Abstract
It is commonly held that substance use comorbidity in schizophrenia represents self-medication, an attempt by patients to alleviate adverse positive and negative symptoms, cognitive impairment, or medication side effects. However, recent advances suggest that increased vulnerability to addictive behavior may reflect the impact of the neuropathology of schizophrenia on the neural circuitry mediating drug reward and reinforcement. We hypothesize that abnormalities in the hippocampal formation and frontal cortex facilitate the positive reinforcing effects of drug reward and reduce inhibitory control over drug-seeking behavior. In this model, disturbances in drug reward are mediated, in part, by dysregulated neural integration of dopamine and glutamate signaling in the nucleus accumbens resulting form frontal cortical and hippocampal dysfunction. Altered integration of these signals would produce neural and motivational changes similar to long-term substance abuse but without the necessity of prior drug exposure. Thus, schizophrenic patients may have a predilection for addictive behavior as a primary disease symptom in parallel to, and in many cases independent from, their other symptoms.
Keywords: Schizophrenia, substance abuse, dependence, dual diagnosis, dopamine, nucleus accumbens, hippocampus
Introduction
Substance disorder comorbidity, although common in many psychiatric illnesses, is particularly prevalent in schizophrenic populations (Selzer and Lieberman 1993). Results from the Epidemiologic Catchment Area (ECA) study show that schizophrenia ranks second only to antisocial personality disorder (which included substance abuse criterion at the time) in rates of substance abuse comorbidity (Reigier et al 1990). These ECA data also found that patients with schizophrenia are 4.6 times more likely to have substance use disorders than persons without mental illness (3 times higher for alcohol, 6 times higher for other illicit drugs). Schizophrenic populations commonly use one or more of several substances, including nicotine, alcohol, cannabis, cocaine, and amphetamines (Cuffel 1992; Dixon et al 1991; Mueser et al 1990; Schottenfeld et al 1993; Selzer and Lieberman 1993; Zisook et al 1992). Nicotine is clearly the most highly self-administered drug in schizophrenic populations, with use rates ranging from 70 to 90%, compared with 26% in the general population (Buckley 1998; Giovino et al 1994; Hughes et al 1986; Masterson and O’Shea 1984; O’Farrell et al 1983). In independent populations of patients with schizophrenia, lifetime prevalence of cocaine abuse or dependence ranges from 15 to 50%; amphetamine abuse from 2 to 25%, alcohol abuse from 20 to 60%, and cannabis abuse from 12 to 42% (Buckley 1998; DeQuardo et al 1994; Mueser et al 1990; Strakowski et al 1994).
Apart from socioeconomic and demographic factors, the most widely held explanation for substance use comorbidity in schizophrenia and other mental illnesses is the self-medication hypothesis (Buckley 1998; Dalack et al 1998; Krystal et al 1999). This hypothesis is based on the psychologic construct of negative reinforcement, defined here as the reinforcing property associated with the “purposeful removal of an aversive stimulus” (March 1999). Self-medication hypotheses assert that patients use drugs to alleviate aversive disease symptoms or medication side effects; however, a growing body of evidence suggests that the neuropathology of schizophrenia may contribute to the vulnerability to addiction by facilitating neural substrates that mediate positive reinforcement. This primary addiction hypothesis is based on basic and clinical neuroscience literature that supports two fundamental tenets:
The putative neuropathology underlying schizophrenia involves alterations in neuroanatomic circuitry that regulate positive reinforcement, incentive motivation, behavioral inhibition, and addictive behavior.
Experimental interventions that model neuropathologic and behavioral aspects of schizophrenia in animals also facilitate positive reinforcement and the incentive motivational effects of reward-related stimuli.
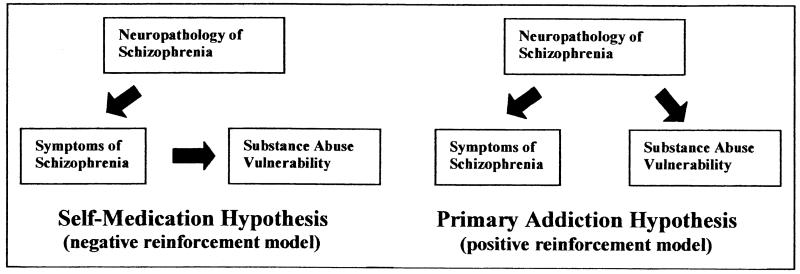
An inherent feature of this hypothesis is that both the schizophrenia syndrome and vulnerability to addiction are primary disease symptoms, each directly caused by common neuropathologic substrates (Figure 1). In contrast, the self-medication hypothesis asserts that vulnerability to addiction is a reaction to the schizophrenia syndrome or medication side effects, and thus represents a secondary symptom. In this scenario, drug use is dependent on the experience or manifestation of symptoms; however, the primary addiction hypothesis posits that both schizophrenia symptoms and addictive behavior occur as independent manifestations of the same disease. This latter feature may account for the failure to find a consistent association of drug addictions with alleviation of general or specific symptoms in schizophrenia as described below.
Figure 1.
The self-medication hypothesis suggests that substance abuse comorbidity is a secondary reaction to primary schizophrenia symptoms, representing a negative reinforcement model of symptom alleviation. The primary addiction hypothesis suggests that propensity for drug addiction is itself a primary symptom in schizophrenia directly resulting from neuropathologic processes that facilitate positive reinforcement, increasing the motivational and behavioral responses to addictive drugs.
First, several studies indicate that substance use rates in schizophrenic populations are substantially higher when compared with other psychiatric populations who would be as likely to self-medicate. For example, although smoking is suggested to alleviate negative emotional states, anxiety, depression, or poor cognitive functioning, nicotine use in patients with schizophrenia is more prevalent than in nonschizophrenic patients who exhibit similar symptomatology as primary features of their disorders. Tobacco use is consistently reported in more than 70% of schizophrenic patients but in less than half of patients with major depression, anxiety disorders, panic disorders, and personality disorders (Glassman et al 1992; Hughes et al 1986; Pohl et al 1992). Even when considering the constellation of symptoms in schizophrenia, there is no consensus among studies for substance use comorbidity to be associated with any particular subset of symptoms in individual patients (Cuffel et al 1993; DeQuardo et al 1994; Lambert et al 1997; Van Ammers et al 1997). Instead, studies on large patient populations find that drug availability is a primary determinant for the type of drugs patients use (Baigent et al 1995; Lambert et al 1997). These findings may suggest an association between heterogeneous schizophrenia presentations and a facilitation of the positive reinforcing and incentive motivational properties of addictive drugs.
A second consideration is that drugs of abuse have a complex array of effects in schizophrenic patients, often producing outcomes that are inconsistent with the view that these drugs serve to medicate their symptoms. For example, although some patients report symptom relief with drug use, others report symptom exacerbation, and yet their drug use persists (Addington and Duchak 1997; Selzer and Lieberman 1993). In many cases, self-reports of symptom improvement with drug use are contradictory to concurrent objective clinical observations that clearly show symptom exacerbation (DeQuardo et al 1994; Seibyl et al 1993). Moreover, drug or alcohol use greatly increases the likelihood of rehospitalization, length of hospitalization, need for greater neuroleptic dosage, and treatment noncompliance in schizophrenic patients (Dixon 1999; Gerding et al 1999; Seibyl et al 1993). Interestingly, the term self-medication is commonly used to explain high rates of drug use in dual-diagnosis patients who are especially medication noncompliant (Agarwal et al 1998; Seibyl et al 1993). These data suggest that similar to the natural course of addictions in nonschizophrenic patients, the incentive motivational properties of drugs that produce chronic drug taking in schizophrenia outweigh motivation to abstain stemming from psychiatric, medical, and financial consequences of drug use.
Another observation indicating a dissociation of schizophrenia symptomatology from addictive behavior is the prevalence of substance abuse before clinical presentation and medication treatment in schizophrenic patients. Studies have found that both drug and alcohol abuse occurs before the onset of psychosis and neuroleptic treatment in 14 to 69% of cases of schizophrenia (Berti 1994; Buckley 1998). One study found that 77% of first-episode patients are already smokers before treatment (McEvoy and Brown 1999). In another study, smoking rates are 54% and 15% in early- versus late-onset schizophrenia respectively, despite a similar duration of neuroleptic treatment (Sandyk and Awerbuch 1993). Although it is difficult to distinguish the initiation of drug use from the onset of prodromal symptoms, in many cases addiction vulnerability may occur as a natural antecedent to onset of other frank signs and symptoms, as a primary consequence of neuropathologic brain development that will later present as schizophrenia.
A final limitation of attributing drug abuse to self-medication of symptomatology is the assignment of treatment value to the effects unique to each type of abused drug while overlooking their common ability to mediate positive reinforcement. Different drugs commonly abused by schizophrenic patients produce highly specific effects on mood, perception, and cognitive functioning, and even mutually opposing subjective experiences via activity on a great diversity of neurotransmitter systems (Ling et al 1996). These facts have lead to attempts to define independent neurobiological mechanisms for self-medication specific to each drug of abuse in association with a particular psychiatric symptom, resulting in a host of relatively disparate theories that are not generalizable to more than one drug. Furthermore, these theories do not explain why standard medication treatments, with well-known efficacy for a variety of symptoms in schizophrenia, are not alone generally effective in reducing substance abuse in dual diagnosis cases (Selzer and Lieberman 1993). The primary addiction hypothesis focuses on the common ability of the abused drugs to produce incentive-motivation and positive reinforcement while remaining unencumbered by their differential ability to worsen or ameliorate certain symptoms of schizophrenia. A pathologic facilitation of incentive-motivational processes is consistent with observations that schizophrenic patients are known to suffer from a variety of apparently “senseless” motivational disturbances in parallel to substance abuse. These include motor and behavioral stereotypies, frequent and inappropriate masturbation, polydipsia, bulimia, pacing, pica, hoarding, and pathologic gambling (Aizengerg et al 1995; Chambers and Potenza 2001; Luchins 1992; Skopec et al 1976).
The primary addiction hypothesis offers a parsimonious explanation for substance use comorbidity in schizophrenia by viewing addiction as an independent process stemming directly from a common neuropathologic root. The hypothesis is synthesized from basic neuroscience literature suggesting that neurodevelopmental alterations in schizophrenia overlay precisely on neural substrates that regulate addictive behavior. We describe the fundamental neurocircuitry implicated in both schizophrenia and addiction and how neurodevelopmental alterations could disrupt normal signal integration within these circuits. Experimental manipulation of key components in this circuitry is shown to directly facilitate brain substrates that mediate positive reinforcement and to impair mechanisms that exert inhibitory control over addictive behavior.
Common Neurocircuitry Implicated in Both Addiction and Schizophrenia
Involvement of the Mesolimbic Dopamine System in Addiction and Schizophrenia
Most drugs abused by humans also are self-administered by laboratory animals. A substantial literature has established the mesolimbic dopamine system as a major neural substrate for the reinforcing effects of psychostimulants, ethanol, nicotine, and cannabinoids in animals (Fibiger et al 1992; Koob 1992; Wise 1990). This system consists of dopamine (DA) neurons in the ventral tegmental area (VTA) and their target neurons in forebrain regions such as the nucleus accumbens (NAc). Rats will self-administer DA, amphetamine (which releases DA), and cocaine (which elevates DA levels by blocking reuptake), all directly into the NAc, suggesting that DA receptors in the NAc mediate reinforcing stimuli (Carlezon et al 1995; Dworkin et al 1986; Hoebel et al 1983). Other drugs used by schizophrenic patients, such as ethanol, nicotine, and cannabinoids, also cause increased DA release in the NAc, possibly through disinhibition of VTA DA neurons (Chen et al 1990; Imperato and Di Chiara 1986; Tanda et al 1997). These findings have led investigators to suggest that DA release in the NAc is a final common mechanism for the reinforcing effects of psychostimulants and other abused drugs (Bozarth and Wise 1986; Di Chiara and Imperato 1988).
The mesolimbic DA system also may be involved in mediating drug craving. This hypothesis is based on the fact that stimuli that induce relapse in animal models and drug craving in human studies are all known to increase DA levels in the NAc (Ito et al 2000; Self 1998; Stewart 2000). These stimuli include small priming doses of drugs, stress, and drug-associated cues. In humans, craving for cocaine, nicotine, and ethanol also is induced by druglike DA agonists (Haney et al 1998; Jaffe et al 1989). In contrast, DA receptor antagonists fail to induce heroin- or cocaine-seeking behavior (Shaham and Stewart 1996; Weissenborn et al 1996), despite their ability to produce withdrawal-like aversive consequences (Shippenberg and Hertz 1987). Moreover, although several studies suggest that drug withdrawal is associated with hypofunction in DA reward substrates, these changes actually are associated with anhedonia rather than increased drug-seeking behavior (Koob and Le Moal 1997). Together these studies support the idea that drug craving is induced by stimuli that facilitate, rather than depress, DA reward systems.
The mesolimbic DA system also pertains to schizophrenia, based on the classic findings that 1) typical neuroleptics exert antipsychotic activity via blockade of DA receptors; and 2) psychostimulants elevate DA levels in the NAc, causing psychotic symptoms in both “healthy” subjects and schizophrenic patients (Crow et al 1977; Lieberman et al 1990). Contemporary revisions of the dopamine hypothesis suggest that schizophrenia symptoms emerge from a functional hyperactivity of DA neurons projecting to the NAc, associated with functional hypoactivity of DA neurons projecting to the frontal cortex (Davis et al 1991; Deutch 1993; Goldstein and Deutch 1992). Other revisions suggest that psychosis and thought disorder may result, in part, from a state of abnormal glutamatergic cortical activity associated with exaggerated DA release or dysregulated DA signaling in the NAc (Csernansky and Bardgett 1998). This imbalance of cortical and DA signaling may contribute to improper gating of perceptual and thought processes (Hoffman and McGlashan 1993; Weinberger et al 1994).
The primary addiction hypothesis incorporates evidence of hyperactivity in NAc DA signaling as it relates both to psychotic symptoms and addictive behavior. The ability of typical antipsychotics to attenuate behavioral sensitization and reinforcement produced by psychostimulants agrees with this idea (Glenthoj et al 1993; Richardson et al 1994; Roberts and Vickers 1987). In rats, an imbalance favoring subcortical over cortical DA signaling, whether biologically inherent or experimentally induced, increases sensitivity to the reinforcing effects of psychostimulants (McGregor et al 1996; Piazza et al 1991; Schenk et al 1991). Stress also activates the mesolimbic DA system and is known to exacerbate psychotic symptoms, stimulate drug craving in human drug abusers, and induce drug-seeking behavior in animals (Lieberman et al 1990; Sinha et al 1999; Stewart 2000; Walker and Diforio 1997). It is possible that an imbalance of cortical-subcortical DA contributes to stress-induced exacerbation of psychosis and drug seeking in patients with schizophrenia. In this regard, the subset of NAc neurons responsive to stress are also thought to be a primary locus of antipsychotic activity (Deutch 1993).
Dysregulation in Cortical, Temporal Limbic, and Mesoaccumbens Circuits Is Implicated in Both Schizophrenia and Substance Abuse Disorders
Although functional hyperactivity in the mesolimbic DA system may be involved in both schizophrenia and addictive behavior, it is important to consider neuropathology in a larger neural network that integrates mesolimbic DA in the NAc with cortical and hippocampal inputs. The integration of these circuits occurs at the level of individual medium spiny neurons in the NAc, which receive convergent excitatory input from the prefrontal cortex (PfC) and the hippocampal formation and DA input from the VTA. Integration of these signals in NAc neurons is thought to contribute substantially to the motivational consequence of drugs or drug-related stimuli. If so, dysfunctional integration of cortical, hippocampal, and DA signals in schizophrenic patients could significantly alter their propensity for addictive behavior.
Network dysregulation in schizophrenia may act similarly to the network dysregulation in drug addiction. Jentsch and Taylor (1999) suggested that chronic drug exposure produces pathophysiologic changes that contribute to loss of cortical control over DA-mediated behavior. This loss of cortical control is thought to sensitize animals to the incentive-motivational effects of drugs, contributing to compulsive drug self-administration (Jentsch and Taylor 1999). Other evidence suggests that chronic, drug-induced neuroadaptations in DA receptor signal transduction in the NAc could alter the impact of DA signals from the VTA and glutamate signals from the PfC and hippocampus (Self 1998; Self and Nestler 1998; White and Kalivas 1998). Interestingly, drug-induced changes include decreases in inhibitory G proteins in the NAc, changes that also have been found in postmortem NAc tissue from schizophrenic patients (Sumiyoshi et al 1995; Yang et al 1998). Notably, decreases in inhibitory G proteins in the NAc have been found to produce escalation in cocaine self-administration in rats (Self et al 1994) and would expectedly produce a similar escalation of drug use in schizophrenic patients.
The primary neuropathology in schizophrenia remains unknown, but current theories are focused on neurodevelopmental abnormalities in cortical and temporal-limbic structures, particularly the hippocampal formation and the PfC (Bunney and Bunney 2000; Selemon and Goldman-Rakic 1999; Weinberger et al 1994). The hippocampus develops over a longer period than any other forebrain structure, and its cell migration paths are long, complex, and particularly vulnerable to genetic or environmental disturbances during the second trimester of gestation (Scheibel and Conrad 1993). Postmortem tissue from schizophrenic patients shows a disruption in the laminar distribution of cells in the CA1-CA3 subfields of the hippocampus (Scheibel and Conrad 1993). In addition, hippocampal neurons have decreased soma size in schizophrenic patients (Arnold et al 1995). Histopathologic abnormalities are corroborated by neuroimaging showing reductions in hippocampal-amygdaloid volume, concomitant with increased lateral ventricular volume, and proportional to severity of symptoms (Bogarts et al 1993). Importantly, these abnormalities are localized to hippocampal regions that are thought to normally provide excitatory input to the PfC and frontal subcortical structures. The resultant dysfunctional circuitry may contribute to a decrement in frontal cortical functioning and effectiveness during executive tasking, as well as a relative hyperactivity of DA function in the NAc correlating with the onset of psychosis (Weinberger and Lipska 1995). Thus, clinical manifestation of these hippocampal abnormalities are thought to become evident after frontal cortical and associated subcortical structures undergo final maturation during and after adolescence (Goldman-Rakic 1994). The validity of this neurodevelopmental theory is supported in rat studies, where postnatal ventral hippocampal damage produces hypersensitivity to amphetamine-induced locomotion but emergent only after adolescence (Lipska et al 1993).
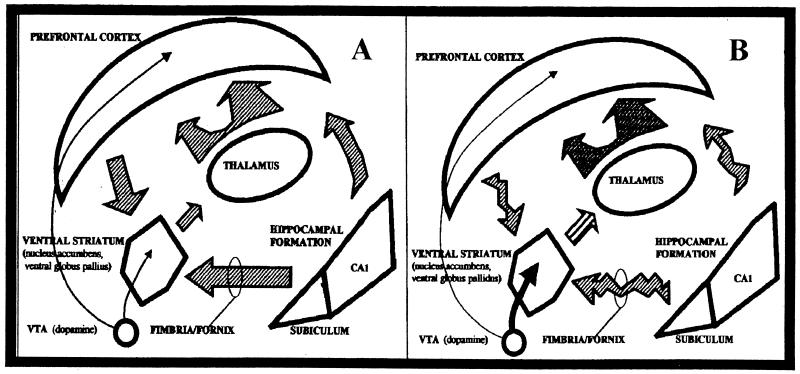
Figure 2 illustrates how developmental abnormalities in hippocampal and PfC projections to the NAc may contribute to an overall functional hyperresponsiveness to mesoaccumbens DA release by reducing cortical and hippocampal regulation over DA-mediated responses in schizophrenia (Weinberger and Lipska 1995). This disinhibition of subcortical DA systems would parallel theories of reduced cortico-striatal tone associated with drug addiction (Jentsch and Taylor 1999; Volkow and Fowler 2000).
Figure 2.
(A) The neural network implicated in reward, drug addiction, and schizophrenia. Excitatory afferents to the nucleus accumbens (NAc) from the hippocampal formation relay contextual information relevant to reward and motivation. Excitatory afferents from the prefrontal cortex (PfC) relay command and inhibitory information to the NAc relevant to regulation of thought and motivation. In the NAc, glutamatergic afferents from hippocampal formation and the PfC interact with dopaminergic afferents from the ventral tegmental area (VTA) to regulate motivational processes. (B) In both schizophrenia and drug addiction, a functional disorganization of afferent excitatory communication to the NAc contributes to relative hyperresponsivity to dopaminergic input from the VTA, increasing motivational salience of drugs and drug-related stimuli. In schizophrenia, dysregulation of hippocampal outputs may disrupt PfC communication to the NAc, weakening executive-inhibitory influence on motivational processes by reducing the impact of PfC inputs (gating), reducing excitatory drive of PfC-NAc projections, or both. Dopamine, black arrow; glutamate, striped arrow.
Abnormal input from cortical and hippocampal sources may cause identifiable changes in markers of DA signaling in the ventral striatum. For instance, amphetamine challenge in schizophrenic patients produces abnormally elevated DA release as marked by heightened displacement of radiotracer from D2 receptors in the striatum (Abi-Dargham et al 1998). Attempts to identify postsynaptic alterations consistent with a functional DA supersensitivity, have been conducted by measurement of striatal D2 receptor densities in living or postmortem brains of schizophrenic patients. A series of these studies variously shows increases, decreases, or no changes, perhaps as a result of variability in patient subgroups, including large differences in neuroleptic exposure (Farde 1997; Haney et al 1998, 1999; Joyce et al 1988; Laruelle 1998; Nordstrom 1995; Martinot et al 1994). The possibility that schizophrenia is associated with changes in striatal D2 receptor density is notable in light of human and animal studies suggesting that D2 receptors are primarily involved in drug craving (DeVries et al 1999; Self et al 1996; Wise 1990). In this vein, Seeman has described evidence that the ratio of D2 receptor densities to D1 receptors is increased in the striatum of drug-naive schizophrenic patients (Seeman et al 1989) and has shown that deficits in the normal coupling of intracellular elements common to D1 and D2 receptors (Seeman and Nitznik 1990). Because D1 receptors are implicated in opposing the drug-seeking effects of D2 receptor agonists (Self et al 1996), an increase in the D2/D1 ratio or disruption in D2-D1 coupling may be associated with increased vulnerability to drug addiction.
A Neurophysiologic Basis for Vulnerability to Addiction in Schizophrenia: In Vivo Studies of the Role of the Hippocampal Formation in Modulating Nucleus Accumbens-Mediated Responses
Anatomy and Neurochemistry of Hippocampal-NAc Connections
The ventral-proximal subiculum (a major output structure of the hippocampal formation) and the CA1 region of the hippocampus send direct projections to the NAc in a topographically organized manner (Groenewegen et al 1987; Kelley and Domesick 1982; Naber and Witter 1998). These hippocampal-subicular axons pass through the fimbria-fornix and diffusely innervate the entire anterior-posterior extent of the NAc (Kelley and Domesick 1982). Hippocampal and VTA DA axons form synapses in close proximity on medium spiny neurons of the NAc, suggesting that physiologic interactions occur at the level of individual dendrites (Sesack and Pickel 1990; Totterdell and Smith 1989).
Hippocampal inputs to NAc release the neurotransmitter glutamate, which acts on postsynaptic NAc neurons, but hippocampal activity also can modulate DA release in the NAc through both “direct” and “indirect” pathways. The direct pathway involves local modulation of DA terminals by hippocampal-subicular afferents to the NAc (Blaha et al 1997). The indirect pathway involves subicular outputs to other brain regions that excite VTA DA neurons and subsequently stimulate DA release in the NAc (Legault et al 2000; Legault and Wise 1999). This latter pathway may involve hippocampal-subicular projections to the PfC or ventral pallidal neurons, which in turn project to the VTA (Legault et al 2000; Todd and Grace 1999). In any event, hippocampal modulation of NAc neurons apparently involves convergent modulation of both gluta-matergic and dopaminergic signaling.
Integration of DA and Glutamatergic Signals in NAc Neurons
Nucleus accumbens neurons are excited by direct mono-synaptic projections from several distal brain regions, including the PfC, amygdala, thalamus, entorhinal cortex, and the subiculum-CA1 region of hippocampal formation (Finch 1996; Lopes da Silva et al 1984; O’Donnell and Grace 1995; Pennartz et al 1994). Excitatory inputs from up to five of these distal regions may converge on a single NAc neuron, resulting in unique spatial-temporal patterns of activity (Finch 1996). For example, concurrent stimulation of the PfC and hippocampal formation produces additive responses in a subset of NAc cells, whereas other unique responses are produced by concurrent stimulation of amygdalar and hippocampal inputs (Finch 1996). Pennartz et al (1994) proposed that specific ensembles of neurons in the NAc perform computations that represent various motivational states. These ensembles receive distinct combinations of convergent information from the hippocampus, PfC, amygdala, and VTA; altered firing patterns in these ensembles are thought to determine behavioral output relating to premotor planning or motivation state (Pennartz et al 1994). Building on this framework, O’Donnell et al (1999) proposed that hippocampal afferents convey spatial information, whereas amygdalar afferents convey emotional information, both of which ultimately interact to gate activity in ventral-striatal-thalamo-prefrontal-cortical circuits. These ventral-striatal-thalamo-prefrontal-cortical circuits are hypothesized to regulate ordered thought and motivational state analogous to the dorsal striatal system, where striatal-thalalmo-cortical circuits are thought to direct ordered movement (Cummings 1993; Groenewegen et al 1997).
The hippocampal inputs to the NAc are of particular importance because they function by gating inputs from other brain regions such as amygdala and PFC (O’Donnell and Grace 1995). For example, NAc neurons display three general patterns of baseline activity: 1) silent, 2) spontaneously firing at low constant rates, or 3) one of two bistable membrane potentials, one of which exhibits a high rate of spontaneous firing (O’Donnell and Grace 1995). Activation of hippocampal afferents— but not cortical, amygdaloid, or thalamic afferents—induces bistable cells to move to the depolarized or active state. Stimulation of PfC inputs to the NAc fails to alter firing of NAc neurons unless they are facilitated by intact hippocampal input, indicating that hippocampal input gates information flow through cortico-ventral-striatal projections (O’Donnell et al 1999; O’Donnell and Grace 1995). Other work suggests that hippocampal input also influences neuroplasticity in NAc neurons. For example, tetanizing stimulation of the fimbria-fornix pathway results in long-term potentiation in NAc neurons but causes long-term depression in NAc neurons receiving concurrent input from the basolateral amygdala (Mulder et al 1998). Therefore, developmental neuropathology in hippocampal function in schizophrenia would produce profound disruption of these gating and neuroplastic processes.
Dopamine generally acts to dampen the excitatory response to cortical and hippocampal afferents in NAc neurons (O’Donnell and Grace 1994; O’Donnell and Grace 1996; O’Donnell et al 1999; Yang and Mogenson 1984). Importantly, this effect would allow fewer bistable NAc neurons to enter the depolarized or active state, thereby reducing the influence of PfC afferents (O’Donnell et al 1999). In the PfC, phasic DA release also can dampen PfC output to the NAc by rendering PfC neurons less responsive to excitatory stimulation from the hippocampal formation (Jay et al 1995). Therefore, a DA flux can act via at least three different mechanisms to take NAc neurons “off-line” from prefrontal executive modulation: 1) reducing activity in PfC-NAc afferents, 2) reducing NAc and PfC responsiviness to hippocampal input, and 3) reducing NAc responsiveness to PfC input. Together these DA effects could reduce inhibitory control over reward-seeking and other DA-mediated behaviors. This idea is supported by a vast neuropsychiatric literaturethat describes behavioral disinhibition following frontal lobe injuries in humans (Cummings 1993). A loss of inhibitory control over DA-mediated behaviors may in turn contribute to both schizophrenia and addictive symptomatology. Interestingly, the dampening effect of DA on cortical and hippocampal inputs to the NAc is mediated primarily by D2 receptors, which are implicated both in exacerbation of psychotic symptoms and in compulsive drug-seeking behavior associated with hyperactivaton of the DA system (DeVries et al 1999; O’Donnell and Grace 1994; Self et al 1996; Sunahara et al 1993).
The Hippocampus Modulates NAc-Mediated Behaviors Associated with Schizophrenia and Substance Abuse
Pharmacologic or surgical compromise of hippocampal function can produce animal behavior consistent with a relative “hyperdopaminergic state” in mesolimbic DA pathways that mimics certain features of the schizophrenia syndrome (Lieberman et al 1990; Lillrank et al 1995; Lipska et al 1992; Reinstein et al 1982; Schaub et al 1997; Whishaw and Mittleman 1991; Wilkinson et al 1993). Notably, lesions of the hippocampus in animals can produce hypersensitivity to dopaminergic stimulation that is greater than that produced by lesions of the PfC or the amygdala, consistent with the hierarchical dominance of hippocampal over PfC and amygdalar inputs to the NAc (Schaub et al 1997). Moreover, the impact on DA-mediated behavior corresponds to the extent of the lesion and is greater with comprehensive lesions of multiple hippocampal substructures (Mittleman et al 1998).
Adult rats with lesions of the hippocampal formation also exhibit greater NAc DA release when exposed to conditioned stimuli associated with foot-shock stress, consistent with a hyperdopaminergic state (Saul’skaya and Gorbachevskaya 1998); however, neonatal hippocampal lesions produce hyperlocomotion to stress and amphetamine, without altering stress- and amphetamine-induced DA release in the NAc (Brake et al 1999; Lillrank et al 1999). This latter effect may represent an acquired postsynaptic or neural network hypersensitivity to NAc DA responses, resulting from disruption of hippocampalcortical inputs during postnatal maturation (Brake et al 1999; Lillrank et al 1999). Neurodevelopmental hippocampal abnormalities underlying schizophrenia could therefore sensitize DA responses at the level of NAc neuronal ensembles, which may mimic sensitization in DA systems that occurs with repeated drug exposure, increasing vulnerability to drug addiction (Jentsch and Taylor 1999; Self and Nestler 1998; White and Kalivas 1998). Interestingly, acute activation of the CA1 region of the hippocampus and subiculum also increases locomotor behavior and DA release in the NAc, possibly via hippocampal regulation of VTA cell firing as discussed earlier (Brudzynski and Gibson 1997; Legault et al 2000; Ma et al 1996). Together these results suggest that although acute activation of the intact hippocampus can produce phasic DA release in the NAc, permanent hippocampal lesions also increase the relative effects of dopaminergic activity on NAc neuronal ensembles. Also, the degree to which this relative dopaminergic effect may be detected as changes in markers of DA function may depend on the developmental stage at the time of the hippocampal insult.
An important behavioral parameter associated with DA system functioning in humans and animals and with cognitive gating deficits in schizophrenia is the disruption of latent inhibition (LI). Normal LI refers to the ability of a stimulus preexposure, before Pavlovian conditioning, to weaken the subsequent association of the stimulus with salient events. Persons with acute, unmedicated psychosis or normal subjects administered dopaminergic psychostimulants both show a reduced capacity for LI (Baruch et al 1988; Gray et al 1992). Loss of LI in schizophrenia has been interpreted as “an inability to screen out irrelevant stimuli” or a “disruption in the normal ability to use past contextual regularities as a guide to current information processing” (Gray et al 1995; Weiner 1990). Notably, patients with schizophrenia treated with typical neuroleptics show a restoration of LI (Baruch et al 1988). Similarly, animals with lesions to the greater hippocampal formation show loss of some forms of LI that is restored with haloperidol administration, suggesting hippocampal involvement in these forms of inhibitory control over behavior (Schmajuk et al 2000). Furthermore, the disruption of LI produced by hippocampal lesions is attributed to hyperactivity in the meso-NAc DA system, suggesting that certain features of schizophrenia may involve a similar mechanism (Gray et al 1995).
Abnormalities in LI associated with hyperdopaminergic states may also produce abnormalities in reward-related learning processes. Dopamine neurons normally fire in response to novel rewards and diminish firing as rewards become more predictable (Hollerman and Schultz 1998). This transition could be disrupted by a hyperdopaminergic state produced by dysfunctional cortical-hippocampal input to NAc and by drug-induced DA release, leading to continuous neural representation of rewards as novel. Thus, in addition to cognitive gating deficits in LI, reward-related learning may be altered by hippocampal dysfunction. Furthermore, lesions of ventral hippocampus in adult rats increase resistance to extinction from food reinforced behaviors and reduce avoidance of rewards paired with conditioned fear (Clark et al 1992). These data suggest that the hippocampus mediates control over behavior associated with inhibitory learning (extinction and conditioned avoidance). In addition, hippocampal lesions decrease food neophobia and increase the reinforcing efficacy of sucrose or electrical stimulation of reward pathways (Burns et al 1996; Kelley and Mittleman 1999; Schmelzeis and Mittleman 1996). Together these behavioral findings suggest that hippocampal dysregulation in schizophrenia could enhance the reinforcing efficacy produced by natural and drug rewards by interfering with normal inhibitory control over compulsive reward-seeking behavior.
Summary
This article reviews evidence that developmental neuropathology in hippocampal and PfC pathways contributes both to symptoms of schizophrenia and to vulnerability to addictive behavior via dysfunctional interactions with the NAc. This hypothesis posits that substance disorder comorbidity represents an independent primary disease symptom in schizophrenia, rather than a form of self-medication secondary to classic schizophrenia symptoms or medication side effects.
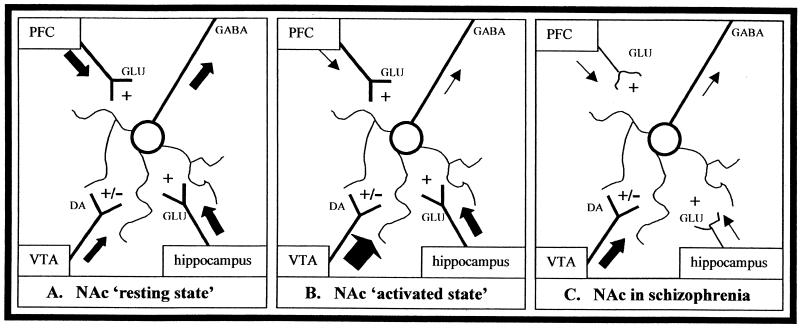
The primary addiction hypothesis is based on substantial data that directly relates deficits in hippocampal-cortical function with neurochemical, neurophysiologic, and neurobehavioral alterations consistent with a facilitation of both schizophrenia and addictive behavior. Figure 3A illustrates this hypothesis by showing that hippocampal and PfC outputs normally exert inhibitory control over DA-mediated behavior via their interaction with NAc neurons. During this “resting state,” homeostasis between hippocampal-cortical inputs and low basal dopaminergic tone contribute to stability in neuronal ensembles and suppression of uncontrolled goal-directed behavior in favor of executive premotor planning. The behaviorally “activated state” is produced by phasic DA surges in response to novelty, natural or drug rewards, stressful situations, or exposure to salient cues associated with rewards (Figure 3B). These DA surges act in the PfC to dampen afferents to the NAc and in the NAc to counteract the glutamatergic influence of hippocampal and PfC inputs. The vast majority of NAc output neurons (many of which receive hippocampal and PfC input) send descending GABAergic projections to the ventral globus pallidus and other brain-stem structures involved in thalamocortical loops that regulate thought, premotor planning, motivation, and movement (Mugnaini and Ortel 1985; Yang and Mogensen 1985). Dopaminergic surges in the NAc could diminish NAc GABAergic outflow to these structures, serving to translate motivation into behavioral action (Lavin and Grace 1994). Thus, DA surge would shift NAc function toward activation of motivated behavior, either in the form of appetitive (approach) or aversive (withdrawal) responses. In schizophrenia (Figure 3C), developmental alterations in hippocampal and PfC ultra-structure impair the ability of hippocampal projections to provide excitatory drive of PfC-NAc projections and provide effective facilitation of PfC inputs to the NAc, resulting in functional hyperresponsiveness to basal and drug-stimulated DA release. Thus, the patient with schizophrenia is particularly sensitive to the reward-activated, relative hyperdopaminergic state induced by addictive drugs in the NAc, by virtue of reduced inhibitory control over DA-mediated behavior. Such cortical-hippocampal dysfunction ultimately could lead to perseveration of drug-seeking and a propensity for relapse, as well as classic schizophrenic symptomatology.
Figure 3.
(A) Nucleus accumbens (NAc): “resting state.” Ensembles of GABAergic neurons are regulated by a balance of glutamatergic afferents from prefrontal cortex (PfC) and other limbic regions and by dopaminergic afferents from the ventral tegmental area (VTA). Hippocampal input to the NAc facilitates PfC glutamatergic modulation of NAc neurons while also provoking low basal dopamine (DA) release to maintain stability of neuronal ensembles. This balance provides ongoing inhibitory tone of GABAergic efferents on striatal output structures. In this state, behavioral inhibition is maintained. (B) Nucleus accumbens: “activated state.” During presentation of novel, rewarding, or stressful stimuli or exposure to addictive drugs, DA surges in the NAc and PfC, dampens PfC influence on NAc neurons. This DA surge also attenuates hippocampal input, further diminishing its ability to facilitate PfC modulation of NAc neurons. Attenuation of PfC tone, coupled with DA surges in the NAc, causes changes in NAc GABAergic outflow, altering neuronal activation of ventral pallidal and thalamic nuclei. In this state, reward-seeking behavior is disinhibited. (C) In schizophrenia, abnormal hippocampal afferents to the NAc and PfC and hyperfunctioning of DA signals result in a relatively inflexible motivation system due to a failure of executive PfC control over NAc neurons. This “relative” hyperfunctioning of DA input is associated with a baseline that operates closer to the “activated state” (Figure 3B) and is less able to enter a “resting state” (Figure 3A). Such dysregulation may create a lower threshold for DA releasing stimuli to recruit motor output programs, and drug reward-seeking behavior is disinhibited.
This hypothesis integrates both clinical and basic neurobiological data and offers a comprehensive heuristic framework to direct future investigations on substance abuse comorbidity in schizophrenia. Its major clinical implications include the need to introduce substance abuse education and other forms of prevention early in the course of treatment. Furthermore, this hypothesis also may suggest an explanation for dual diagnosis cases in other psychiatric disorders thought to involve hippocampal dysfunction, including posttraumatic stress disorder, border-line personality disorder, and major affective disorders (Chambers et al 1999). Further studies are needed to determine whether animal models of hippocampal dysfunction in schizophrenia can produce certain core features of the addicted phenotype, such as escalating drug intake and a propensity for relapse. These studies potentially could identify both convergent and divergent mechanisms for comorbidity between addictive behavior and schizophrenia. A better understanding of these mechanisms will inevitably provide the basis for better treatment strategies for motivational disturbances that are pervasively present in the mentally ill.
Acknowledgments
Support for this work was provided by National Research Service Award, Department of Health and Human Services of the Public Health Service, Veterans Administration Special Neuroscience Research Fellowship Training Grant, Department of Veteran’s Affairs and Alcohol Research Center, Schizophrenia Biological Research Center, and National Center for PTSD (NIAA Grant No. KOZAA00261).
References
- Abi-Dargham A, Gil R, Krystal J, et al. Increased striatal dopamine transmission in schizophrenia: Confirmation in a second cohort. Am J Psychiatry. 1998;155:761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- Addington J, Duchak V. Reasons for substance use in schizophrenia. Acta Psychiatr Scand. 1997;96:329–333. doi: 10.1111/j.1600-0447.1997.tb09925.x. [DOI] [PubMed] [Google Scholar]
- Agarwal MR, Sharma VK, Kishore Kumar KV, Lowe D. Non-compliance with treatment in patients suffering from schizophrenia: A study to evaluate possible contributing factors. Int J Soc Psychiatry. 1998;44:92–106. doi: 10.1177/002076409804400202. [DOI] [PubMed] [Google Scholar]
- Aizengerg D, Zemishlany Z, Dorfman-Etrog P, Weizman A. Sexual dysfunction in male schizophrenic patients. J Clin Psychiatry. 1995;56:137–141. [PubMed] [Google Scholar]
- Arnold SE, Franz BR, Gur RC, et al. Smaller neuron size in hippocampal subfields that mediate cortical-hippocampal interactions. Am J Psychiatry. 1995;152:738–748. doi: 10.1176/ajp.152.5.738. [DOI] [PubMed] [Google Scholar]
- Baigent M, Holme G, Hafner RJ. Self-reports of the interaction between substance abuse and schizophrenia. Aust N Z J Psychiatry. 1995;29:69–74. doi: 10.3109/00048679509075894. [DOI] [PubMed] [Google Scholar]
- Baruch I, Hemsley DR, Gray JA. Differential performance of acute and chronic schizophrenics in a latent inhibition task. J Nerv Ment Dis. 1988;176:598–606. doi: 10.1097/00005053-198810000-00004. [DOI] [PubMed] [Google Scholar]
- Berti A. Schizophrenia and substance abuse: The interface. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:279–284. doi: 10.1016/0278-5846(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci. 1997;9:902–911. doi: 10.1111/j.1460-9568.1997.tb01441.x. [DOI] [PubMed] [Google Scholar]
- Bogarts B, Lieberman JA, Ashtari M, et al. Hippocampus-amygdala volumes and psychopathology in schizophrenia. Biol Psychiatry. 1993;33:236–246. doi: 10.1016/0006-3223(93)90289-p. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Involvement of the ventral tegmental dopamine system in opioid and psychomotor stimulant reinforcement [Harris L, editor] NIDA Res Monogr. 1986;67:190–196. [PubMed] [Google Scholar]
- Brake WG, Sullivan RM, Flores G, Srivastava LK, Gratton A. Neonatal ventral hippocampal lesions attenuate the nucleus accumbens dopamine response to stress: An electrochemical study on the adult rat. Brain Res. 1999;831:25–32. doi: 10.1016/s0006-8993(99)01477-8. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Gibson CJ. Release of dopamine in the nucleus accumbens caused by stimulation of the subiculum in freely moving rats. Brain Res Bull. 1997;42:303–308. doi: 10.1016/s0361-9230(96)00290-0. [DOI] [PubMed] [Google Scholar]
- Buckley PF. Substance abuse in schizophrenia: A review. J Clin Psychiatry. 1998;59:26–30. [PubMed] [Google Scholar]
- Bunney WE, Bunney BG. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res Brain Res Rev. 2000;31:138–146. doi: 10.1016/s0165-0173(99)00031-4. [DOI] [PubMed] [Google Scholar]
- Burns LH, Annett L, Everett BJ, Robbins TW, Kelley AE. Effects of lesions to amygdala, ventral subiculum, medial prefrontal cortex, and nucleus accumbens on the reaction to novelty: Implication for limbic-striatal interactions. Behav Neurosci. 1996;110:60–73. doi: 10.1037//0735-7044.110.1.60. [DOI] [PubMed] [Google Scholar]
- Carlezon WAJ, Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology. 1995;122:194–197. doi: 10.1007/BF02246095. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Bremner JD, Moghaddam B, Southwick SM, Charney DS, Krystal JH. Glutamate and post-traumatic stress disorder: Toward a psychobiology of dissociation. Sem Clin Neuropsychiatry. 1999;4:274–281. doi: 10.153/SCNP00400274. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Potenza MN. Schizophrenia and pathological gambling (letter) Am J Psychiatry. 2001;158:497–498. doi: 10.1176/appi.ajp.158.3.497-a. [DOI] [PubMed] [Google Scholar]
- Chen J, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta-9-tetrahydrocannabinol produces naloxone blockable enhancement of presynaptic dopamine eflux in nucleus accumbens of conscious, freely-moving rats as measured by intracranial microdialysis. Psychopharmacology. 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Clark AJM, Feldon J, Rawlins JNP. Aspiration lesions of rat ventral hippocampus disinhibit responding in conditioned suppression or extinction, but spare latent inhibition and the partial reinforcement extinction effect. Neuroscience. 1992;48:821–829. doi: 10.1016/0306-4522(92)90270-c. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Deakin JFW, Longden A. The nucleus accumbens—possible site of action of antipsychotic action of neuroleptic drugs? Physiolog Med. 1977;7:213–221. doi: 10.1017/s0033291700029287. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Bardgett ME. Limbic-cortical neuronal damage and the pathophysiology of schizophrenia. Schizophr Bull. 1998;24:231–248. doi: 10.1093/oxfordjournals.schbul.a033323. [DOI] [PubMed] [Google Scholar]
- Cuffel BJ. Prevalence estimates of substance abuse in schizophrenia and their correlates. J Nerv Ment Dis. 1992;180:589–592. doi: 10.1097/00005053-199209000-00008. [DOI] [PubMed] [Google Scholar]
- Cuffel BJ, Heithoff KA, Lawson W. Correlates of patterns of substance abuse among patients with schizophrenia. Hosp Comm Psychiatry. 1993;44:247–251. doi: 10.1176/ps.44.3.247. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: Clinical phenomena and laboratory findings. Am J Psychiatry. 1998;155:1490–1501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine and schizophrenia: A review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- DeQuardo JR, Carpenter CF, Tandon R. Patterns of substance abuse in schizophrenia: Nature and significance. J Psychiatr Res. 1994;28:267–275. doi: 10.1016/0022-3956(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Deutch AY. Prefrontal cortical dopamine systems and the elaboration of functional corticostriatal circuits: Implications for schizophrenia and Parkinson’s disease. J Neural Transm. 1993;91:197–221. doi: 10.1007/BF01245232. [DOI] [PubMed] [Google Scholar]
- DeVries TJ, Schoffelmeer ANM, Binnekade R, Vanderschuren LJMJ. Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal of IV drug self-administration. Psychopharmacology. 1999;143:254–260. doi: 10.1007/s002130050944. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L. Dual diagnosis of substance abuse in schizophrenia: Prevalence and impact on outcomes. Schizophr Res. 1999;35(suppl):S93–S100. doi: 10.1016/s0920-9964(98)00161-3. [DOI] [PubMed] [Google Scholar]
- Dixon L, Haas G, Weidon PJ, Sweeney J, Frances AJ. Drug use in schizophrenic patients: Clinical correlates and reasons for use. Am J Psychiatry. 1991;148:224–230. doi: 10.1176/ajp.148.2.224. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Goeders NE, Smith JE. The reinforcing and rate effects of intracranial dopamine administration [Harris L, editor] NIDA Res Monogr. 1986;67:242–248. [PubMed] [Google Scholar]
- Farde L. Brain imaging of schizophrenia—the dopamine hypothesis. Schizophr Res. 1997;28:157–162. doi: 10.1016/s0920-9964(97)00121-7. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, Phillips AG, Brown EE. The neurobiology of cocaine-induced reinforcement. Ciba Found Symp. 1992;166:96–124. doi: 10.1002/9780470514245.ch7. [DOI] [PubMed] [Google Scholar]
- Finch DM. Neurophysiology of converging synaptic inputs from the rat prefrontal cortex, amygdala, midline thalamus, and hippocampal formation onto single neurons of the caudat/putamen and nucleus accumbens. Hippocampus. 1996;6:495–512. doi: 10.1002/(SICI)1098-1063(1996)6:5<495::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gerding LB, Labbate LA, Meason MO, Santos AB, Arana GW. Alcohol dependence and hospitalization in schizophrenia. Schizophr Res. 1999;38:71–75. doi: 10.1016/s0920-9964(98)00177-7. [DOI] [PubMed] [Google Scholar]
- Giovino GA, Schooley MW, Zhu B, et al. Surveillance for selected tobacco-use behaviors—United States, 1990–1994. Surveillance Summaries/Morbidity and Mortality Weekly Report. 1994;43:1–43. [PubMed] [Google Scholar]
- Glassman AH, Covey LS, Dalack GW, Stetner F. Cigarette smoking, major depression and schizophrenia. Clin Neuropharmacol. 1992;15:560A–561A. doi: 10.1097/00002826-199201001-00291. [DOI] [PubMed] [Google Scholar]
- Glenthoj B, Mogensen J, Laursen H, Holm S, Hemmingsen R. Electrical sensitization of the meso-limbic dopaminergic system in rats: A pathogenic model of schizophrenia. Brian Res. 1993;619:39–54. doi: 10.1016/0006-8993(93)91594-i. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Goldstein M, Deutch AY. Dopaminergic mechanisms in the pathogenesis schizophrenia. FASEB J. 1992;6:2413–2421. [PubMed] [Google Scholar]
- Gray JA, Joseph MH, Hemsley DR, et al. The role of mesolimbic dopaminergic and retrohippocampal afferents to the nucleus accumbens in latent inhibition: Implications for schizophrenia. Behav Brain Res. 1995;71:19–31. doi: 10.1016/0166-4328(95)00154-9. [DOI] [PubMed] [Google Scholar]
- Gray NS, Pickering AD, Hemsley DR, Dawling S, Gray JA. Abolition of latent inhibition by a single 5 mg dose of d-amphetamine in man. Psychopharmacology. 1992;107:425–430. doi: 10.1007/BF02245170. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat: A study using anterograde transport of phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Uylings HBM. The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J Psychopharmacol. 1997;11:99–106. doi: 10.1177/026988119701100202. [DOI] [PubMed] [Google Scholar]
- Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW. Effect of a selective D1 agonist (ABT-431) on smoked cocaine self-administration in humans. Psychopharmacology. 1999;143:102–110. doi: 10.1007/s002130050925. [DOI] [PubMed] [Google Scholar]
- Haney M, Foltin RW, Fischman MW. Effects of pergolide on intravenous cocaine self-administration in men and women. Psychopharmacology. 1998;137:15–24. doi: 10.1007/s002130050588. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Monaco AP, Hernandez L, Aulisi EF, Stanley BG, Lenard L. Self-injection of amphetamine directly into the brain. Psychopharmacology. 1983;81:158–163. doi: 10.1007/BF00429012. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, McGlashan TH. Parallel distributed processing and the emergence of schizophrenic symptoms. Schizophr Bull. 1993;19:119–139. doi: 10.1093/schbul/19.1.119. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nature Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaineinduced cocaine craving. Psychopharmacology. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Jay TM, Glowinski J, Thierry AM. Inhibition of hippocampo-prefrontal cortex excitatory responses by the mesocortical DA system. NeuroReport. 1995;6:1845–1848. doi: 10.1097/00001756-199510020-00006. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Lexow N, Bird E, Winokur A. Organization of dopamine D1 and D2 receptors in human striatum: Receptor autoradiographic studies in Huntington’s disease and schizophrenia. Synapse. 1988;2:546–557. doi: 10.1002/syn.890020511. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: An anterograde and retrograde-horseradish peroxidase study. Neuroscience. 1982;7:2321–2335. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Mittleman G. Effects of hippocampal damage on reward threshold and response rate during self-stimulation of the ventral tegmental area in the rat. Behav Brain Res. 1999;99:133–141. doi: 10.1016/s0166-4328(98)00097-7. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: Anatomy, pharmacology and function of reward pathways. Trend Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Madonick S, Petrakis IL. Toward a rational pharmacotherapy of comorbid substance abuse in schizophrenic patients. Schizophr Res. 1999;35(suppl):S35–S49. doi: 10.1016/s0920-9964(98)00162-5. [DOI] [PubMed] [Google Scholar]
- Lambert M, Haasen C, Mass R, Krausz M. Consumption patterns and motivation for use of addictive drugs in schizophrenic patients. Psychiatr Prax. 1997;24:185–189. [PubMed] [Google Scholar]
- Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q J Nucl Med. 1998;42:211–221. [PubMed] [Google Scholar]
- Lavin A, Grace AA. Modulation of dorsal thalamic cell activity by the venral pallidum: Its role in the regulation of thalamocortical activity by the basal ganglia. Synapse. 1994;18:104–127. doi: 10.1002/syn.890180205. [DOI] [PubMed] [Google Scholar]
- Legault M, Rompre PP, Wise RA. Chemical stimulation of the ventral hippocampus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. J Neurosci. 2000;20:1635–1642. doi: 10.1523/JNEUROSCI.20-04-01635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault M, Wise RA. Injections of N-Methyl-D-Aspartate into the ventral hippocampus increase extracellular dopamine in the ventral tegmental area and nucleus accumbens. Synapse. 1999;31:241–249. doi: 10.1002/(SICI)1098-2396(19990315)31:4<241::AID-SYN1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Kinon BJ, Loebel AD. Dopaminergic mechanisms in idiopathic and drug- induced psychosis. Schizophr Bull. 1990;16:97–110. doi: 10.1093/schbul/16.1.97. [DOI] [PubMed] [Google Scholar]
- Lillrank SM, Lipska BK, Kolachana BS. Attenuated extracellular dopamine levels after stress and amphetamine in the nucleus accumbens of rats with neonatalventral hippocampal damage. J Neural Transm. 1999;106:183–196. doi: 10.1007/s007020050150. [DOI] [PubMed] [Google Scholar]
- Lillrank SM, Lipska BK, Weinberger DR. Neurodevelopmental animal models of schizophrenia. Clin Neurosci. 1995;3:98–104. [PubMed] [Google Scholar]
- Ling W, Compton P, Rawson R, Wesson DR. Neuropsychiatry of alcohol and drug abuse. In: Fogel B, Schiffer R, Rao S, editors. Neuropsychiatry. Williams and Wilkins; Baltimore: 1996. pp. 679–721. [Google Scholar]
- Lipska BK, Jaskiw GE, Chrapusta S, Karoum F, Weinberger DR. Ibotenic acid lesion of the ventral hippocampus differentially affects dopamine and its metabolites in the nucleus accumbens and prefrontal cortex in the rat. Brain Res. 1992;585:1–6. doi: 10.1016/0006-8993(92)91184-g. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: A potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva FH, Arnolds DEAT, Neijt HC. A functional link between the limbic cortex and ventral striatum: Physiology of the subiculum accumbens pathway. Exp Brain Res. 1984;55:205–214. doi: 10.1007/BF00237271. [DOI] [PubMed] [Google Scholar]
- Luchins DJ. Repetitive behaviors in chronically institutionalized schizophrenic patients. Schizophr Res. 1992;8:119–123. doi: 10.1016/0920-9964(92)90027-3. [DOI] [PubMed] [Google Scholar]
- Ma J, Brudzynski SM, Leung LS. Involvement of the nucleus accumbens-ventral pallidal pathway in postictal behavior induced by a hippocampal after discharge in rats. Brain Res. 1996;739:26–35. doi: 10.1016/s0006-8993(96)00793-7. [DOI] [PubMed] [Google Scholar]
- March JS. Cognitive-behavioral psychotherapy. In: Sadock BJ, Sadock VA, editors. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry. 7th ed II. Lippincott Williams & Wilkins; New York: 1999. p. 2810. [Google Scholar]
- Martinot JL, Paillere-Martinot ML, Loc’h C, et al. Central D2 receptors and negative symptoms of schizophrenia. Br J Psychiatry. 1994;164:27–34. doi: 10.1192/bjp.164.1.27. [DOI] [PubMed] [Google Scholar]
- Masterson E, O’Shea B. Smoking and malignancy in schizophrenia. Br J Psychiatry. 1984;145:429–432. doi: 10.1192/bjp.145.4.429. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Brown S. Smoking in first-episode patients with schizophrenia. Am J Psychiatry. 1999;156:1120–1121. doi: 10.1176/ajp.156.7.1120a. [DOI] [PubMed] [Google Scholar]
- McGregor A, Baker G, Roberts DC. Effects of 6-hydroxydopamine lesions of the medial prefrontal cortex on intravenous cocaine self-administration under a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 1996;53:5–9. doi: 10.1016/0091-3057(95)00192-1. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Bratt AM, Chase R. Heterogeneity of the hippocampus: Effects of subfield lesions on locomotion elicited by dopaminergic agonists. Behav Brain Res. 1998;92:31–45. doi: 10.1016/s0166-4328(97)00124-1. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Yarnold PR, Levinson DF, et al. Prevalence of substance abuse in schizophrenia: Demographics and clinical correlates. Schizophr Bull. 1990;16:31–54. doi: 10.1093/schbul/16.1.31. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Ortel WH. An atlas of the distribution of GABA-ergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. Vol. 4. Elsevier; Amsterdam: 1985. [Google Scholar]
- Mulder AB, Hodenpijl MG, Lopez de, Silva FH. Electrophysiology of the hippocampal and amygdaloid projections to the nucleus accumbens of the rat: Convergence, segregation, and interactions of inputs. J Neurosci. 1998;18:5095–5102. doi: 10.1523/JNEUROSCI.18-13-05095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber PA, Witter MP. Subicular efferents are organized mostly as parallel projections: A double-labeling, retrogradetracing study in the rat. J Comp Neurol. 1998;393:284–297. [PubMed] [Google Scholar]
- Nordstrom AL, Farde L, Eriksson L, Halldin C. No elevated DZ dopamine receptors in neuroleptic-naive schizophrenic patients revealed by positron emission tomography and [nc] N-methylspiperone. Psychiatry Res. 1995;61:67–83. doi: 10.1016/0925-4927(95)02732-d. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Tonic D2-mediated attenuation of cortical excitation in nucleus accumbens neurons recorded in vitro. Brain Res. 1994;634:105–112. doi: 10.1016/0006-8993(94)90263-1. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Dopaminergic reduction of excitability in nucleus accumbens neurons recorded in vitro. Neuropsychopharmacology. 1996;15:87–97. doi: 10.1016/0893-133X(95)00177-F. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Greene J, Pabello N, Lewis BL, Grace AA. Modulation of cell firing in the nucleus accumbens. Ann N Y Acad Sci. 1999;877:157–175. doi: 10.1111/j.1749-6632.1999.tb09267.x. [DOI] [PubMed] [Google Scholar]
- O’Donnell PO, Grace AA. Synaptic interactions among excitatory afferents to the nucleus accumbens neurons: Hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Farrell TJ, Conners GJ, Upper D. Addictive behaviors among hospitalized psychiatric patients. Addict Behav. 1983;8:329–333. doi: 10.1016/0306-4603(83)90032-1. [DOI] [PubMed] [Google Scholar]
- Pennartz CMA, Groenewegen HJ, Lopez da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: An integration of behavioral, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Moal M, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991;567:169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Pohl R, Yergani VK, Balon R, Lycaki H, McBride R. Smoking in patients with panic disorder. Psychiatry Res. 1992;43:253–262. doi: 10.1016/0165-1781(92)90058-b. [DOI] [PubMed] [Google Scholar]
- Reigier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drugs of abuse. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Reinstein DK, Hannigan JH, Isaacson RL. Time course of certain behavioral changes after hippocampal damage and their alteration by dopaminergic intervention into nucleus accumbens. Pharmacol Biochem Behav. 1982;17:193–202. doi: 10.1016/0091-3057(82)90069-7. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Smith AM, Roberts DC. A single injection of either flupenthixol decanoate or haloperidol decanoate produces long-term changes in cocaine self-administration in rats. Drug Alcohol Depend. 1994;36:23–25. doi: 10.1016/0376-8716(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Vickers G. The effects of haloperidol on cocaine self-administration is augmented with repeated administrations. Psychopharmacology. 1987;93:526–528. doi: 10.1007/BF00207247. [DOI] [PubMed] [Google Scholar]
- Sandyk R, Awerbuch GI. Late onset schizophrenia: Relationship to awareness of abnormal involuntary movements and tobacco addiction. Int J Neurosci. 1993;71:9–19. doi: 10.3109/00207459309000587. [DOI] [PubMed] [Google Scholar]
- Saul’skaya NB, Gorbachevskaya AI. Conditioned reflex release of dopamine in the nucleus accumbens after disruption of the hippocampal formation in rats. Neurosci Behav Physiol. 1998;28:380–385. doi: 10.1007/BF02464791. [DOI] [PubMed] [Google Scholar]
- Schaub CL, Schnelzeis MC, Mittleman G. The effects of limbic lesions on locomotion and stereotypy elicited by dopamine agonists in the rat. Behav Brain Res. 1997;84:129–143. doi: 10.1016/s0166-4328(96)00142-8. [DOI] [PubMed] [Google Scholar]
- Scheibel AB, Conrad AS. Hippocampal dysgenesis and schizophrenic man: Is there a relationship? Schizophr Bull. 1993;19:21–33. doi: 10.1093/schbul/19.1.21. [DOI] [PubMed] [Google Scholar]
- Schenk S, Horger BA, Peltier R, Shelton K. Supersensitivity to the reinforcing effects of cocaine following 6-hydroxydopamine lesions to the medial prefrontal cortex in rats. Brain Res. 1991;543:227–235. doi: 10.1016/0006-8993(91)90032-q. [DOI] [PubMed] [Google Scholar]
- Schmajuk NA, Christiansen B, Cox L. Haloperidol reinstates latent inhibition impaired by hippocampal lesions: Data and theory. Behav Neurosci. 2000;114:659–670. doi: 10.1037//0735-7044.114.4.659. [DOI] [PubMed] [Google Scholar]
- Schmelzeis MC, Mittleman G. The hippocampus and reward: Effects of hippocampal lesions on progressive-ratio responding. Behav Neurosci. 1996;110:1049–1066. doi: 10.1037//0735-7044.110.5.1049. [DOI] [PubMed] [Google Scholar]
- Schottenfeld R, Carol K, Rounsaville B. Comorbid psychiatric disorders and cocaine abuse. NIDA Res Monogr. 1993;135:31–47. [PubMed] [Google Scholar]
- Seeman P, Nitznik HB. Dopamine receptors and transporters in Parkinson’s disease and schizophrenia. FASEB J. 1990;4:2737–2744. doi: 10.1096/fasebj.4.10.2197154. [DOI] [PubMed] [Google Scholar]
- Seeman P, Niznik HB, Guan HC, Booth G, Ulpian C. Link between D1 and D2 dopamine receptors is reduced in schizophrenia and Huntington diseased brain. Proc Natl Acad Sci U S A. 1989;86:10156–10160. doi: 10.1073/pnas.86.24.10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibyl JP, Satel SL, Anthony D, Southwick SM, Krystal JH, Charney DS. Effects of cocaine on hospital course in schizophrenia. J Nerv Ment Dis. 1993;181:31–37. doi: 10.1097/00005053-199301000-00006. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: A circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Self DW. Neural substrates of drug craving and relapse in drug addiction. Ann Med. 1998;30:379–389. doi: 10.3109/07853899809029938. [DOI] [PubMed] [Google Scholar]
- Self DW, Bernhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: Neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Self DW, Terwilliger RZ, Nestler EJ, Stein L. Inactivation of Gi and Go proteins in nucleus accumbens reduces both cocaine and heroin reinforcement. J Neurosci. 1994;14:6239–6247. doi: 10.1523/JNEUROSCI.14-10-06239.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer JA, Lieberman JA. Schizophrenia and substance abuse. Psychiatr Clin N A. 1993;16:401–412. [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminal converge on spiny neurons and are in apposition to each other. Brain Res. 1990;527:266–279. doi: 10.1016/0006-8993(90)91146-8. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and reexposure to heroin in rats. Psychopharmacology. 1996;125:385–391. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Hertz A. Place preference reveals the involvement of D-1dopamine receptors in the motivational properties of mu and kappa-opioid agonists. Brain Res. 1987;436:169–172. doi: 10.1016/0006-8993(87)91571-x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Skopec M, Rosenberg SD, Tucker GJ. Sexual behavior in schizophrenia. Med Aspects Hum Sex. 1976;10:32–47. [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: The neurobiology of drug- and stress-induced relapse to drug taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Tohen M, Flaum M, Amador X. Substance abuse in psychotic disorders: Associations with affective syndromes. Schizophr Res. 1994;14:73–81. doi: 10.1016/0920-9964(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Stockmeier CA, Overholser JC, Thompson PA, Meltzer HY. Dopamine D4 receptors and effects of guanine nucleotides on (3H) raclopride binding in postmortem caudate nucleus of subjects with schizophrenia or major depression. Brain Res. 1995;681:109–116. doi: 10.1016/0006-8993(95)00301-6. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Seeman P, Van Tol HH, Niznik HB. Dopamine receptors and antipsychotic drug response. Br J Psychiatry. 1993;22:31–38. [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Todd C, Grace AA. Modulation of ventral tegmental area dopamine cell activity by the ventral subiculum and entorhinal cortex. Ann N Y Acad Sci. 1999;877:688–690. doi: 10.1111/j.1749-6632.1999.tb09302.x. [DOI] [PubMed] [Google Scholar]
- Totterdell S, Smith AD. Convergence of hippocampal and dopaminergic input onto identified neurons in the nucleus accumbens of the rat. J Chem Neuroanat. 1989;2:285–298. [PubMed] [Google Scholar]
- Van Ammers EC, Sellman JD, Mulder RT. Temperament and substance abuse in schizophrenia: Is there a relationship? J Nerv Ment Dis. 1997;185:283–288. doi: 10.1097/00005053-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: A neural diathesis-stress model. Psychol Rev. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Aloa MS, Goldberg TE, Berman KF. The frontal lobes and schizophrenia. J Neuropsychiatry. 1994;6:419–427. doi: 10.1176/jnp.6.4.419. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Lipska BK. Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: A search for common ground. Schizophr Res. 1995;16:87–110. doi: 10.1016/0920-9964(95)00013-c. [DOI] [PubMed] [Google Scholar]
- Weiner I. Neural substrates of latent inhibition: The switching model. Psychol Bull. 1990;108:442–461. doi: 10.1037/0033-2909.108.3.442. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Deroche V, Koob G, Weiss F. Effects of dopamine agonists and antagonists on cocaine-induced operant responding for a cocaine-associated stimulus. Psychopharmacology. 1996;126:311–322. doi: 10.1007/BF02247382. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Mittleman G. Hippocampal modulation of nucleus accumbens: Behavioral evidence from amphetamine-induced activity profiles. Behav Neural Biol. 1991;55:289–306. doi: 10.1016/0163-1047(91)90633-2. [DOI] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS, Mittleman G, Torres E, Humbly T, Hall FS, Robbins TW. Enhancement of amphetamine-induced locomotor activity and dopamine release in nucleus accumbens following excitotoxic lesions of the hippocampus. Behav Brain Res. 1993;55:143–150. doi: 10.1016/0166-4328(93)90110-c. [DOI] [PubMed] [Google Scholar]
- Wise RA. The role of reward pathways in the development of drug dependence. In: Balfour D, editor. Psychotropic Drugs of Abuse. Pergamon Press; Oxford, UK: 1990. pp. 23–57. [Google Scholar]
- Yang CQ, Kitamura N, Nishino N, Shirakawa O, Nakai H. Isotype-specific G protein abnormalities in the left superior temporal cortex and limbic structures of patients with chronic schizophrenia. Biol Psychiatry. 1998;43:12–19. doi: 10.1016/s0006-3223(97)80250-8. [DOI] [PubMed] [Google Scholar]
- Yang C, Mogenson GJ. Electrophysiological responses of neurons in the nucleus accumbens to hippocampal stimulation and the attenuation of the excitatory responses by the mesolimbic dopaminergic system. Brain Res. 1984;324:69–84. doi: 10.1016/0006-8993(84)90623-1. [DOI] [PubMed] [Google Scholar]
- Yang CR, Mogensen GJ. An electrophysiological study of the neural projections from the hippocampus to the ventral pallidum and the subpallidal areas by way of the nucleus accumbens. Neuroscience. 1985;15:1015–1024. doi: 10.1016/0306-4522(85)90250-7. [DOI] [PubMed] [Google Scholar]
- Zisook S, Heaton R, Moranville J, Kuck J, Jernigan T, Braff D. Past substance abuse and clinical course of schizophrenia. Am J Psychiatry. 1992;149:552–553. doi: 10.1176/ajp.149.4.552. [DOI] [PubMed] [Google Scholar]