Abstract
Neuropilin-1 (Nrp1) is a cell surface molecule originally identified for its role in neuronal development. Recently, Nrp1 has been implicated in several aspects of immune function including maintenance of the immune synapse and development of regulatory T (Treg) cells. In this study, we provide evidence for a central role of Nrp1 in the regulation of CD4 T-cell immune responses in experimental autoimmune encephalitis (EAE). EAE serves as an animal model for the central nervous system (CNS) inflammatory disorder multiple sclerosis (MS). EAE is mediated primarily by CD4+ T cells that migrate to the CNS and mount an inflammatory attack against myelin components, resulting in CNS pathology. Using a tissue-specific deletion system, we observed that the lack of Nrp1 on CD4+ T cells results in increased EAE severity. These conditional knockout mice exhibit preferential TH-17 lineage commitment and decreased Treg-cell functionality. Conversely, CD4+ T cells expressing Nrp1 suppress effector T-cell proliferation and cytokine production both in vivo and in vitro independent of Treg cells. Nrp1-mediated suppression can be inhibited by TGF-β blockade but not by IL-10 blockade. These results suggest that Nrp1 is essential for proper maintenance of peripheral tolerance and its absence can result in unchecked autoreactive responses, leading to diseases like EAE and potentially MS.
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) characterized by progressive demyelination of the brain and spinal cord (1). MS patients develop paralysis because of immune-mediated axonal damage. MS is generally considered to be an autoimmune disease orchestrated by TH-1 and TH-17 lymphocytes, although various genetic and environmental factors also play a part in disease etiology (2, 3). Evidence for the role of immune cells in MS pathogenesis is provided by studies using the mouse model experimental autoimmune encephalomyelitis (EAE). In EAE, myelin-specific CD4+ T lymphocytes migrate into the CNS and mediate neuronal demyelination and destruction similar to that seen in MS patients (4), leading to loss of motor function and paralysis.
Comparisons between the immune system and the CNS began with the naming of dendritic cells (5). For example, the term immunological synapse describes the junction formed between T cells and antigen-presenting cells (APCs), which resembles the synapse between neurons in both formation and architecture (6). In the nervous system, chemorepulsive factors, such as semaphorins, are required for guiding the formation of neuronal synapses. Several reports have also suggested important roles for semaphorins in the immune system (7, 8). Neuropilin-1 (Nrp1) is a type 1 transmembrane protein, originally identified for its role in the development of growing neurons, which can serve as a receptor for semaphorin-3A in combination with plexin molecules to regulate growth cone collapse (9–11). In addition, Nrp1 is involved in the process of angiogenesis through interactions with vascular endothelial growth factor (VEGF) (12). Nrp1 has been recently implicated to play a role in the immunological synapse (13) and has been reported to be constitutively expressed on murine CD4+CD25+ regulatory T (Treg) cells, suggesting a potential role for Nrp1 in the attenuation of autoreactive immune responses (14).
We have shown that mice epicutaneously immunized (ECi) with myelin peptide before induction of EAE show a significant degree of protection compared with non-ECi mice (15). Myelin-specific CD4+ T cells from these ECi mice are able to confer protection from EAE to naïve recipient mice upon adoptive transfer (15). Through gene analysis, we observed that Nrp1 is highly expressed on CD4+ T suppressor cells from mice protected from EAE development by ECi with myelin antigen. We therefore examined the role of Nrp1 in the immune response in EAE, because we hypothesized that Nrp1 may have a protective function in EAE development.
Here, we show that overexpression of Nrp1 attenuates EAE progression and, conversely, the lack of Nrp1 results in disease aggravation. This increase in disease severity occurs in a CD4+ T-cell–dependent manner (that skews the balance of helper T cells away from regulatory subtypes toward inflammatory TH-17 subtypes). We demonstrate that the suppressive effect of CD4+ T cells from myelin antigen-ECi mice appears to be independent of Foxp3, because the lack of Nrp1 impairs immune suppression without altering Foxp3 expression. Because of the complex relationship among Foxp3, Nrp1, and Treg cells in general, CD4+Nrp1+ cells, such as those studied in the ECi model, are hereafter referred to as simply “suppressor T cells” so as not to erroneously place them into a specific area of the current Treg cell paradigm. These results demonstrate a specific role for Nrp1 in CD4+ T-cell immune response.
Results
Nrp1 Expression Is Protective Against EAE.
We have shown that mice with T-cell receptor transgenic for the peptide Ac1-11 of myelin basic protein, when epicutaneously immunized (ECi) with the same peptide, are protected from EAE (15). Further, C57BL/6 mice ECi with myelin oligodendrocyte glycoprotein peptide (MOG35–55, referred to as MOG) are resistant to EAE pathogenesis (Fig. 1A). CD4+ T cells from these mice can confer dominant suppression against EAE (ref. 15 and Fig. S1A). To determine the basis of this protection, we performed microarray gene analysis to assess the gene expression profile of CD4+ T cells from MOG ECi mice compared with PBS control or unimmunized control mice. One of the most up-regulated genes in this study was Nrp1, exhibiting greater than fivefold induction in the MOG ECi mice compared with control mice. Because Nrp1 has been proposed to be a constitutive marker of Treg cells (14), we compared Nrp1 mRNA expression in CD4+ T cells of both naïve and MOG ECi mice to naïve CD4+CD25+ T cells. As expected, Nrp1 expression was substantially higher (≥7-fold) in naive CD4+CD25+ T cells compared with naïve WT CD4+ T cells (Fig. S1B). Consistent with increased mRNA expression, Nrp1 expression was almost threefold higher in CD4+ T cells from MOG ECi mice compared with naïve CD4+ CD25+ T cells or PBS control (Fig. S1C). These results demonstrate higher expression of Nrp1 on MOG ECi CD4+ T “suppressor” cells compared with traditional Treg cells.
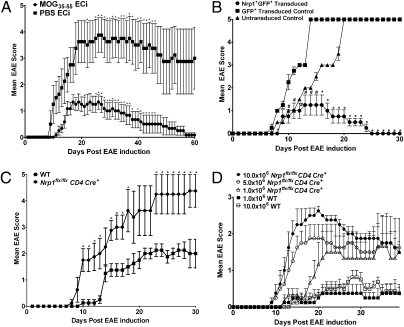
Fig. 1.
Nrp1 expression is protective against EAE, whereas the lack of Nrp1 increases disease severity. (A) WT mice were ECi with 100 μg of MOG35–55 (n = 6) or PBS (n = 4) and immunized with MOG35–55/CFA plus pertussis toxin to induce EAE. Representative (1 of 6) results are expressed as mean EAE score (±SEM, *P < 0.05). (B) CD4+CD25− T cells were isolated from Ac1-11–activated MBP-TCR-Tg mice, transduced with a retroviral GFP construct containing Nrp1 (circle, n = 3) or an empty vector (square, n = 3), and 106 cells were adoptively transferred into B10.Pl TCRα−/− recipient mice concomitant with 106 (untransduced) Ac1-11–activated CD4+CD25− cells. Untransduced cells served as a control (triangle, n = 3). Results from one experiment are expressed as mean EAE score (±SEM, #P < 0.05 for Nrp1+GFP+ transduced vs. GFP+ transduced controls; *P < 0.05 for both Nrp1+GFP+ transduced vs. GFP+ transduced and Nrp1+GFP+ transduced vs. untransduced controls). (C) EAE was induced by using MOG35–55/CFA plus pertussis toxin in Nrp1flx/flxCD4Cre+ (n = 5) and WT mice (n = 5). Representative (1 of 4) results are expressed as mean EAE score (±SEM, *P < 0.05). (D) CD4+ cells from Nrp1flx/flxCD4Cre+ (n = 20) and WT mice (n = 5) primed s.c. with a MOG35–55/CFA emulsion were isolated and transferred into C57BL/6-TCRα−/− recipient mice (n = 4 mice each) followed by immunization using MOG35–55/CFA plus pertussis toxin to induce EAE. Results from one experiment are displayed as mean (±SEM) EAE score.
To determine whether the protection seen in MOG or Ac1-11 ECi mice could be explained solely by the up-regulation of Nrp1, we overexpressed Nrp1 in vivo and followed EAE progression. We first constructed a retroviral GFP vector containing mouse Nrp1 cDNA. We then isolated CD4+ T cells from naïve myelin basic protein (MBP)-T-cell receptor (TCR)-Transgenic (Tg) mice (MBP-TCR-Tg) (15), activated them in vitro with Ac1-11 peptide of MBP, and transduced them with either the Nrp1 construct or an empty vector. Successfully transduced CD4+ T cells were then cotransferred with untransduced, Ac1-11 activated, MBP-TCR-Tg, CD4+ T cells into syngeneic recipient (B10.PL-TCRα−/−) mice. Mice receiving T cells transduced with the Nrp1 expression vector exhibited significant resistance to EAE pathogenesis compared with those receiving the GFP null vector or the naïve CD4+ T cells alone (Fig. 1B and Table S1). This result suggests that increased expression of Nrp1 alone is sufficient to recapitulate the EAE protection previously observed in mice ECi with myelin peptide.
Nrp1 Conditional Knockout Mice Exhibit Severe EAE.
We next asked whether the lack of Nrp1 would result in increased susceptibility to EAE by using a T-cell specific Nrp1 knockout mouse (Nrp1flx/flxCD4Cre+). We compared the development of EAE in WT and Nrp1flx/flxCD4Cre+ mice and demonstrated that Nrp1flx/flxCD4Cre+ mice developed significantly more severe disease than WT mice (Fig. 1C). Disease onset was more rapid in the Nrp1flx/flxCD4Cre+ mice, appearing as early as 8 d after immunization (Fig. 1C) compared with 10 d in WT mice (Fig. 1C and Table S2). Examination of brains on day 30 of EAE and enumeration of CD4+ T-cell infiltration in the brain parenchyma showed considerably more CD4+ T cells in brains of Nrp1flx/flxCD4Cre+ mice compared with WT mice (Fig. S2).
To determine whether the aggravated disease in Nrp1flx/flxCD4Cre+ mice is due specifically to the effects of CD4+ T cells, we isolated CD4+ T cells from both WT and Nrp1flx/flxCD4Cre+ mice primed with MOG and adoptively transferred them into TCRα−/− recipients. After transfer, we induced EAE in recipients and recorded disease progression. Indeed, mice receiving cells from Nrp1flx/flxCD4Cre+ donors exhibited greater disease severity than mice receiving cells from WT donors (Fig. 1D). Moreover, by injecting recipient mice with increasing amounts of CD4+ T cells from Nrp1flx/flxCD4Cre+ mice, we observed that EAE severity is directly correlated with the total number of Nrp1-deficient CD4+ T cells (Fig. 1D and Table S3).
CD4+ T Cells from Nrp1flx/flxCD4Cre+ Mice Display a Skewed TH-17 Response and Are More Proliferative than Wild-Type CD4+ T Cells.
CD4+ T cells can increase EAE severity in two general ways. First, CD4+ T cells may perpetuate increased autoreactivity if suppressive subtypes become functionally impaired. Second, CD4+ T cells may lead to more severe pathogenesis if they possess enhanced inflammatory capacity.
To investigate whether Nrp1flx/flxCD4Cre+ CD4+ T cells possess greater inflammatory capability than WT CD4+ T cells, we assessed proliferation and TH-17 cell differentiation. Under all culture conditions, CD4+ T cells from Nrp1flx/flxCD4Cre+ mice were more proliferative in response to antigen-specific and nonantigen-specific stimuli. Naive WT CD4+ T cells and Nrp1flx/flxCD4Cre+ cells skewed to TH-17 and stimulated with MOG, or anti-CD3/anti-CD28 proliferated more than WT cells (Fig. 2A). Similarly, CD4+ T cells obtained from Nrp1flx/flxCD4Cre+ mice on day 30 after EAE induction also proliferated significantly more than day 30 CD4+ T cells from WT mice (Fig. 2B). Because IL-23 is required for expansion of TH-17 cells, we cultured Nrp1flx/flxCD4Cre+ and WT cells under TH-17 conditions in the absence of IL-23. Notably, Nrp1flx/flxCD4Cre+ cells skewed to TH-17 proliferated more than WT cells even in the absence of IL-23 (Fig. 2B). Further, the frequency of TH-17 cells was significantly greater in Nrp1flx/flxCD4Cre+ mice compared with WT mice under TH-17 polarizing conditions (Fig. 2C) (with IL-23) (P = 0.035), as well as under neutral (no skewing) conditions (P = 0.0077) (Fig. 2D). Consistent with these observations, Nrp1flx/flxCD4Cre+ CD4+ T cells secrete significantly more IL-17 than their WT counterparts under both TH-17 polarizing (P = 0.0017) and neutral (P = 0.0003) conditions (Fig. 2E). Furthermore, CD4+ T cells from Nrp1flx/flxCD4Cre+ mice expressed increased levels of the transcription factor RORγt, which is important for TH-17 differentiation (Fig. S3). Because a subset of TH-17 cells have been identified that can produce IL-10 or IFN-γ in addition to IL-17 (16), we next determined whether altered IL-10 production by TH-17 may be the cause for increased EAE pathogenicity. IL-10 production by CD4+IL-17+ T cells from Nrp1flx/flxCD4Cre+ mice is significantly less than that from CD4+IL-17+ T cells from WT mice (P = 0.044) (Fig. S4). Together, these data indicate that cells lacking Nrp1 are biased toward a TH-17 phenotype and that Nrp1 regulates CD4+ T-cell expansion.
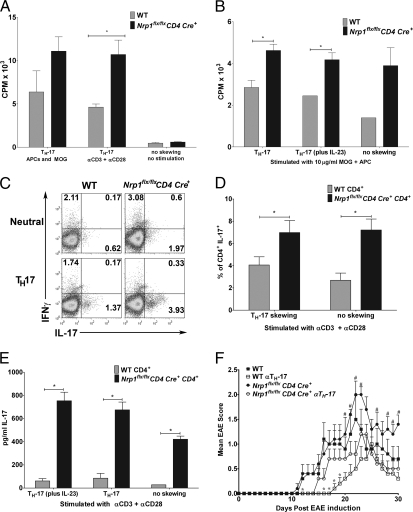
Fig. 2.
Nrp1-deficient CD4+ T cells display an increased TH-17 phenotype and are more proliferative than WT. (A) Naïve CD4+ cells [WT (gray bars) and Nrp1flx/flxCD4Cre+ (black bars), n = 5 each] were skewed toward TH-17. After 7 d, skewed cells were stimulated with either APC and MOG, or with anti-CD3 and anti-CD28, or were left unskewed and unstimulated. Representative (1 of 5) results are displayed as mean (±SEM) CPM × 103 (*P < 0.05). (B) EAE was induced by using MOG35–55/CFA plus pertussis toxin in WT (gray bars) and Nrp1flx/flxCD4Cre+ (black bars) mice (n ≥ 3 each). On day 30, CD4+ cells were stimulated with 10 μg/mL MOG and cultured under neutral, TH-17 polarizing, or TH-17 polarizing with supplemental IL-23 conditions. Representative results (1 of 3) are displayed as mean (±SEM) cpm × 103. (*P < 0.05). (C) Naïve CD4+ T cells (WT and Nrp1flx/flxCD4Cre+, n = 4 mice each), were cultured under neutral or TH-17 polarizing conditions. Cells were stained for CD4, IFNγ, and IL-17 and analyzed by flow cytometry. Data are representative of three experiments. (D) Naïve CD4+ cells [WT (gray bars) and Nrp1flx/flxCD4Cre+ (black bars)] were cultured under neutral or TH-17 polarizing conditions, stained for CD4 and IL-17 expression, and analyzed by flow cytometry. Representative (1 of 5) results are presented as mean (±SEM) percent of total CD4+ cells, which are IL-17+ (*P < 0.05). (E) Naïve CD4+ cells [WT (gray bars) and Nrp1flx/flxCD4Cre+ (black bars), n = 5 each] were cultured under neutral or TH-17 polarizing conditions, and cell culture supernatant was analyzed by ELISA for IL-17. Representative (1 of 5) results are displayed as mean (±SEM) IL-17 pg/mL (*P ≤ 0.005). (F) WT (squares) or Nrp1flx/flxCD4Cre+ (circles) mice (n = 5 each) immunized for EAE by using MOG35–55/CFA plus pertussis toxin were treated with (open symbols) or without (filled symbols) anti–TH-17 antibodies (anti–IL-6, anti–IL-23, anti–TGF-β). Representative (1 of 2) results are presented as mean EAE score ± SEM (#P < 0.05 for the Nrp1flx/flxCD4Cre+ versus the Nrp1flx/flxCD4Cre+-anti–TH-17 treated group. *P < 0.05 for the WT versus WT anti–TH-17 group.)
In Vivo Blockade of TH-17 Cell Development Ameliorates EAE.
To further pinpoint the role of TH-17 cells in EAE, we treated Nrp1flx/flxCD4Cre+ or WT mice with antibodies to cytokines involved in TH-17 cell polarization and expansion. Because TH-17 cells play a critical role in EAE pathogenesis, we reasoned that blocking TH-17 cell development at the initiation phase of disease might protect both WT and Nrp1flx/flxCD4Cre+ mice from EAE. Both Nrp1flx/flxCD4Cre+ and WT mice treated with the TH-17 anti-cytokine antibody regimen show significant disease amelioration compared with controls (Fig. 2F and Table S4). These results confirm TH-17 involvement in EAE pathogenesis in Nrp1flx/flxCD4 Cre+ mice.
Nrp1-Deficient Treg Cells Are Impaired in Their Ability to Suppress CD4 Autoreactive Cells.
CD4+ T cells could lead to increased EAE pathogenesis through reduced Treg cell function. The lack of Nrp1 might impair the ability of the immune system to suppress autoreactive cells, indirectly leading to increased autoinflammatory cell proliferation. Previous findings support this hypothesis because Nrp1 has been reported to be constitutively expressed on Treg cells (14). Therefore, we asked whether immune suppression in Nrp1flx/flxCD4Cre+ mice is altered, in addition to the predisposition toward inflammatory subtypes, as demonstrated earlier (Fig. 2). We assessed the ability of WT CD4+ T cells expressing Nrp1 (CD4+Nrp1+) to suppress the proliferation of target cells in vitro compared with CD4+CD25+ T cells. We also compared the suppressive capabilities of these WT CD4+Nrp1+ T cells to CD4+CD25+ T cells from Nrp1flx/flxCD4Cre+ mice. Responder CD4+ T cells were isolated from MOG-TCR-transgenic mice (2D2-Tg) (17) and primed with MOG. WT CD4+CD25+ T or CD4+Nrp1+ T suppressor cells, or Nrp1flx/flxCD4Cre+ CD4+CD25+ T suppressor cells were cultured with responder cells, APCs, and MOG. We observed a statistically significant decrease in the proliferation of target cells cultured with CD4+CD25+Nrp1+ T cells or with CD4+CD25+ T cells from WT mice at all ratios (Fig. 3A). Interestingly, at all suppressor to responder cell ratios, CD4+Nrp1+ T cells suppressed effector cell proliferation more efficiently than CD4+CD25+ T cells (Fig. 3A). In contrast, CD4+CD25+ T cells from Nrp1flx/flxCD4Cre+ mice were not suppressive at the same cell ratios as WT CD4+CD25+ T or CD4+Nrp1+ T cells (Fig. 3A). These results indicate that the corresponding Nrp1flx/flxCD4Cre+ CD4+ T cells are impaired in their ability to curb immune proliferation.
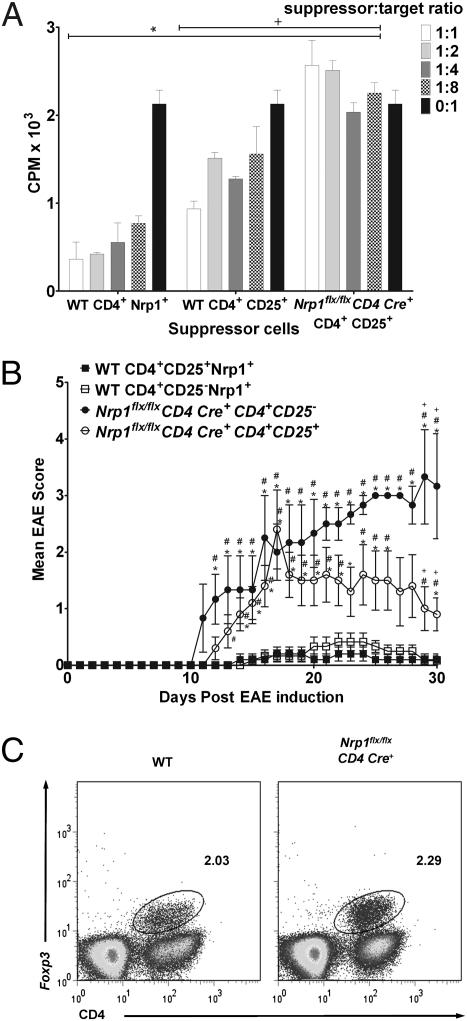
Fig. 3.
Nrp1 deficiency impairs Treg cell function. (A) The 2D2-Tg (17) CD4+ cells (n ≥ 3 mice) were used as target cells. Naïve Nrp1flx/flxCD4Cre+ or WT CD4+CD25+ cells (n ≥ 7 each), as well as WT CD4+CD25−Nrp1+ cells (all purified by using magnetic beads), were used as suppressor T cells. Cells were stimulated with 10 μg/mL MOG and APC (5:1 APC:target ratio), cultured for 48 h, then pulsed with 0.5 μCi/well Td-3H for 18 h. P values compare either WT CD4+Nrp1+ (*P < 0.05) or WT CD4+CD25+ (+P < 0.05) suppressor cells to Nrp1flx/flxCD4Cre+ CD4+CD25+ cells of the same ratio. Representative (1 of 4) results are expressed in mean (±SEM) CPM × 103. (B) The 2D2-Tg CD4+ T cells (n = 5 mice) were skewed to TH-17 in vitro, and 107 cells were transferred into C57BL/6-TCR-α−/− recipients along with: 106 naïve WT Nrp1+CD4+CD25+, or Nrp1+CD4+CD25−; or, naïve Nrp1flx/flxCD4Cre+ CD4+CD25+ or CD4+CD25− cells (n = 10 mice each). EAE was then induced in recipients by using MOG35–55/CFA plus pertussis toxin. Results from one experiment are expressed as mean (± SEM) EAE score. P values compare: WT CD4+CD25−Nrp1+ versus either Nrp1flx/flxCD4Cre+CD4+CD25+ or Nrp1flx/flxCD4Cre+CD4+CD25−, #P < 0.05; WT CD4+CD25+Nrp1+ versus either Nrp1flx/flxCD4Cre+CD4+CD25+ or Nrp1flx/flxCD4Cre+CD4+CD25−, *P < 0.05; Nrp1flx/flxCD4Cre+CD4+CD25+ versus Nrp1flx/flxCD4Cre+CD4+CD25−, +P < 0.05). (C) CD4+ T cells from WT and Nrp1flx/flxCD4Cre+ mice (n ≥ 3) were isolated, stained for CD4 and Foxp3, and analyzed by FACS. Results are representative of three experiments.
To determine whether this difference in suppression is significant in vivo and to more specifically define the role of Nrp1 in immune suppression, four different populations of CD4+ T cells from WT or Nrp1 conditional knockout mice were adoptively transferred into naïve TCR-α−/− recipients with concomitant adoptive transfer of MOG-stimulated, TH-17–polarized CD4+ responder cells. WT CD4+CD25+Nrp1+ and CD4+CD25−Nrp1+ T cells exhibited similar disease profiles and significantly suppressed EAE (Fig. 3B and Table S5). Recipients of Nrp1flx/flxCD4Cre+ CD4+CD25+ T cells exhibited a much more severe EAE disease profile than their WT CD4+CD25+Nrp1+ counterparts (Fig. 3B and Table S5). From these observations, we conclude that Nrp1-expressing CD4+ T cells are capable of suppressing EAE inflammatory response independent of naturally occurring Treg cell involvement. We also conclude that naturally occurring Treg cells expressing Nrp1 are more efficient suppressors than Treg cells lacking Nrp-1 expression. These findings suggest that a major role of Nrp1 in the immune response is in regulating inflammatory responses by CD4+ effector T cells.
Foxp3 Expression Is Unaffected by the Lack of Nrp1 in Nrp1flx/flxCD4Cre+ Mice.
Because Nrp1 appears to play an important role in T-cell–mediated immune suppression, we examined the effect of Nrp1 on the expression of another gene associated with the archetypal Treg cell, Foxp3. CD4+ T cells from Nrp1flx/flxCD4Cre+ mice express Foxp3 at a similar frequency to WT mice (Fig. 3C). Consistent with these data, the dominant immune suppression induced in CD4+ T cells in mice ECi with myelin antigen does not correspond to an increase in Foxp3 expression. These results suggest that Nrp1 suppression is independent of Foxp3.
TGF-β, but Not IL-10, Is Important for Nrp1-Mediated Suppression.
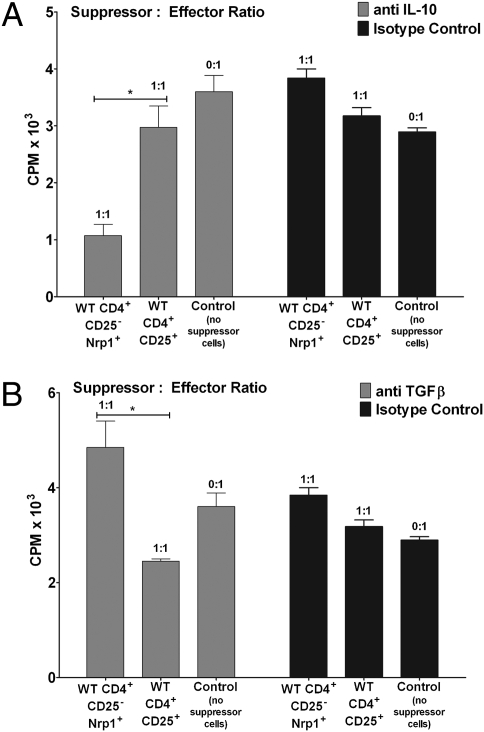
One of the mechanisms by which suppressor T cells suppress autoimmunity is by the induction of anti-inflammatory cytokines such as IL-10 or TGF-β. Because IL-10 is decreased in TH-17–skewed CD4+ T cells from Nrp1flx/flxCD4Cre+ mice (Fig. S4), we asked whether IL-10 is involved in Nrp1 suppression of target cell proliferation by neutralizing IL-10 in vitro (18). CD4+CD25+ T cells or CD4+CD25−Nrp1+ cells from WT mice were used as suppressors in the presence or absence of anti–IL-10 (Fig. 4A). Interestingly, anti–IL-10 abrogated WT CD4+CD25+ cell suppression of target cell proliferation (Fig. 4A), but had little effect on WT CD4+CD25−Nrp1+ cell suppression (Fig. 4A). These results suggest that Nrp1 suppressor function does not depend on IL-10. We next asked whether TGF-β is involved in Nrp1-mediated suppression by evaluating it in the presence or absence of anti–TGF-β (18). As shown in Fig. 4B, anti–TGF-β significantly inhibited WT CD4+CD25−Nrp1+ T-cell suppression of effector cell proliferation but had little effect on WT CD4+CD25+ T-cell suppression. These data strongly indicate that Nrp1 suppression of CD4+ T-cell effector function depends on TGF-β.
Fig. 4.
Suppression by CD4+Nrp1+ cells is abrogated in the presence of anti–TGF-β but not anti–IL-10. (A) The 2D2-Tg CD4+ T cells (n ≥ 3 mice) were primed in vivo, isolated by using magnetic beads, and used as target cells. Naïve WT and Nrp1flx/flxCD4Cre+ CD4+CD25+ T cells (n = 10) and naïve WT CD4+CD25−Nrp1+ T cells (n = 10) were isolated by using magnetic beads and used as suppressor T cells. Suppressors and targets were combined at a 1:1 ratio. Cells were treated with anti–IL-10 (10 μg/mL) or an isotype control (10 μg/mL) and stimulated with 10 μg/mL MOG and APC (5:1 APC:target ratio). Representative (1 of 3) results are expressed as mean (±SEM) CPM × 103. (WT CD4+CD25+ versus WT CD4+CD25−Nrp1+, *P < 0.05.) (B) Cell populations were purified, combined, and cultured as in Fig. 4A, except cells were treated with anti–TGF-β (10 μg/mL) or an isotype control (10 μg/mL). Representative (1 of 3) results are expressed as mean (±SEM) cpm × 103. (WT CD4+CD25+ versus WT CD4+CD25−Nrp1+, *P < 0.05.)
Discussion
The goal of this study was to elucidate the role of Nrp1 in EAE pathogenesis. As shown in Fig. 1A, initial ECi with MOG before induction of EAE results in complete protection against disease progression. Through global gene expression analysis, we found that Nrp1 is one of the most highly expressed genes in this protective response. These results, along with previous data suggesting a role for Nrp1 in immune suppression, led us to hypothesize that Nrp1 is important for preventing autoinflammatory conditions such as EAE. Our hypothesis is supported by data showing that overexpression of Nrp1 is sufficient to protect mice from EAE pathogenesis as well as the converse finding that the lack of Nrp1 results in increased disease severity. Protection and disease aggravation are CD4+ T-cell–dependent, because these disease states can be recapitulated in T-cell–deficient recipients when exogenous T cells overexpressing or lacking Nrp1, respectively, are transferred. Furthermore, our data strongly demonstrate that Nrp1 plays a critical role in regulating the expansion and cytokine production of TH-17 cells both in vitro and in vivo.
Because of the discovery of the TH-17 lineage of CD4+ T cells, many autoimmune disorders previously described as TH-1–mediated, including MS and EAE, have been reattributed to TH-17 cells (19). Accordingly, we demonstrate that Nrp1-deficient CD4+ T cells are poised to differentiate into the TH-17 lineage. Furthermore, blockade of TH-17 cell development with an anti–TH-17 antibody regimen suppressed EAE in Nrp1flx/flxCD4Cre+ mice. These results further support our hypothesis that one mechanism by which Nrp1 controls autoreactivity is by regulating TH-17 cell expansion and cytokine production.
As of yet, no signaling pathway has been attributed specifically to Nrp1. Although Nrp1 comprises one chain of the semaphorin-3A receptor, the second chain, plexin-A, is responsible for initiating cell signaling as a result of receptor ligation (20). Studies show that dendritic cells produce large amounts of semaphorin-3A (8). Moreover, the side chain of plexin-A is expressed on CD4+ T cells and plexin-A4−/− mice exhibit increased EAE pathogenicity (21). Together with our findings, these results suggest that secretion of semaphorin-3A by DCs may represent a negative feedback loop to reduce the duration of T-cell–APC interaction and, consequently, reduce the expression of inflammatory molecules such as IL-17. In neuronal cells, semaphorin-3 signaling through the Nrp1:plexin-A complex acts through a rho/rac-dependent pathway leading to actin depolymerization and growth cone collapse (10). Similarly, Nrp1:plexin-A signaling in T cells could lead to similar actin depolymerization, causing the disassembly of molecular scaffolding supporting cellular polarization and the supramolecular activation complex at the immune synapse.
Nrp1flx/flxCD4Cre+ mice may be susceptible to increased EAE severity because the lack of Nrp1 impairs suppression of autoreactive cells by the immune system, indirectly leading to increased autoinflammatory cell proliferation. Such a hypothesis is supported by previous findings that Nrp1 is constitutively expressed on Treg cells (14). Indeed, we found that suppressor cells from Nrp1flx/flxCD4Cre+ mice have an impaired ability to suppress effector cells both in vitro and in vivo. Moreover, we found that WT CD4+ T cells sorted specifically for Nrp1 expression were as capable of or more effective at suppressing target cells than suppressors sorted specifically as CD4+CD25+, indicating that Nrp1 plays an important role in immune suppression.
Our result is consistent with findings that show that Nrp1 expression allows Treg cells to supplant effector T cells for the limited space available on primed APCs (22). Along with our data showing that antigen and APCs are required for successful Treg cell function (as opposed to contact independent PMA/ionomycin stimulation), this result supports the notion of antigen-dependent regulatory T-cell suppression. As described by Sarris et al. (22), Nrp1 greatly contributes to the fidelity of the immune synapse, thus favoring APC interactions with T cells that express Nrp1.
Alternatively, Nrp1 may function at the level of Treg cell stimulation. Several reports have shown that Nrp1 is important for Treg cell development (23, 24), and Nrp1 has been proposed as a receptor for TGF-β (25). Whether this interaction actually contributes to signaling, the functional activity of Treg cells is enhanced by Nrp1:TGF-β ligation. Because of the shared requirement of TGF-β for both Treg and TH-17 cells (26, 27), Nrp1 may function to sequester TGF-β, simultaneously preventing the development of inflammatory TH-17 cells and promoting the differentiation of peripheral Treg cells. Consistent with this idea, neutralization of TGF-β inhibits Nrp1 suppressor capacity, whereas blockade of IL-10 had little or no impact.
In addition to naturally occurring Treg (nTreg) cells, additional Treg subtypes emerge from the thymus as CD4+CD25−Foxp3− cells. Although appearing phenotypically similar to a naïve CD4+ T cell, these inducible Treg (iTreg) cells can be induced to express Foxp3 and CD25 in the periphery (28). Such a notion of Treg cell heterogeneity is consistent with our results. We demonstrate that EAE-tolerant CD4+ cells from MOG ECi mice, with highly up-regulated Nrp1 levels, have only modestly up-regulated Foxp3 levels. Moreover, Nrp1flx/flxCD4Cre+ mice display virtually no variation in the Foxp3+ cell population. Despite this lack of fluctuation in Foxp3 expression, Nrp1-deficient suppressor T cells still have an impaired ability to attenuate immune proliferation. These results suggest that Nrp1 may simply be a mechanism used by cells capable of immune suppression and not specific to any regulatory cell lineage in particular. A cell capable of immune suppression would alter Nrp1 expression depending on inflammatory cues. As a result, any suppressive cell incapable of expressing Nrp1, whether a Foxp3+ nTreg cell or a type of iTreg cell, would have an impaired ability to curb autoinflammation.
In summary, we demonstrate that Nrp1 plays a critical role in the pathogenesis of EAE. Mice that lack Nrp1-expressing CD4+ T cells exhibit increased degenerative signs and CNS infiltration associated with EAE, preferential TH-17 cell commitment, enhanced proliferation and cytokine production, and impaired suppressor capacity. Our study provides evidence for a direct role of Nrp1 in an immune-mediated disease. This phenomenon is the combined result of increased inflammatory lineage commitment and impaired regulatory T-cell function. This study supports previous findings that Nrp1 may be essential for proper immune suppression. As the body of knowledge surrounding Nrp1 and Treg cell function expands, this molecule may prove to be a novel target for new treatments and therapies for diseases like MS.
Materials and Methods
Mice.
Nrp1 conditional knockout mice were generated by crossing Nrp1flx/flx mice [graciously provided by David Ginty, (The Solomon H. Snyder Department of Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD)] (12) on the C57BL/6 background with CD4Cre+ mice (Taconic), generating Nrp1flx/flxCD4Cre+ mice (SI Materials and Methods). MBP-TCR-Tg mice on the B10.PL background were described (29). C57BL/6-Tg(Tcra2D2,Tcrb2D2)1Kuch/J mice, which have a transgenic T-cell receptor recognizing the MOG35–55 peptide (MOG-TCR-Tg), were kindly provided by Vijay Kuchroo (Harvard Medical School, Center for Neurologic Diseases, Brigham & Women's Hospital, Boston, MA) (17). C57BL/6 mice and TCRα−/− (B6.129S2-Tcrαtm1Mom/J or B10.PL-TCRα−/−) mice were purchased from Jackson Laboratory. Experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee of Cornell University.
EAE Induction and Scoring.
EAE was induced by injecting 50 μL s.c. in each flank a 1:1 CFA (Thermo Scientific): MOG (Anaspec) [3 mg/mL in PBS (Mediatech)] emulsion on day one, in parallel with i.v. pertussis toxin (List Biological Laboratories) (200 ng) on days 0 and 2. Mice were scored daily for EAE based on a numerical score of disease sign severity: 0 = no disease, 0.5–1 = weak/limp tail, 2 = limp tail and partial hind limb paralysis, 3 = total hind limb paralysis, 4 = both hind limb and fore limb paralysis, and 5 = death.
Adoptive Transfer and in Vivo Suppression.
Mice were primed with s.c. CFA:MOG peptide. After 1 wk, CD4+ T cells were isolated from spleen and lymph nodes and negatively selected for using magnetic separation (SI Materials and Methods). CD4+ cells were transferred to TCRα−/− mice at the indicated dose in a total of 200 μL of sterile PBS. For in vivo suppression, CD4+CD25+ cells from unimmunized mice were sorted by using a magnetic regulatory T-cell separation kit (Miltenyi Biotech). Either 107 WT or Nrp1flx/flxCD4Cre+ CD4+CD25+ cells were injected i.v. into WT recipients in a total dose of 200 μL of sterile PBS.
T-Cell Polarization.
Purified naïve CD4+ T cells (SI Materials and Methods) were cultured in Bruff's media (Invitrogen) and stimulated with immobilized mouse anti-CD3 and soluble anti-mouse CD28 (BD Biosciences) in the presence of TH-17–polarizing (with IL-23 where indicated) or neutral conditions (SI Materials and Methods). At day 3 after stimulation, cells were expanded for an additional 4 d in fresh media containing 25 U/mL mouse IL-2. At day 7, cells were washed and restimulated with either anti-mouse CD3/CD28 (1 μg/mL each) plus mouse IL-2 (25 U/mL), or with MOG35–55 and APC plus mouse IL-2 (25 U/mL) for 48 h. Cell culture supernatant was collected for ELISA, and differentiated T cells were collected for either proliferation or intracellular cytokine staining.
Flow Cytometry.
Cell suspensions were stained with fluorochrome-conjugated antibodies for CD4, IFN-γ, Foxp3, IL-10, IL-4, and IL-17 (BD Biosciences and eBioscience) or with rabbit anti-Nrp1 (AbCam) and then with anti-rabbit AF488 (Invitrogen) (SI Materials and Methods). Samples were acquired on a FACSCalibur (BD Biosciences) by using CellQuest (BD Biosciences) software and analyzed with FlowJo software (Tree Star).
T-Cell Suppression Assay.
For a full description, please refer to SI Materials and Methods. Briefly, CD4+ responder cells were primed in vivo by immunization of WT mice with a 1:1 CFA:MOG (3 mg/mL in PBS) emulsion (50 μg in both flanks of the mouse) on day 0 and day 5 and then isolated on day 7. CD4+ responder cells from 2D2-Tg mice were also used in certain assays (17). For suppressor cells, CD4+ cells were first isolated from naïve WT or Nrp1flx/flxCD4Cre+ mice. Then, positive magnetic selection was used to isolate CD25+ suppressor cells, and WT CD4+CD25−Nrp1+ cells were finally selected from the CD4+CD25− population. Primed CD4+ responder cells (105) were cultured with suppressor cells and irradiated APCs (1:5 T-cell:APC) in the presence of 10 μg/mL MOG. In certain experiments, anti–IL-10 (eBioscience), sIL-10 receptor (R&D Systems) or anti–TGF-β (eBioscience) were used. Proliferation was measured by 3H-thymidine incorporation.
Epicutaneous Immunization, Retroviral Overexpression, ELISA, and RT-PCR.
A description of the methods used for epicutaneous immunization, retroviral overexpression, ELISA, and RT-PCR can be found in SI Materials and Methods.
Statistics.
P values are calculated by using the Student's t test.
Supplementary Material
Acknowledgments
We thank Dr. David Ginty for his generous gift of Nrp1flx/flx mice, Dr. Vijay Kuchroo for 2D2 MOG-TCR-transgenic mice, Dr. Stephen Strittmatter for graciously providing Nrp1 cDNA, and Dr. Jeffrey H. Mills for help with statistics for the manuscript. This study was supported by National Institutes of Health Grants AI 57854 and AI 072434-01A2 (to M.S.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008721108/-/DCSupplemental.
References
- 1.Keegan BM, Noseworthy JH. Multiple sclerosis. Annu Rev Med. 2002;53:285–302. doi: 10.1146/annurev.med.53.082901.103909. [DOI] [PubMed] [Google Scholar]
- 2.Niino M, Fukazawa T, Kikuchi S, Sasaki H. Recent advances in genetic analysis of multiple sclerosis: Genetic associations and therapeutic implications. Expert Rev Neurother. 2007;7:1175–1188. doi: 10.1586/14737175.7.9.1175. [DOI] [PubMed] [Google Scholar]
- 3.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol. 2007;61:504–513. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- 4.Brown DA, Sawchenko PE. Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J Comp Neurol. 2007;502:236–260. doi: 10.1002/cne.21307. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dustin ML, et al. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- 7.Czopik AK, Bynoe MS, Palm N, Raine CS, Medzhitov R. Semaphorin 7A is a negative regulator of T cell responses. Immunity. 2006;24:591–600. doi: 10.1016/j.immuni.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Kikutani H, Kumanogoh A. Semaphorins in interactions between T cells and antigen-presenting cells. Nat Rev Immunol. 2003;3:159–167. doi: 10.1038/nri1003. [DOI] [PubMed] [Google Scholar]
- 9.Kolodkin AL, et al. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 10.Kolodkin AL. Semaphorins: Mediators of repulsive growth cone guidance. Trends Cell Biol. 1996;6:15–22. doi: 10.1016/0962-8924(96)81033-6. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi T, et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 12.Gu C, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tordjman R, et al. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat Immunol. 2002;3:477–482. doi: 10.1038/ni789. [DOI] [PubMed] [Google Scholar]
- 14.Bruder D, et al. Neuropilin-1: A surface marker of regulatory T cells. Eur J Immunol. 2004;34:623–630. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- 15.Bynoe MS, Evans JT, Viret C, Janeway CA., Jr Epicutaneous immunization with autoantigenic peptides induces T suppressor cells that prevent experimental allergic encephalomyelitis. Immunity. 2003;19:317–328. doi: 10.1016/s1074-7613(03)00239-5. [DOI] [PubMed] [Google Scholar]
- 16.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 17.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu S, et al. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 - precursors. Am J Transplant. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 19.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 20.Fujisawa H. From the discovery of neuropilin to the determination of its adhesion sites. Adv Exp Med Biol. 2002;515:1–12. doi: 10.1007/978-1-4615-0119-0_1. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto M, et al. Plexin-A4 negatively regulates T lymphocyte responses. Int Immunol. 2008;20:413–420. doi: 10.1093/intimm/dxn006. [DOI] [PubMed] [Google Scholar]
- 22.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28:402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corbel C, et al. Neuropilin 1 and CD25 co-regulation during early murine thymic differentiation. Dev Comp Immunol. 2007;31:1082–1094. doi: 10.1016/j.dci.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X, et al. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glinka Y, Prud'homme GJ. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J Leukoc Biol. 2008;84:302–310. doi: 10.1189/jlb.0208090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morishima N, Mizoguchi I, Takeda K, Mizuguchi J, Yoshimoto T. TGF-beta is necessary for induction of IL-23R and Th17 differentiation by IL-6 and IL-23. Biochem Biophys Res Commun. 2009;386:105–110. doi: 10.1016/j.bbrc.2009.05.140. [DOI] [PubMed] [Google Scholar]
- 27.Huber S, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173:6526–6531. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 28.Mills KH. Regulatory T cells: Friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 29.Hardardottir F, Baron JL, Janeway CA., Jr T cells with two functional antigen-specific receptors. Proc Natl Acad Sci USA. 1995;92:354–358. doi: 10.1073/pnas.92.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.