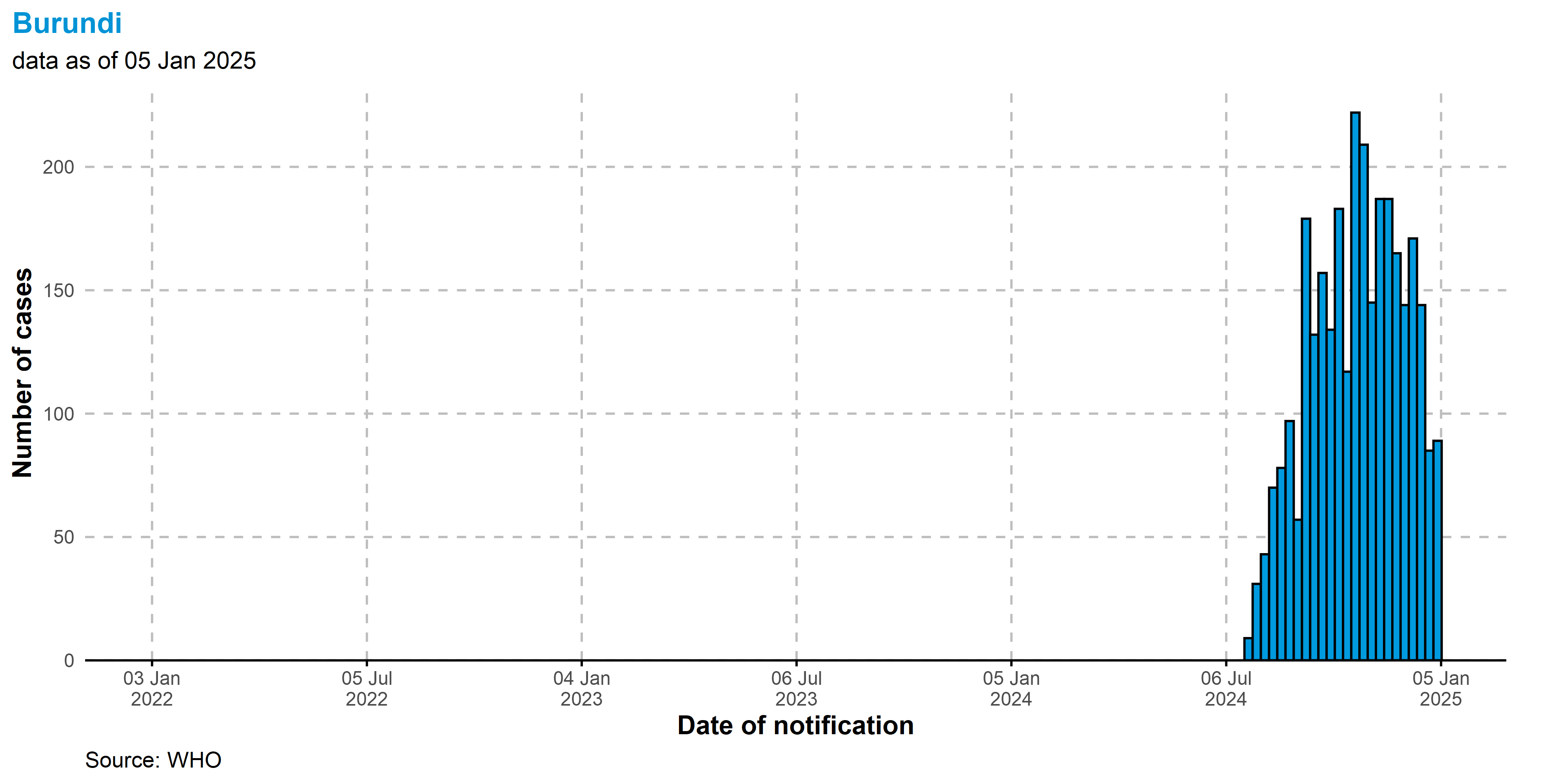
Burundi
Cameroon

Central African Republic

Congo

Côte d’Ivoire

Democratic Republic of the Congo

Egypt

Gabon

Ghana

Guinea

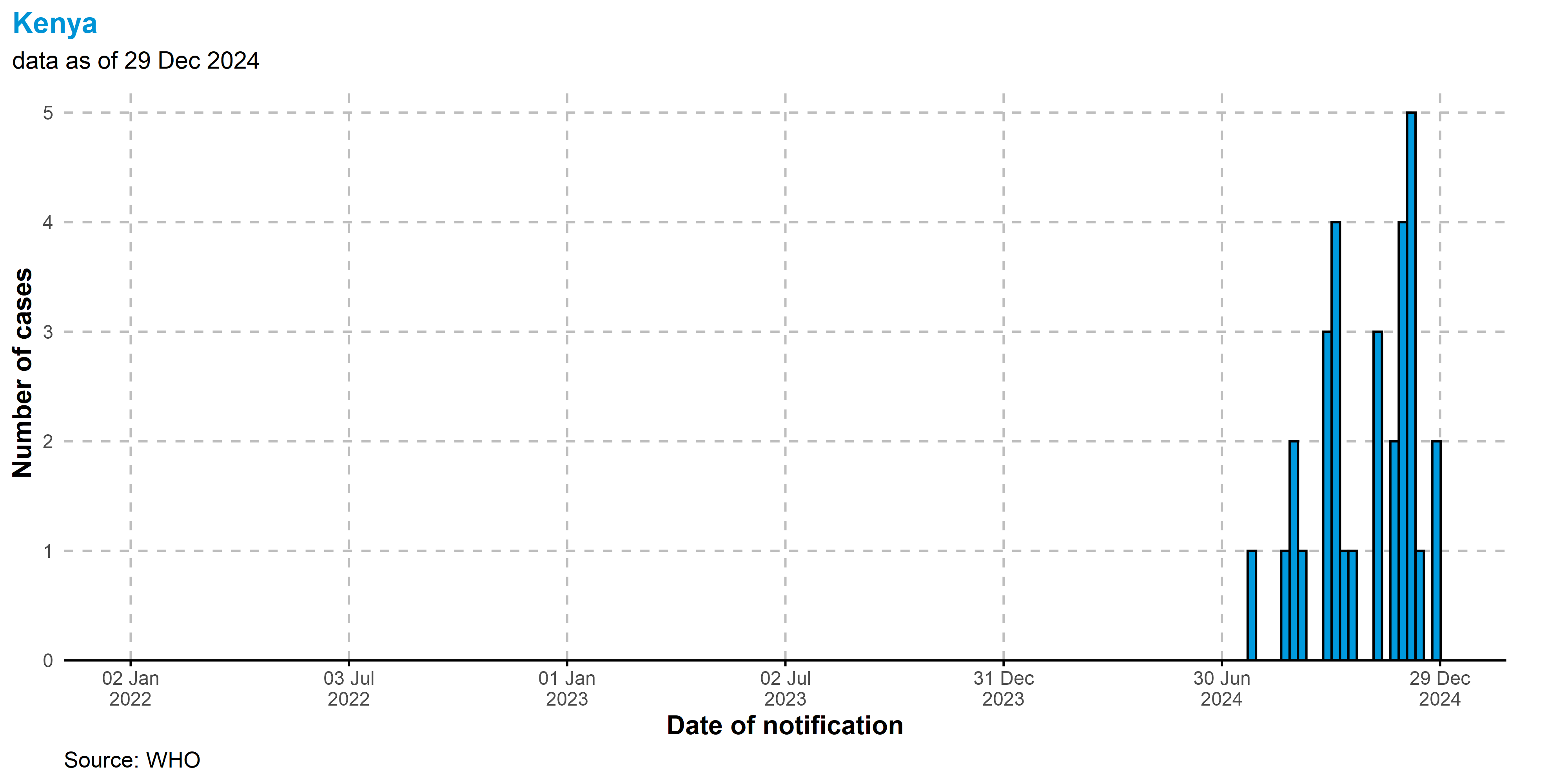
Kenya
Liberia

Mauritius

Morocco

Mozambique

Nigeria

Rwanda

South Africa

Sudan

Uganda

Zambia

Zimbabwe

2.6 Case definitions
This section includes the national case definition used in African countries in order to provide more context for the interpretation of data, especially of suspected cases.
Case definitions for suspected cases are shown for the following countries below:
2.6.1 Burundi
Suspected Case:
Any person presenting with sudden onset of fever >38.3°C (101°F), intense headaches, adenopathy, back pain, myalgia, and intense weakness, followed 1-3 days later by a vesiculopustular skin rash that develops progressively, often starting on the face (more dense) and then spreading to other parts of the body, including the soles of the feet and palms of the hands.
Confirmed Case:
Any case that has been clinically and epidemiologically diagnosed with mpox and laboratory confirmed.
2.6.2 Democratic Republic of the Congo
Suspected case:
A person with sudden onset of high fever followed by a vesiculopustular rash predominantly on the face and present on the palms of the hands and soles of the feet; OR presence of at least 5 smallpox-like scars,
OR
Any person with fever > 38.3 °C (101 F), severe headache, lymphadenopathy, back pain, myalgia, and severe weakness, followed 1-3 days later by a progressive rash that often begins on the face (more dense) and then spreads elsewhere on the body, including the soles of the feet and palms of the hands.
Confirmed case:
Any case for which the clinical and epidemiological diagnosis of mpox has been laboratory confirmed.
Data for download
Data by country can be downloaded as a csv file by clicking the button below. The data include the number of new cases and deaths reported each week, as well as the total number of cases and deaths reported to date. The data are current as of 05 Jan 2025.
3 Situation in focus - Democratic Republic of the Congo
Given the recent increase in cases in the Democratic Republic of the Congo, and the emergence of clade Ib in the Sud-Kivu province, we exceptionally present an overview of the ongoing situation in the Democratic Republic of the Congo, in agreement with the national Ministry of Public Health.
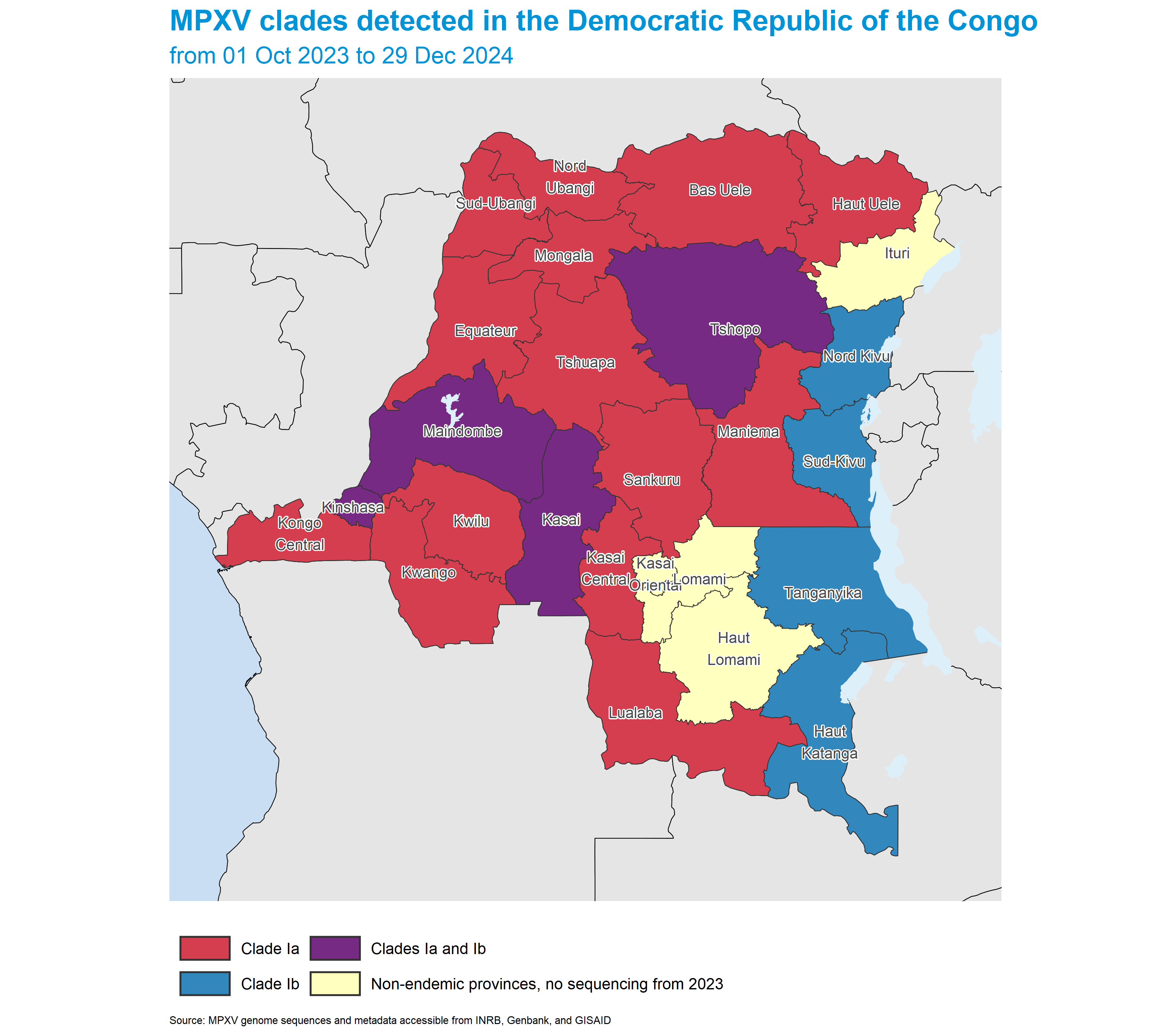
Based on currently available information, the current spread of mpox cases in the Democratic Republic of the Congo is attributed to two main outbreaks - spread of MPXV clade Ia in historically affected endemic provinces of the country, and the spread of MPXV clade Ib in the provinces of North and South Kivu (Nord Kivu and Sud-Kivu). Cases of clade Ib have also been detected in the Kinshasa, Kasai, Tanganyika, and Tshopo provinces. Clade Ia is known to be associated with zoonotic spillover events from animal reservoirs. In contrast, clade Ib is thought to be solely associated with human to human transmission.
In this section, clade distribution across provinces is based on sequencing data provided by the national Ministry of Public Health. While around 10% of positive samples nationwide have been sequenced, the geographical representation may be incomplete, and the actual clade distribution could be broader and more nuanced than currently presented.
The report presents trends based on three key data sources:
Weekly aggregate syndromic surveillance data of suspected cases (via Integrated Disease Surveillance database [IDS]). This is used for overall case numbers and trends in cases. Note that syndromic cases that go on to test negative are included in these numbers.
Laboratory data from a subset of suspected cases who undergo testing (currently about 45% in 2024, with approximately 50% of these testing positive, via laboratory database). This is used for confirmed case numbers and trends in confirmed cases.
Case-based data, available for a subset of suspected and confirmed cases, provide more detailed insights, such as the demographic characteristics of cases (via provincial case-based datasets). This is used for demographic data.
Sequencing metadata made available by the Democratic Republic of the Congo National Institute of Biomedical Research (INRB), provinding information on the clade distribution of MPXV in the country.
Case definitions for both suspected and confirmed cases can be found above.
It is important to note that all surveillance data are subject to delays, and data cleaning is ongoing. As a result, sub-national case counts in this section may lag behind those reported at the national level. Mpox surveillance coverage and completeness varies in the Democratic Republic of the Congo over geographic space and time, and in some cases, trends in reported cases may be affected by changes in surveillance.
Compte tenu de l’augmentation récente des cas en République démocratique du Congo et de l’émergence du clade Ib dans la province du Sud-Kivu, nous présentons exceptionnellement un aperçu de la situation actuelle en République démocratique du Congo, en accord avec le ministère national de la Santé publique.
Sur la base des informations actuellement disponibles, la propagation actuelle des cas de mpox en République démocratique du Congo est attribuée à deux principales épidémies - la propagation du MPXV clade Ia dans les provinces endémiques historiquement touchées du pays, et la propagation du MPXV clade Ib dans les provinces du Nord et du Sud-Kivu (Nord-Kivu et Sud-Kivu). Des cas de clade Ib ont également été détectés dans les provinces de Kinshasa, du Kasaï, du Tanganyika et de la Tshopo. Le clade Ia est connu pour être associé à des événements de propagation zoonotique à partir de réservoirs animaux. En revanche, le clade Ib serait uniquement associé à la transmission interhumaine.
Dans cette section, la répartition des clades dans les provinces est basée sur les données de séquençage fournies par le ministère national de la Santé publique. Bien qu’environ 10 % des échantillons positifs à l’échelle nationale aient été séquencés, la représentation géographique peut être incomplète et la répartition réelle des clades pourrait être plus large et plus nuancée que celle présentée actuellement.
Le rapport présente les tendances basées sur trois sources de données clés :
Données de surveillance syndromique agrégées hebdomadaires des cas suspects (via la base de données de surveillance intégrée des maladies [IDS]). Ces données sont utilisées pour le nombre total de cas et les tendances des cas. Notez que les cas syndromiques qui se révèlent négatifs au test sont inclus dans ces chiffres.
Données de laboratoire provenant d’un sous-ensemble de cas suspects qui subissent des tests (actuellement environ 45 % en 2024, dont environ 50 % seront positifs, via la base de données du laboratoire). Ces données sont utilisées pour le nombre de cas confirmés et les tendances des cas confirmés.
Données basées sur les cas, disponibles pour un sous-ensemble de cas suspects et confirmés, fournissent des informations plus détaillées, telles que les caractéristiques démographiques des cas (via des ensembles de données basés sur les cas provinciaux). Ces données sont utilisées pour les données démographiques.
Métadonnées de séquençage mises à disposition par l’Institut national de recherche biomédicale de la République démocratique du Congo (INRB), fournissant des informations sur la distribution des clades du MPXV dans le pays.
Les définitions de cas pour les cas suspects et confirmés se trouvent ci-dessus.
Il est important de noter que toutes les données de surveillance sont sujettes à des retards et que le nettoyage des données est en cours. Par conséquent, le nombre de cas infranationaux dans cette section peut être inférieur à celui rapporté au niveau national. La couverture et l’exhaustivité de la surveillance du Mpox varient en République démocratique du Congo dans l’espace géographique et dans le temps, et dans certains cas, les tendances des cas signalés peuvent être affectées par des changements dans la surveillance.
3.1 Situation summary
3.1.1 Distribution of MPXV sequences
The map below shows the distribution of MPXV sequences from the Democratic Republic of the Congo from 2023. The color of each province represents the proportion of sequences that are clade Ib. Sequences are obtained from metadata shared by the Democratic Republic of the Congo National Institute of Biomedical Research (INRB), and from publicly available sequences on Genbank and GISAID.
La carte ci-dessous montre la distribution des séquences MPXV de la République démocratique du Congo à partir de 2023. La couleur de chaque province représente la proportion de séquences qui sont du clade Ib. Les séquences sont obtenues à partir de métadonnées partagées par l’Institut national de recherche biomédicale de la République démocratique du Congo (INRB) et à partir de séquences accessibles au public sur Genbank et GISAID.

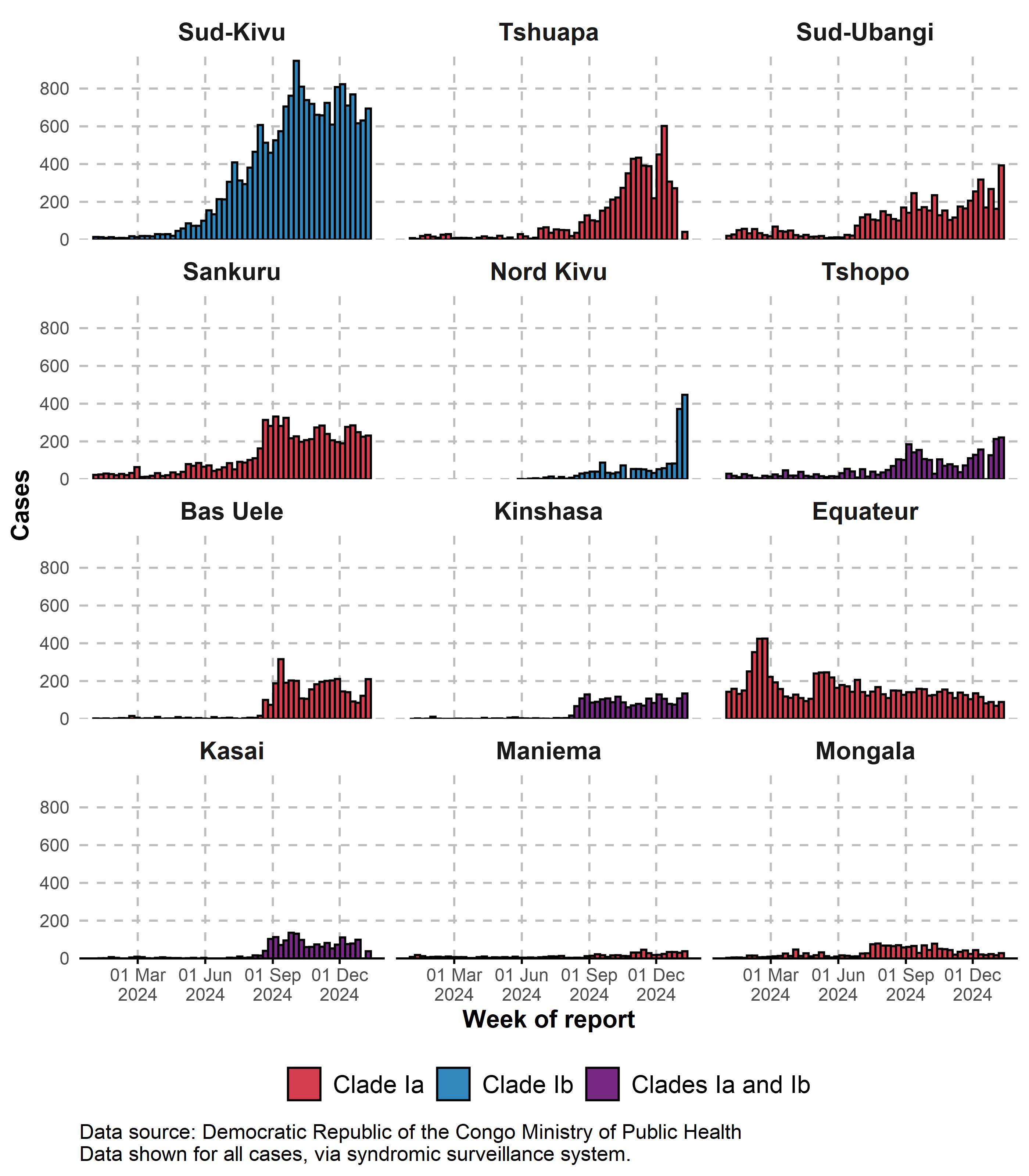
3.1.2 Trends in all cases by province
Trends shown for the twelve provinces reporting the highest numbers in the past six weeks. Data shown for all cases, via syndromic surveillance system.
Tendances présentées pour les douze provinces ayant signalé les chiffres les plus élevés au cours des six dernières semaines. Data shown for all cases, via syndromic surveillance system.

3.1.3 Recent cases by health zone
Data shown for all cases, via syndromic surveillance system.

3.2 Genomic surveillance
In this subsection, we present visualizations of sequences that are publicly available in the INRB, GISAID and Genbank databases. Publicly available sequence metadata from the Democratic Republic of the Congo National Institute of Biomedical Research (INRB) were also used, as well as information from a post pertaining to Nord Kivu on virological.org. MPXV sequences from Democratic Republic of the Congo are available from as early as 1970, with the latest sequence sampled on 2024-11-15.
When interpreting these data, it is important to note that the availability of sequences is not uniform across time and space, and that the distribution of sequences may not be representative of the true distribution of clades in the country.
It should be noted that samples associated with clade Ib were first collected in late 2023 - the designation of this lineage from 2024 retrospectively classified all previously identified sequences as clade Ia (previously they would be considered clade I). Therefore, all sequences identified before 2023 are retrospectively attributable to ancestral clade Ia. For this reason, we highlight endemic provinces in these analysis, given the known history of zoonotic spillover events associated with clade Ia.
Dans cette sous-section, nous présentons des visualisations de séquences accessibles au public dans les bases de données INRB, GISAID et Genbank. Des métadonnées de séquence accessibles au public provenant de l’Institut national de recherche biomédicale de la République démocratique du Congo (INRB) ont également été utilisées, ainsi que des informations provenant d’un article concernant le Nord-Kivu sur virological.org. Les séquences MPXV de la République démocratique du Congo sont disponibles dès 1970, la dernière séquence ayant été échantillonnée le 2024-11-15.
Lors de l’interprétation de ces données, il est important de noter que la disponibilité des séquences n’est pas uniforme dans le temps et l’espace, et que la distribution des séquences peut ne pas être représentative de la véritable distribution des clades dans le pays.
Il convient de noter que les échantillons associés au clade Ib ont été collectés pour la première fois fin 2023 - la désignation de cette lignée en 2024 a classé rétrospectivement toutes les séquences précédemment identifiées comme clade Ia (auparavant, elles étaient considérées comme clade I). Par conséquent, toutes les séquences identifiées avant 2023 sont rétrospectivement attribuables au clade ancestral Ia. Pour cette raison, nous mettons en évidence les provinces endémiques dans ces analyses, compte tenu de l’historique connu des événements de débordement zoonotique associés au clade Ia.
3.2.1 Sequencing from 2023

3.2.2 Classification of provinces
3.3 Data tables
All tables show data for 2024.
Tous les tableaux affichent les données pour 2024.
3.3.1 All cases
3.3.2 Laboratory confirmed cases
3.3.3 All cases by Health Zone
The following table is shown for the health zones with the highest numbers of recorded cases in the past six weeks.
Le tableau suivant présente les zones de santé ayant enregistré le plus grand nombre de cas au cours des six dernières semaines.
3.4 Maps of cases
Note: Maps can be clicked to view on a larger scale.
Remarque: les cartes peuvent être cliquées pour être visualisées à une plus grande échelle.:::
3.4.1 All cases by province
Data shown for all cases, via syndromic surveillance system.

3.4.2 Recent cases by province
Data shown for all cases, via syndromic surveillance system.

3.4.3 Confirmed cases by province

3.4.4 Recent confirmed cases by province

3.4.5 Deaths by province
Data shown for all deaths, via syndromic surveillance system.

3.4.6 Recent deaths by province
Data shown for all deaths, via syndromic surveillance system.

3.4.7 All cases by health zone
Data shown for all cases, via syndromic surveillance system.

3.4.8 Recent cases by health zone
Data shown for all cases, via syndromic surveillance system.

3.4.9 Confirmed cases by health zone

3.4.10 Recent confirmed cases by health zone

3.5 Trends by province
3.5.1 Trends in all cases by province
Trends shown for the twelve provinces reporting the highest numbers of cases from 2024. Data shown for all cases, via syndromic surveillance system.
Tendances présentées pour les douze provinces signalant le plus grand nombre de cas en 2024. Data shown for all cases, via syndromic surveillance system.
3.5.2 Trends in confirmed cases by province
Trends in confirmed cases shown for the twelve provinces reporting the highest numbers of syndromic cases in the past six weeks. Note that confirmed cases are highly sensitive to the availability of testing which is variable over space and time due.
Tendances des cas confirmés présentées pour les douze provinces ayant signalé le plus grand nombre de cas syndromiques au cours des six dernières semaines. Il convient de noter que les cas confirmés sont très sensibles à la disponibilité des tests, qui est variable dans l’espace et dans le temps.

3.5.3 Trends in individual provinces - all cases
Trends shown for individual province, with the top five health zones within the province shown as individual colours. Province can be selected from the dropdown menu. Data shown for all cases, via syndromic surveillance system.
Les tendances sont présentées pour chaque province, les cinq principales zones de santé de la province étant représentées par des couleurs individuelles. La province peut être sélectionnée dans le menu déroulant. Data shown for all cases, via syndromic surveillance system.
Bas Uele

Equateur

Haut Katanga

Haut Lomami

Haut Uele

Ituri

Kasai

Kasai Central

Kasai Oriental

Kinshasa

Kongo Central

Kwango

Kwilu

Lomami

Lualaba

Maindombe

Maniema

Mongala

Nord Kivu

Nord Ubangi

Sankuru

Sud-Kivu

Sud-Ubangi

Tanganyika

Tshopo

Tshuapa

3.5.4 Trends in individual provinces - confirmed cases
Trends shown for individual provinces via the laboratory database. Province can be selected from the dropdown menu. Due to the lack of testing in some health zones, we do not separate the epidemic curve by individual health zones.
Tendances présentées pour les provinces individuelles via la base de données des laboratoires. La province peut être sélectionnée dans le menu déroulant. En raison du manque de tests dans certaines zones de santé, nous ne distinguons pas la courbe épidémique par zone de santé individuelle.
Bas Uele
Data shown for all cases, via syndromic surveillance
system.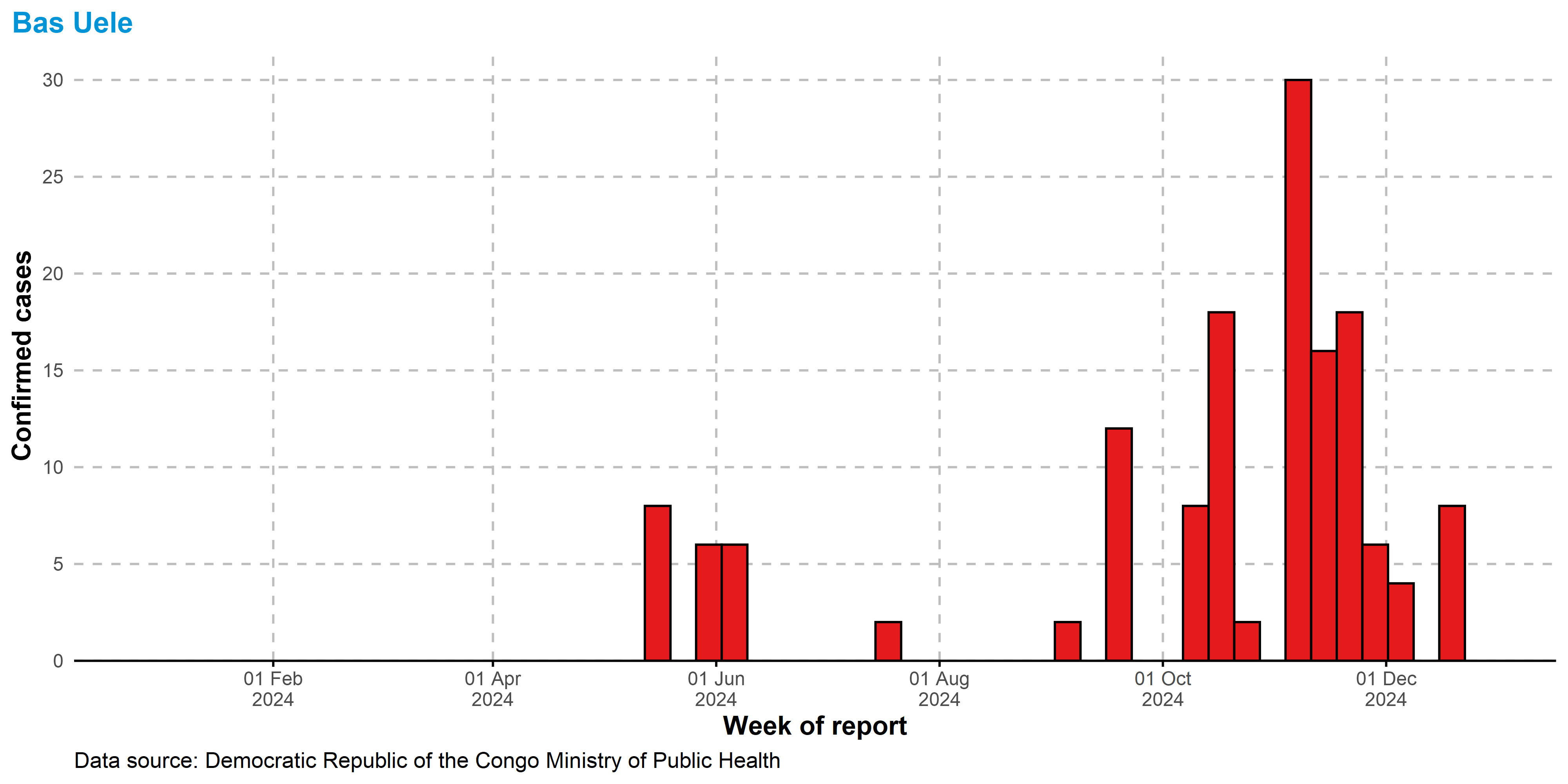
Equateur
Data shown for all cases, via syndromic surveillance
system.
Haut Katanga
Data shown for all cases, via syndromic surveillance
system.
Haut Lomami
Data shown for all cases, via syndromic surveillance
system.
Haut Uele
Data shown for all cases, via syndromic surveillance
system.
Ituri
Data shown for all cases, via syndromic surveillance
system.
Kasai
Data shown for all cases, via syndromic surveillance
system.
Kasai Central
Data shown for all cases, via syndromic surveillance
system.
Kasai Oriental
Data shown for all cases, via syndromic surveillance
system.
Kinshasa
Data shown for all cases, via syndromic surveillance
system.
Kongo Central
Data shown for all cases, via syndromic surveillance
system.
Kwango
Data shown for all cases, via syndromic surveillance
system.
Kwilu
Data shown for all cases, via syndromic surveillance
system.
Lomami
Data shown for all cases, via syndromic surveillance
system.
Lualaba
Data shown for all cases, via syndromic surveillance
system.
Maindombe
Data shown for all cases, via syndromic surveillance
system.
Maniema
Data shown for all cases, via syndromic surveillance
system.
Mongala
Data shown for all cases, via syndromic surveillance
system.
Nord Kivu
Data shown for all cases, via syndromic surveillance
system.
Nord Ubangi
Data shown for all cases, via syndromic surveillance
system.
Sankuru
Data shown for all cases, via syndromic surveillance
system.
Sud-Kivu
Data shown for all cases, via syndromic surveillance
system.
Sud-Ubangi
Data shown for all cases, via syndromic surveillance
system.
Tanganyika
Data shown for all cases, via syndromic surveillance
system.
Tshopo
Data shown for all cases, via syndromic surveillance
system.
Tshuapa
Data shown for all cases, via syndromic surveillance
system.
3.6 Severity
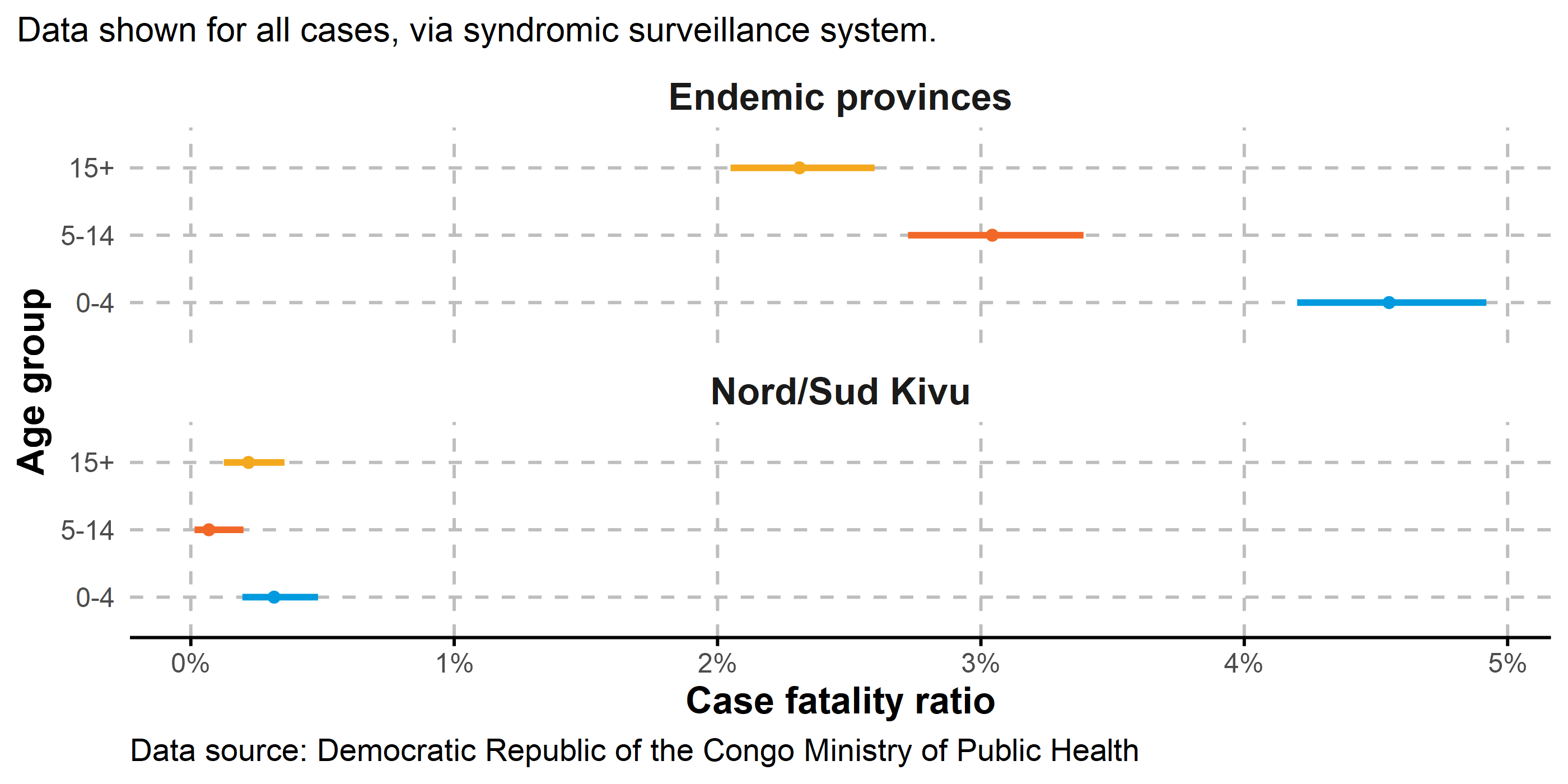
At present, case fatality ratio (CFR) is highest in endemic provinces (Equateur, Sankuru, Tshuapa, Tshopo, Nord Ubangi, Bas Uele, Sud-Ubangi, Mongala, Kwilu, Maindombe, and Maniema).
These provinces are associated with a dominance of clade Ia, although CFR may be affected by demographic factors, healthcare access, reporting practices, and comorbidities. CFR is calculated as the number of deaths divided by the number of cases, with 95% confidence intervals shown.
À l’heure actuelle, le taux de létalité (TLC) est le plus élevé dans les provinces d’endémie (Equateur, Sankuru, Tshuapa, Tshopo, Nord Ubangi, Bas Uele, Sud-Ubangi, Mongala, Kwilu, Maindombe, et Maniema).
Ces provinces sont associées à une dominance du clade Ia, bien que le TLC puisse être affecté par des facteurs démographiques, l’accès aux soins de santé, les pratiques de déclaration et les comorbidités. Le TLC est calculé comme le nombre de décès divisé par le nombre de cas, avec des intervalles de confiance à 95 % indiqués.
3.6.1 All cases

3.6.2 By age group
3.7 Case demographics
3.7.1 Age-sex pyramids by location
3.7.1.1 Nord/Sud Kivu
The age-sex pyramids for North and South Kivu show a higher concentration of confirmed cases among adults, while suspected cases are primarily reported among children. These variations have changed substantially over time, and are likely attributable to distinct regional transmission dynamics, confounding diseases in suspected cases, and differences in healthcare access or reporting practices across space and time.
Les pyramides des âges et des sexes du Nord-Kivu et du Sud-Kivu montrent une concentration plus élevée de cas confirmés chez les adultes, tandis que les cas suspects sont principalement signalés chez les enfants. Ces variations ont considérablement changé au fil du temps et sont probablement attribuables à des dynamiques de transmission régionales distinctes, à des maladies confondantes dans les cas suspects et à des différences dans l’accès aux soins de santé ou dans les pratiques de déclaration dans l’espace et dans le temps.

3.7.1.2 Kinshasa
Cases in Kinshasa are primarily reported among adults, with a distinct bias towards male cases, especially with regards to confirmed cases. The outbreak in Kinshasa is relatively new compared to other provinces, and dynamics are more subject to founder effects.
À Kinshasa, les cas sont principalement signalés chez les adultes, avec une nette préférence pour les cas de sexe masculin, notamment en ce qui concerne les cas confirmés. L’épidémie à Kinshasa est relativement nouvelle par rapport aux autres provinces et sa dynamique est davantage sujette aux effets fondateurs.

3.7.1.3 Endemic provinces
In endemic provinces, age and gender proportions remain relatively consistent across confirmed, and suspected cases. Cases are generally consistently distributed between males than females. Incidence is highest in children under 15 years of age.
Endemic provinces are: Equateur, Sankuru, Tshuapa, Tshopo, Nord Ubangi, Bas Uele, Sud-Ubangi, Mongala, Kwilu, Maindombe, and Maniema.
Dans les provinces d’endémie, les proportions d’âge et de sexe restent relativement constantes entre les cas confirmés et suspects. Les cas sont généralement répartis de manière cohérente entre les hommes et les femmes. L’incidence est la plus élevée chez les enfants de moins de 15 ans.
Les provinces d’endémie sont : Equateur, Sankuru, Tshuapa, Tshopo, Nord Ubangi, Bas Uele, Sud-Ubangi, Mongala, Kwilu, Maindombe, et Maniema.

3.7.2 Age and sex in recent weeks
3.7.2.1 Nord/Sud Kivu

3.7.2.2 Kinshasa

3.7.2.3 Endemic provinces
Endemic provinces are: Equateur, Sankuru, Tshuapa, Tshopo, Nord Ubangi, Bas Uele, Sud-Ubangi, Mongala, Kwilu, Maindombe, and Maniema.
Les provinces endémiques sont : Equateur, Sankuru, Tshuapa, Tshopo, Nord Ubangi, Bas Uele, Sud-Ubangi, Mongala, Kwilu, Maindombe, and Maniema.

4 Global situation update
All of the following data in this report are presented in the context of the ongoing global mpox outbreak. The data presented here are based on the most recent complete month of data reported to WHO as of 30 November 2024.
Since 1 January 2022, cases of mpox have been reported to WHO from 127 Member States across all 6 WHO regions. As of 30 November 2024 , a total of 117 663 laboratory confirmed cases and 2 probable cases, including 263 deaths, have been reported to WHO.
As of November 2024, the number of monthly reported new cases has decreased by 13.2%, compared to the previous month. The majority of cases reported in the past month were notified from the African Region (71.2%) and the Western Pacific Region (10.8%).
The 10 most affected countries globally since 1 January 2022 are: United States of America (n = 34 349), Brazil (n = 13 236), Democratic Republic of the Congo (n = 10 492), Spain (n = 8 443), France (n = 4 371), Colombia (n = 4 280), Mexico (n = 4 192), The United Kingdom (n = 4 146), Germany (n = 4 040), and Peru (n = 3 949). Together, these countries account for 77.8% of the cases reported globally.
In the most recent month of reporting, 42 countries have reported cases, 20, of which reported an increase in monthly case counts.
In the past month, 1 country reported their first case. Countries which reported their first case in the past month are: Angola.
Global aggregated data are collected through direct reporting from Member States to WHO and its partners or from official country sources. The below epidemic curve shows the aggregated number of cases by month according to the date of case reporting.
4.1 Epidemic curves
4.1.1 Global (cases)
Epidemic curve shown by month for cases reported up to 30 Nov 2024 to avoid showing incomplete months of data.

4.1.2 Global (deaths)
Epidemic curve shown by month for deaths reported up to 30 Nov 2024 to avoid showing incomplete months of data.

4.1.3 By WHO Region (cases)
Epidemic curve shown by month for cases reported up to 30 Nov 2024 to avoid showing incomplete months of data. Note different y-axis scales.

4.1.4 By WHO Region (deaths)
Epidemic curve shown by month for deaths reported up to 30 Nov 2024 to avoid showing incomplete months of data. Note different y-axis scales.

4.1.5 Top 10 countries (cases)
Epidemic curve shown by month for cases reported up to 30 Nov 2024 to avoid showing incomplete months of data. Note different y-axis scales.

4.1.6 By country
Andorra

Angola
Argentina

Aruba

Australia

Austria

Bahamas

Bahrain

Barbados

Belgium

Benin

Bermuda

Bolivia (Plurinational State of)

Bosnia and Herzegovina

Brazil

Bulgaria

Burundi

Cambodia

Cameroon

Canada

Central African Republic

Chile

China

Colombia

Congo

Costa Rica

Croatia

Cuba

Curaçao

Cyprus

Czechia

Côte d’Ivoire

Democratic Republic of the Congo

Denmark

Dominican Republic

Ecuador

Egypt

El Salvador

Estonia

Finland

France

Gabon

Georgia

Germany

Ghana

Gibraltar

Greece

Greenland

Guadeloupe

Guam

Guatemala

Guinea

Guyana

Honduras

Hungary

Iceland

India

Indonesia

Iran (Islamic Republic of)

Ireland

Israel

Italy

Jamaica

Japan

Jordan

Kenya

Lao People’s Democratic Republic

Latvia

Lebanon

Liberia

Lithuania

Luxembourg

Malaysia

Malta

Martinique

Mauritius

Mexico

Monaco

Montenegro

Morocco

Mozambique

Nepal

Netherlands

New Caledonia

New Zealand

Nigeria

Norway

Oman

Pakistan

Panama

Paraguay

Peru

Philippines

Poland

Portugal

Qatar

Republic of Korea

Republic of Moldova

Romania

Russian Federation

Rwanda

Saint Martin

San Marino

Saudi Arabia

Serbia

Singapore

Slovakia

Slovenia

South Africa

Spain

Sri Lanka

Sudan

Sweden

Switzerland

Thailand

The United Kingdom

Trinidad and Tobago

Türkiye

Uganda

Ukraine

United Arab Emirates

United States of America

Uruguay

Venezuela (Bolivarian Republic of)

Viet Nam

Zambia

Zimbabwe

4.2 Recent trends
In the past twelve months, the number of cases reported monthly has declined substantially from the global peak of 30,968 cases observed in August 2022. In the past twelve months (01 Dec 2023 - 30 Nov 2024):
On average, at the global level, 1 940 cases have been observed monthly
The most affected region was the African Region, where 12 973 cases and 55 deaths have been reported. This is followed by the Region of the Americas (5 539 cases, 8 deaths), and the Western Pacific Region (2 267 cases, 10 deaths)
4.2.1 Global (cases)
Epidemic curve shown by month for cases reported up to 30 Nov 2024 to avoid showing incomplete months of data.

4.2.2 Global (deaths)
Epidemic curve shown by month for deaths reported up to 30 Nov 2024 to avoid showing incomplete weeks of data.

4.2.3 By WHO Region (cases)
Epidemic curve shown by month for cases reported up to 30 Nov 2024 to avoid showing incomplete weeks of data. Note different y-axis scales.

4.2.4 Top 10 countries (cases)
Epidemic curve shown by month for cases reported up to 30 Nov 2024 to avoid showing incomplete weeks of data. Note different y-axis scales.

4.3 Maps
Note: Maps can be clicked to view on a larger scale
4.3.1 Cumulative cases

4.3.2 Cumulative deaths

4.3.3 Cases in the past 12 months
Epidemic curve shown by month for cases reported up to 30 Nov 2024 to avoid showing incomplete months of data.

4.3.4 Deaths in the past 12 months
Epidemic curve shown by month for cases reported up to 30 Nov 2024 to avoid showing incomplete months of data.

4.3.5 Cases in the past month

4.3.6 Monthly change in cases

4.4 Tables
4.4.1 Cumulative cases and deaths by WHO Region
| Total mpox cases, by WHO region | ||||||
| Data as of November 2024 | ||||||
| WHO Region | Total cases1 | Total deaths1 | Cases in Oct 2024 | Cases in Nov 2024 | Monthly % change in cases | Month most recent cases reported |
|---|---|---|---|---|---|---|
| Region of the Americas | 66,806 | 151 | 586 | 206 | −65.0% | Nov 2024 |
| European Region | 28,682 | 9 | 229 | 277 | 21.0% | Nov 2024 |
| African Region | 15,267 | 77 | 1,935 | 1,941 | 0.3% | Nov 2024 |
| Western Pacific Region | 5,039 | 12 | 375 | 294 | −22.0% | Nov 2024 |
| South-East Asia Region | 991 | 11 | 13 | 7 | −46.0% | Nov 2024 |
| Eastern Mediterranean Region | 878 | 3 | 3 | 1 | −67.0% | Nov 2024 |
| 1 From Jan 2022 | ||||||
4.4.2 Cumulative cases and deaths by country
| Total Mpox cases, by WHO region | |||
| From 1 Jan 2022. Data as of 30 Nov 2024 | |||
| Total Confirmed Cases | Total Probable Cases | Total Deaths | |
|---|---|---|---|
| Region of the Americas | |||
| United States of America | 34,349 | 0 | 63 |
| Brazil | 13,236 | 0 | 16 |
| Colombia | 4,280 | 0 | 0 |
| Mexico | 4,192 | 0 | 35 |
| Peru | 3,949 | 0 | 23 |
| Canada1 | 1,836 | 0 | 0 |
| Chile | 1,480 | 0 | 3 |
| Argentina | 1,250 | 0 | 2 |
| Ecuador | 621 | 0 | 3 |
| Guatemala | 407 | 0 | 2 |
| Bolivia (Plurinational State of) | 266 | 0 | 0 |
| Panama | 242 | 0 | 1 |
| Costa Rica | 226 | 0 | 1 |
| Paraguay | 126 | 0 | 0 |
| Dominican Republic | 110 | 0 | 1 |
| El Salvador | 104 | 0 | 0 |
| Honduras | 44 | 0 | 0 |
| Jamaica | 21 | 0 | 0 |
| Uruguay | 20 | 0 | 0 |
| Venezuela (Bolivarian Republic of) | 12 | 0 | 0 |
| Cuba | 8 | 0 | 1 |
| Martinique | 7 | 0 | 0 |
| Trinidad and Tobago | 4 | 0 | 0 |
| Bahamas | 4 | 0 | 0 |
| Aruba | 3 | 0 | 0 |
| Curaçao | 3 | 0 | 0 |
| Guyana | 2 | 0 | 0 |
| Barbados | 1 | 0 | 0 |
| Bermuda | 1 | 0 | 0 |
| Guadeloupe | 1 | 0 | 0 |
| Saint Martin | 1 | 0 | 0 |
| African Region | |||
| Democratic Republic of the Congo | 10,492 | 0 | 45 |
| Burundi | 2,334 | 0 | 1 |
| Nigeria | 1,006 | 0 | 9 |
| Uganda | 784 | 0 | 2 |
| Ghana | 133 | 0 | 4 |
| Central African Republic | 126 | 0 | 3 |
| Côte d’Ivoire | 101 | 0 | 1 |
| Liberia | 68 | 0 | 0 |
| Rwanda | 59 | 0 | 0 |
| Cameroon | 54 | 0 | 5 |
| Congo | 48 | 0 | 2 |
| South Africa | 30 | 0 | 3 |
| Kenya | 19 | 0 | 1 |
| Benin | 3 | 0 | 0 |
| Angola | 2 | 0 | 0 |
| Gabon | 2 | 0 | 0 |
| Zimbabwe | 2 | 0 | 0 |
| Guinea | 1 | 0 | 0 |
| Mozambique | 1 | 0 | 1 |
| Zambia | 1 | 0 | 0 |
| Mauritius | 1 | 0 | 0 |
| European Region | |||
| Spain | 8,443 | 0 | 3 |
| France | 4,371 | 0 | 0 |
| The United Kingdom | 4,146 | 0 | 0 |
| Germany | 4,040 | 0 | 0 |
| Netherlands | 1,412 | 0 | 0 |
| Portugal | 1,227 | 0 | 2 |
| Italy | 1,079 | 0 | 0 |
| Belgium | 845 | 0 | 2 |
| Switzerland | 588 | 0 | 0 |
| Austria | 363 | 0 | 1 |
| Israel | 325 | 0 | 0 |
| Sweden | 305 | 0 | 0 |
| Ireland | 265 | 0 | 0 |
| Poland | 225 | 0 | 0 |
| Denmark | 209 | 0 | 0 |
| Norway | 119 | 0 | 0 |
| Greece | 107 | 0 | 0 |
| Czechia | 92 | 0 | 1 |
| Hungary | 85 | 0 | 0 |
| Luxembourg | 62 | 0 | 0 |
| Romania | 48 | 0 | 0 |
| Slovenia | 47 | 0 | 0 |
| Finland | 43 | 0 | 0 |
| Serbia | 40 | 0 | 0 |
| Malta | 38 | 0 | 0 |
| Croatia | 35 | 0 | 0 |
| Slovakia | 18 | 0 | 0 |
| Iceland | 17 | 0 | 0 |
| Türkiye | 12 | 0 | 0 |
| Estonia | 11 | 0 | 0 |
| Bosnia and Herzegovina | 9 | 0 | 0 |
| Bulgaria | 8 | 0 | 0 |
| Gibraltar | 6 | 0 | 0 |
| Lithuania | 6 | 0 | 0 |
| Latvia | 6 | 0 | 0 |
| Cyprus | 5 | 0 | 0 |
| Ukraine | 5 | 0 | 0 |
| Andorra | 4 | 0 | 0 |
| Russian Federation | 4 | 0 | 0 |
| Monaco | 3 | 0 | 0 |
| Georgia | 2 | 0 | 0 |
| Montenegro | 2 | 0 | 0 |
| Republic of Moldova | 2 | 0 | 0 |
| Greenland | 2 | 0 | 0 |
| San Marino | 1 | 0 | 0 |
| Western Pacific Region | |||
| China2 | 2,755 | 0 | 1 |
| Australia | 1,436 | 0 | 0 |
| Japan | 252 | 0 | 1 |
| Viet Nam | 209 | 0 | 9 |
| Republic of Korea | 172 | 0 | 0 |
| Singapore | 67 | 0 | 0 |
| New Zealand | 66 | 2 | 0 |
| Philippines | 56 | 0 | 1 |
| Cambodia | 13 | 0 | 0 |
| Malaysia | 10 | 0 | 0 |
| Guam | 1 | 0 | 0 |
| New Caledonia | 1 | 0 | 0 |
| Lao People's Democratic Republic | 1 | 0 | 0 |
| South-East Asia Region | |||
| Thailand | 865 | 0 | 10 |
| Indonesia | 88 | 0 | 0 |
| India | 33 | 0 | 1 |
| Sri Lanka | 4 | 0 | 0 |
| Nepal | 1 | 0 | 0 |
| Eastern Mediterranean Region | |||
| Saudi Arabia | 764 | 0 | 0 |
| United Arab Emirates | 30 | 0 | 1 |
| Lebanon | 28 | 0 | 0 |
| Sudan | 19 | 0 | 1 |
| Pakistan | 13 | 0 | 1 |
| Morocco | 7 | 0 | 0 |
| Qatar | 5 | 0 | 0 |
| Oman | 4 | 0 | 0 |
| Egypt | 3 | 0 | 0 |
| Bahrain | 2 | 0 | 0 |
| Jordan | 2 | 0 | 0 |
| Iran (Islamic Republic of) | 1 | 0 | 0 |
| - | |||
| Total | 117,663 | 2 | 263 |
| 1 Date information is unavailable for 45 cases in Canada | |||
| 2 Cases shown include those in mainland China (2203), Hong Kong SAR (68), Taipei (441), and Macao (2) | |||
4.4.3 Recent trends - African Region
| Mpox cases in last month, by country | ||||||
| November 2024 | ||||||
| Country | Total cases1 | Total deaths1 | Cases in Oct 2024 | Cases in Nov 2024 | Monthly % change in cases | Month most recent cases reported |
|---|---|---|---|---|---|---|
| Burundi | 2,334 | 1 | 656 | 825 | 26.0% | Nov 2024 |
| Democratic Republic of the Congo | 10,492 | 45 | 940 | 497 | −47.0% | Nov 2024 |
| Uganda | 784 | 2 | 241 | 494 | 100.0% | Nov 2024 |
| Rwanda | 59 | 0 | 20 | 33 | 65.0% | Nov 2024 |
| Liberia | 68 | 0 | 6 | 31 | 420.0% | Nov 2024 |
| Nigeria | 1,006 | 9 | 30 | 27 | −10.0% | Nov 2024 |
| Central African Republic | 126 | 3 | 12 | 12 | 0.0% | Nov 2024 |
| Côte d’Ivoire | 101 | 1 | 19 | 10 | −47.0% | Nov 2024 |
| Kenya | 19 | 1 | 6 | 5 | −17.0% | Nov 2024 |
| Cameroon | 54 | 5 | 0 | 3 | – | Nov 2024 |
| Angola | 2 | 0 | 0 | 2 | – | Nov 2024 |
| Ghana | 133 | 4 | 1 | 2 | 100.0% | Nov 2024 |
| Benin | 3 | 0 | 0 | 0 | – | Jun 2022 |
| Congo | 48 | 2 | 0 | 0 | – | Sep 2024 |
| Gabon | 2 | 0 | 0 | 0 | – | Sep 2024 |
| Guinea | 1 | 0 | 0 | 0 | – | Sep 2024 |
| Mauritius | 1 | 0 | 1 | 0 | – | Oct 2024 |
| Mozambique | 1 | 1 | 0 | 0 | – | Oct 2022 |
| South Africa | 30 | 3 | 0 | 0 | – | Sep 2024 |
| Zambia | 1 | 0 | 1 | 0 | – | Oct 2024 |
| Zimbabwe | 2 | 0 | 2 | 0 | – | Oct 2024 |
| 1 From Jan 2022 | ||||||
4.4.4 Recent trends - Region of the Americas
| Mpox cases in last month, by country | ||||||
| November 2024 | ||||||
| Country | Total cases1 | Total deaths1 | Cases in Oct 2024 | Cases in Nov 2024 | Monthly % change in cases | Month most recent cases reported |
|---|---|---|---|---|---|---|
| Brazil | 13,236 | 16 | 330 | 117 | −65.0% | Nov 2024 |
| United States of America | 34,349 | 63 | 157 | 61 | −61.0% | Nov 2024 |
| Argentina | 1,250 | 2 | 15 | 14 | −6.7% | Nov 2024 |
| Chile | 1,480 | 3 | 9 | 7 | −22.0% | Nov 2024 |
| Canada | 1,836 | 0 | 55 | 6 | −89.0% | Nov 2024 |
| Colombia | 4,280 | 0 | 7 | 1 | −86.0% | Nov 2024 |
| Aruba | 3 | 0 | 0 | 0 | – | Sep 2022 |
| Bahamas | 4 | 0 | 0 | 0 | – | May 2023 |
| Barbados | 1 | 0 | 0 | 0 | – | Jul 2022 |
| Bermuda | 1 | 0 | 0 | 0 | – | Jul 2022 |
| Bolivia (Plurinational State of) | 266 | 0 | 0 | 0 | – | Feb 2024 |
| Costa Rica | 226 | 1 | 0 | 0 | – | Mar 2024 |
| Cuba | 8 | 1 | 0 | 0 | – | Nov 2022 |
| Curaçao | 3 | 0 | 0 | 0 | – | Sep 2022 |
| Dominican Republic | 110 | 1 | 0 | 0 | – | Apr 2024 |
| Ecuador | 621 | 3 | 1 | 0 | – | Oct 2024 |
| El Salvador | 104 | 0 | 0 | 0 | – | Apr 2023 |
| Guadeloupe | 1 | 0 | 0 | 0 | – | Aug 2022 |
| Guatemala | 407 | 2 | 1 | 0 | – | Oct 2024 |
| Guyana | 2 | 0 | 0 | 0 | – | Aug 2022 |
| Honduras | 44 | 0 | 0 | 0 | – | Apr 2023 |
| Jamaica | 21 | 0 | 0 | 0 | – | Mar 2023 |
| Martinique | 7 | 0 | 0 | 0 | – | Jan 2023 |
| Mexico | 4,192 | 35 | 10 | 0 | – | Oct 2024 |
| Panama | 242 | 1 | 0 | 0 | – | Sep 2024 |
| Paraguay | 126 | 0 | 0 | 0 | – | May 2023 |
| Peru | 3,949 | 23 | 1 | 0 | – | Oct 2024 |
| Saint Martin | 1 | 0 | 0 | 0 | – | Aug 2022 |
| Trinidad and Tobago | 4 | 0 | 0 | 0 | – | Jul 2023 |
| Uruguay | 20 | 0 | 0 | 0 | – | Sep 2024 |
| Venezuela (Bolivarian Republic of) | 12 | 0 | 0 | 0 | – | Dec 2022 |
| 1 From Jan 2022 | ||||||
4.4.5 Recent trends - Eastern Mediterranean Region
| Mpox cases in last month, by country | ||||||
| November 2024 | ||||||
| Country | Total cases1 | Total deaths1 | Cases in Oct 2024 | Cases in Nov 2024 | Monthly % change in cases | Month most recent cases reported |
|---|---|---|---|---|---|---|
| Pakistan | 13 | 1 | 0 | 1 | – | Nov 2024 |
| Bahrain | 2 | 0 | 0 | 0 | – | Apr 2023 |
| Egypt | 3 | 0 | 0 | 0 | – | Dec 2022 |
| Iran (Islamic Republic of) | 1 | 0 | 0 | 0 | – | Aug 2022 |
| Jordan | 2 | 0 | 0 | 0 | – | Sep 2024 |
| Lebanon | 28 | 0 | 0 | 0 | – | Mar 2024 |
| Morocco | 7 | 0 | 1 | 0 | – | Oct 2024 |
| Oman | 4 | 0 | 1 | 0 | – | Oct 2024 |
| Qatar | 5 | 0 | 0 | 0 | – | Sep 2022 |
| Saudi Arabia | 764 | 0 | 0 | 0 | – | Aug 2024 |
| Sudan | 19 | 1 | 0 | 0 | – | Apr 2023 |
| United Arab Emirates | 30 | 1 | 1 | 0 | – | Oct 2024 |
| 1 From Jan 2022 | ||||||
4.4.6 Recent trends - Table - European Region
| Mpox cases in last month, by country | ||||||
| November 2024 | ||||||
| Country | Total cases1 | Total deaths1 | Cases in Oct 2024 | Cases in Nov 2024 | Monthly % change in cases | Month most recent cases reported |
|---|---|---|---|---|---|---|
| Spain | 8,443 | 3 | 64 | 97 | 52.0% | Nov 2024 |
| Germany | 4,040 | 0 | 48 | 60 | 25.0% | Nov 2024 |
| Netherlands | 1,412 | 0 | 25 | 38 | 52.0% | Nov 2024 |
| The United Kingdom | 4,146 | 0 | 10 | 36 | 260.0% | Nov 2024 |
| France | 4,371 | 0 | 29 | 8 | −72.0% | Nov 2024 |
| Austria | 363 | 1 | 3 | 7 | 130.0% | Nov 2024 |
| Italy | 1,079 | 0 | 10 | 7 | −30.0% | Nov 2024 |
| Belgium | 845 | 2 | 17 | 6 | −65.0% | Nov 2024 |
| Greece | 107 | 0 | 0 | 5 | – | Nov 2024 |
| Ireland | 265 | 0 | 8 | 4 | −50.0% | Nov 2024 |
| Norway | 119 | 0 | 7 | 3 | −57.0% | Nov 2024 |
| Czechia | 92 | 1 | 3 | 2 | −33.0% | Nov 2024 |
| Slovakia | 18 | 0 | 0 | 2 | – | Nov 2024 |
| Bulgaria | 8 | 0 | 0 | 1 | – | Nov 2024 |
| Croatia | 35 | 0 | 0 | 1 | – | Nov 2024 |
| Andorra | 4 | 0 | 0 | 0 | – | Aug 2022 |
| Bosnia and Herzegovina | 9 | 0 | 0 | 0 | – | Oct 2022 |
| Cyprus | 5 | 0 | 0 | 0 | – | Aug 2022 |
| Denmark | 209 | 0 | 0 | 0 | – | Sep 2024 |
| Estonia | 11 | 0 | 0 | 0 | – | Sep 2022 |
| Finland | 43 | 0 | 0 | 0 | – | Feb 2024 |
| Georgia | 2 | 0 | 0 | 0 | – | Aug 2022 |
| Gibraltar | 6 | 0 | 0 | 0 | – | Aug 2022 |
| Greenland | 2 | 0 | 0 | 0 | – | Aug 2022 |
| Hungary | 85 | 0 | 0 | 0 | – | Jun 2024 |
| Iceland | 17 | 0 | 0 | 0 | – | Sep 2023 |
| Israel | 325 | 0 | 0 | 0 | – | Sep 2024 |
| Latvia | 6 | 0 | 0 | 0 | – | Oct 2022 |
| Lithuania | 6 | 0 | 0 | 0 | – | Sep 2024 |
| Luxembourg | 62 | 0 | 1 | 0 | – | Oct 2024 |
| Malta | 38 | 0 | 1 | 0 | – | Oct 2024 |
| Monaco | 3 | 0 | 0 | 0 | – | Aug 2022 |
| Montenegro | 2 | 0 | 0 | 0 | – | Aug 2022 |
| Poland | 225 | 0 | 0 | 0 | – | Aug 2024 |
| Portugal | 1,227 | 2 | 0 | 0 | – | Sep 2024 |
| Republic of Moldova | 2 | 0 | 0 | 0 | – | Aug 2022 |
| Romania | 48 | 0 | 0 | 0 | – | Aug 2024 |
| Russian Federation | 4 | 0 | 0 | 0 | – | Jan 2024 |
| San Marino | 1 | 0 | 0 | 0 | – | Oct 2022 |
| Serbia | 40 | 0 | 0 | 0 | – | Sep 2022 |
| Slovenia | 47 | 0 | 0 | 0 | – | Sep 2022 |
| Sweden | 305 | 0 | 3 | 0 | – | Oct 2024 |
| Switzerland | 588 | 0 | 0 | 0 | – | Aug 2024 |
| Türkiye | 12 | 0 | 0 | 0 | – | Oct 2022 |
| Ukraine | 5 | 0 | 0 | 0 | – | Oct 2022 |
| 1 From Jan 2022 | ||||||
4.4.7 Recent trends - South-East Asia Region
| Mpox cases in last month, by country | ||||||
| November 2024 | ||||||
| Country | Total cases1 | Total deaths1 | Cases in Oct 2024 | Cases in Nov 2024 | Monthly % change in cases | Month most recent cases reported |
|---|---|---|---|---|---|---|
| Thailand | 865 | 10 | 13 | 7 | −46.0% | Nov 2024 |
| India | 33 | 1 | 0 | 0 | – | Sep 2024 |
| Indonesia | 88 | 0 | 0 | 0 | – | Jun 2024 |
| Nepal | 1 | 0 | 0 | 0 | – | Jun 2023 |
| Sri Lanka | 4 | 0 | 0 | 0 | – | Jun 2023 |
| 1 From Jan 2022 | ||||||
4.4.8 Recent trends - Western Pacific Region
| Mpox cases in last month, by country | ||||||
| November 2024 | ||||||
| Country | Total cases1 | Total deaths1 | Cases in Oct 2024 | Cases in Nov 2024 | Monthly % change in cases | Month most recent cases reported |
|---|---|---|---|---|---|---|
| Australia | 1,436 | 0 | 296 | 214 | −28.0% | Nov 2024 |
| China | 2,755 | 1 | 61 | 45 | −26.0% | Nov 2024 |
| Philippines | 56 | 1 | 0 | 24 | – | Nov 2024 |
| Viet Nam | 209 | 9 | 0 | 6 | – | Nov 2024 |
| Republic of Korea | 172 | 0 | 1 | 3 | 200.0% | Nov 2024 |
| Japan | 252 | 1 | 3 | 1 | −67.0% | Nov 2024 |
| New Zealand | 66 | 0 | 12 | 1 | −92.0% | Nov 2024 |
| Cambodia | 13 | 0 | 0 | 0 | – | Feb 2024 |
| Guam | 1 | 0 | 0 | 0 | – | Sep 2022 |
| Lao People's Democratic Republic | 1 | 0 | 0 | 0 | – | Sep 2023 |
| Malaysia | 10 | 0 | 0 | 0 | – | Sep 2024 |
| New Caledonia | 1 | 0 | 0 | 0 | – | Jul 2022 |
| Singapore | 67 | 0 | 2 | 0 | – | Oct 2024 |
| 1 From Jan 2022 | ||||||
4.4.9 Countries reporting cases in the previous month
| Countries reporting cases in November | ||
| Country | New cases | New deaths |
|---|---|---|
| African Region | ||
| Burundi | 825 | 1 |
| Democratic Republic of the Congo | 497 | 0 |
| Uganda | 494 | 1 |
| Rwanda | 33 | 0 |
| Liberia | 31 | 0 |
| Nigeria | 27 | 0 |
| Central African Republic | 12 | 0 |
| Côte d’Ivoire | 10 | 0 |
| Kenya | 5 | 0 |
| Cameroon | 3 | 0 |
| Angola | 2 | 0 |
| Ghana | 2 | 0 |
| Western Pacific Region | ||
| Australia | 214 | 0 |
| China | 45 | 0 |
| Philippines | 24 | 1 |
| Viet Nam | 6 | 1 |
| Republic of Korea | 3 | 0 |
| Japan | 1 | 0 |
| New Zealand | 1 | 0 |
| Region of the Americas | ||
| Brazil | 117 | 0 |
| United States of America | 61 | 0 |
| Argentina | 14 | 0 |
| Chile | 7 | 0 |
| Canada | 6 | 0 |
| Colombia | 1 | 0 |
| European Region | ||
| Spain | 97 | 0 |
| Germany | 60 | 0 |
| Netherlands | 38 | 0 |
| The United Kingdom | 36 | 0 |
| France | 8 | 0 |
| Austria | 7 | 0 |
| Italy | 7 | 0 |
| Belgium | 6 | 0 |
| Greece | 5 | 0 |
| Ireland | 4 | 0 |
| Norway | 3 | 0 |
| Czechia | 2 | 0 |
| Slovakia | 2 | 0 |
| Bulgaria | 1 | 0 |
| Croatia | 1 | 0 |
| South-East Asia Region | ||
| Thailand | 7 | 0 |
| Eastern Mediterranean Region | ||
| Pakistan | 1 | 0 |
5 Detailed case data
Detailed case data are acquired via direct reporting of case-based data from Member States to WHO. Data from cases are reported1 according to the WHO minimum dataset under the International Health Regulations (IHR 2005) Article 6. Completeness of records is variable, meaning denominators for variables may be different from one another. All of the following is derived from detailed case data, and as a result, overall numbers may not be reflective of figures shown with aggregate case numbers. All detailed cases shown are confirmed cases, where the reporting date occurred after 01 January 2022.
- Note that a small number of detailed case reports are constructed from official public reports about individual cases.
5.1 Reporting coverage
The detailed case dataset was last updated on November 2024. As of this date, the total number of detailed confirmed cases reported is 98 671, representing 83.9% of all aggregated cases reported.
The table below indicates the reporting coverage between reported aggregated confirmed cases and detailed confirmed cases by countries and per region.
5.1.1 Table - Coverage by region
| Mpox reporting completeness | |||
| As of 30 Nov 2024 | |||
| Total Confirmed Cases | Total Detailed Confirmed Cases1 | % Detailed Cases reported | |
|---|---|---|---|
| Region of the Americas | 66,806 | 65,683 | 98.3% |
| European Region | 28,682 | 28,394 | 99.0% |
| African Region | 15,267 | 672 | 4.4% |
| Western Pacific Region | 5,039 | 2,902 | 57.6% |
| South-East Asia Region | 991 | 923 | 93.1% |
| Eastern Mediterranean Region | 878 | 97 | 11.0% |
| 1 Note that in rare cases total detailed cases may exceed total confirmed cases due to ongoing data cleaning issues | |||
5.2 Trends in cases
Trends in cases are shown for all submitted detailed cases. These are shown by:
- Date of symptom onset
- Date of lab or clinical diagnosis (if date of symptom onset is not available)
- Date of reporting (if date of symptom onset and date of diagnosis are not available)
Reporting of detailed cases is subject to some delay. The epidemic curves shown are not right-censored, and therefore trends in the most recent weeks shown should be interpreted with caution. It should be additionally noted that date of report does not reflect the date of reporting to WHO, but rather reporting to national or regional authorities.
Delay between date of onset and date of diagnosis were calculated for all countries where reporting quality passed criteria. Delays were only shown when the time between onset and diagnosis was between 0 and 50 days.
The median delay between onset and diagnosis was 7 days (interquartile range: 4-10 days)
Data by date of onset and country can be downloaded below.
5.2.1 Cases by date of symptom onset

5.2.2 All cases by date of onset and by WHO region

5.2.3 Cases by date of onset and by country (top 10 total cases)
Note different y-axis scales per country.

5.2.4 Cases by date of onset and by age group
Note different y-axis scales per age group

5.2.5 Cases by date of onset and by sexual behaviour
Note that reported sexual behaviour does not necessarily reflect who the case has had recent sexual history with nor does it imply sexual activity. Note different y-axis scales.

5.2.6 Proportion of men who have sex with men and proportion of males by date of report
Proportion of cases who are men who have sex with men, and proportion of cases who are male are used to monitor the spillover of cases from networks of men who have sex with men to the general population. In both plots below, the denominator is defined as all detailed cases where sexual behavior (above) or sex (below) has been reported. Shaded areas represent 95% confidence intervals.
Note that reported sexual behavior does not necessarily reflect who the case has had recent sexual history with nor does it imply sexual activity.


5.2.7 Epidemic curves by country
Select country of interest from the dropdown link below:
Andorra

Argentina

Aruba

Australia

Austria

Bahamas

Bahrain

Barbados
Belgium
Benin

Bermuda

Bolivia (Plurinational State of)

Bosnia and Herzegovina

Brazil

Bulgaria

Cambodia

Cameroon

Canada

Central African Republic

Chile

China

Colombia

Congo

Costa Rica

Croatia

Cuba

Curaçao

Cyprus

Czechia

Democratic Republic of the Congo

Denmark

Dominican Republic

Ecuador

El Salvador

Estonia

Finland

France

Georgia

Germany

Ghana

Greece

Guam

Guatemala

Guyana

Honduras

Hungary

Iceland

India

Indonesia

Ireland

Israel

Italy

Jamaica

Japan

Lao People’s Democratic Republic

Latvia

Lebanon

Liberia

Lithuania

Luxembourg

Malaysia

Malta

Mexico

Morocco

Nepal

Netherlands

New Caledonia

New Zealand

Nigeria

Norway

Oman

Pakistan

Panama

Paraguay

Peru

Philippines

Poland

Portugal

Qatar

Republic of Korea

Republic of Moldova

Romania

Saint Martin

San Marino

Saudi Arabia

Serbia

Singapore

Slovakia

Slovenia

South Africa

Spain

Sri Lanka

Sudan

Sweden

Switzerland

Thailand

The United Kingdom

Türkiye

Ukraine

United Arab Emirates

United States of America

Uruguay

Venezuela (Bolivarian Republic of)

Viet Nam

5.3 Case profile (overall)
As of 30 Nov 2024, the vast majority of cases for which detailed case based data are available are not associated with the outbreak of clade Ib, or the African region. For this reason the following analysis is overwhelmingly reflective of the global situation outside of Africa and clade Ib.
Key features of these cases are as follows:
96.3% (90 760/94 248) of cases with available data are male, the median age is 34 years (IQR: 29 - 41).
Males between 18-44 years old continue to be disproportionately affected by this outbreak as they account for 79.3% of reported cases.
Of all cases with available data, 3.7% (3 488/94 248) are female:
The majority of these cases are reported from the Region of the Americas (2 645/3 488; 76%) and the European Region (473/3 488; 14%)
The most commonly reported form of transmission is via sexual encounters (270/522; 52%)
Of the 98 232 cases where age was available, there were 1 267 (1.3%) cases reported aged 0-17, out of which 360 (0.4%) were aged 0-4:
- The majority of cases aged 0-17 are reported from the Region of the Americas (816 /1 267; 64%).
60 cases were reported to be pregnant or recently pregnant. Of these:
3, 12, and 9 cases were in their first, second, and third trimesters respectively. 33 were in an unknown trimester, and 0 were six weeks or less post-partum.
The median age was 26.5 years old (IQR: 22 - 31).
13 of these cases were known to be hospitalized. 0 were known to be admitted to ICU and 0 were known to have died.
The most common mode of transmission was sexual encounter (4/7 cases where route was known).
Among cases with known data on sexual behaviour, 86.7% (30 846/35 575) identified as men who have sex with men.
Among those with known HIV status, 51.8% (19 118/36 893) were people living with HIV. Note that information on HIV status is not available for the majority of cases.
1 401 cases were reported to be health workers. However, most were exposed in the community and further investigation is ongoing to determine which were due to occupational exposure.
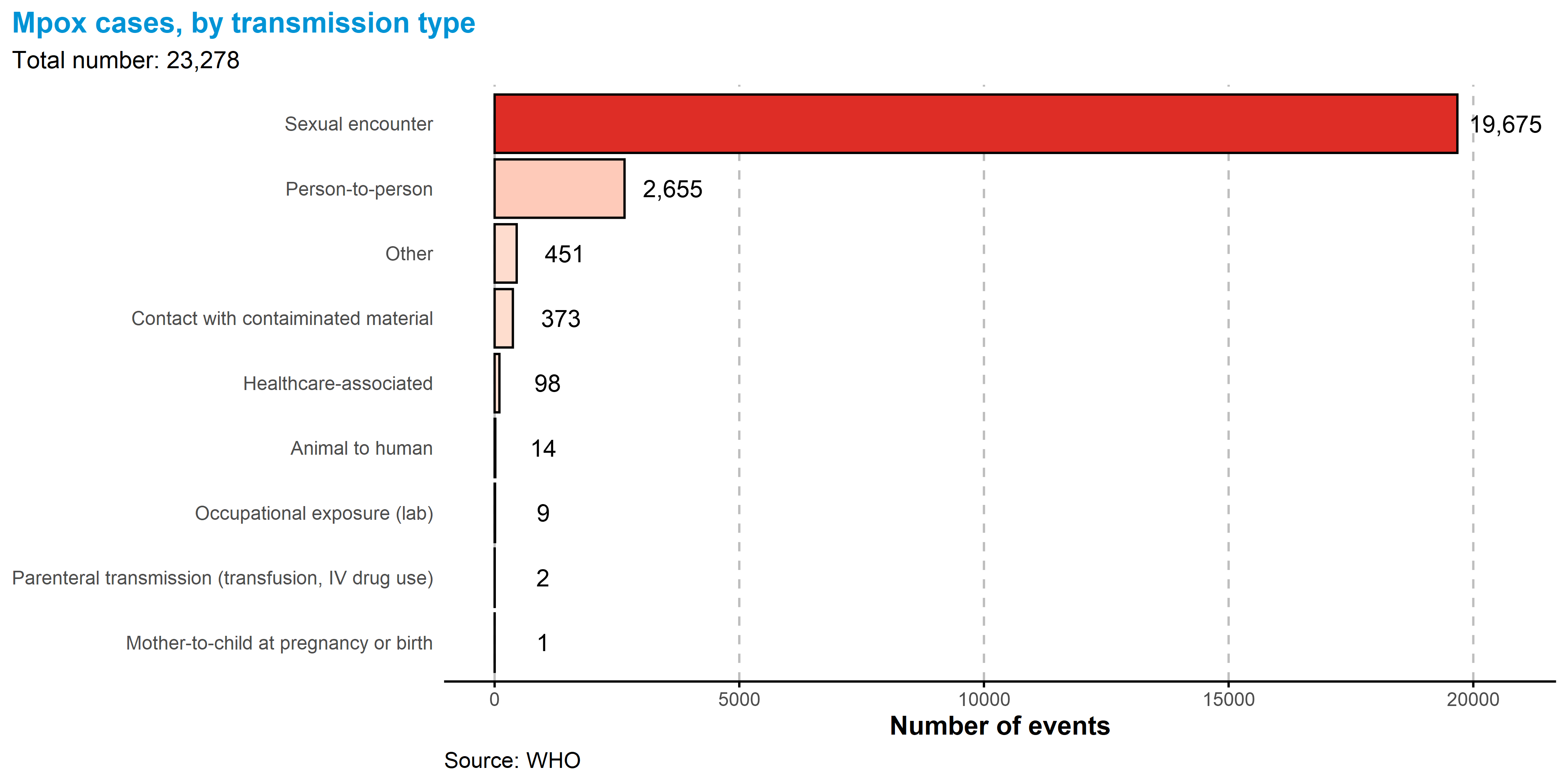
Of all reported types of transmission, a sexual encounter was reported most commonly, with 19 675 of 23 278 (84.5%) of all reported transmission events.
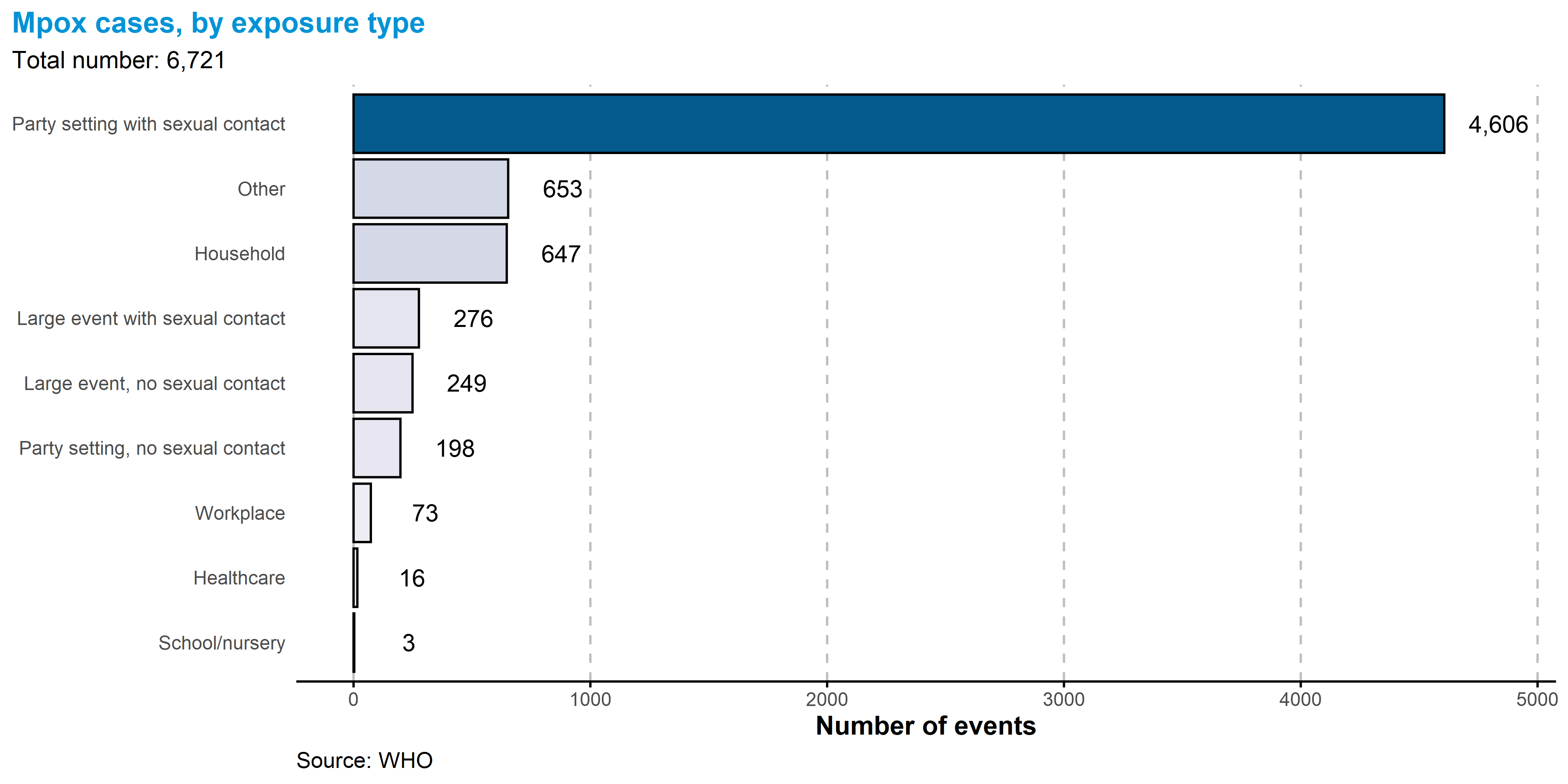
Of all settings in which cases were likely exposed, the most common was in party setting with sexual contacts, with 4 606 of 6 721 (68.5%) of all reported exposure events.
As of 6 October 2023, the updated case reporting form no longer requires collection of exposure setting as an aspect of the case-based data. While we longer receive this information, we continue to present these data for the historical record.
5.3.1 Demographic Information
Note that the proportions shown below should be interpreted with caution. In some cases, a variable may be more likely to be filled in if the answer is yes than if the answer is no. This is most likely to be true for variables such as HIV status, health worker status, travel history, hospitalization, ICU, and death.
| Case profiles | |||
| As of 30 Nov 2024 | |||
|
Reported values
|
Unknown or Missing Value | ||
|---|---|---|---|
| Yes | No | ||
| Men who have sex with men | 30,846 (86.7%) | 4,729 (13.3%) | 63,087 |
| Persons living with HIV | 19,118 (51.8%) | 17,775 (48.2%) | 61,769 |
| Health worker | 1,401 (4.1%) | 33,172 (95.9%) | 64,089 |
| Travel History | 4,256 (15.4%) | 23,297 (84.6%) | 71,109 |
| Sexual Transmission | 19,674 (84.5%) | 3,603 (15.5%) | 75,385 |
| Hospitalized1 | 6,326 (10.7%) | 52,667 (89.3%) | 39,669 |
| ICU | 50 (0.4%) | 11,331 (99.6%) | 87,281 |
| Died | 200 (0.3%) | 60,128 (99.7%) | 38,334 |
| 1 May be hospitalized for isolation or medical treatment | |||
5.3.2 Age-sex pyramid

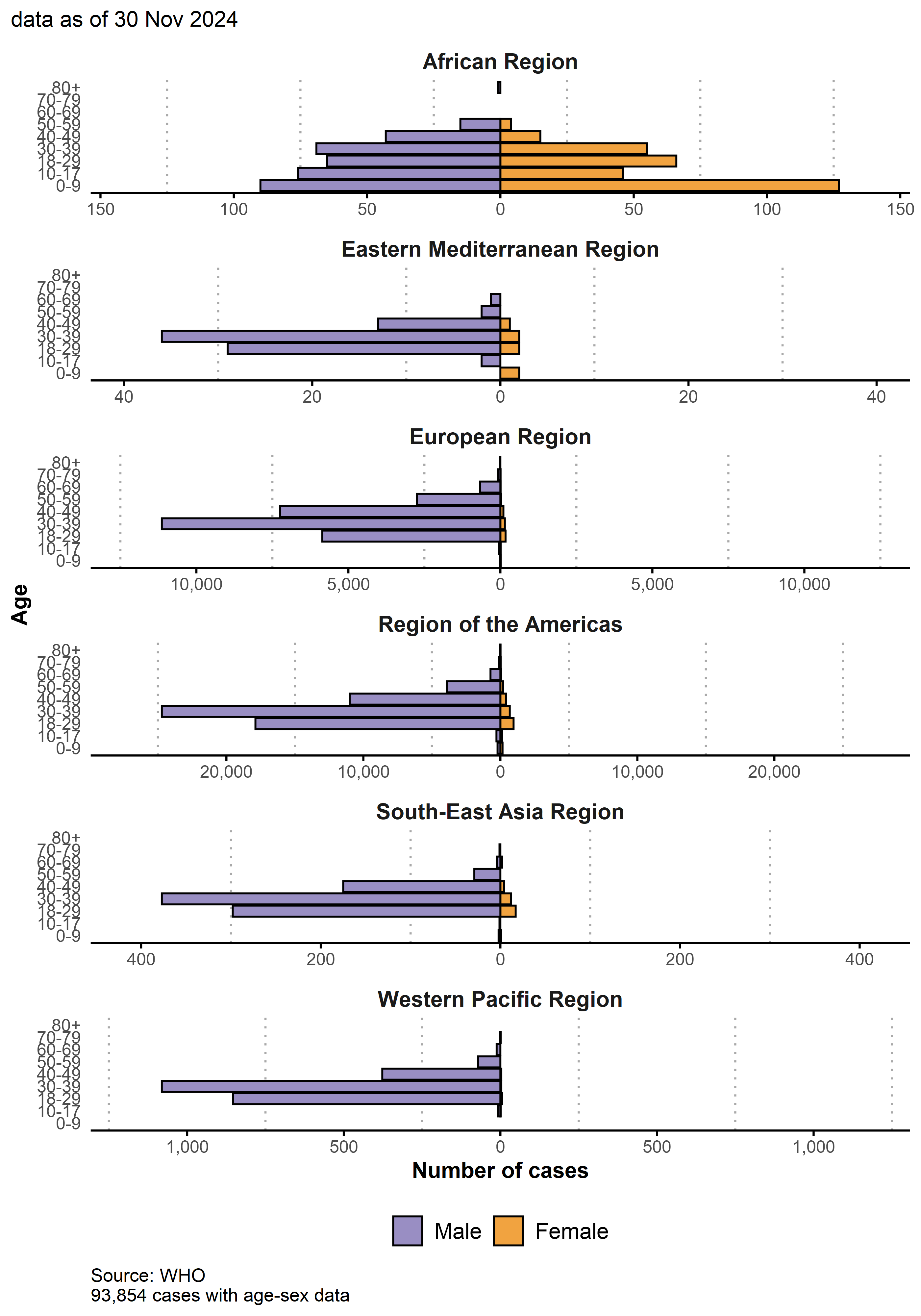
5.3.3 Age-sex pyramid by region
Note different x-axis scales.
5.3.4 Age-sex pyramid (Hospitalized cases)
Note that some cases represented below may be hospitalized for isolation rather than treatment purposes.

5.3.5 Age-sex pyramid (ICU cases)

5.3.6 Sexual behaviour by region

5.3.7 Transmission type
Transmission data were available for 23 278/98 671 (23.6%) of cases.
Transmission can occur during sex via skin-to-skin contact as well as via bodily fluids. While skin-to-skin contact with lesions remains an important transmission route, monkeypox virus has been isolated from semen samples and rectal swabs from confirmed cases. Note that person to person contact excludes known sexual, healthcare-associated, and mother to child transmission.
5.3.8 Exposure settings
Exposure setting data were available for 7 986/98 671 (8.1%) of cases.
Note that multiple exposure settings can be attributed to a single case. Here, differentiation between party settings and large events is determined by size of the event, although there is no formal size threshold to distinguish the two.
5.3.9 Transmission by exposure type

5.4 Case profile (excluding men who have sex with men)
As of this date in time, with regards to the outbreak of clade IIb, the multi-country mpox outbreak has been overwhelmingly concentrated in sexual networks of men who have sex with men. For this reason, understanding events in which individuals having other sexual behaviours have acquired mpox is important to monitor potential of sustained spillover into the general population. Note that the demographics of cases affected with clade Ib are not represented here.
The following outputs apply to cases with sexual behaviour reported as other than men who have sex with men. As above, note that reported sexual behaviour does not necessarily reflect persons who the case has had recent sexual history with nor does it imply sexual activity.
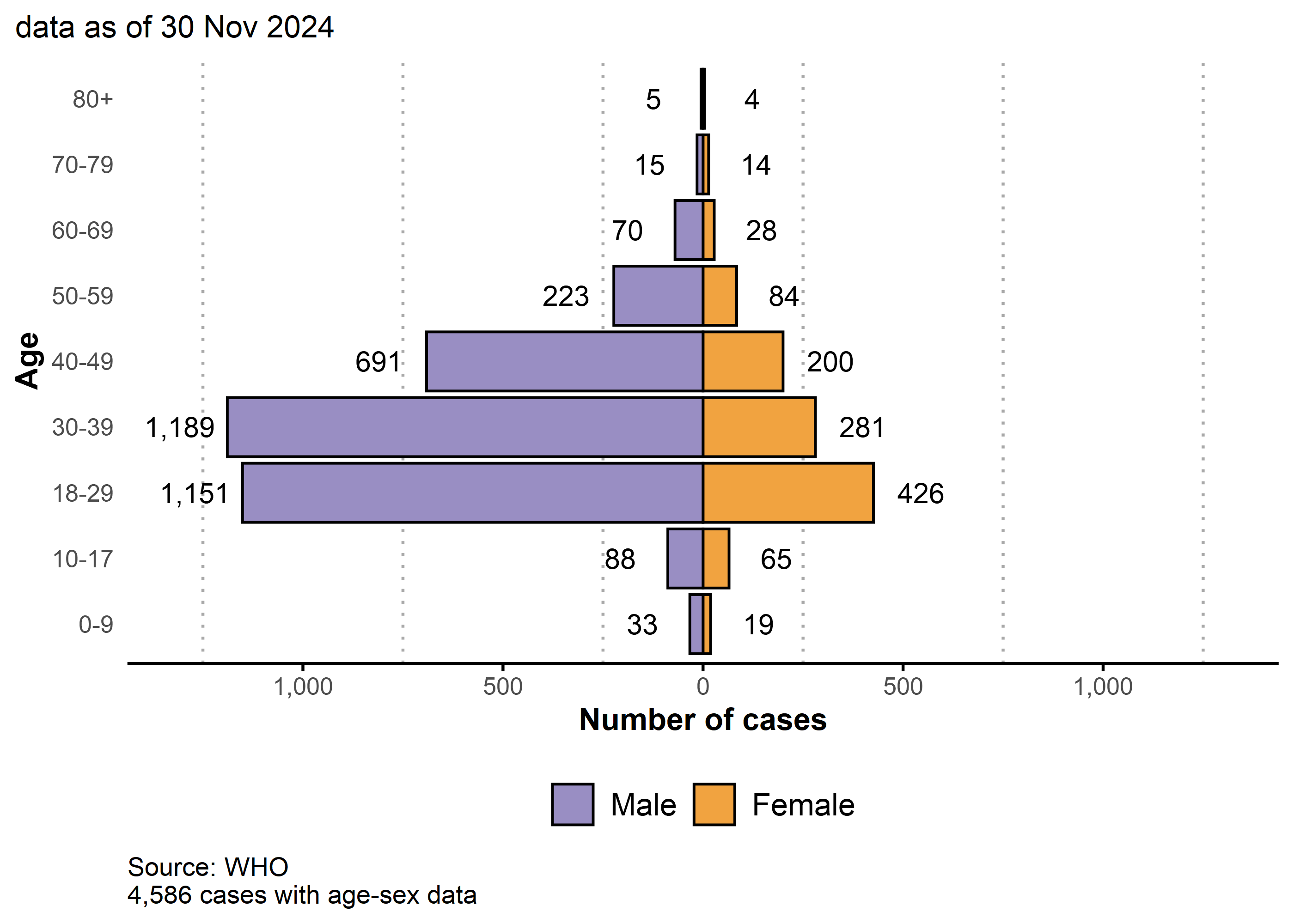
75.6% (3 465/4 586) of cases with available data are male; the median age is 33 years (IQR: 26-41).
Males between 18-44 years old account for 60.1% of cases.
Among those with known HIV status 22.9% (800/3 500) were people living with HIV. Note that information on HIV status is not available for the majority of cases.
99 cases were reported to be health workers. However, most were exposed in the community.
Of all reported types of transmission, sexual encounter was reported most commonly, with 676 of 1 144 (59.1%) of all reported transmission events.
Of all settings in which cases were likely exposed, the most common was in party setting with sexual contacts, with 138 of 337 (40.9%) of all likely exposure categories.
5.4.1 Demographic Information
Note that the proportions shown below should be interpreted with caution. In some cases, a variable may be more likely to be filled in if the answer is yes than if the answer is no. This is most likely to be true for variables such as HIV status, health worker status, travel history, hospitalization, ICU, and death.
| Case profiles (excluding men who have sex with men) | |||
| As of 30 Nov 2024 | |||
|
Reported values
|
Unknown or Missing Value | ||
|---|---|---|---|
| Yes | No | ||
| Men who have sex with men | 0 | 4,729 (100.0%) | 0 |
| Persons living with HIV | 800 (22.9%) | 2,700 (77.1%) | 1,229 |
| Health worker | 99 (5.4%) | 1,747 (94.6%) | 2,883 |
| Travel History | 288 (13.1%) | 1,916 (86.9%) | 2,525 |
| Sexual Transmission | 676 (59.1%) | 468 (40.9%) | 3,585 |
| Hospitalized1 | 315 (11.8%) | 2,345 (88.2%) | 2,069 |
| ICU | 13 (2.0%) | 628 (98.0%) | 4,088 |
| Died | 19 (0.6%) | 2,978 (99.4%) | 1,732 |
| 1 May be hospitalized for isolation or medical treatment | |||
5.4.2 Age-sex pyramid excluding men who have sex with men
5.4.3 Transmission type
Transmission data were available for 1 144/4 729 (24.2%) of cases.
Transmission can occur during sex via skin-to-skin contact as well as via bodily fluids. While skin-to-skin contact with lesions remains an important transmission route, monkeypox virus has been isolated from semen samples and rectal swabs from confirmed cases. Note that person to person contact excludes known sexual, healthcare-associated, and mother to child transmission.
All Genders

Male

Female

5.4.4 Exposure settings
Exposure setting data were available for 389/4 729 (8.2%) of cases that were not men who have sex with men.
Note that multiple exposure settings can be attributed to a single case. Here, differentiation between party settings and large events is determined by size of the event, although there is no formal size threshold to distinguish the two.
All Sexes

Male

Female

5.5 Case profile (recent cases)
This section of the report pertains specifically to the most recent six months of the outbreak, and case report forms that were reported in that time period (01 Jun 2024 - 30 Nov 2024).
In the last six months:
Of all cases with available information, 97% (2 219 / 2 280) of cases were male, and 89% (683 / 769) reported being as men who have sex with men.
Of all reported types of transmission, a sexual encounter was reported most commonly, with 580 of 619 (93.7%) of all reported transmission events.
5.5.1 Demographic information
Note that the proportions shown below should be interpreted with caution. In some cases, a variable may be more likely to be filled in if the answer is yes than if the answer is no. This is most likely to be true for variables such as HIV status, health worker status, travel history, hospitalization, ICU, and death.
| Case profiles | |||
| From 01 Jun 2024 to 30 Nov 2024 | |||
|
Reported values
|
Unknown or Missing Value | ||
|---|---|---|---|
| Yes | No | ||
| Men who have sex with men | 683 (88.8%) | 86 (11.2%) | 1,603 |
| Persons living with HIV | 272 (40.6%) | 398 (59.4%) | 1,702 |
| Health worker | 20 (2.6%) | 758 (97.4%) | 1,594 |
| Travel History | 145 (22.4%) | 503 (77.6%) | 1,724 |
| Sexual Transmission | 580 (93.7%) | 39 (6.3%) | 1,753 |
| Hospitalized1 | 128 (8.2%) | 1,429 (91.8%) | 815 |
| ICU | 1 (0.4%) | 245 (99.6%) | 2,126 |
| Died | 4 (0.3%) | 1,446 (99.7%) | 922 |
| 1 May be hospitalized for isolation or medical treatment | |||
5.5.2 Age-sex pyramid, recent cases

5.5.3 Transmission type
Transmission data were available for 619/2 372 (26.1%) of cases.
Transmission can occur during sex via skin-to-skin contact as well as via bodily fluids. While skin-to-skin contact with lesions remains an important transmission route, monkeypox virus has been isolated from semen samples and rectal swabs from confirmed cases. Note that person to person contact excludes known sexual, healthcare-associated, and mother to child transmission.

5.6 Symptomatology
Although most cases in current outbreaks have presented with mild disease symptoms, monkeypox virus (MPXV) may cause severe disease in certain population groups (young children, pregnant women, immunosuppressed persons).
Among the cases who reported at least one symptom, the most common symptom is any rash and is reported in 91% of cases with at least one reported symptom. Note that identifying true denominators for symptomatology is difficult due to a general lack of negative reporting and symptom definitions that may vary between countries’ reporting systems.
A bar chart and table showing symptoms is shown below. Here any rash refers to one or more rash symptoms (systemic, oral, genital, or unknown location), and any lymphadenopathy refers to either general or local lymphadenopathy. Systemic rash included rash on the body, excluding mucosal and genital rash. Symptom information is shown for all cases where information was available reported from January 2022.
5.6.1 Bar chart - Symptoms
All cases

Male cases

Female cases

5.6.2 Table - Symptoms
| Summary of symptoms | |||
| As of 30 Nov 2024 | |||
| All | Male | Female | |
|---|---|---|---|
| Any rash | 32,544 (91.3%) | 30,188 (92.0%) | 1,115 (86.7%) |
| Systemic rash | 18,670 (52.4%) | 17,722 (54.0%) | 914 (71.1%) |
| Genital rash | 18,504 (51.9%) | 16,974 (51.7%) | 383 (29.8%) |
| Fever | 17,727 (49.7%) | 16,928 (51.6%) | 627 (48.8%) |
| Any lymphadenopathy | 9,262 (26.0%) | 9,004 (27.4%) | 196 (15.2%) |
| Headache | 8,756 (24.6%) | 8,253 (25.1%) | 451 (35.1%) |
| Muscle ache | 8,594 (24.1%) | 7,455 (22.7%) | 352 (27.4%) |
| General lymphadenopathy | 7,344 (20.6%) | 7,163 (21.8%) | 120 (9.3%) |
| Fatigue | 5,227 (14.7%) | 5,111 (15.6%) | 109 (8.6%) |
| Sore throat | 4,620 (13.0%) | 4,372 (13.3%) | 197 (15.3%) |
| Local lymphadenopathy | 4,148 (11.6%) | 4,052 (12.3%) | 95 (7.4%) |
| Rash, unknown location | 3,621 (10.2%) | 3,594 (10.9%) | 25 (1.9%) |
| Oral rash | 2,972 (8.3%) | 2,654 (8.1%) | 82 (6.4%) |
| Chills | 2,306 (6.5%) | 2,161 (6.6%) | 112 (8.7%) |
| Cough | 834 (2.3%) | 763 (2.3%) | 56 (4.4%) |
| Vomiting | 783 (2.2%) | 688 (2.1%) | 51 (4.0%) |
| Lymphadenopathy, location unknown | 479 (1.3%) | 465 (1.4%) | 14 (1.1%) |
| Anogenital pain and/or bleeding | 418 (1.2%) | 411 (1.3%) | 6 (0.5%) |
| Asymptomatic | 298 (0.8%) | 273 (0.8%) | 18 (1.4%) |
| Other | 254 (0.7%) | 249 (0.8%) | 5 (0.4%) |
| Conjunctivitis | 211 (0.6%) | 172 (0.5%) | 13 (1.0%) |
| Diarrhea | 125 (0.4%) | 103 (0.3%) | 2 (0.2%) |
| Genital oedema | 46 (0.1%) | 45 (0.1%) | 0 |
6 Genomic epidemiology
MPXV genetic sequences are routinely shared within NCBI GenBank and GISAID databases. Based on mutations and phylogenetic clustering, MPXV is currently divided into two major clades, clade I (one, formally Congo Basin clade) and clade II (two, formally West Africa clade). Each of these clades is further subdivided into two subclades: clade Ia and clade Ib within clade I; clade IIa and clade IIb within clade II.
Clade Ia circulates within multiple countries in Central Africa and is associated with regular spillover from an animal reservoir(s) with some onward person-to-person transmission. Clade Ia has been sampled in Cameroon, Central African Republic, Congo, Democratic Republic of the Congo, South Sudan and Sudan. Mixing of virus sequences from these countries within the clade Ia phylogenetic tree shows international movement of clade Ia viruses.
Clade Ib has recently emerged in eastern regions of the Democratic Republic of the Congo and is undergoing sustained person-to-person transmission. Recent cases of clade Ib have also been detected in Belgium, Burundi, Canada, China, France, Germany, India, Kenya, Pakistan, Rwanda, Sweden, Thailand, The United Kingdom, Uganda, United States of America, Zambia and Zimbabwe. There is limited mutational diversity among clade Ib sequences. However, recent sequences from Kenya, Uganda, Sweden and Thailand share several mutations.
Clade IIa has rarely been isolated in humans with most available genetic sequences coming from animal species. Clade IIb has undergone sustained circulation within humans since at least 2016 and has caused a large ongoing outbreak from 2022 to present. The 2022-24 clade IIb outbreak is currently divided into 33 lineages which enable fine scale tracking.
The following phylogenetic visualisations were generated in R using the ggtree package.
6.1 Phylogeny of all MPXV clades
6.2 Phylogeny of clade 1b
6.3 Phylogeny of clade 1a
7 Literature summary & epidemic parameters
In order to promote a better understanding of the dynamics of the mpox outbreak and to support forecasting work, in 2022, WHO undertook an effort to extract epidemiological parameters (incubation period, serival interval and generation interval) from the literature. The initial literature screening was performed and maintained by the Public Health Agency of Canada (PHAC). The overall search strategy was as follows:
- Inclusion criteria: monkeypox and monkeypox virus
- Study design:
- Any study design including primary and secondary studies (both animal and human)
- Guidelines and commentaries are not excluded but are not searched systematically.
- Publication language: no restriction for peer-reviewed articles, grey literature is focused on English
- Publication date: from April 14, 2022 – January 19, 2023
- Bibliographic databases and other sources searched:
- PubMed Scopus
- Pre-print servers: Europe PMC, arXiv and SSRN
- WHO, PHAC, CDC, ECDC, UKHSA
The tables below provide an overview of the most relevant estimates for incubation period and generation interval extracted from the literature where the following criteria are met:
- Studies with a sample size greater than 5
- Clear estimate of the specific parameter
The epidemic parameter tables are no longer updated, as the literature screening is no longer carried out.
7.1 Parameters
| Incubation Period | ||||||||||
| As of 19 Jan 2023 | ||||||||||
| Reference | N | Mean1 | 95% CrI (mean)1 | 95% CI (mean)1 | SD1 | Median1 | 95% CrI (median)1 | IQR1 | Range1 | Distribution |
|---|---|---|---|---|---|---|---|---|---|---|
| Miura et al. [1] | 18 | 8.5 | 6.6 - 10.9 | - | - | - | - | - | - | Log-normal |
| Charniga et al. [2] | 40 | 7.6 | 6.2 - 9.7 | - | 1.8 | 6.4 | 5.1 - 7.9 | - | - | Log-normal |
| Rodríguez et al. [3] | 45 | - | - | - | - | - | - | - | - | - |
| Thornhill et al. [4] | 23 | - | - | - | - | 7.0 | - | - | 3 - 20 | - |
| Català et al. [5] | 77 | - | - | - | - | 6.0 | - | - | 4 - 9 | - |
| Tarín-Vicente et al. [6] | 144 | - | - | - | - | 7.0 | - | 5 - 10 | 1 - 19 | - |
| Guzzetta et al. [7] | 30 | 9.1 | - | 6.5 - 10.9 | - | - | - | - | - | Gamma |
| Mailhe et al. [8] | 112 | - | - | - | - | 6.0 | - | 3 - 8 | - | - |
| Moschese et al. [9] | 16 | - | - | - | - | 11.0 | - | 11 - 16 | - | - |
| Gomez-Garberi et al. [10] | 14 | - | - | - | - | 13.0 | - | - | 3 - 30 | - |
| O'Laughlin et al. [11] | 527 | 7.0 | - | - | - | - | - | 4 - 9 | - | - |
| Angelo et al. [12] | 78 | - | - | - | - | 8.0 | - | 5 - 11 | 2 - 40 | - |
| Madewell et al. [14] | 35 | 5.6 | 4.3 - 7.8 | - | - | - | - | - | - | - |
| Ward et al. [15] | 54 | 7.8 | 6.6 - 9.2 | - | - | - | - | - | - | Weibull |
| Besombes et al. [16] | 29 | - | - | - | - | 7.0 | - | 1 - 13 | 0 - 17 | - |
| Kröger et al. [17] | 209 | 8.2 | - | - | 4.7 | - | - | - | - | Log-normal |
| Source: PHAC | ||||||||||
| 1 Units are in days | ||||||||||
| Serial Interval | |||||||
| As of 19 Jan 2023 | |||||||
| Reference | N | Mean1 | 95% CrI (mean)1 | SD1 | Median1 | 95% CrI (median)1 | Distribution |
|---|---|---|---|---|---|---|---|
| Guo et al. [13] | 21 | 5.6 | 1.7 - 10.4 | 1.5 | 5.5 | 1.4 - 10.4 | - |
| Madewell et al. [14] | 57 | 8.5 | 7.3 - 9.9 | - | - | - | Gamma |
| Ward et al. [15] | 79 | 9.5 | 7.4 - 12.3 | - | - | - | Gamma |
| Miura et al. [18] | 34 | 9.4 | - | 6.2 | - | - | Normal |
| Source: PHAC | |||||||
| 1 Units are in days | |||||||
| Generation Interval | ||||
| As of 19 Jan 2023 | ||||
| Reference | N | Mean1 | 95% CrI1 | Distribution |
|---|---|---|---|---|
| Guzzetta et al. [7] | 16 | 12.5 | 7.5 - 17.3 | Gamma |
| Source: PHAC | ||||
| 1 Units are in days | ||||
7.2 Bibliography
The incubation period for monkeypox cases confirmed in the Netherlands, May 2022 ( 658) medRxiv . Miura, Fuminari, van Ewijk, Catharina Else, Backer, Jantien A., Xiridou, Maria, Franz, Eelco, de Coul, Eline Op, Brandwagt, Diederik, van Cleef, Brigitte, van Rijckevorsel, Gini, Swaan, Corien, van den Hof, Susan, Wallinga, Jacco. #volume# (2022): 2022.06.09.22276068–> 10.1101/2022.06.09.22276068 ; http://medrxiv.org/content/early/2022/06/13/2022.06.09.22276068.abstract
Estimating the incubation period of monkeypox virus during the 2022 multi-national outbreak ( 722) medRxiv . Charniga, Kelly, Masters, Nina B., Slayton, Rachel B., Gosdin, Lucas, Minhaj, Faisal S., Philpott, David, Smith, Dallas, Gearhart, Shannon, Alvarado-Ramy, Francisco, Brown, Clive, Waltenburg, Michelle A., Hughes, Christine M., Nakazawa, Yoshinori. #volume# (2022): 2022.06.22.22276713–> 10.1101/2022.06.22.22276713 ; http://medrxiv.org/content/early/2022/06/23/2022.06.22.22276713.abstract
Epidemiologic Features and Control Measures during Monkeypox Outbreak, Spain, June 2022 ( 888) Emerg Infect Dis . Rodríguez, B. S., Herrador, B. R. G., Franco, A. D., Fariñas, M. P. S., Del Amo Valero, J., Llorente, A. H. A., de Agreda, Jpap, Malonda, R. C., Castrillejo, D., Chirlaque López, M. D., Chong, E. J., Balbuena, S. F., García, V. G., García-Cenoz, M., Hernández, L. G., Montalbán, E. G., Carril, F. G., Cortijo, T. G., Bueno, S. J., Sánchez, A. L., Linares Dópido, J. A., Lorusso, N., Martins, M. M., Martínez Ochoa, E. M., Mateo, A. M., Peña, J. M., Antón, A. I. N., Otero Barrós, M. T., Martinez, Mdcp, Jiménez, P. P., Martín, O. P., Rivas Pérez, A. I., García, M. S., Soria, F. S., Sierra Moros, M. J.. 28,2022/07/13 (2022): #pages#–> 10.3201/eid2809.221051 ; https://wwwnc.cdc.gov/eid/article/28/9/22-1051_article
Monkeypox Virus Infection in Humans across 16 Countries - April-June 2022 ( 933) N Engl J Med . Thornhill, J. P., Barkati, S., Walmsley, S., Rockstroh, J., Antinori, A., Harrison, L. B., Palich, R., Nori, A., Reeves, I., Habibi, M. S., Apea, V., Boesecke, C., Vandekerckhove, L., Yakubovsky, M., Sendagorta, E., Blanco, J. L., Florence, E., Moschese, D., Maltez, F. M., Goorhuis, A., Pourcher, V., Migaud, P., Noe, S., Pintado, C., Maggi, F., Hansen, A. E., Hoffmann, C., Lezama, J. I., Mussini, C., Cattelan, A., Makofane, K., Tan, D., Nozza, S., Nemeth, J., Klein, M. B., Orkin, C. M.. 2022/07/23 (2022): #pages#–> 10.1056/NEJMoa2207323 ; #URL#
Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases ( 1008) Br J Dermatol . Català, A., Clavo Escribano, P., Riera, J., Martín-Ezquerra, G., Fernandez-Gonzalez, P., Revelles Peñas, L., Simón Gozalbo, A., Rodríguez-Cuadrado, F. J., Guilera Castells, V., De la Torre Gomar, F. J., Comunión Artieda, A., Fuertes de Vega, L., Blanco, J. L., Puig, S., García Miñarro Á, M., Fiz Benito, E., Muñoz-Santos, C., Repiso-Jiménez, J. B., Ceballos-Rodriguez, C., García Rodríguez, V., Castaño Fernández, J. L., Sánchez-Gutiérrez, I., Calvo López, R., Berna-Rico, E., de Nicolás-Ruanes, B., Corella Vicente, F., Tarín Vicente, E. J., Fernández de la Fuente, L., Riera-Martí, N., Descalzo-Gallego, M., Grau-Perez, M., García-Doval, I., Fuertes, I.. 2022/08/03 (2022): #pages#–> 10.1111/bjd.21790 ; #URL#
Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study ( 1074) Lancet . Tarín-Vicente, E. J., Alemany, A., Agud-Dios, M., Ubals, M., Suñer, C., Antón, A., Arando, M., Arroyo-Andrés, J., Calderón-Lozano, L., Casañ, C., Cabrera, J. M., Coll, P., Descalzo, V., Folgueira, M. D., García-Pérez, J. N., Gil-Cruz, E., González-Rodríguez, B., Gutiérrez-Collar, C., Hernández-Rodríguez, Á., López-Roa, P., de Los Ángeles Meléndez, M., Montero-Menárguez, J., Muñoz-Gallego, I., Palencia-Pérez, S. I., Paredes, R., Pérez-Rivilla, A., Piñana, M., Prat, N., Ramirez, A., Rivero, Á., Rubio-Muñiz, C. A., Vall, M., Acosta-Velásquez, K. S., Wang, A., Galván-Casas, C., Marks, M., Ortiz-Romero, P., Mitjà, O.. 2022/08/12 (2022): #pages#–> 10.1016/s0140-6736(22)01436-2 ; #URL#
Early Estimates of Monkeypox Incubation Period, Generation Time, and Reproduction Number, Italy, May-June 2022 ( 1189) Emerg Infect Dis . Guzzetta, G., Mammone, A., Ferraro, F., Caraglia, A., Rapiti, A., Marziano, V., Poletti, P., Cereda, D., Vairo, F., Mattei, G., Maraglino, F., Rezza, G., Merler, S.. 28,2022/08/23 (2022): #pages#–> 10.3201/eid2810.221126 ; https://wwwnc.cdc.gov/eid/article/28/10/22-1126_article
Clinical characteristics of ambulatory and hospitalised patients with monkeypox virus infection: an observational cohort study ( 1238) Clin Microbiol Infect . Mailhe, M., Beaumont, A. L., Thy, M., Le Pluart, D., Perrineau, S., Houhou-Fidouh, N., Deconinck, L., Bertin, C., Ferré, V. M., Cortier, M., C.,De La Porte Des Vaux,, Phung, B. C., Mollo, B., Cresta, M., Bouscarat, F., Choquet, C., Descamps, D., Ghosn, J., Lescure, F. X., Yazdanpanah, Y., Joly, V., Peiffer-Smadja, N.. 2022/08/27 (2022): #pages#–> 10.1016/j.cmi.2022.08.012 ; #URL#
Natural history of Human Monkeypox in individuals attending a sexual health clinic in Milan, Italy ( 1262) J Infect . Moschese, D., Pozza, G., Giacomelli, A., Mileto, D., Cossu, M. V., Beltrami, M., Rizzo, A., Gismondo, M. R., Rizzardini, G., Antinori, S.. 2022/08/26 (2022): #pages#–> 10.1016/j.jinf.2022.08.019 ; #URL#
Genitourinary Lesions Due to Monkeypox ( 1440) Eur Urol . Gomez-Garberi, M., Sarrio-Sanz, P., Martinez-Cayuelas, L., Delgado-Sanchez, E., Bernabeu-Cabezas, S., Peris-Garcia, J., Sanchez-Caballero, L., Nakdali-Kassab, B., Egea-Sancho, C., Olarte-Barragan, E., Ortiz-Gorraiz, M.. 2022/09/13 (2022): #pages#–> 10.1016/j.eururo.2022.08.034 ; #URL#
Clinical Use of Tecovirimat (Tpoxx) for Treatment of Monkeypox Under an Investigational New Drug Protocol - United States, May-August 2022 ( 1486) MMWR Morb Mortal Wkly Rep . O’Laughlin, K., Tobolowsky, F. A., Elmor, R., Overton, R., O’Connor, S. M., Damon, I. K., Petersen, B. W., Rao, A. K., Chatham-Stephens, K., Yu, P., Yu, Y.. 71,2022/09/16 (2022): 1190–> 10.15585/mmwr.mm7137e1 ; #URL#
Epidemiological and clinical characteristics of patients with monkeypox in the GeoSentinel Network: a cross-sectional study ( 1748) Lancet Infect Dis . Angelo, K. M., Smith, T., Camprubí-Ferrer, D., Balerdi-Sarasola, L., Díaz Menéndez, M., Servera-Negre, G., Barkati, S., Duvignaud, A., Huber, K. L. B., Chakravarti, A., Bottieau, E., Greenaway, C., Grobusch, M. P., Mendes Pedro, D., Asgeirsson, H., Popescu, C. P., Martin, C., Licitra, C., de Frey, A., Schwartz, E., Beadsworth, M., Lloveras, S., Larsen, C. S., Guagliardo, S. A. J., Whitehill, F., Huits, R., Hamer, D. H., Kozarsky, P., Libman, M.. 2022/10/11 (2022): #pages#–> 10.1016/s1473-3099(22)00651-x ; #URL#
Estimation of the serial interval of monkeypox during the early outbreak in 2022 ( 1895) J Med Virol . Guo, Z., Zhao, S., Sun, S., He, D., Chong, K. C., Yeoh, E. K.. 2022/10/23 (2022): #pages#–> 10.1002/jmv.28248 ; #URL#
Serial interval and incubation period estimates of monkeypox virus infection in 12 U.S. jurisdictions, May - August 2022 ( 2007) medRxiv . Madewell, Zachary, Charniga, Kelly, Masters, Nina, Asher, Jason, Fahrenwald, Lily, Still, William, Chen, Judy, Kipperman, Naama, Bui, David, Shea, Meghan, Saathoff-Huber, Lori, Johnson, Shannon, Harbi, Khalil, Berns, Abby, Perez, Taidy, Gateley, Emily, Spicknall, Ian, Nakazawa, Yoshinori, Gift, Thomas. #volume# (2022): #pages#–> 10.1101/2022.10.26.22281516 ; http://europepmc.org/abstract/PPR/PPR564657 https://doi.org/10.1101/2022.10.26.22281516
Transmission dynamics of monkeypox in the United Kingdom: contact tracing study ( 2069) Bmj . Ward, T., Christie, R., Paton, R. S., Cumming, F., Overton, C. E.. 379,2022/11/03 (2022): e073153–> 10.1136/bmj-2022-073153 ; #URL#
National Monkeypox Surveillance, Central African Republic, 2001-2021 ( 2104) Emerg Infect Dis . Besombes, C., Mbrenga, F., Schaeffer, L., Malaka, C., Gonofio, E., Landier, J., Vickos, U., Konamna, X., Selekon, B., Dankpea, J. N., Von Platen, C., Houndjahoue, F. G., Ouaïmon, D. S., Hassanin, A., Berthet, N., Manuguerra, J. C., Gessain, A., Fontanet, A., Yandoko, E. N.. 28,2022/11/04 (2022): #pages#–> 10.3201/eid2812.220897 ; https://wwwnc.cdc.gov/eid/article/28/12/22-0897_article
Monkeypox outbreak 2022 – an overview of all cases reported to the Cologne Health Department ( 2181) Research Square . Kröger, Sophia Toya, Lehmann, Max Christian, Treutlein, Melanie, Fiethe, Achim, Kossow, Annelene, Küfer-Weiß, Annika, Nießen, Johannes, Grüne, Barbara. #volume# (2022): #pages#–> 10.21203/rs.3.rs-2251751/v1 ; http://europepmc.org/abstract/PPR/PPR570380 https://doi.org/10.21203/rs.3.rs-2251751/v1
Time scales of human monkeypox transmission in the Netherlands ( 2405) medRxiv . Miura, Fuminari, Backer, Jantien, van Rijckevorsel, Gini, Bavalia, Roisin, Raven, Stijn, Petrignani, Mariska, Ainslie, Kylie E. C., Wallinga, Jacco. #volume# (2022): #pages#–> 10.1101/2022.12.03.22283056 ; http://europepmc.org/abstract/PPR/PPR579534 https://doi.org/10.1101/2022.12.03.22283056
8 Disclaimers
8.1 Data Overview and Visualizations
The WHO 2022-25 mpox global trends report aims to provide frequently updated data visualizations. Caution must be taken when interpreting all data presented, and differences between information products published by WHO, national public health authorities, and other sources using different inclusion criteria and different data cut-off times are to be expected. While steps are taken to ensure accuracy and reliability, all data are subject to continuous verification and change. All counts are subject to variations in case detection, definitions, laboratory testing, and reporting strategies between countries, states and territories.
Data are compiled and shared with WHO by national public health authorities. Data compilation and submission to WHO Headquarters is done by the WHO Regional Offices and WHO Country Offices.
WHO makes no warranties or representations regarding the contents, appearance, completeness, technical specifications, or accuracy of the report. WHO disclaims all responsibility relating to, and shall not be liable for, any use of the report, the results of such use, or the reliance thereon.
WHO reserves the right to make updates and changes to the report without notice, and accepts no liability for any errors or omissions in this regard.
The user of the report is responsible for the interpretation and use of the analysis and outputs performed by the report. The submission of content to the report does not imply WHO’s approval or endorsement of that content, or that the content is appropriate for any purpose or meets any established standard or requirement
Any designations employed or presentation by the user in its use of the app, including tables and maps, do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers and boundaries.
All references to Kosovo should be understood to be in the context of the United Nations Security Council resolution 1244 (1999).
A dispute exists between the Governments of Argentina and the United Kingdom of Great Britain and Northern Ireland concerning sovereignty over the Falkland Islands (Malvinas).
8.2 Copyright, Permissions, and Referencing
© World Health Organization 2025, All rights reserved.
WHO supports open access to the published output of its activities as a fundamental part of its mission and a public benefit to be encouraged wherever possible. Permission from WHO is not required for the use of the WHO Mpox global trends report or data available for download.
The user shall not, in connection with use of the app, state or imply that WHO endorses or is affiliated with the user, its use of the app, or any content, output, or analysis resulting from or related to the app, or that WHO endorses any entity, organization, company, or product.
The use of the WHO emblem / logo by a user of the report in connection with its use is not permitted. For further information, please visit WHO Copyright, Licencing and Permissions.
Suggested citation: 2022-24 Mpox Outbreak: Global Trends. Geneva: World Health Organization, 2025. Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (last cited: [date]).
9 Acknowledgements
We gratefully acknowledge the input of national public health staff involved in surveillance activities and data submission to WHO, the WHO regional and country offices for the timely compilation of data, and the European Centre for Disease Prevention and Control (ECDC) for the provision of surveillance data collected via the TESSy platform, as well as external partners who contributed additional insights and contextual information on the data.