: Powdery mildew of apple
Podosphaera leucotricha (Ell. and Ev.) Salm. apples and flowering crabapples (Malus spp.) and pear (Pyrus spp.)
 |
 |
| Powdery mildew on fruit and cluster leaves. (Courtesy K. Yoder and S. Marine) |
Powdery mildew of apple occurs in all apple-producing regions of the world. The disease causes economic damage by reducing tree vigor, flower bud production, and fruit quality.
Symptoms and Signs
Powdery mildew of apple produces symptoms on young shoots, leaves, blossoms, and fruit. In general, symptoms are most noticeable on the leaves and fruit.
Apple shoots
Overwintered infections in dormant flower and shoot buds provide inoculum for the following year. In spring when the terminal buds begin to grow, the fungus colonizes the young, green tissue as it emerges (Figure 1). These infected “flag shoots” have a silver-gray appearance and may exhibit defoliation, stunted growth, and die-back (Figure 2). In the following weeks, as the growing season progresses, the primary infections on the flag shoots produce inoculum, which causes secondary infections on leaves, blossoms, and fruit. Heavily infected trees become weakened and are more likely to be invaded by secondary pathogens. By midsummer, the mycelium darkens and numerous brown fruiting bodies (ascocarps) form (Figure 3).

Figure 1. |

Figure 2. |

Figure 3. |
Apple leaves and fruit
In contrast to primary infections, in which the leaf is colonized as it emerges from the bud, secondary infections occur when windborne spores land on young leaves as they unfurl and expand. Fungal colonies composed of mycelium and spores appear as white, felt-like patches (Figure 4). Secondary infections commonly appear first on the lower leaf surface, and may be detectable on the upper leaf surface as chlorotic spots. Leaves infected along the leaf margin may become curled, crinkled, or folded longitudinally. As the disease progresses, affected tissues develop the powdery, silver-gray appearance typical of powdery mildews (Figure 5). Infections on the blossom receptacle or of young fruit will cause netlike russetting and discoloration as the fruit matures (Figure 6). Fruit may also become distorted and/or dwarfed. Mildew reduces both apple yield and quality.

Figure 4. |

Figure 5. |

Figure 6. |
Apple blossoms
Infected flower buds have a silver-gray appearance and open 5-8 days later than healthy ones, if at all. Petals are distorted and pale yellow or light green (Figure 7). Blossoms may become shriveled and fail to produce fruit (Figure 8). Secondary infections may occur on newly forming flower buds, which will remain dormant until the following spring. Since these buds will be diseased when they open, severe infection can eliminate the crop the following season.

Figure 7. |

Figure 8. |
Pathogen Biology
Podosphaera leucotricha is an ascomycete fungus in the Erysiphaceae family and is found in all apple-producing regions. (Other powdery mildew species have occasionally been recorded on Malus species, but appear to be of no economic significance.) During the growing season, this fungal obligate parasite continuously produces asexual spores (conidia) on specialized short stalks called conidiophores (Figure 9). Conidia are hyaline (clear, without color), measure 20-38 × 12 µm, and contain distinct fibrosin bodies. Fibrosin bodies are refractive inclusion bodies that exhibit varied shapes including rods and cones, and that can aid in the recognition of this group of powdery mildews. Conidia are wind-dispersed and do not require free moisture to germinate. If they land on susceptible tissue, they initiate infection and produce colonies of mycelium. Infected lateral and terminal apple buds serve as overwintering sites and provide the earliest source of inoculum the following spring. However, extremely low winter temperatures will negatively impact the survival of P. leucotricha as infected buds are more vulnerable to winter kill.
P. leucotricha also produces sexual spores (ascospores) in sac-like asci enclosed in fruiting bodies (ascocarps) (Figure 10). Each ascocarp contains a single ascus with eight ascospores, each of which is elliptical and measures 22-36 x 12-15 µm. Ascocarps are recognized as distinct black dots on the surface of a mycelial mat (Figure 3). Ascocarps are densely grouped together, measure 75-96 µm in diameter and have apical and basal appendages. Ascocarps form late in the growing season and serve as overwintering structures, but don’t play any known role in initiating new epidemics, as the ascospores fail to germinate readily. In the past, the ascocarps of P. leucotricha were called cleistothecia (reflecting the closed nature and lack of a preformed opening), perithecia (reflecting the arrangement of the asci of many powdery mildew fungi in a layer [hymenium]), and most recently, chasmothecia. All three terms can be found in the literature. Since cleistothecia in other groups of ascomycetes lack a hymenium (i.e., the asci are randomly scattered throughout the enclosed structure), the term chasmothecia has been recently introduced to distinguish powdery mildew ascocarps from other cleistothecia. The word is derived from the vertical chasm that is formed during ascospore discharge.

Figure 9. |

Figure 10. |
Disease Cycle and Epidemiology
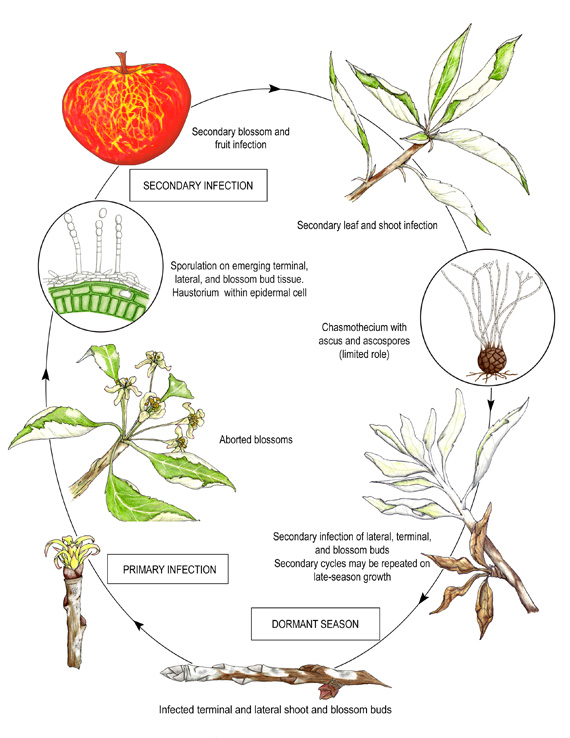
Disease cycle of apple powdery mildew.
Disease Cycle
P. leucotricha overwinters as mycelium in dormant flower and shoot buds infected the previous year. In spring, the infected buds break dormancy and the fungus resumes growth, colonizing the developing shoots and young leaf tissue. From these primary infections, asexual conidia are produced on conidiophores and dispersed by wind. Conidia will germinate at high relative humidity (greater than 70%, which is commonly available in the microclimate of the lower leaf surface) at temperatures between 10 and 25°C; in contrast to most foliar fungal pathogens, leaf wetting is a deterrent to infection. The youngest leaves are the most susceptible, but become increasingly resistant as they mature.
Mildew colonies generally appear first on the lower leaf surface as white felt-like patches. Conidia germinate to form hyphal outgrowths, which traverse the leaf surface, swell and then flatten to form appressoria. These structures release enzymes, which allow fungal infection pegs to penetrate the plant’s epidermal cells and then enlarge to form haustoria (Figure 11). Haustoria are specialized organs formed inside living plant cells, which absorb nutrients and anchor the fungus. As the mildew colony expands or as secondary infections lead to new colony formation, the infection process (hyphal outgrowth > appressorium > infection peg > haustorium) is repeated until susceptible tissue is no longer available. Late-season growth may result in a sudden increase in mildew activity. In addition to contributing toward a rapid inoculum buildup, secondary disease cycles are also responsible for infecting lateral and terminal buds that will carry the fungus through the winter.
Primary infections in flower buds can produce conidia as early as tight-cluster, the stage of apple development when flower cluster leaves start to separate but flower buds remain aggregated (Figure 12). Infected buds usually open later than healthy ones, ensuring the presence of susceptible tissue (expanding and unfurling leaves, open blossoms, and immature fruit) for the fungus to colonize. Secondary infection of the blossom receptacle occurs from 3 weeks before to 3 weeks after bloom. Infected receptacles may shrivel and fail to produce fruit or may mature to produce fruit that is discolored, russetted, dwarfed, and/or distorted.
In late summer and early fall, overwintering structures (ascocarps) are formed within the mycelial mat on leaves and shoots. However, the sexual spores (ascospores) contained in these ascocarps are seldom viable, and no role in survival and infection has been established.
Mildew is a chronic recurrent problem. High disease levels at the end of a season may (i) increase the percentage of infected buds, leading to high levels of primary inoculum the next spring and/or (ii) inhibit flower bud formation, reducing or eliminating the fruit crop the following season. Therefore, management of the disease must focus on reducing the primary inoculum and protecting the trees from secondary inoculum.

Figure 11. |

Figure 12. |
Disease Management
Cultivar Selection
The use of less susceptible apple cultivars is perhaps the most effective means of preventing mildew. Apple cultivars are available that demonstrate natural resistance to mildew and need control only under high disease pressure; these include Jonafree, Prima, and Enterprise, but they are not widely grown. Planting cultivars that have some level of resistance to several common apple diseases (apple scab, powdery mildew, fireblight, and cedar apple rust) can reduce the number of fungicide treatments and the total cost of the spray program in a given growing season. Cultivar selection is influenced more by commercial appeal, fruit qualities, marketability, and pollination characteristics than by disease resistance. As a result, growers typically interplant cultivars of different susceptibilities in an orchard. Cultivars such as Golden Delicious, Idared, and Granny Smith are widely grown, but are moderately to highly susceptible to mildew and may require chemical disease management. Charts of apple cultivars and their susceptibility to mildew are available to aid growers in cultivar selection (Table 1).
Table 1. Apple cultivar susceptibility to mildew.
| apple cultivar |
mildew susceptibility |
| Braeburn |
R |
| Britegold |
R |
| Cortland |
HS |
| Delicious |
R |
| Empire |
S |
| Enterprise |
R |
| Fuji |
R |
| Gala |
R |
| Ginger Gold |
HS |
| Golden Delicious |
S |
| Granny Smith |
HS |
| Idared |
HS |
| Jonafree |
R |
| Jonathan |
HS |
| Liberty |
S |
| McIntosh |
S |
| Nittany |
R |
| Rome Beauty |
HS |
| Stayman Winesap |
HS |
| Winesap |
R |
| HS |
= |
highly susceptible |
(control always needed when disease is present) |
| S |
= |
susceptible |
(control usually needed when disease is present) |
| R |
= |
resistant |
(control needed only under high disease pressure) |
Cultural Practices
Primary infections can be controlled by removal of the primary inoculum sources (i.e., flower and shoot buds infected the previous year). Growers should note any whitened terminal shoots and prune them out during winter or early spring. Unfortunately, this is hard to accomplish effectively. Removal of inoculum by pruning, especially in large commercial orchards, would be labor-intensive and may interfere with tree-structure training. Complete removal of this type of inoculum is just not economically feasible. The best candidates to use this control practice are small young orchards with low numbers of primary infections per tree.
Chemical Control
Secondary infections and fruit infections can be controlled by foliar fungicide applications. In commercial orchards, fungicides are almost always used to control mildew, as well as other apple diseases. Fungicides are usually applied at 7- to 10-day intervals from the tight-cluster stage until terminal shoot growth ends (about midsummer). This ensures that fungicide application coincides with rapid leaf development and the post-bloom period, and that the new growth does not remain unprotected for long. For highly susceptible cultivars, this could mean as many as 18 sprays.
A variety of compounds are registered in the U. S. for control of mildew including: inorganics (sulfur), sterol-inhibitors (such as fenbuconazole and myclobutanil), and strobilurins (such as trifloxystrobin and kresoxim-methyl). All of these can provide effective control, but growers should not rely solely on one class of fungicides. Whenever possible, growers should rotate or alternate with different mode of action groups, use multi-site fungicides (like sulfur) at times of low risk, and plant less susceptible cultivars. Benzimidazoles had activity against mildew, but their utility in the apple disease management program was reduced due to widespread resistance development in Venturia inaequalis (apple scab). Horticultural oils, waxes, and biological compounds produced by Bacilllus strains are also available, but their effectiveness is somewhat inconsistent.
Failure to include pre-bloom sprays is one of the most common mistakes growers make in mildew management. When P. leucotricha resumes growth in spring, large numbers of conidia are produced in uncontrolled secondary cycles. These asexual spores infect healthy flower and shoot buds, which serve as the primary inoculum source next year. Control is difficult to achieve during the growing season if it has been neglected early on. Growers may be tempted to relax spray programs during dry conditions when other apple diseases cannot develop, but mildew thrives in dry weather and protection needs to be maintained.
Studies have also found that control is more enhanced by shortening the spray interval than by increasing the fungicide rate. However, this is mostly done in severe disease situations, as labor and fuel cost may become prohibitive if 3- to 4-day intervals are used. Chemical control programs must be developed with pesticide compatibility, phytotoxicity, and registration restrictions in mind.
Significance
Before the 1940’s, mildew was considered a disease primarily of nursery stock and was of relatively minor importance to most commercial apple growers. Its rise to prominence within the apple disease spectrum can be partially attributed to the replacement of sulfur fungicides with organic fungicides, whose spectrum of activity focused more on apple scab and rust control. Initial efforts in breeding programs focused on “wet weather” disease resistance, but mildew infections do not require free moisture. The limited number of fungicide options available and the lack of satisfactory control helped ensure mildew’s significance among other apple diseases.
Currently, mildew is a persistent problem wherever apples are grown. Serious outbreaks can be a consequence of inadequate early-season spray programs, lax spray programs during dry spells, or development of resistance to some of the fungicides used. Mildew’s chronic effect on tree vigor and yield is detrimental to both the longevity and profitability of the orchard. Successful control of mildew is dependent on grower education and a management strategy that incorporates resistant apple cultivars, cultural practices, and effective fungicide application.
Selected References
Glawe, D. A. 2008. The powdery mildews: A review of the world’s most familiar (yet poorly known) plant pathogens. Annual Review of Phytopathology 46: 27-51.
Hickey, K. D. and K. S. Yoder. 1990. Apple powdery mildew. Pages 9-10 in A. L. Jones and H. S. Aldwinckle, ed. Compendium of Apple and Pear Diseases. American Phytopathological Society, St. Paul, MN.
Pfeiffer, D. G., J. C. Bergh, R. D. Fell, R. Yuan, C. S. Walsh, K. S. Yoder, A. R. Biggs, J. B. Kotcon, J. F. Derr, R. S. Chandran, M. J. Weaver, J. F. Baniecki, A. Brown, and J. Parkhurst. 2010. 2010 spray bulletin for commercial tree fruit growers. Virginia Cooperative Extension Publication.
Turechek, W. W., J. E. Carroll, and D. A. Rosenberger. 2004. Powdery mildew of apple. Tree Fruit Factsheet, Cornell University.
Yoder, K. S. 1992. Powdery mildew of apple. Pages 66-89. In: J. Kumar, H. S. Chaube, U. S. Singh, and A. N. Mukhopadhyay (eds.) Plant Diseases of International Importance, Vol. 3. Diseases of Fruit Crops, Prentice Hall, NJ.
Yoder, K. S., and A. R. Biggs. 1997. Apple cultivar susceptibility to the powdery mildew fungus.
Related WWW Links
Biggs, A. R., K. S. Yoder, and D. A. Rosenberger. 2009. Relative susceptibility of selected apple cultivars to powdery mildew caused by Podosphaera leucotricha. Plant Health Progress. doi:10.1094/PHP-2009-1119-01-RS.
Commodity Apple Profile
