Abstract
CTCL is a cancer of skin homing T cells with variants that include leukemic CTCL (L-CTCL), a malignancy of central memory T cells (TCM), and mycosis fungoides (MF), a malignancy of skin resident effector memory T cells (TEM). We report that low-dose alemtuzumab (αCD52) effectively treated patients with refractory L-CTCL but not MF. Alemtuzumab depleted all T cells in blood and depleted both benign and malignant TCM from skin, but a diverse population of skin resident TEM remained in skin after therapy. T-cell depletion with alemtuzumab required the presence of neutrophils, a cell type frequent in blood but rare in normal skin. These data suggest that TCM were depleted because they recirculate between the blood and skin whereas skin resident TEM were spared because they are sessile and non-recirculating. After alemtuzumab treatment, skin T cells produced lower amounts of IL-4 and higher amounts of IFNγ. Moreover, there was a marked lack of infections in alemtuzumab-treated L-CTCL patients despite the complete absence of T cells in blood, suggesting that skin resident TEM can protect the skin from pathogens even in the absence of T cell recruitment from the circulation. Together, these data suggest that alemtuzumab may treat refractory L-CTCL without severely compromising the immune response to infection by depleting circulating TCM but sparing the skin resident TEM that provide local immune protection of the skin.
Introduction
Cutaneous T cell lymphomas (CTCL) are a heterogeneous group of non-Hodgkin’s lymphomas that represent malignancies of skin homing T cells (1). CTCL encompasses both skin limited variants such as mycosis fungoides (MF) and leukemic forms of the disease (L-CTCL) including Sézary syndrome. In MF, malignant cells are confined to fixed skin lesions and many patients have indolent disease with a normal life expectancy (2). Patients with progressive MF can develop skin tumors and lymph node involvement, but blood involvement is rare. L-CTCL patients often present with lymphadenopathy and diffuse erythema: Malignant T cells in these patients are frequently present in the blood, skin, and lymph nodes. L-CTCL is often refractory to multiple therapies; patients have a median survival of 3 years and most die from infections. Hematopoietic stem cell transplantation is the only potentially definitive cure for both advanced MF and L-CTCL (3). We report here findings that low dose alemtuzumab (Campath), a T cell-depleting antibody directed against CD52, can induce clinical responses in all patients and complete remission in 50% of patients with refractory L-CTCL.
Although early-stage MF and L-CTCL have previously been considered to be points in a disease continuum, differing molecular profiles and responses to therapy suggest these disorders may arise from two distinct T cell subsets (2, 4–6). We have found that the malignant T cells in L-CTCL are L-selectin/CCR7+ and have a central memory T cell (TCM) phenotype, whereas the malignant T cells in MF have a phenotype of skin resident effector memory T cells (TEM) (6). In mouse models of T cell memory, TCM and TEM have distinct migratory patterns and effector potential but these issues have not been studied in human beings. We present here findings that human cutaneous TCM recirculate into blood, whereas TEM are a non-recirculating skin resident population. Moreover we provide evidence from our treated patients that cutaneous TEM can provide immunologic protection against skin infection even in the absence of TCM.
Results
Malignant T cells have a TCM phenotype in L-CTCL and a TEM phenotype in MF
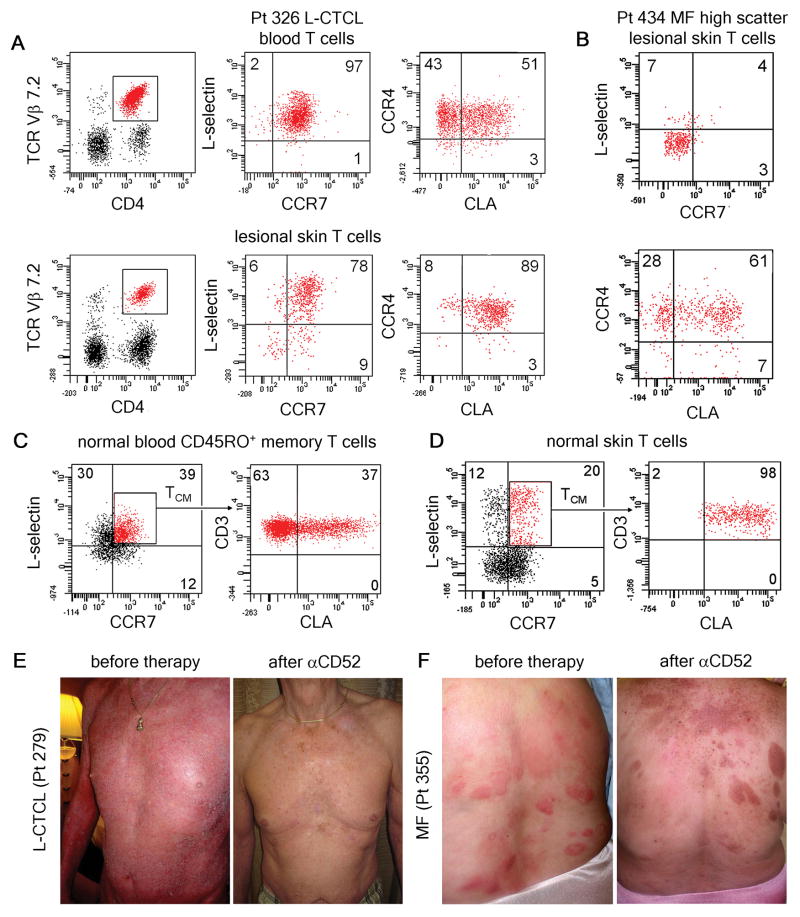
Clonal malignant T cells can be identified in some CTCL patients by staining with commercially available antibodies to TCR Vβ subfamilies. By identifying the malignant T cell clone, researchers can assess disease burden and monitor for recurrence (7). As reported previously, clonal malignant T cells from both the blood and skin of L-CTCL patients co-expressed L-selectin and CCR7, a phenotype characteristic of TCM (6)(Fig. 1A). Greater than 90% of malignant T cells in blood expressed CCR4, but separate populations of CLA− and CLA+ clonal T cells existed in the blood of most patients. However, malignant T cells expressing CLA were the predominant population observed in lesional skin (Fig. 1A, Table S1).
Fig. 1.
Low dose alemtuzumab is effective in the treatment of L-CTCL, a malignancy of TCM, but is ineffective in MF, a malignancy of TEM. (A) Clonal malignant T cells isolated from the blood and lesional skin of patients with L-CTCL co-expressed L-selectin and CCR7, a phenotype consistent with TCM. A subset of circulating malignant cells also expressed the skin homing addressins CCR4 and CLA. Malignant T cells co-expressing CCR4 and CLA predominated in skin lesions. A representative patient is shown; expression of CLA and CCR4 was confirmed in 5 additional L-CTCL patients with identifiable T cell clones (Table S1). (B) High side/forward scatter can be used to identify the malignant T cells in CTCL. High scatter cells isolated from the fixed skin lesions of MF expressed skin homing addressins CLA and CCR4 but lacked expression of L-selectin and CCR7, consistent with the claim that MF is a malignancy of skin resident effector memory T cells (TEM). Findings have been replicated in 17 additional MF patients. (C) A subset of circulating TCM in the blood of healthy individuals co-express the skin homing addressin CLA. (D) These CLA-expressing TCM make up approximately 20% of the T cells present in normal human skin. As observed in L-CTCL, only CLA-expressing TCM were found in skin. (E) Alemtuzumab is effective in the treatment of refractory L-CTCL. Patient 279 received six weeks of low dose alemtuzumab (αCD52) and had complete clearance of skin disease and loss of the malignant T cell clone from his blood. (F) In contrast, two MF patients (including the patient shown) experienced no improvement in inflammatory skin lesions with alemtuzumab therapy.
A high forward/side scatter phenotype by flow cytometry analysis can be used to identify the malignant T cell population in MF (8). The high scatter T cell population from MF skin lesions expressed CLA and CCR4 but lacked CCR7 and L-selectin co-expression, a phenotype consistent with TEM (Fig. 1B)(6).
A population of skin tropic TCM are present in human skin and blood
Although TCM were first described as L-selectin+/CCR7+ T cells that lacked expression of tissue specific adhesion receptors, our findings in CTCL suggested that a population of CLA+ skin tropic TCM may exist in blood that can enter skin (9). Indeed, analysis of CD45RO+ memory TCM from normal human blood demonstrated that a mean 40% (st. dev. 6.4) of circulating CD45RO+/L-selectin+/CCR7+ TCM also expressed the skin homing addressin CLA (Fig 1C). In normal human skin, L-selectin/CCR7+ TCM comprised approximately 20% of total T cells (Fig. 1D)(10). Similar to our observations in L-CTCL, the TCM present in normal human skin expressed CLA, suggesting that only CLA+ TCM are capable of migrating into the skin under non-inflamed conditions.
Low dose alemtuzumab is a highly effective, well tolerated therapy for L-CTCL
Alemtuzumab (10 mg) was administered subcutaneously to patients with L-CTCL three times a week for six weeks. The duration of therapy was determined by patient response, and therapy was discontinued when the skin was clinically clear. Patients were maintained on valacyclovir and trimethoprim/sulfamethoxazole for HSV and Pneumocystis jirovecii prophylaxis until six months after completion of therapy. Characteristics and clinical responses of 18 patients are shown in Table I. All patients had had CTCL for greater than 3 years with consistent skin biopsies, elevated absolute CD4 counts, and CD4/CD8 ratios >10 and were refractory to at least two prior therapies. Responding patients treated with alemtuzumab experienced rapid depletion of lymphocytes from blood and gradual clearing of skin disease. Clearance of skin disease lagged behind depletion of T cells from blood and required at least three weeks of additional therapy after loss of detectable circulating T cells (Table I). Peripheral blood disease improved in 100% of patients and completely cleared in 89%. Skin disease showed a partial response in 89% of patients and a complete response in 50%. With respect to clearing of both blood and skin disease, we observed partial responses in 89% of patients and complete remissions in 50% of patients. Responses were often durable. Patient 279 experienced complete remission for 18 months after completing a single six-week course of αCD52 (Fig. 1E) and patient 119 had a 16 month full remission after a similar course. In contrast to its effectiveness in L-CTCL, we found alemtuzumab therapy to be completely ineffective in the treatment of MF (Fig. 1F).
Table I.
Patient characteristics and clinical responsiveness to alemtuzumab therapy
| Pt | Age/G | CD4/8 | AbsCD4 | Prior Rx | Response (Blood/Skin) | D. Duration | Skin Clear | Infection |
|---|---|---|---|---|---|---|---|---|
| 84 | 69M | 11 | 4570 | I,P,V,B10, | CR/CR | 15y | 5 wks | no |
| 119 | 77F | 14.5 | 2800 | I,P,V,B | CR/CR | 9yr | 5 wks | no |
| 279 | 69M | 13.5 | 2504 | I,P,V,B,G,D | CR/CR | 12yr | 5 wks | no |
| 288 | 59M | 12.7 | 2431 | I,P,V,B | CR/PR | 11yr | 6 wks | no |
| 292 | 67M | 32.3 | 5356 | I,P,B,GND | CR/CR | 5yr | 3 wks | no |
| 295 | 73M | 93.0 | 6190 | I,P,B,O | CR/CR* | 6yr | 6 wks | no |
| 326 | 78M | 10.2 | 2893 | I,P,B | CR/PR | 4yr | 4 wks | no |
| 333 | 65M | 23 | 2314 | I,P,V,G,D | CR/PR | 3yr | 6 wks | no |
| 372 | 62F | 94 | 11083 | I,P,M | CR/CR** | 3yr | 6 wks** | no |
| 330 | 70M | 18.6 | 5028 | I, P, | CR/CR | 4yr | 5 wks | no |
| 056 | 69M | 12.4 | 7431 | I, P, B | CR/CR | 9yr | 5 wks | pneumonia |
| 413 | 64F | 23 | 2902 | I, P | CR/PR | 2yr | 6 wks | no |
| 414 | 72M | 45.5 | 5385 | I,P,B | CR/PR | 2yr | 6 wks | no |
| 042 | 69M | 6.64 | 1936 | I,O,V, | PR/PR*** | 5yr | 3 wks | no |
| 044 | 59F | 13.17 | 2944 | I,P,O, | PR/PD | 11yr | NA | no |
| 438 | 86M | **** | 9884**** | I,P | CR/CR | 1yr | 4 wks | no |
| 428 | 66F | 21 | 8077 | I,P,V,B | CR/PR | 3yr | 6 wks | no |
| 395 | 64M | 31 | 8323 | I, O, G | CR/PD | 2yr | NA | no |
D. Duration=disease duration, Skin Clear=duration of alemtuzumab therapy before clearing of skin disease CR=complete response, PR=partial response, PD=persistent disease, yr=years, wks=weeks, NA=not applicable I=IFN2a, P=extracorporeal photochemotherapy, V=vorinostat, B=bexarotene, O=Ontak, G=gemcitabine, D=Doxil, GND=gemcitabine/navelbine/doxil
Patient had CR to first two courses but developed resistance during the third course.
At 3 wks low dose alemtuzumab, patient was PR/PR; dose was increased to 30 mg three times a week and patient had CR/CR at 6 weeks.
Patient developed resistance and did not have T cell depletion.
Patient’s malignant clone was CD3+CD4−CD8−, the absolute number of malignant clonal cells, is shown as calculated from flow cytometry results. The CD4/CD8 T cell ratio was 3 but this is not clinically relevant.
In general, patients treated with alemtuzumab did not develop serious infections, despite the fact that they had no circulating T or B cells (Table I). The one observed exception was a patient who had been treated with lung irradiation for a prior diagnosis of Hodgkin’s disease. This patient had multiple medical problems including radiation-induced lung fibrosis and recurrent pulmonary infections. He succumbed to respiratory distress two months after starting alemtuzumab therapy, probably secondary to pneumonia. We saw no evidence for reactivation of CMV in any of our patients treated with low dose alemtuzumab.
Alemtuzumab therapy selectively depletes the skin of malignant and benign TCM
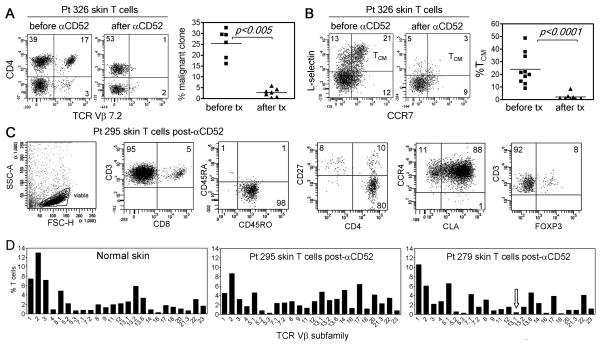
To gain insight into why alemtuzumab is effective in L-CTCL but not in MF, we studied T cells from the skin of L-CTCL patients before and after treatment with alemtuzumab. In L-CTCL patients in whom clonal malignant T cells could be identified, we observed depletion of the malignant T cell clone from the skin of patients after treatment with alemtuzumab; this depletion of clonal T cells from the skin correlated with clearance of skin disease and cutaneous symptoms (Table I and Fig. 2A). Further studies revealed that, in addition to loss of the malignant T cell clone, treatment with alemtuzumab depleted TCM from skin (Fig. 2B).
Fig. 2.
Alemtuzumab therapy depletes TCM from both blood and skin but does not affect skin resident TEM. (A) In patient 326, the TCR Vβ7.2-expressing malignant T cell clone was prominent in the skin before treatment but not detectable after alemtuzumab therapy. Similar results in additional patients with identifiable malignant T cell clones are shown. (B) All TCM were depleted from skin by alemtuzumab in patient 326. Studies in additional patients demonstrated that depletion of both benign and malignant TCM from skin was a common feature of alemtuzumab therapy. (C) TEM remaining in the skin of a patient on alemtuzumab therapy are shown. Skin T cells were CD4+ or CD8+ CD45RO+ memory T cells that expressed the skin homing addressins CLA and CCR4. Most CD4+ skin resident T cells were CD27low, a phenotype suggestive of TEM, although a subpopulation of FOXP3+ regulatory T cells was also evident. A representative patient is shown; the TEM phenotype of T cells remaining in skin was confirmed in 6 additional patients (Table S2). (D) The TCR diversity of T cells remaining in skin after alemtuzumab therapy was assessed by flow cytometry and found to be comparable to that of normal skin. The malignant clone in patient 295 was not identified by commercially available TCR Vβ antibodies. The malignant clonotype in patient 279 (TCR Vβ 13.1) is indicated by an arrow. Similar findings were observed in 4 additionalalemtuzumab treated patients.
Alemtuzumab therapy leaves intact a diverse population of skin resident TEM
Skin biopsies from L-CTCL patients after alemtuzumab therapy revealed the presence of viable T cells in skin despite a complete absence of circulating T cells in these patients (Fig. 2A,B and Table S2). The surviving T cells were CD45RO+ memory T cells, either CD4+ or CD8+, and co-expressed the skin homing addressins CLA and CCR4 (Fig 2C). The majority of these cells were CCR7−/L-selectin−/CD27lo, consistent with a TEM phenotype, although a subpopulation of FOXP3+ regulatory T cells was also evident (Fig. 2B,C, Table S2). In general, the phenotype of T cells remaining in the skin after alemtuzumab therapy resembled T cells found in healthy skin (10). When TCR diversity was assayed by flow cytometry after staining with TCR Vβ antibodies, the TCR diversity of T cells present in skin after alemtuzumab therapy was found to be comparable to the diversity of T cells from the skin of healthy individuals (Fig. 2D).
Neutrophils participate in alemtuzumab-mediated T cell depletion
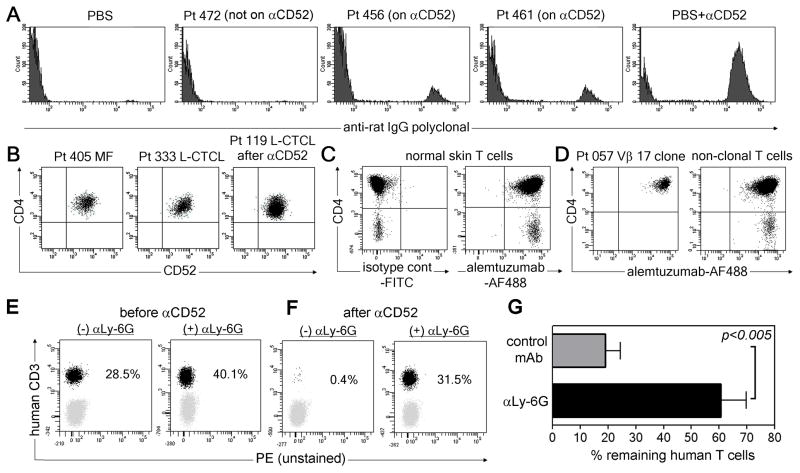
Our findings suggested that alemtuzumab depletes T cells in the circulation but not in the skin. Sparing of skin resident T cells could result from poor skin penetration of alemtuzumab, a lack of CD52 expression by skin resident TEM, differential post-translational modification of CD52 on skin TEM that abrogated binding, or a cell type critical for T cell depletion that is not present in the skin. We analyzed the extracellular fluid of skin samples from CTCL patients and detected the drug in the skin of patients on alemtuzumab but not in patients on other therapies (Fig. 3A), confirming that alemtuzumab enters the skin. CD52 was uniformly expressed by skin T cells from both MF and L-CTCL patients, both before and after alemtuzumab therapy (Fig 3B). We conjugated alemtuzumab to the fluorochrome Alexa Fluor 488 (AF488) and used this reagent to directly stain the T cells from the skin of both normal and CTCL patients. Alemtuzumab bound well to skin resident T cells from healthy individuals, and to both the malignant and benign T cells from CTCL skin lesions, demonstrating there were no post-translational modifications that blocked binding (Fig. 3C,D). In two mouse models, alemtuzumab has been shown to deplete CD52+ cells via antibody dependent cell mediated cytotoxicity (ADCC) mediated by neutrophils and to a lesser extent NK cells (11, 12). Therefore, we engrafted NOD/SCID/IL2 receptor γ chainnull mice with human T cells and treated them with alemtuzumab in the presence or absence of the neutrophil depleting Ab αLy-6G. Human T cell depletion occurred in mice treated with control antibody but not in neutrophil-depleted mice, suggesting neutrophils contribute to T cell depletion (Fig. 3E–G).
Fig. 3.
Neutrophils can support alemtuzumab-mediated T cell depletion. (A) Alemtuzumab was detected in the extracellular fluid of skin from patients on alemtuzumab therapy (456, 461) but not in a patient not receiving alemtuzumab (472). Negative (PBS) and positive control (PBS with alemtuzumab; PBS+αCD52) samples are also shown. (B) CD52 is expressed by skin T cells in CTCL patients before and after alemtuzumab therapy. Gates are set based on isotype-matched negative control staining. Representative patients are shown, similar results were observed in seven additional MF patients, 12 additional L-CTCL patients prior to therapy and five additional patients after alemtuzumab therapy (Table S3). (C, D) Alemtuzumab conjugated to AF488 bound to T cells from (C) normal skin and (D) both benign and malignant T cells from CTCL skin lesions. (E–G) NOD/SCID/IL2 receptor γ chainnull mice were engrafted with human PBMC and treated with alemtuzumab in the presence of either the neutrophil depleting Ab αLy-6G ((+) αLy-6G) or control mAb ((−) αLy-6G). The % of human T cells in blood before (E) and after (F) alemtuzumab was determined by flow cytometry. A representative set of mice is shown. (G) Aggregate data (mean+/− SEM) from eight mice treated with control Ab and nine treated with αLy-6G.
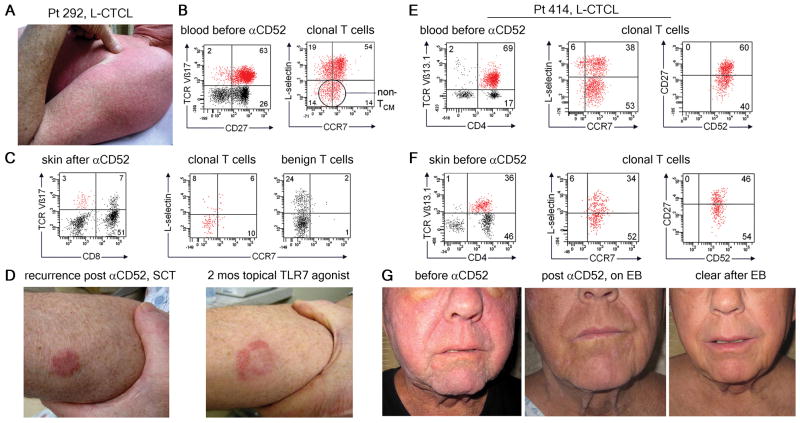
Alemtuzumab clears central memory (TCM) but not effector memory (TEM) disease
After antigen stimulation, TCM normally undergo proliferation and differentiation into tissue tropic TEM (13). In a subset of patients, clonal malignant T cells with both TCM and TEM phenotypes were observable in the circulation. In these patients, alemtuzumab therapy tended to improve pruritus and diffuse cutaneous erythema, clinical features of L-CTCL, but patients often had persistent or emergent fixed lesions more suggestive of MF. In one example, patient 292 had highly refractory L-CTCL and cutaneous disease that included both diffuse erythema and smaller discrete inflammatory skin lesions (Fig 4A). Malignant T cells expressed TCR Vβ17 and consisted of a mixed population of L-selectin+/CCR7+ TCM-like cells and non-TCM L-selectin−/CCR7−/+ cells (Fig. 4B). The patient’s cutaneous symptoms improved markedly after alemtuzumab therapy but skin biopsy after treatment demonstrated residual Vβ17+ T cells with a TEM phenotype. The patient underwent allogeneic stem cell transplantation (SCT) and subsequently recurred with fixed inflammatory skin lesions characteristic of MF (Fig. 4C). Topical therapy with the TLR7 agonist imiquimod, an effective treatment for MF, caused an initial central clearing and gradual resolution of these fixed lesions (Fig. 4D) (14). He remains clear of leukemic disease. Similarly, patient 414 presented with diffuse erythema, leonine facies (a clinical presentation of L-CTCL characterized by dense infiltration of the skin with malignant T cells), and more well defined nodular lesions (Fig. 4G). Analysis of T cells from the blood and skin prior to alemtuzumab therapy demonstrated the presence of both TCM and TEM-like subpopulations within the Vβ13.1-expressing malignant T cell pool (Fig. 4E,F). After alemtuzumab therapy, there was loss of the malignant T cell clone from blood as well as clearing of the patient’s diffuse erythema and leonine facies. However, the more discrete inflammatory nodules persisted and then worsened, requiring the addition of skin directed electron beam therapy (Fig. 4G). After electron beam treatment of his fixed lesions, the patient went into complete remission.
Fig. 4.
Alemtuzumab clears central memory (TCM) but not effector memory (TEM) disease. (A) Patient 292 had both diffuse erythema and discrete papules on presentation. (B) Clonal T cells in blood were TCR Vβ17+ with both TCM and TEM phenotypes. (C) After alemtuzumab, skin biopsy showed clearance of TCM from skin but detectable Vβ17+ TEM remained. (D) The patient underwent stem cell transplantation and disease then recurred with isolated skin lesions that responded to topical TLR7 agonist therapy. In a second patient, malignant T cells from the (E) blood and (F) skin were TCR Vβ13.1+ with both TCM and TEM phenotypes.(G) Clinical presentation with diffuse erythema and leonine facies. Well demarcated lesions remained after alemtuzmab. Skin directed electron beam therapy cleared remaining skin disease.
Skin T cells have decreased Th2 and increased Th1 responses after alemtuzumab therapy
Patients with L-CTCL have clinical abnormalities characteristic of Th2-biased immunity, including decreased antigen-specific T cell responses, impaired cell-mediated cytotoxicity, peripheral eosinophilia, and elevated levels of serum IgE and IgA (15–17). Malignant T cells in L-CTCL have been shown to be Th2 biased (18). One prior study demonstrated improved Th1 responses in peripheral blood T cells after the immunomodulatory therapies extracorporeal photopheresis and/or IFNα therapy (19). We assayed cytokine production of skin T cells before and after alemtuzumab (Fig. 5). IL-4 was produced by greater percentages of T cells in L-CTCL lesions than in normal skin (Fig. 5A). Alemtuzumab therapy reduced IL-4 production to levels observed in normal skin. Production of IFNγ was slightly higher in lesional L-CTCL skin than in normal controls, but alemtuzumab therapy further enhanced IFNγ to levels significantly higher than those observed in normal skin. In contrast, IL-17 production in both untreated and alemtuzumab-treated L-CTCL patients was significantly lower than in normal skin. TNFα is produced by most T cells in healthy skin and although alemtuzumab enhanced TNFα production, increases were not statistically significant. TSLP levels were not increased in the lesional skin of patients with MF or L-CTCL (Fig. S1).
Fig. 5.
Patients treated with alemtuzumab have decreased Th2 cytokine production and enhanced Th1 responses after therapy. (A) IL-4 was produced by greater percentages of T cells from L-CTCL skin lesions as compared to normal skin, and production was decreased after alemtuzumab therapy. Total T cells from L-CTCL skin lesions are shown. Representative histograms and results from multiple donors before (Pre) and after (Post) alemtuzumab are shown, compared to normal skin (NS). (B) Production of IFNγ was enhanced after alemtuzumab therapy. (C) Production of IL-17 was low in untreated L-CTCL patients and did not recover after alemtuzumab therapy. (D) TNFα production trended upward after alemtuzumab therapy but this change was not statistically significant.
Down-regulation of CD52 and development of a blocking antibody can lead to alemtuzumab resistance
Two patients developed resistance to alemtuzumab therapy. Patient 295 achieved two complete remissions but only a partial response to a third course. T cells were not fully depleted during this third course and three weeks after beginning therapy, he had both circulating CD4+ and CD8+ T cells (Fig. 6A). Malignant T cells were not recognized by available TCR Vβ antibodies but could be identified by their CD4+/CD3low phenotype. Peripheral blood disease worsened after discontinuation of alemtuzumab and analysis demonstrated loss of CD52 expression on malignant T cells and a subset of CD8 cells (Fig. 6B). Loss of CD52 expression was durable; six months after alemtuzumab therapy, lymphocytes remained CD52− (Fig. 6C).
Fig. 6.
Down-regulation of CD52 and development of a blocking antibody can lead to alemtuzumab resistance. (A) Malignant T cells from the blood of patient 295 were identifiable by their distinct CD4+CD3low phenotype. At 3 weeks of therapy, both benign (gray) and malignant (black) T cells remained in blood. (B) There was loss of CD52 expression on clonal malignant T cells. (C) Down-regulation of CD52 expression was persistent. (D) Patient 042 showed only transient loss of lymphocytes and monocytes. (E) CD3 T cells and monocytes expressed CD52. (F) Patient plasma blocked binding of alemtuzumab to normal blood T cells. (G) Loss of alemtuzumab binding was titratable and (H) depleted by protein G sepharose.
Patient 042 experienced an initial drop and then a rebound in the number of circulating monocytes and T cells on alemtuzumab therapy (Fig. 6D). After six weeks, normal numbers of T cells and monocytes expressing CD52 were demonstrable in the peripheral blood (Fig. 6E). To assay for the presence of an inhibitory factor in serum, AF488-conjugated alemtuzumab was used to stain normal blood T cells in the presence and absence of plasma from patient 042 or healthy donors. Plasma from patient 042 blocked binding of alemtuzumab to T cells (Fig. 6F). Inhibition of binding was titratable (Fig. 6G). Preclearance of patient 042’s plasma with protein G-conjugated sepharose beads led to a loss of binding inhibition, suggesting the presence in this patient plasma of an antibody that blocked alemtuzumab binding to CD52.
Discussion
Alemtuzumab (Campath) is a humanized monoclonal antibody directed against CD52, a protein expressed on T cells, B cells, monocytes, eosinophils, and a subset of dendritic cells. Alemtuzumab leads to rapid and prolonged depletion of T and B cells from blood (20). Alemtuzumab is FDA-approved for the treatment of chronic lymphocytic leukemia (CLL) but is used off-label to treat autoimmune and inflammatory diseases including multiple sclerosis (MS), graft-versus-host disease, and rheumatoid arthritis (20–22). In CLL patients, alemtuzumab therapy is immunosuppressive and associated with reactivation of systemic CMV (23). However, CLL patients have marked immune abnormalities and most CLL patients have received other systemic immunosuppressive therapies prior to receiving alemtuzumab (24). Indeed, otherwise healthy individuals with MS treated with alemtuzumab are not more prone to infections and sometimes develop secondary autoimmune diseases, suggesting some aspects of T cell immunity are spared (25).
Alemtuzumab has been previously used at higher, more conventional dosages for the treatment of CTCL, with variable reports of infectious complications (26–37). Infections were more common in heavily pretreated patients (33) and the lower doses used in this study have not been associated with increased infections (38).
We report that alemtuzumab, used at 15–30% the dosage used in CLL, led to clinical responses in 100% and complete remission in 50% of patients with refractory L-CTCL. Other systemic therapies for advanced L-CTCL, including extracorporeal photopheresis, IFNα, IFNγ, denileukin diftitox, bexarotene, and HDAC inhibitors such as vorinostat all induce partial responses in only 30% of patients (39)..
We found that alemtuzumab depleted T cells in blood but not in skin, explaining why it effectively treated L-CTCL but not MF. These data contrast with zanolimumab, an antibody to CD4 that depletes CD4+ T cells, which is effective in MF (40). Although both antibodies are human IgG1, they differ in effector mechanisms. Zanolimumab inhibits and depletes CD4+ T cells in two ways—first by uncoupling CD4 from the TCR, which activates inhibitory pathways mediated by PDK-1 and SHIP-1, and second, by depleting CD4+ T cells through NK cell-mediated ADCC (41). There is no evidence for neutrophil-mediated ADCC with Zanolimumab. In contrast, two mouse models using hCD52-expressing cells as targets showed that alemtuzumab depletes T cells only by ADCC mediated by neutrophils, and to a lesser extent, NK cells (11, 12). In our experiments, immunodeficient mice engrafted with human T cells only experienced T cell depletion in the presence of neutrophils (Fig. 3E–G). These mice lack NK cells so these experiments do not evaluate NK cell-mediated ADCC. However, both neutrophils and NK cells are abundant in blood but rare in normal skin. The absence of these cell types may explain why alemtuzumab does not deplete T cells in skin.
TEM and TCM are two distinct subsets of memory T cells (9). TEff are generated early in primary immune responses, up-regulate tissue homing addressins, develop effector functions, migrate into peripheral tissues and orchestrate the clearance of pathogens (42, 43). In mouse models, a subset of these effector T cells differentiate into TEM, long lived, highly protective T cells that persist long term in peripheral tissues (44, 45). In contrast, TCM appear in the blood after resolution of the acute immune response (46), co-express the lymph node homing addressins L-selectin and CCR7, and have less potent effector functions than TEM. However, TCM can proliferate and give rise to new populations of tissue-tropic effector cells when rechallenged with antigen (13).
In mouse models of cutaneous HSV infection, TCM recirculated between the skin, blood, and lymph nodes, whereas TEM were sessile, non-recirculating cells (47). Our findings suggest these distinct migratory patterns also occur in human beings. Alemtuzumab depleted T cells from blood and over time purged the skin of TCM, leaving skin resident TEM unaffected. Because alemtuzumab only depleted T cells in the circulation, the depletion of TCM from skin by alemtuzumab suggests that TCM actively recirculate between the blood and skin. By the same argument, the survival of a diverse population of skin resident TEM argues that these T cells are non-recirculating and remain resident long-term within the skin.
Patients with progressive MF can develop lymph node involvement, most often of the skin draining lymph nodes, without evidence of peripheral blood disease. In these patients, malignant T cells may undergo changes in addressin expression that allow them to exit the skin and enter draining lymph nodes via afferent lymph. The observation that at least some human TEM are nonmigratory has the potential to explain some puzzling dermatologic observations, including fixed drug eruption and the fixed and recurrent nature of psoriatic plaques.
Research in mice and humans suggests that skin and other epithelial tissues are progressively colonized by a sessile population of TEM that increase in number and diversity following infections and represent an accumulation of local immune memory (44, 48). In one series of experiments in mice, skin infection with vaccinia was used to generate a long-lived skin resident population of TEM. TCM were then depleted from the skin and blood by FTY720 and the mice were re-infected with vaccinia (45). These mice were fully protected, suggesting that skin resident TEM can provide immune protection in the absence of TCM.
Although similar experiments cannot be performed in humans, the biology of L-CTCL patients treated with alemtuzumab is striking in its similarity. L-CTCL patients have accumulated skin resident TEM as a result of cutaneous infections. Alemtuzumab depletes all TCM, including malignant T cells, from the skin and blood but leaves behind skin resident TEM. Although these patients have no circulating T or B cells, neither our patients nor the many MS patients treated with alemtuzumab develop infections (25). Although indirect, this is evidence in humans that skin resident TEM can function to protect the skin from infection in the absence of circulating T cells. The lack of pulmonary and GI infections in all but one of our patients suggests that TEM may also persist in these tissues, although this remains to be demonstrated. The one patient who developed pulmonary infection after alemtuzumab had a history of pulmonary irradiation, a therapy that would be expected to deplete lung resident TEM.
In summary, our observations of L-CTCL patients treated with low dose alemtuzumab provide evidence in humans that cutaneous TCM recirculate, TEM are nonmigratory, and resident TEM can function alone to provide front-line immunologic protection. We find that low dose alemtuzumab is a safe and effective therapy for carefully selected L-CTCL patients that depletes malignant cells, produces improvement in all patients and full remission in 50% of patients while at the same time sparing benign tissue resident TEM that protect against infection.
Materials and Methods
Skin and blood samples
The protocols of this study were performed in accordance with the Declaration of Helsinki, and were approved by the Institutional Review Board of the Partners Human Research Committee (Partners Research Management). Skin from healthy patients was obtained from patients undergoing cosmetic surgery procedures and blood from healthy individuals was obtained as discarded tissue after leukopheresis. Blood and lesional skin from patients with CTCL were obtained from patients seen at the Dana-Farber/Brigham and Women’s Cancer Center Cutaneous Lymphoma Program. L-CTCL and MF patients described in this manuscript met the WHO-EORTC criteria for L-CTCL/SS or MF (1). Since 2002, 443 CTCL patients have been enrolled in studies that permit the collection of blood and/or skin; of these, 427 patients (96%) have consented to provide samples. There have been 11 publications to date on this patient population. Patients are assigned a unique study number (e.g. Pt 188) that is used to identify their experimental data in this and other manuscripts arising from studies of this patient population. PBMC were isolated by ficoll centrifugation and T cells were isolated from skin using short-term explant cultures (1–3 weeks) as described (49).
Flow cytometry
Analysis of T cells was performed using directly conjugated monoclonal antibodies obtained from BD Biosciences, eBioscience, Biolegend, or R&D Systems. Vβ staining was performed using the IOtest Beta Mark TCR V beta Repertoire kit (Beckman Coulter) as per manufacturer’s instructions. FOXP3 staining utilized the PCH101 antibody (eBioscience). Isotype-matched negative control antibodies were used to set the gates for positive staining. Alemtuzumab was conjugated to AF488 using Alexa Fluor 488 Microscale Protein Labeling Kit (Invitrogen) as per manufacturer’s instructions. For analysis of cytokine production, T cells were stimulated with either control medium or 50 ng/ml PMA and 750 ng/ml ionomycin plus 10 μg/ml Brefeldin A (Calbiochem) for four hours. Cells were surface stained, fixed, permeabilized, stained with anti-cytokine antibodies, and examined by flow cytometry. Analysis of flow cytometry samples was performed on Becton Dickinson FACSCanto instruments and data was analyzed using FACSDiva software (V5.1).
Detection of alemtuzumab in the extracellular fluid of skin
Alemtuzumab is a humanized monoclonal antibody that retains amino acid sequences derived from the original rat IgG2a antibody (50). These residual rat sequences can be recognized by polyclonal anti-rat antibodies, allowing the detection of alemtuzumab in biological specimens (51). Briefly, skin biopsies from patients were minced in 100 μl of medium and the resulting supernatant was denatured in the presence of 2% SDS for 5 min at 96°C to expose the rat-derived amino acid sequence present in alemtuzumab. This denatured supernatant was then incubated with 6.9 μM carboxylated polystyrene beads (Bangs Laboratories) coated with rabbit anti-rat IgG antibodies (Sigma Aldrich). PE-labeled rabbit anti-human IgG (BioLegend) was then added to the beads and binding was assessed by flow cytometry. 0.01 ug/ml alemtuzumab in PBS was used as a positive control (Fig. 3A, far right).
T-cell depletion studies in immunodeficient mice engrafted with human T cells
T cells from the blood of healthy donors were obtained using the Pan T Cell Isolation Kit (Miltenyi Biotec) and the autoMACS™ Separator (Miltenyi Biotec) according to manufacturer’s instructions. 6- to 8-week-old NOD/SCID/IL2 receptor γ chainnull mice were injected I.V. with 2.5×106 human T cells in 250 μL of saline. Successfully engrafted mice were those that had greater than 500/μL human CD3+ cells in blood as determined by flow cytometry staining for CD3 and direct cell counting. Mice were then injected I.P. with 0.5 mg anti-mouse Ly-6G abs (1A8; BioXCell) or control rat IgG2a (BioXCell) and 72 hours later, 2.5 μg alemtuzumab or control human IgG (Jackson ImmunoResearch) was administrated I.P.. The efficacy of T cell depletion was measured by comparing the absolute numbers of CD3 positive cells/μL blood before and 24 hrs after alemtuzumab administration. The non-parametric Wilcoxon rank-sum test was used for comparisons between groups.
Depletion of blocking antibody from plasma using protein G
To deplete immunoglobulins from the plasma of patient 042, plasma was incubated with Pierce® Protein G Agarose (Thermo Scientific, Fisher) at 4 °C overnight. Ig-depleted and non-depleted plasma from patient 042 and plasma from healthy donors were then assessed for the ability to block alemtuzumab binding. Plasma samples were mixed at the indicated concentrations with Alexa Fluor 488-labeled alemtuzumab and this mixture was used to label PBMC derived from the blood from healthy individuals. Binding of fluorochrome-labeled alemtuzumab to cells was then assessed by flow cytometry.
Statistical analyses
The non-parametric Wilcoxon rank-sum test was used for comparisons between two groups. Statistical analysis of three comparison groups was performed using the Kruskal-Wallis test and Dunn’s test. A significance level of P<0.05 was chosen for all analyses.
Supplementary Material
Acknowledgments
The authors would like to thank the patients who made this work possible, both for entrusting us with their clinical care and for donating skin and blood samples. Dr. Thomas Cochran of the Boston Center for Plastic Surgery and Dr. Elof Eriksson of Brigham and Women’s Hospital generously provided adult human skin samples.
Funding: This work was supported by a generous charitable contribution from Edward P. Lawrence, Esq., a Damon Runyon Clinical Investigator Award (to R.A.C.) and R01 AR056720 (to R.A.C.), A Special Fellow Award from the Leukemia & Lymphoma Society (to R.W.), the SPORE in Skin Cancer P50 CA9368305 NIH/NCI (to T.S.K.) and R01 A1025082 NIH/NIAID (to T.S.K). Salary support for C.S. was provided by the Swiss National Science Foundation and the Fondation Rene Touraine.
Footnotes
Competing interests: C.S.C. has served on an advisory board for Genzyme. R.A.C. served on an expert panel for Biogen Idec. The other authors declare that they have no competing interests.
List of supplementary material
Fig. S1. TSLP levels are not increased in the skin lesions of MF or L-CTCL. Table SI. The majority of clonal malignant T cells in L-CTCL skin lesions express skin homing addressins.
Table S2. T cells remaining in skin after alemtuzumab therapy have a skin resident TEM phenotype.
Table S3. T cells in skin express CD52.
Reference
Author contributions: R.A.C. planned, carried out and supervised experiments, analyzed data, prepared figures, and drafted the manuscript. R.W. carried out experiments, analyzed data, helped prepare figures, and provided clinical data, J.E.T. and C.S. carried out experiments and provided editorial assistance. D.C.F., M.C.T., N.A., A.A.D., K.S.C., C.S.C., N.R.L., and J.B.C. provided clinical samples and information, helped to recruit patients and assisted in editing the manuscript. T.S.K. planned experiments, funded the collection of clinical specimens, recruited patients, interpreted results, and contributed to drafting and editing of the manuscript. The research program was led by R.A.C. and T.S.K. who are joint senior authors.
References and Notes
- 1.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, Grange F, Harris NL, Kempf W, Kerl H, Kurrer M, Knobler R, Pimpinelli N, Sander C, Santucci M, Sterry W, Vermeer MH, Wechsler J, Whittaker S, Meijer CJLM. WHO-EORTC classification for cutaneous lymphomas 10.1182/blood-2004-09-3502. Blood. 2005;105:3768. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 2.Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Archives of dermatology. 2003;139:857. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology™ Non-Hodgkin’s Lymphomas Version 3.2009 Vol. 2009 To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. (© 2009 National Comprehensive Cancer Network, Inc., 2009)..
- 4.Shin J, Monti S, Aires DJ, Duvic M, Golub T, Jones DA, Kupper TS. Lesional gene expression profiling in cutaneous T-cell lymphoma reveals natural clusters associated with disease outcome. Blood. 2007;110:3015. doi: 10.1182/blood-2006-12-061507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Doorn R, van Kester MS, Dijkman R, Vermeer MH, Mulder AA, Szuhai K, Knijnenburg J, Boer JM, Willemze R, Tensen CP. Oncogenomic analysis of mycosis fungoides reveals major differences with Sezary syndrome. Blood. 2009;113:127. doi: 10.1182/blood-2008-04-153031. [DOI] [PubMed] [Google Scholar]
- 6.Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010;116:767. doi: 10.1182/blood-2009-11-251926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferenczi K, Yawalkar N, Jones D, Kupper TS. Monitoring the decrease of circulating malignant T cells in cutaneous T-cell lymphoma during photopheresis and interferon therapy. Archives of dermatology. 2003;139:909. doi: 10.1001/archderm.139.7.909. [DOI] [PubMed] [Google Scholar]
- 8.Clark RA, Shackelton JB, Watanabe R, Calarese A, Yamanaka K, Campbell JJ, Teague JE, Kuo HP, Hijnen D, Kupper TS. High-scatter T cells: a reliable biomarker for malignant T cells in cutaneous T-cell lymphoma. Blood. 2011;117:1966. doi: 10.1182/blood-2010-05-287664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 10.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 11.Siders WM, Shields J, Garron C, Hu Y, Boutin P, Shankara S, Weber W, Roberts B, Kaplan JM. Involvement of neutrophils and natural killer cells in the anti-tumor activity of alemtuzumab in xenograft tumor models. Leukemia & lymphoma. 2010;51:1293. doi: 10.3109/10428191003777963. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y, Turner MJ, Shields J, Gale MS, Hutto E, Roberts BL, Siders WM, Kaplan JM. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology. 2009;128:260. doi: 10.1111/j.1365-2567.2009.03115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallusto F, Geginat J, Lanzavecchia A. Central Memory and Effector Memory T Cell Subsets: Function, Generation, and Maintenance. Annual review of immunology. 2004;22:745. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 14.Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-Cell lymphoma with topical application of the immune response modifier imiquimod. Archives of dermatology. 2002;138:1137. doi: 10.1001/archderm.138.9.1137. [DOI] [PubMed] [Google Scholar]
- 15.Rook AH, Heald P. The immunopathogenesis of cutaneous T-cell lymphoma. Hematology/oncology clinics of North America. 1995;9:997. [PubMed] [Google Scholar]
- 16.Rook AH, Vowels BR, Jaworsky C, Singh A, Lessin SR. The immunopathogenesis of cutaneous T-cell lymphoma. Abnormal cytokine production by Sezary T cells. Archives of dermatology. 1993;129:486. [PubMed] [Google Scholar]
- 17.Tendler CL, Burton JD, Jaffe J, Danielpour D, Charley M, McCoy JP, Pittelkow MR, Waldmann TA. Abnormal cytokine expression in Sezary and adult T-cell leukemia cells correlates with the functional diversity between these T-cell malignancies. Cancer research. 1994;54:4430. [PubMed] [Google Scholar]
- 18.Dummer R, Heald PW, Nestle FO, Ludwig E, Laine E, Hemmi S, Burg G. Sezary syndrome T-cell clones display T-helper 2 cytokines and express the accessory factor-1 (interferon-gamma receptor beta-chain) Blood. 1996;88:1383. [PubMed] [Google Scholar]
- 19.Yoo EK, Cassin M, Lessin SR, Rook AH. Complete molecular remission during biologic response modifier therapy for Sezary syndrome is associated with enhanced helper T type 1 cytokine production and natural killer cell activity. Journal of the American Academy of Dermatology. 2001;45:208. doi: 10.1067/mjd.2001.116345. [DOI] [PubMed] [Google Scholar]
- 20.Coles AJ, Cox A, Le Page E, Jones J, Trip SA, Deans J, Seaman S, Miller DH, Hale G, Waldmann H, Compston DA. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. Journal of neurology. 2006;253:98. doi: 10.1007/s00415-005-0934-5. [DOI] [PubMed] [Google Scholar]
- 21.Isaacs JD, Watts RA, Hazleman BL, Hale G, Keogan MT, Cobbold SP, Waldmann H. Humanised monoclonal antibody therapy for rheumatoid arthritis. Lancet. 1992;340:748. doi: 10.1016/0140-6736(92)92294-p. [DOI] [PubMed] [Google Scholar]
- 22.Hale G, Waldmann H. Recent results using CAMPATH-1 antibodies to control GVHD and graft rejection. Bone marrow transplantation. 1996;17:305. [PubMed] [Google Scholar]
- 23.Martin SI, Marty FM, Fiumara K, Treon SP, Gribben JG, Baden LR. Infectious complications associated with alemtuzumab use for lymphoproliferative disorders. Clin Infect Dis. 2006;43:16. doi: 10.1086/504811. [DOI] [PubMed] [Google Scholar]
- 24.Nosari A, Tedeschi A, Ricci F, Montillo M. Characteristics and stage of the underlying diseases could determine the risk of opportunistic infections in patients receiving alemtuzumab. Haematologica. 2008;93:e30. doi: 10.3324/haematol.12465. [DOI] [PubMed] [Google Scholar]
- 25.Jones JL, Coles AJ. Spotlight on alemtuzumab. International MS journal/MS Forum. 2009;16:77. [PubMed] [Google Scholar]
- 26.Querfeld C, Mehta N, Rosen ST, Guitart J, Rademaker A, Gerami P, Kuzel TM. Alemtuzumab for relapsed and refractory erythrodermic cutaneous T-cell lymphoma: a single institution experience from the Robert H. Lurie Comprehensive Cancer Center. Leukemia & lymphoma. 2009;50:1969. doi: 10.3109/10428190903216770. [DOI] [PubMed] [Google Scholar]
- 27.Alinari L, Geskin L, Grady T, Baiocchi RA, Bechtel MA, Porcu P. Subcutaneous alemtuzumab for Sezary Syndrome in the very elderly. Leukemia research. 2008;32:1299. doi: 10.1016/j.leukres.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Ure UB, Ar MC, Salihoglu A, Guner SI, Baran A, Oguz O, Ferhanoglu B. Alemtuzumab in Sezary syndrome: efficient but not innocent. Eur J Dermatol. 2007;17:525. doi: 10.1684/ejd.2007.0269. [DOI] [PubMed] [Google Scholar]
- 29.Roch N, Salameire D, Gressin R, Morand P, Epaulard O, Pavese P, Brion JP, Stahl JP. Fatal adenoviral and enteroviral infections and an Epstein-Barr virus positive large B-cell lymphoma after alemtuzumab treatment in a patient with refractory Sezary syndrome. Scandinavian journal of infectious diseases. 2008;40:343. doi: 10.1080/00365540701684817. [DOI] [PubMed] [Google Scholar]
- 30.Gautschi O, Blumenthal N, Streit M, Solenthaler M, Hunziker T, Zenhausern R. Successful treatment of chemotherapy-refractory Sezary syndrome with alemtuzumab (Campath-1H) European journal of haematology. 2004;72:61. doi: 10.1046/j.0902-4441.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- 31.Capalbo S, Delia M, Dargenio M, Liso A, Diomede D, Garofalo L, Liso V. Mycosis fungoides/Sezary syndrome: a report of three cases treated with Campath-1H as salvage treatment. Medical oncology (Northwood, London, England) 2003;20:389. doi: 10.1385/MO:20:4:389. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy GA, Seymour JF, Wolf M, Januszewicz H, Davison J, McCormack C, Ryan G, Prince HM. Treatment of patients with advanced mycosis fungoides and Sezary syndrome with alemtuzumab. European journal of haematology. 2003;71:250. doi: 10.1034/j.1600-0609.2003.00143.x. [DOI] [PubMed] [Google Scholar]
- 33.Lundin J, Hagberg H, Repp R, Cavallin-Stahl E, Freden S, Juliusson G, Rosenblad E, Tjonnfjord G, Wiklund T, Osterborg A. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood. 2003;101:4267. doi: 10.1182/blood-2002-09-2802. [DOI] [PubMed] [Google Scholar]
- 34.Ferrajoli A, O’Brien SM, Cortes JE, Giles FJ, Thomas DA, Faderl S, Kurzrock R, Lerner S, Kontoyiannis DP, Keating MJ. Phase II study of alemtuzumab in chronic lymphoproliferative disorders. Cancer. 2003;98:773. doi: 10.1002/cncr.11551. [DOI] [PubMed] [Google Scholar]
- 35.Lundin J, Osterborg A, Brittinger G, Crowther D, Dombret H, Engert A, Epenetos A, Gisselbrecht C, Huhn D, Jaeger U, Thomas J, Marcus R, Nissen N, Poynton C, Rankin E, Stahel R, Uppenkamp M, Willemze R, Mellstedt H. CAMPATH-1H monoclonal antibody in therapy for previously treated low-grade non-Hodgkin’s lymphomas: a phase II multicenter study. European Study Group of CAMPATH-1H Treatment in Low-Grade Non-Hodgkin’s Lymphoma. J Clin Oncol. 1998;16:3257. doi: 10.1200/JCO.1998.16.10.3257. [DOI] [PubMed] [Google Scholar]
- 36.Lenihan DJ, Alencar AJ, Yang D, Kurzrock R, Keating MJ, Duvic M. Cardiac toxicity of alemtuzumab in patients with mycosis fungoides/Sezary syndrome. Blood. 2004;104:655. doi: 10.1182/blood-2003-07-2345. [DOI] [PubMed] [Google Scholar]
- 37.Gibbs SD, Herbert KE, McCormack C, Seymour JF, Prince HM. Alemtuzumab: effective monotherapy for simultaneous B-cell chronic lymphocytic leukaemia and Sezary syndrome. European journal of haematology. 2004;73:447. doi: 10.1111/j.1600-0609.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 38.Bernengo MG, Quaglino P, Comessatti A, Ortoncelli M, Novelli M, Lisa F, Fierro MT. Low-dose intermittent alemtuzumab in the treatment of Sezary syndrome: clinical and immunologic findings in 14 patients. Haematologica. 2007;92:784. doi: 10.3324/haematol.11127. [DOI] [PubMed] [Google Scholar]
- 39.Lansigan F, Foss FM. Current and emerging treatment strategies for cutaneous T-cell lymphoma. Drugs. 2010;70:273. doi: 10.2165/11532190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Kim YH, Duvic M, Obitz E, Gniadecki R, Iversen L, Osterborg A, Whittaker S, Illidge TM, Schwarz T, Kaufmann R, Cooper K, Knudsen KM, Lisby S, Baadsgaard O, Knox SJ. Clinical efficacy of zanolimumab (HuMax-CD4): two phase 2 studies in refractory cutaneous T-cell lymphoma. Blood. 2007;109:4655. doi: 10.1182/blood-2006-12-062877. [DOI] [PubMed] [Google Scholar]
- 41.Rider DA, Havenith CE, de Ridder R, Schuurman J, Favre C, Cooper JC, Walker S, Baadsgaard O, Marschner S, vandeWinkel JG, Cambier J, Parren PW, Alexander DR. A human CD4 monoclonal antibody for the treatment of T-cell lymphoma combines inhibition of T-cell signaling by a dual mechanism with potent Fc-dependent effector activity. Cancer research. 2007;67:9945. doi: 10.1158/0008-5472.CAN-07-1148. [DOI] [PubMed] [Google Scholar]
- 42.Mackay C, Marston W, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitton JL, Zhang J. Principles of cytotoxic T lymphocyte induction and recognition. Current topics in microbiology and immunology. 1995;202:247. doi: 10.1007/978-3-642-79657-9_16. [DOI] [PubMed] [Google Scholar]
- 44.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 45.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nature medicine. 2010;16:224. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Razvi ES, Jiang Z, Woda BA, Welsh RM. Lymphocyte apoptosis during the silencing of the immune response to acute viral infections in normal, lpr, and Bcl-2-transgenic mice. The American journal of pathology. 1995;147:79. [PMC free article] [PubMed] [Google Scholar]
- 47.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature immunology. 2009;10:524. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 48.Clark RA. Skin resident T cells: the ups and downs of on site immunity. The Journal of investigative dermatology. 2010;130:362. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark RA, Chong BF, Mirchandani N, Yamanaka K, Murphy GF, Dowgiert RK, Kupper TS. A novel method for the isolation of skin resident T cells from normal and diseased human skin. The Journal of investigative dermatology. 2006;126:1059. doi: 10.1038/sj.jid.5700199. [DOI] [PubMed] [Google Scholar]
- 50.Riechmann L, Clark M, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature. 1988;332:323. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- 51.Chan HE, Jilani I, Chang R, Albitar M. Measuring humanized antibodies in plasma of patients treated with antibody-based therapy using bead-based flow cytometry: the story of alemtuzumab. Methods in molecular biology (Clifton, NJ. 2007;378:159. doi: 10.1007/978-1-59745-323-3_11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.