Abstract
Drug use disorders are often accompanied by deficits in the capacity to efficiently process reward-related information and to monitor, suppress, or override reward-controlled behavior when goals are in conflict with aversive or immediate outcomes. This emerging deficit in behavioral flexibility and impulse control may be a central component of the progression to addiction, as behavior becomes increasingly driven by drugs and drug-associated cues at the expense of more advantageous activities. Understanding how neural mechanisms implicated in impulse control are affected by addictive drugs may therefore prove a useful strategy in the search for new treatment options. Animal models of impulsivity and addiction could make a significant contribution to this endeavor. Here, some of the more common behavioral paradigms used to measure different aspects of impulsivity across species are outlined, and the importance of the response to reward-paired cues in such paradigms is discussed. Naturally occurring differences in forms of impulsivity have been found to be predictive of future drug self-administration, but drug exposure can also increase impulsive responding. Such data are in keeping with the suggestion that impulsivity may contribute to multiple stages within the spiral of addiction. From a neurobiological perspective, converging evidence from rat, monkey, and human studies suggest that compromised functioning within the orbitofrontal cortex may critically contribute to the cognitive sequelae of drug abuse. Changes in gene transcription and protein expression within this region may provide insight into the mechanism underlying drug-induced cortical hypofunction, reflecting new molecular targets for the treatment of uncontrolled drug-seeking and drug-taking behavior.
Keywords: Orbitofrontal Cortex, Cocaine, Delay Discounting, Five-Choice Serial Reaction Time Task, Rat, Monkey
DEFICITS IN ASPECTS of impulse control are a key feature of psychiatric disorders, including bipolar disorder and hypomania, borderline personality disorder and attention-deficit hyperactivity disorder (APA DSM IV, 1994). Furthermore, it is increasingly recognized that high levels of impulsivity are associated with vulnerability to substance abuse and can contribute to relapse to drug-seeking and treatment failure (Jentsch and Taylor, 1999b; Moeller et al., 2001b; Volkow and Fowler, 2000). Consequently, the need for a better understanding of the neurobiological basis of impulsivity is growing, as such information could significantly contribute to improved treatment options for these illnesses. One of the biggest challenges facing researchers in this field is defining impulsivity in a manner that allows its empirical measurement in laboratory-based tests. Through this process, it has become clear that the term “impulsivity” covers a range of actions that may recruit different brain circuitries and be susceptible to diverse pharmacological influences; despite this growing knowledge, it is unclear what specific dimensions of impulsivity play the most important role in substance use disorders (see Torregrossa et al., 2008).
One of the most important issues for the fields of psychiatry and neuroscience to address is the reasons why some individuals are susceptible to psychiatric disorders whereas others are resilient. This review will focus on the role played by impulsivity in mediating drug abuse, and how preclinical models may help in the generation of biomarkers for addiction. Using nonhuman subjects enables higher throughput pharmacological challenges and ex vivo molecular analysis of brain tissue. Such experiments could help to determine whether individuals showing higher levels of impulsivity respond differently to certain drugs and what the molecular basis of such variation might be. Furthermore, the environment of laboratory animals is easier to control and manipulate, providing the opportunity to test for causal links between environmental factors and the expression of impulsive behaviors. Finally, it is theoretically possible to selectively breed animals for high levels of impulsivity, which should provide insight into the genetic and epigenetic inheritance of impulse control deficits. The first part of this review therefore aims to outline some of the ways in which impulsivity has been measured in the laboratory, and why it might be important to consider the differences between these behavioral measures when mapping the relationship between impulsivity and addiction. Literature from both rats and monkeys, indicating an important role for the orbitofrontal cortex (OFC) in the interaction between impulsivity and cocaine abuse, will then be considered.
Phylogenetic Conservation of Individual Differences in Impulsivity
Although impulsivity, in most of its forms, is typically envisioned as inefficient, unadaptive or suboptimal, this is clearly not universally the case. The remarkable conservation of “impulsive” traits across phylogeny suggests that manifestations of rapid action, quick decision making, and hasty reward seeking can represent a phenotypic advantage for many species. This is true across vertebrate species, at the least. The observation that the rapid, inflexible approach to potentially risky social stimuli in adolescent vervet monkeys predicts adult dominance to a significant, albeit limited, degree is a particularly powerful illustration of this point (Fairbanks et al., 2004a). Although the full impact of laboratory studies of impulsive temperament in rodents must be considered in light of the restricted phenotypic and genotypic variability that has been attained through selective breeding, as well as because of the very different manifestations of impulsivity that may stem from the different ecological niche occupied by rodents, they nevertheless represent efficient models for investigating the neural systems underlying aspects of impulse control. Many species of nonhuman primates, including those commonly used in laboratory investigations, retain a good deal of natural variation in these traits (Fairbanks, 2001; Fairbanks et al., 2004b; Higley and Linnoila, 1997; Manuck et al., 2003). Hence, these animals represent a unique opportunity to investigate the genetic, molecular, and neural basis of impulsivity and its neurocognitive correlates (James et al., 2007) in a manner that may directly inform our understanding of these processes in humans.
It appears that genetic and neurochemical determinants of impulsive temperament are conserved at least across Old World monkeys and humans (Bailey et al., 2007; Forbes et al., 2009; James et al., 2007; Keltikangas-Jarvinen et al., 2003). Remarkably, a 48-basepair variable number tandem repeat polymorphism in exon 3 of the dopamine D4 receptor (DRD4) gene is found across a broad number of mammals, and this similar allelic variant is responsible for phenotypic variation in aspects of impulsive temperament in several species. This result further endorses the notion that the forces acting upon impulsivity are phylogenetically very old, using common mechanisms in species having diverged millions of years ago. Although genetic factors mediating impulsivity may not be uniformly conserved across all mammals, there is considerable evidence suggesting that shared neurochemical mechanisms contribute to these processes. For example, relatively low levels of brain serotonin turnover (as indicated by cerebrospinal fluid levels of 5-hydroxyindoleacetic acid, the primary acidic catabolite of serotonin) are predictive of a variety of impulsive behaviors in both monkeys and humans (Brown and Linnoila, 1990; Fairbanks et al., 1999, 2001; Faustman et al., 1991; Spreux-Varoquaux et al., 2001; Westergaard et al., 2003). Clearly, this particular phenomenon dovetails nicely with the observation that some aspects of impulsivity in rodents are also affected by serotonergic manipulations (Soubrie, 1986; Winstanley et al., 2004b), showing how rodents can be used to understand mechanistic relationships discovered in more complex species in a directed and hypothesis-driven way.
Fractionating Impulsivity
In keeping with the diverse nature of impulsive behaviors, there are currently a variety of very different tests used in both human and nonhuman subjects to measure forms of impulsivity, leading some to question whether “impulsivity” is even a valid or useful construct. However, its nonunitary nature is actually more typical than atypical when comparing it with other psychological concepts. For example, there are many different classes of memory, short-term, long-term, iconic, episodic, semantic, procedural, verbal, working, reference, spatial, to name but a few (see Kesner and Rogers, 2004; Squire, 2004 for recent reviews). In this way, memory is an umbrella term that usefully covers these subclasses of mnemonic processes, and the fact that these mental faculties are distributed across different brain regions does not invalidate the term.
It may be important to note that isolating the exact nature of the memory or attentional deficit associated with certain psychiatric or neurological condition has improved, rather than hindered, diagnostic tools (Brooks and Baddeley, 1976; Budson, 2009). In addition, it has been possible for research into the nonunitary nature of such processes to contribute to more fundamental hypotheses regarding how the brain works. For example, the realization that there is no one “memory centre” supports the view that our mental operations are conducted within a distributed network of “multi-tasking” brain regions rather than a discrete “one region—one function” model (e.g. Poldrack and Foerde, 2008). Rather than fearing the plethora of tasks claiming to measure impulsivity, researchers may be better served by embracing the diversity of impulsive behaviors and considering what can be learned from their comparison.
The majority of research into impulsivity at a clinical level involves the use of self-report questionnaires designed to measure impulsive tendencies, the most widely used of which is the Barratt Impulsivity Scale (BIS-11) (Patton et al., 1995). Factor analysis of the output from this 30-item scale indicates that 3 separate dimensions of impulsivity can be identified: attentional, motor, and nonplanning. The attentional/cognitive domain is reflective of the degree to which an individual can focus on the task at hand or tolerate cognitive complexity, the motor component reflects spontaneity or action without due consideration, whereas nonplanning impulsivity reflects a lack of regard for the future. Although useful indicators of cognitive well-being, self-report questionnaires are always limited by the subjects’ insight and view of the self (Moeller et al., 2001a). Behavioral tests of impulsivity have therefore been developed which are a better medium for neurobiological experiments, in terms of both clinical fMRI and pharmacological challenges and preclinical modeling studies. Such tests use as their base the ideas encapsulated within the self-report questionnaires. However, despite the best efforts of researchers in the field, it is not always possible to provide a 1:1 relationship with the dimensions of impulsivity extracted from the BIS-11 and the psychological processes measured in a behavioral paradigm.
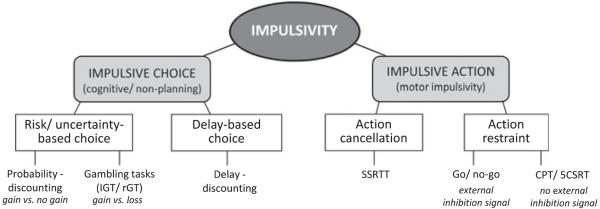
Behavioral tasks that measure impulsivity have often been classified into two, rather than 3, camps: those measuring impulsive action (motor impulsivity) and those measuring impulsive choice or decision making (Dalley et al., 2008; Pattij and Vanderschuren, 2008; Winstanley et al., 2006). A diagram illustrating how distinct aspects of impulsivity are reflected in different behavioral paradigms is provided in Fig. 1. The concept of impulsive action, or behavioral disinhibition, maps well onto the BIS-11 factor of motor impulsivity, and there are a number of behavioral tasks that appear to provide valid measurements of this phenomenon in both human and nonhuman subjects. Nevertheless, it may be unwise to consider them all as measuring identical processes. In contrast, the concept of impulsive choice appears to span both the cognitive/attentive and nonplanning domains of the BIS-11 (Patton et al., 1995). However, the majority of tests used to assess this construct focus on delayed gratification and discounting functions and therefore probably reflect non-planning more directly. The inability to process complex tasks overlaps with a wide range of executive functions and is therefore difficult to isolate. The matching familiar figures test of “reflection” impulsivity is probably the task most directly aligned with this BIS-11 factor. However, this is fundamentally difficult to model in animals (see Evenden, 1999) which has hindered exploration of its biological basis from this perspective. Indeed, the degree to which the application of “cognitive effort” or the analysis of cost-benefit decisions overlaps with impulsivity is an open question that merits further discussion (see Floresco et al., 2008; Pattij and Vanderschuren, 2008 for reviews of both literatures).
Fig. 1.
Different aspects of impulsivity can be measured using a variety of different paradigms. Examples of different behavioral tests that measure distinct aspects of impulse control are provided in this illustration, although this is by no means an exhaustive list. In terms of modeling impulsivity in laboratory animals, the construct of impulsivity has been broadly divided into impulsive choice and impulsive action. The former most likely reflects the cognitive-attentive and nonplanning factors within the Barratt Impulsivity Scale, whereas the latter closely equates to the motor impulsivity factor. Within the domain of impulsive choice, delay-discounting paradigms have been developed to assess decision making based on delayed gratification, whereas tests of probability-discounting and gambling-related decision making, such as the Iowa gambling task (IGT) and the rat gambling task (rGT), are used to measure decisions based on risk or uncertainty. In probability-discounting paradigms, subjects choose either a small but certain reward or a larger but increasingly uncertain reward. As such, on each trial, the subject either gains or fails to gain reward. In the gambling-like models, an element of loss is also incorporated, such that it is possible to finish the trial worse off than at the start if the gamble does not end favorably. Within the domain of impulsive action, it has been suggested that tasks that require cancelation of an already-initiated action, such as the stop-signal reaction time task, are distinct from those in which subjects must withhold from initiating a response. Within the latter category, there are tasks that require subjects to attend to an externally provided inhibition signal, such as a light or a tone, and use that cue to guide their behavior. An example of such a paradigm is the go/no-go task. Alternatively, tasks such as the continuous performance test or five-choice serial reaction time task require subjects to inhibit their behavior in the absence of an explicit inhibition signal and to refrain from making a motor response until the appropriate stimulus has been presented.
It is worth noting, however, that dimensions of temperament or personality are likely to be secondary to individual differences in more basic psychological processes, including aspects of cognition, reward sensitivity, and approach/avoidance. In both humans and monkeys, it is now clear that individuals exhibiting relatively high naturally occurring impulsivity exhibit poor working memory capabilities (Cools et al., 2007; Dowson et al., 2007; James et al., 2007; Romer et al., 2009) and that this association may be mediated by impairments in the function of the frontal cortex (Cools et al., 2007). Perhaps the basic mechanism mediating this relationship is that poor working memory leads to deficiencies in developing and maintaining goal-directed plans related to behavior—the result being a more myopic behavioral phenotype. If correct, this would suggest that working memory and its related component processes should also be considered as a focus on endophenotype-oriented working for disorders of impulse control (Loo et al., 2008). Of particular relevance to the current review, recent data suggest that working memory performance is impaired following chronic, escalating administration of cocaine, and that such impairments are correlated with cell loss within the frontal cortex (George et al., 2008). Furthermore, the authors suggest that the working memory impairments observed may reflect aspects of increased perseveration, compulsivity, and incentive motivation induced by repeated drug intake, and these processes may likewise influence impulsive behaviors.
IMPULSIVE ACTION VERSUS IMPULSIVE CHOICE
The level of motor impulsivity has largely been conceptualized as the inability to withhold from making a motor response. Three of the most widely used laboratory-based tests used to assess this process are the stop-signal reaction time task (SSRTT), the continuous performance test (CPT), and the go/no-go paradigms. In the SSRTT, subjects are required to make a rapid response upon the presentation of a go signal (Logan and Cowan, 1984). On a subset of trials, a stop signal is presented at varying delays after the presentation of the go signal, and the subject must then cancel their planned response. Go/no-go paradigms likewise use 2 signals, one indicating that a go response is required, and the other that this response should be inhibited. However, only one signal is presented on any trial, and the signal occurs at the beginning of each trial, before any action has been initiated. Both SSRTT and go/no-go tasks have been developed for use in rodents and nonhuman primates (SSRTT: Eagle and Robbins, 2003; Feola et al., 2000; Liu et al., 2009; go/no-go Iversen and Mishkin, 1970; Terman and Terman, 1973).
In contrast, the CPT is ostensibly a test of attentional function that incorporates an aspect of motor impulsivity (Rosvold et al., 1956; Wilkinson, 1963). In this task, subjects are required to scan a 5-digit sequence and respond when the numbers match a target stimulus. “False alarm” errors occur when the subject responds positively to a sequence which is identical to the target, with the exception of the final number. When making such errors, the subject therefore responds prematurely before processing the full sequence, hence providing an index of motor impulsivity. The five-choice serial reaction time task (5CSRT) was developed as a rodent analog of the CPT (Carli et al., 1983). Animals learn to respond to a stimulus light which is briefly presented in 1 of 5 spatially distinct apertures. However, subsequent to initiating a trial and prior to presentation of the light, there is a 5-s inter-trial interval during which the animal must withhold from responding at the aperture array. Any responses made during this time are described as premature responses and are punished by time-out periods. Such premature responses are thought to be analogous to the false alarms errors made in the CPT.
Despite the fact that all 3 of these tests purport to measure motor impulsivity and are often referred to interchangeably, there are subtle but perhaps critical differences in the inhibitory processes involved (see Eagle et al., 2008 for a recent review). In particular, it appears possible to dissociate action restraint (the inhibition of a motor response prior to its initiation, as in the go/no-go paradigm) and action cancelation (the inhibition of an already-initiated motor response, as in the SSRTT) in terms of their neurochemical modulation. As one example, the 5-HT system is strongly implicated in action restraint but not action cancelation, whereas noradrenaline may play a more fundamental role in action cancelation. Furthermore, although performance of action restraint and cancelation tasks was correlated in control subjects, there was no such relationship between task scores in a group of attention-deficit hyperactivity disorder (ADHD) patients, despite the fact that ADHD patients were collectively worse on both inhibitory measures (Schachar et al., 2007). Such findings suggest that patients with different inhibitory deficits may obtain therapeutic benefit from different pharmacological treatments and highlights the importance of considering individual differences in impulsive symptomatology. Within this framework, premature responding on the 5CSRT may have more in common with errors on go/no-go tasks rather than deficits in responding to stop signals on SSRTT. However, in the 5CSRT there is no “no-go” signal: the subjects regulate their own behavior in anticipation of the presentation of a “go” signal. Whether this turns out to be an important factor when considering translation between tasks and species remains to be determined, but the response to reward-related stimuli is clearly affected by addictive drugs.
In addition to these motor-based paradigms, impulsivity is also reflected in more cognitive tests of choice and preference. One of the most widely used tests of impulsive decision making in both animals and humans is the delay-discounting paradigm. Delay discounting describes the process by which the subjective value of a reward diminishes as the delay to its delivery increases, and rates of discounting are assessed by measuring choice between smaller rewards delivered relatively soon versus larger rewards delivered later in time (e.g. Rachlin et al., 1991). Selection of the smaller-sooner reward is indicative of a more impulsive choice, reflecting an intolerance to delayed gratification that results in a negative outcome i.e. less reward in the long run. The delay-discounting curves for both human and nonhuman subjects are best represented by the same form of hyperbolic equation (Ainslie, 1975; Mazur, 1987), and there is a general concordance between the effects of brain damage and drug administration on delay-discounting behavior between species (see Winstanley et al., 2006 for a review).
Just as delay to its delivery can devalue a larger reward, so can the probability that such a reward will actually be received. It has been suggested that perceived uncertainty regarding the stability of an environment can lead to enhanced delay discounting, as the delayed reward appears probabilistic rather than guaranteed (Mischel and Grusec, 1967). The question of whether decision making related to delayed versus probabilistic rewards is controlled by similar psychological and neural processes can be addressed empirically using simple probability-discounting schedules (Adriani and Laviola, 2006; Cardinal and Howes, 2005; Mobini et al., 2002) and also more complex gambling-like paradigms (e.g. Rivalan et al., 2009; Zeeb et al., 2009). The extent to which decision making under risk or uncertainty is analogous to delay-discounting tests more commonly used to assess impulsive decision making is an important question, particularly given both the association with pathological gambling, impulsivity, and substance abuse. Whether engaging in impulsive-like behaviors is itself rewarding and the contribution such processes make to the development of pathological gambling are likewise key issues in terms of understanding the relationship between behavioral and chemical addictions. However, in the interests of brevity and focus, we will restrict our discussion to the more conventional, delay-discounting models of impulsive choice, as more data is available regarding the relationship between impulsive choice on such models and the response to addictive drugs.
WHY MIGHT ADDICTIVE DRUGS INFLUENCE IMPULSIVITY AND VICE VERSA?
One of the reasons why addictive drugs exert such a powerful control over the behavior of people affected by substance use disorders is that the dopamine response to drug-paired cues is potentiated, such that these cues trigger craving for drug and drug seeking (Bossert et al., 2005; Everitt et al., 2003; Parkinson et al., 2001, 1999; Shaham et al., 2003; Taylor and Robbins, 1986; Wyvell and Berridge, 2000). Both acute and repeated administration of cocaine and other addictive drugs leads to the enhancement of conditioned reinforcement in rodents (Jentsch and Taylor, 2001; Mead et al., 2004; Olausson et al., 2004; Taylor and Horger, 1999), see also (Mead et al., 2004; Miles et al., 2004; Wyvell and Berridge, 2001). This is also true in nonhuman primates as monkeys that had previously been exposed to cocaine showed augmented responding to a reward-associated cue when tested on the acquisition of a second-order schedule of reinforcement (Olausson et al., 2007). Conditioned stimuli (lights, tones, etc.) are also thought to make a significant contribution to the addictive nature of gambling behavior (Kushner et al., 2008). Understanding how manipulation of the salience of reward-paired cues influences different forms of impulsive responding may prove fruitful when considering how addictive drugs influence impulsivity under different circumstances. For example, it has been suggested that animals that respond more to reward-paired cues in autoshaping (“sign-tracking”) paradigms, theoretically as a result of individual differences in the attribution of incentive salience to cues, are more sensitive to cocaine and may likewise be more impulsive (Flagel et al., 2009; Tomie, 1996).
With these considerations in mind, it may be particularly important to understand what drives impulsive behavior within different tests of impulse control, particularly if impulsive responding is generated by the reaction to an external cue. For example, it could be argued that inhibitory failure in the SSRTT and go/no-go task arise through the failure to process or attend to the stop/no-go signal, whereas premature responding in the 5CSRT is “self-generated”: there is no inhibitory signal that is ignored. Alternatively, elevated impulsive responding in all 3 tests could arise through a hyper-response to the perceived or anticipated “go” signal. Drug-induced potentiation of the power of reward-related cues to elicit a behavioral response may therefore directly contribute to high impulsivity on these motor tasks.
It is also worth considering how such a heightened response to reward-paired cues might influence delay-discounting processes. When an animal selects the small immediate reward over a larger delayed one, such a decision is regarded as an impulsive choice, because the desire for immediacy outweighs the larger reward on offer following a delay. However, it could also be argued that elevated choice of the larger reward is indicative of an increase in incentive motivation for the large reward, such that the delay fails to sufficiently devalue its subjective value, and it is selected despite the negative consequences (Uslaner and Robinson, 2006; Winstanley et al., 2004a). This view shares similarities with the idea that persistent drug-taking results in a short-sighted view of the future such that decisions are made with little regard for their future consequences. If choice of the large reward is signaled by a cue, immediate feedback is provided that could be amplified by exposure to psychostimulant drugs, or alcohol (Grant and Macdonald, 2005; Olmstead et al., 2006). It has been observed that using a cue light to bridge the gap between responding for the large reward and its delivery can facilitate the acquisition of delay-discounting performance and alter the response to amphetamine (Cardinal et al., 2000). Recent unpublished findings also suggest that use of such a cue leads to virtually exclusive selection of the larger delayed reward in a subset of individuals, and that this may reflect enhanced dopamine signaling within the OFC (Zeeb et al., 2010; see Floresco et al., 2008 for further discussion of cues in delay-discounting tasks). Whether these “cue-sensitive” rats are like-wise more vulnerable to addictive behaviors remains to be determined.
The inability to withhold from responding to a reward-paired stimulus may also impact the performance of reversal learning tasks, where subjects are required to inhibit responding to a previously rewarded option, and instead select a new or previously unrewarded stimulus (see Birrell and Brown, 2000; Boulougouris et al., 2007; Chudasama and Robbins, 2003; Cools et al., 2002; Dias et al., 1997; Seu et al., 2009 for recent studies using such methodology). Although deficits in shifting away from the previously rewarded stimulus are generally regarded as evidence for cognitive inflexibility or perseverative/habitual responding, such behavior could also reflect a difficulty in inhibiting a prepotent reward-directed response (Groman et al., 2009; Tait and Brown, 2007). Such comparisons may help explain the pattern of behavioral impairments reported following chronic intake of addictive drugs.
HIGH IMPULSIVITY AS A PREDICTOR OF DRUG ABUSE
Given the diversity of impulsive behavioral tests just described, an important question to consider is whether it matters what kind of test is used to probe the relationship between addiction and impulsivity: do all kinds of impulsivity show similar relationships with substance abuse, or is there a particular impulsive subtype that stands out? Furthermore, is there a particular phase in the development of addiction where impulsivity is more prominent, and where interventions aimed at reducing impulsivity may be maximally effective? There is also the similarly critical question of whether the specific drug used or the sex or age of the subjects influences the importance of different impulsive behaviors in the development of addiction. However, for brevity, we will largely restrict our discussion to alcohol and cocaine, as the links between impulsivity and addiction appear particularly strong for these drugs, and their use has been heavily studied.
Does Impulsivity Predict Alcohol Abuse?
Alcoholics can be divided into Type 1 or Type 2 classes, depending on personality variables and drinking patterns. As such, Type 1 alcoholics show low levels of novelty seeking, high levels of harm avoidance and generally start drinking later in life, whereas Type 2 alcoholics are highly novelty seeking, show low levels of harm avoidance, and start drinking in their teens (Cloninger, 1987). It is this latter group that experiences problems with impulse control, and Type 2 alcoholism is also highly heritable (e.g., Cloninger et al., 1981). Hence, if animal models are to accurately model the relationship between impulsivity and alcoholism in animals, it could be argued that impulsive tendencies and high alcohol preferences should be co-inherited and evident at an early age. Given that the behavioral tests of impulsivity outlined here take weeks to train and that the adolescence of rats only lasts around 14 days, it is notoriously difficult to obtain a measurement of impulsivity in juvenile animals. Nevertheless, data from adult animals exploring these issues are encouraging.
Considerable individual differences exist in rats’ propensity to consume alcohol, providing the potential opportunity to identify biological markers for susceptibility to alcoholism. Whether levels of motor impulsivity are predictive of alcohol abuse is currently unclear, as the studies to date have not used “clean” tests of impulsivity, but tests that could reflect anxiety, perseveration, or hyperactivity (e.g., Johansson et al., 1999; Johansson and Hansen, 2000; McMillen et al., 1998). However, there is now a substantial body of literature supporting the hypothesis that high levels of impulsive choice, as assessed using a variety of delay-discounting paradigms, are associated with high levels of alcohol drinking. Animals identified as highly impulsive on these tasks (HI-DD) drank significantly more of the higher concentrations of alcohol presented within a limited access self-administration procedure (Poulos et al., 1995). Furthermore, among rats with “intermediate” levels of impulsive choice, there was significant variability in the acute response to alcohol on-task: “high reactive” rats showed a dose-dependent increase in impulsive choice when given the drug, whereas “low reactive” rats were largely unaffected. Critically, the high reactive animals drank a significantly greater amount of the more concentrated alcohol solutions (Poulos et al., 1998), again affirming the relationship between high levels of impulsivity on the delay-discounting paradigm and subsequent alcohol intake. Rodents bred for high levels of alcohol intake, but which were alcohol naive, also showed steeper discounting curves for both delayed and probabilistic rewards (Oberlin and Grahame, 2009; Wilhelm and Mitchell, 2008). Hence, in terms of demonstrating that high levels of impulsive choice may be a vulnerability factor in predisposition to a higher preference for alcohol, the data appear quite strong.
Does Impulsivity Predict Cocaine Abuse?
There is some indication that deficits in impulsive control may be particularly pronounced in crack cocaine users. For example, a clinical study observed higher levels of impulsive decision making, as measured by the delay-discounting task, in those using crack compared to those using heroin (Bornovalova et al., 2005), although heroin addicts choose more impulsively on delay-discounting questionnaires than healthy controls (Kirby et al., 1999). Furthermore, long-term abusers of the psychostimulant amphetamine showed maladaptive decision making on a gambling task similar to that observed in OFC-damaged patients, whereas opiate abusers did not show such changes (Rogers et al., 1999). The reasons why the use of different drugs is associated with varying degrees to changes in impulsive behavior can arise through both pharmacological and contextual reasons or an interaction between the two. For example, preferential crack cocaine use is consistently associated with higher levels of risky sexual behavior compared with populations of other drug users (Baseman et al., 1999; Ross et al., 2002). This seems largely because of the sex-for-drugs black market economy that has evolved with respect to crack, but why the association is so strong for this particular drug is unclear. The low socioeconomic status of many crack users is likely one contributing factor, but whether a predisposition to impulsive acts in those who use crack cocaine is also involved remains to be determined. Likewise, drug reinforcement of impulsive decisions may lead to further engagement in maladaptive behavior.
Considering tests of motor impulsivity, it is perhaps surprising that the rodent SSRTT has not been used to explore the relationship between inhibitory control and addiction, as a number of clinical reports have been published suggesting SSRTT impairments in cocaine users (e.g., Fillmore and Rush, 2002; Li et al., 2008, 2006). However, a number of studies have been performed in rats looking at the relationship between premature responding on the 5CSRT and the response to addictive drugs. Animals that were identified as highly impulsive (HI-5CSRT) on this task showed an escalation of cocaine intake in the binge administration model, indicative of the loss of control over drug consumption typical in addicted subjects (Dalley et al., 2007). These HI-5CSRT rats also showed higher break points under progressive ratio schedules for cocaine, and persistent responding for the drug even when doing so resulted in delivery of electric shocks (Belin et al., 2008). Furthermore, HI-5CSRT rats showed higher levels of reinstatement of cocaine seeking even after this response was extinguished by shock administration (Economidou et al., 2009). HI-5CSRT rats were therefore highly motivated to take the drug despite negative consequences, again reproducing key features of substance abuse. In a more recent study (Diergaarde et al., 2008), it has been reported that distinct aspects of impulsivity (premature responding on the 5-CSRT vs. impulsive choice in a delay-discounting paradigm) predict dissociable aspects of inflexible nicotine self-administration. Moreover, different aspects of craving have been associated with distinct types of impulsivity in human nicotine addicts (Doran et al., 2009), further highlighting the need to examine distinct aspects of impulsivity and to relate them to the variable dimensions of drug-maintained behavior.
In parallel to earlier work on alcohol (Poulos et al., 1995), it has also been suggested that rats that show high levels of impulsive choice on the delay-discounting paradigm (HI-DD) are more susceptible to the addictive-like effects of cocaine. Both male and female rats classed as HI-DD were faster to acquire cocaine self-administration than their less impulsive counterparts (Perry et al., 2005, 2008). However, whether faster acquisition of the self-administration paradigm necessarily indicates that animals are more addiction-prone is open to debate (see Belin et al., 2008, for example). However, female, but not male, HI-DD rats showed elevated levels of reinstatement to cocaine seeking following a priming injection of cocaine. In as much as the 5CSRT and delay-discounting data sets can be compared, it would therefore appear that impulsive decision making may facilitate initial contact with the drug, but motor impulsivity is more predictive of the development of drug dependency. However, intolerance to delayed gratification may again play a role in reinstatement of drug seeking.
IMPULSIVITY AND COGNITIVE DEFICITS AS A CONSEQUENCE OF COCAINE ABUSE: FOCUS ON THE OFC
Although high levels of impulsivity appear to predispose individuals to cocaine abuse, data from both rats and monkeys indicate that drug exposure can likewise affect a range of cognitive behaviors, including impulse control, and such behavioral change may be integral to the development and maintenance of addiction. Short-term cocaine exposure is sufficient to increase subsequent cocaine self-administration in rodents (Vezina, 2004), indicating that the cocaine-induced neuroadaptations that occur early during drug experimentation can promote further cocaine consumption. One hypothesis is therefore that drug exposure can dysregulate the inhibitory control functions dependent on regions of the prefrontal cortex (PFC) to facilitate cue-motivated drug seeking and the development of an “addictive” state in vulnerable individuals (Everitt et al., 2007; Jentsch and Taylor, 1999; Robbins and Everitt, 1999; Robinson and Berridge, 2003). A significant challenge is therefore to determine the neurobiological substrates associated with cocaine-induced alterations in behavior and motivation.
In addition to the nucleus accumbens and striatum (e.g., Belin and Everitt, 2008; Calu and Schoenbaum, 2008; Cornish and Kalivas, 2001; Everitt et al., 2008; Nestler, 2004; Robinson and Berridge, 1993), one major focus of such research has been the OFC. Hypoactivity within this region has been observed repeatedly in human cocaine addicts (Volkow et al., 1993, 1991). Cocaine self-administration reduces glucose utilization in the primate OFC and PFC (Beveridge et al., 2006; Lyons et al., 1996), and these alterations progressively impact cortical-striatal regions with increased exposure (Porrino et al., 2007). It has been suggested that this reduced function contributes to impulse-control deficits in addiction (Rogers et al., 1999), as OFC-damaged patients score more highly on the BIS-11 and show high levels of impulsive responding on gambling tasks (Bechara et al., 1999; Berlin et al., 2005) but see (Fellows and Farah, 2005). In rats, damage to this region slows SSRTT and transiently increases premature responses, indicating an increase in motor impulsivity (Chudasama et al., 2003; Eagle and Baunez, 2009). Interestingly, both increases and decreases in impulsive choice have been observed following OFC lesions, which may reflect differences in the design of the delay-discounting tasks used (Rudebeck et al., 2006; Winstanley et al., 2004a; see Floresco et al., 2008 for discussion). In addition, OFC damage across species leads to cognitive inflexibility and perseveration, as evidenced by impairments on reversal-learning tasks (Boulougouris et al., 2007; Chudasama and Robbins, 2003; McAlonan and Brown, 2003; Schoenbaum et al., 2003). Such deficits have also been observed in rats, monkeys, and humans following repeated cocaine intake (Calu et al., 2007; Fillmore and Rush, 2002; Jentsch et al., 2002; Schoenbaum et al., 2004), again indicating that compromised OFC function may be central to the cognitive dysfunction associated with cocaine addiction.
OFC lesions reduce the ability of reward-associated cues to elicit or maintain reward-motivated behavior (Pears et al., 2003) and play an important role in the ability of reward-associated stimuli to influence behavioral output (O’Doherty et al., 2003; Roesch and Olson, 2004; Schoenbaum et al., 1998). The OFC may therefore play a critical role in mediating the motivational effects of drug-associated stimuli. This hypothesis is supported by observations that OFC lesions disrupt cocaine self-administration under a second-order schedule of reinforcement (Everitt et al., 2007; Hutcheson and Everitt, 2003), and reversible inactivation of the lateral OFC reduced the motivational effects of cocaine-associated cues in a relapse model (Fuchs et al., 2004). As outlined above, such increased sensitivity to reward-paired cues could promote drug seeking, but also influence impulsive responding.
In a recent experiment, 5CSRT testing and cocaine self-administration were combined in a concurrent design such that animals self-administered cocaine in the afternoons and were tested on the 5CSRT in the mornings (Winstanley et al., 2009). Thus, it was possible to track the development of any changes in impulsive behavior as drug use developed. During the initial phase of acquisition of cocaine self-administration, premature responding increased, but this was no longer evident after 4 weeks of drug exposure. In contrast, when animals were withdrawn from drug, premature responding sharply increased. Furthermore, these deficits could be exacer-bated by overexpression of the transcription factor ΔFosB, a member of the Fos family of immediate early genes, within the OFC. ΔFosB has previously been associated with the generation and maintenance of the addicted state through its drug-dependent induction within the striatum (Nestler, 2004), but self-administration of cocaine also increases ΔFosB within the OFC (Winstanley et al., 2007). Furthermore, microarray data suggests that this increase leads to upregulation of local inhibitory networks within this region, potentially providing a mechanism by which OFC function is reduced in human addicts (Volkow and Fowler, 2000; Winstanley et al., 2007).
It is worth noting that animals with a history of cocaine self-administration showed a tolerance to the ability of an acute cocaine challenge to increase premature responding, and this tolerance could be induced by overexpression of ΔFosB within the OFC (Winstanley et al., 2009, 2007). Such data somewhat parallels the observation that HI-5CSRT rats become less impulsive following binge access to cocaine (Dalley et al., 2007). One possibility is that the level of impulsivity exhibited by individuals changes as addiction develops, with levels of impulsivity decreasing after initial exposure to drug, potentially, because of adaptive neuroplastic changes that allow the addict to function while taking large quantities of stimulant drugs. However, when drug is withdrawn, impulse control deficits are unmasked as the brain can no longer function optimally without drug onboard (see Winstanley, 2007 for discussion).
Understanding the biochemical changes that occur within the OFC, and other regions of the frontal cortex, after cocaine exposure could potentially provide valuable information into the mechanisms responsible for deficits in impulse control and behavioral flexibility associated with addiction. Using genomics and proteomics, it is possible to identify both the effects of chronic drug exposure and, by simultaneously sampling hundreds or thousands of gene products and proteins, also the global patterns of changes that are more relevant to the neurobiology of addiction than any individual alteration alone. An increasing number of studies have, however, demonstrated limited correlations between changes in mRNA and protein expression using these techniques, indicating that mechanisms that control protein translation are important in determining the biochemical effects of drug exposure. As proteins represent functional components of the cellular machinery, methods for unbiased proteomic analysis therefore offer substantial advantages. Initial proteomics studies in cocaine-exposed monkeys, which show established deficits in reversal learning deficits (Jentsch et al., 2002), have found alterations in metabolic proteins, protein folding and turnover, and cyto-skeletal rearrangements in striatum and OFC when measured 4 weeks after the final cocaine injection (Krueger et al., 2005; Olausson et al., 2007). The prominence of alterations in proteins related to energy metabolism at this time point was striking. It is possible that these changes reflect an alteration in synapse number or function as neurotransmission is a highly energy-intensive process, possibly consistent with the structural changes observed in the OFC in abstinent cocaine users (Franklin et al., 2002; Matochik et al., 2003).
In cocaine-dependent individuals, decreased metabolic activity within the OFC was associated with reduced striatal D2 receptor availability (Volkow et al., 1993); studies in monkeys demonstrate that this effect is most likely rapidly induced by cocaine intake itself (Nader et al., 2006). Similarly, quantitative real-time PCR data show that chronic cocaine exposure reduces D2, but not D1, receptor mRNA in the caudate nucleus in monkeys (P. Olausson, N.C. Tronson, D. Krueger, A.T. Kedves, A.C. Nairn, J.R. Taylor, unpublished observations). This observation may be directly comparable with the reduction in D2/3 receptor binding observed in the ventral striatum of highly impulsive rats (Dalley et al., 2007). In rats, extended cocaine self-administration was reported to reduce the levels of D2 receptors in OFC (Briand et al., 2008) and to disrupt D2-dependent modulation of prefrontal cortical neuron excitability (Nogueira et al., 2006). Whether cocaine exposure also reduces D2 receptor levels in the primate OFC and whether such alterations contribute to the reversal learning deficits observed in cocaine-exposed animals remains to be determined, although this link is generally supported by behavioral pharmacological studies (Lee et al., 2007).
Collectively, these data converge to support an important role for D2-like receptor function in aspects of impulsivity in the context of drug addiction. Furthermore, considering impulsivity as part of a spectrum of neurocognitive functioning, highly impulsive volunteers showed improved working memory performance following administration of a D2/3 agonist (bromocriptine) which was accompanied by the modulation of frontostriatal activity, yet low impulsive people did not (Cools et al., 2007). Hence, genetically or environmentally determined differences in dopaminergic transmission, particularly through the D2 receptor, could alter neurocognitive mechanisms, and this may increase engagement in hasty, risky, and/or impulsive behaviors.
IMPULSIVITY AND COMPULSIVITY
The precise nature of the relationship between impulsive and compulsive behaviors remains an area of active debate. One conceptual distinction between these forms of behavior may lie in the goal-directed nature of incentively motivated impulsive action and choice, as opposed to the habitual nature of relatively outcome-insensitive compulsions (Balleine and O’Doherty, 2009). In other words, impulsive behavior, as we have characterized it here, often depends very much on motivational systems that mediate voluntary reward seeking, while compulsive behaviors are usually thought of as almost reflexive actions triggered by contextual stimuli paired with outcome availability. These stimulus-response actions are also thought to be relatively independent of input from the prefrontal cortices and are largely mediated by areas within the striatum, thalamus, and basal ganglia.
Prospectively, it appears that impulsive tendencies are the predictors of risk for substance abuse and that impulsive behavior is fueled in the early stages of drug use. More recently, however, increased attention has been paid to the idea that—as drug use behavior continues—a “drug habit” may form (Belin et al., 2009; Everitt et al., 2008; Torregrossa et al., 2008). For example, recent preclinical studies have suggested that drug-taking behavior in rats becomes increasingly inflexible with longer experience (Deroche-Gamonet et al., 2004; Vanderschuren and Everitt, 2004). As such, the transition from impulsive to compulsive behaviors may reflect a progressive decline in the influence the prefrontal cortices exert over behavioral output and a shift in emphasis from the ventral to the dorsal striatum (Kalivas, 2008; Robbins and Everitt, 1999). Moreover, impulsive animals that are at risk for the rapid development of drug-taking behavior are subsequently more likely to exhibit evidence of the transition to “compulsive” drug taking (Belin et al., 2008). Conceptually, this type of research has a lot of appeal for our understanding of addiction processes, but a limitation of these models is that it is assumed that inflexible drug taking is synonymous with compulsive actions. This relationship is simply not clear. For that reason, more work in this area is required.
SUMMARY
Data across different species is generally convergent in that high levels of a number of forms of impulsivity are not only predictive of addiction, but can also be affected by drug intake, and that the increases in impulsivity and other cognitive deficits associated with drug dependency may reflect compromised function within the frontal cortex (see Fig. 2 for a summary of this position). Based on current findings looking at cocaine abuse, it would appear that motor impulsivity in particular is associated with the development of drug dependence, whereas intolerance to delayed gratification may contribute to relapse to drug use. Such high levels of delay discounting also appear to be predictive of increased alcohol intake. Such a hypothesis is open to verification by future studies in which different forms of impulsivity are systematically compared, and it would be useful to determine whether pharmacological interventions to reduce different forms of impulsivity would likewise decrease drug taking or seeking. However, as indicated at various points throughout this review, impulsive behavior does not exist in a vacuum, but is part of a wider set of cognitive vulnerabilities for addictive and risky behavior. Key nodes within the frontostriatal circuitry regulating impulsivity are likewise implicated in other cognitive processes that relate to addiction. The studies discussed here largely focused on the OFC, and it is clear that this region not only plays an important role in impulse control deficits, but also the response to reward-paired stimuli and cognitive flexibility. By considering how these behaviors relate to one another, we may be better positioned to identify biomarkers for susceptibility to addiction.
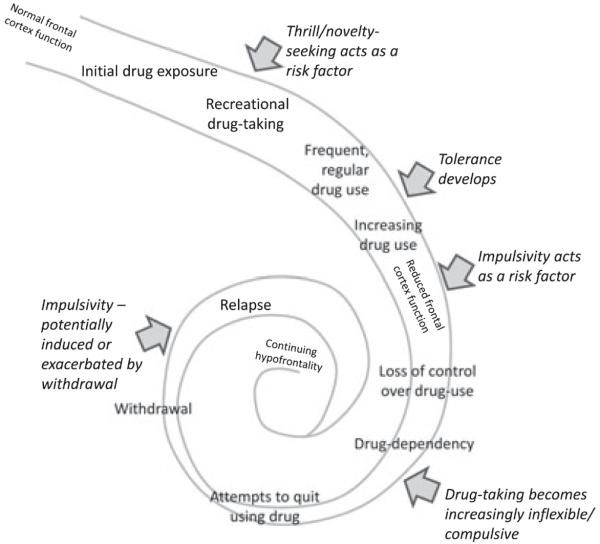
Fig. 2.
The downward spiral of addiction. Sensitivity to novelty or thrill seeking is thought to facilitate initial contact with addictive drugs and the development of regular or recreational drug use. As tolerance develops, drug intake increases, and evidence suggests that highly impulsive individuals are more likely to lose control of their drug taking, leading to escalating patterns of drug use and drug dependency. Dysfunction within areas of prefrontal cortex such as the OFC, as well as alterations in synaptic plasticity throughout the mesocortical dopamine system and striatum, contributes significantly to these behavioral changes. As the addicted state becomes more advanced, drug taking becomes more compulsive and inflexible. Attempts to quit using drug are followed by an aversive withdrawal state during which high levels of impulsivity may precipitate relapse to drug seeking, thus perpetuating the cycle of addiction and prefrontal damage.
ACKNOWLEDGMENTS
This work was supported by grants from the following US Public Health Service grants: RL1-MH083270 and P50-MH007248 (DJD), RO1-DA011717 (JRT) as well as operating grants from NSERC, CIHR, and the Institute for Research into Gambling Disorders (CAW). Salary support for CAW is also provided by the CIHR New Investigator program and the Michael Smith Foundation for Health Research.
REFERENCES
- Adriani W, Laviola G. Delay aversion but preference for large and rare rewards in two choice tasks: implications for the measurement of self-control parameters. BMC Neurosci. 2006;7:52. doi: 10.1186/1471-2202-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–498. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- American Psychiatry Association . Diagnostic and Statistical Manual IV. 4th ed. American Psychiatric Press; Washington DC: 1994. [Google Scholar]
- Bailey JN, Breidenthal SE, Jorgensen MJ, McCracken JT, Fairbanks LA. The association of DRD4 and novelty seeking is found in a nonhuman primate model. Psychiatr Genet. 2007;17:23–27. doi: 10.1097/YPG.0b013e32801140f2. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2009;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J, Ross M, Williams M. Sale of sex for drugs and drugs for sex: an economic context of sexual risk behavior for STDs. Sex Transm Dis. 1999;26:444–449. doi: 10.1097/00007435-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Iversen SD. Borderline personality disorder, impulsivity, and the orbitofrontal cortex. Am J Psychiatry. 2005;162:2360–2373. doi: 10.1176/appi.ajp.162.12.2360. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci. 2006;23:3109–3118. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Daughters SB, Hernandez GD, Richards JB, Lejuez CW. Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Exp Clin Psychopharmacol. 2005;13:311–318. doi: 10.1037/1064-1297.13.4.311. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology. 2008;33:2969–2980. doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DN, Baddeley AD. What can amnesic patients learn? Neuropsychologia. 1976;14:111–122. doi: 10.1016/0028-3932(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Brown GL, Linnoila MI. CSF serotonin metabolite (5-HIAA) studies in depression, impulsivity, and violence. J Clin Psychiatry. 1990;51(Suppl):31–41. discussion 42–3. [PubMed] [Google Scholar]
- Budson AE. Understanding memory dysfunction. Neurologist. 2009;15:71–79. doi: 10.1097/NRL.0b013e318188040d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Schoenbaum G. Cocaine-paired cues activate aversive representations in accumbens neurons. Neuron. 2008;57:633. doi: 10.1016/j.neuron.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem. 2007;14:325–328. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Howes NJ. Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neurosci. 2005;6:37. doi: 10.1186/1471-2202-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha- flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology. 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurons on performance of a 5-choice serial reaction time task in rats – implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5 choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D’Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27:5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Cocaine sensitization and craving: differing roles for dopamine and glutamate in the nucleus accumbens. J Addict Dis. 2001;20:43–54. doi: 10.1300/J069v20n03_05. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from “on-line” processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Doran N, Cook J, McChargue D, Spring B. Impulsivity and cigarette craving: differences across subtypes. Psychopharmacology (Berl) 2009;207:365–373. doi: 10.1007/s00213-009-1661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowson JH, Blackwell AD, Turner DC, Harvey E, Malhotra T, Robbins TW, Sahakian BJ. Questionnaire ratings of attention-deficit/hyperactivity disorder (ADHD) in adults are associated with spatial working memory. Eur Psychiatry. 2007;22:256–263. doi: 10.1016/j.eurpsy.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl) 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev. 2009;34:50–72. doi: 10.1016/j.neubiorev.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Evenden JL. The pharmacology of impulsive behaviour in rats VII: the effects of serotonergic agonists and antagonists on responding under a discrimination task using unreliable visual stimuli. Psychopharmacology. 1999;146:422–431. doi: 10.1007/pl00005487. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Ann N Y Acad Sci. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA. Individual differences in response to a stranger: social impulsivity as a dimension of temperament in vervet monkeys (Cercopithecus aethiops sabaeus) J Comp Psychol. 2001;115:22–28. doi: 10.1037/0735-7036.115.1.22. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Fontenot MB, Phillips-Conroy JE, Jolly CJ, Kaplan JR, Mann JJ. CSF monoamines, age and impulsivity in wild grivet monkeys (Cercopithecus aethiops aethiops) Brain Behav Evol. 1999;53:305–312. doi: 10.1159/000006601. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Jorgensen MJ, Huff A, Blau K, Hung YY, Mann JJ. Adolescent impulsivity predicts adult dominance attainment in male vervet monkeys. Am J Primatol. 2004a;64:1–17. doi: 10.1002/ajp.20057. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Melega WP, Jorgensen MJ, Kaplan JR, McGuire MT. Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vervet monkeys. Neuropsychopharmacology. 2001;24:370–378. doi: 10.1016/S0893-133X(00)00211-6. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Newman TK, Bailey JN, Jorgensen MJ, Breidenthal SE, Ophoff RA, Comuzzie AG, Martin LJ, Rogers J. Genetic contributions to social impulsivity and aggressiveness in Vervet monkeys. Biol Psychiatry. 2004b;55:642–647. doi: 10.1016/j.biopsych.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Faustman WO, King RJ, Faull KF, Moses JA, Jr, Benson KL, Zarcone VP, Csernansky JG. MMPI measures of impulsivity and depression correlate with CSF 5-HIAA and HVA in depression but not schizophrenia. J Affect Disord. 1991;22:235–239. doi: 10.1016/0165-0327(91)90069-5. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Feola TW, de Wit H, Richards JB. Effects of d-amphetamine and alcohol on a measure of behavioral inhibition in rats. Behav Neurosci. 2000;114:838–848. doi: 10.1037/0735-7044.114.4.838. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alchohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cogn Affect Behav Neurosci. 2008;8:375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology. 2008;33:2474–2482. doi: 10.1038/sj.npp.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant NK, Macdonald TK. Can alcohol lead to inhibition or disinhibition? Applying alcohol myopia to animal experimentation. Alcohol Alcohol. 2005;40:373–378. doi: 10.1093/alcalc/agh177. [DOI] [PubMed] [Google Scholar]
- Groman SM, James AS, Jentsch JD. Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2009;33:690–698. doi: 10.1016/j.neubiorev.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Linnoila M. Low central nervous system serotonergic activity is traitlike and correlates with impulsive behavior. A nonhuman primate model investigating genetic and environmental influences on neurotransmission. Ann N Y Acad Sci. 1997;836:39–56. doi: 10.1111/j.1749-6632.1997.tb52354.x. [DOI] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ. The effects of selective orbitofrontal cortex lesions on the acquisition and performance of cue-controlled cocaine seeking in rats. Ann N Y Acad Sci. 2003;1003:410–411. doi: 10.1196/annals.1300.038. [DOI] [PubMed] [Google Scholar]
- Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- James AS, Groman SM, Seu E, Jorgensen M, Fairbanks LA, Jentsch JD. Dimensions of impulsivity are associated with poor spatial working memory performance in monkeys. J Neurosci. 2007;27:14358–14364. doi: 10.1523/JNEUROSCI.4508-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by rewardrelated stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impaired inhibition of conditioned responses produced by subchronic administration of phencyclidine to rats. Neuropsychopharmacology. 2001;24:66–74. doi: 10.1016/S0893-133X(00)00174-3. [DOI] [PubMed] [Google Scholar]
- Johansson AK, Bergvall AH, Hansen S. Behavioral disinhibition following basal forebrain excitotoxin lesions: alcohol consumption, defensive aggression, impulsivity and serotonin levels. Behav Brain Res. 1999;102:17–29. doi: 10.1016/s0166-4328(98)00159-4. [DOI] [PubMed] [Google Scholar]
- Johansson AK, Hansen S. Increased alcohol intake and behavioral disinhibition in rats with ventral striatal neuronal loss. Physiol Behav. 2000;70:453–463. doi: 10.1016/s0031-9384(00)00284-5. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Keltikangas-Jarvinen L, Elovainio M, Kivimaki M, Lichtermann D, Ekelund J, Peltonen L. Association between the type 4 dopamine receptor gene polymorphism and novelty seeking. Psychosom Med. 2003;65:471–476. doi: 10.1097/01.psy.0000041547.31072.25. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Rogers J. An analysis of independence and interactions of brain substrates that subserve multiple attributes, memory systems, and underlying processes. Neurobiol Learn Mem. 2004;82:199–215. doi: 10.1016/j.nlm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Krueger DD, Olausson P, Wu T, Williams K, Taylor JR, Nairn AC. Proteomic analysis of persistent cortico-limbic-striatal neuroadaptations following repeated cocaine exposure in Vervet monkeys; Paper presented at the Society for Neuroscience; Washington, DC. 2005. [Google Scholar]
- Kushner M, Thurus P, Sletten S, Frye B, Abrams K, Adson D, Demark JV, Maurer E, Donahue C. Urge to gamble in a simulated gambling environment. J Gambl Stud. 2008;24:219–227. doi: 10.1007/s10899-007-9083-3. [DOI] [PubMed] [Google Scholar]
- Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology. 2007;32:2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology. 2008;33:1798–1806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Milivojevic V, Kemp K, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006;85:205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Liu S, Heitz RP, Bradberry CW. A touch screen based stop signal response task in rhesus monkeys for studying impulsivity associated with chronic cocaine self-administration. J Neurosci Methods. 2009;177:67–72. doi: 10.1016/j.jneumeth.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action- a theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Loo SK, Rich EC, Ishii J, McGough J, McCracken J, Nelson S, Smalley SL. Cognitive functioning in affected sibling pairs with ADHD: familial clustering and dopamine genes. J Child Psychol Psychiatry. 2008;49:950–957. doi: 10.1111/j.1469-7610.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- Lyons D, Friedman DP, Nader MA, Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci. 1996;16:1230–1238. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Kaplan JR, Rymeski BA, Fairbanks LA, Wilson ME. Approach to a social stranger is associated with low central nervous system serotonergic responsivity in female cynomolgus monkeys (Macaca fascicularis) Am J Primatol. 2003;61:187–194. doi: 10.1002/ajp.10118. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Mazur J. In: An adjusting procedure for studying delayed reinforcement, in Quantitative Analyses of Behaviour: The Effect of Delay and Intervening Events on Reinforcement Value. Commons ML, Nevin JA, Rachlin H, editors. Vol. 5. Erlbaum; Hillsdale, NJ: 1987. pp. 55–73. [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Means LW, Matthews JN. Comparison of the alcohol-preferring P rat to the Wistar rat in behavioral tests of impulsivity and anxiety. Physiol Behav. 1998;63:371–375. doi: 10.1016/s0031-9384(97)00442-3. [DOI] [PubMed] [Google Scholar]
- Mead AN, Crombag HS, Rocha BA. Sensitization of psychomotor stimulation and conditioned reward in mice: differential modulation by contextual learning. Neuropsychopharmacology. 2004;29:249–258. doi: 10.1038/sj.npp.1300294. [DOI] [PubMed] [Google Scholar]
- Miles FJ, Everitt BJ, Dalley JW, Dickinson A. Conditioned activity and instrumental reinforcement following long-term oral consumption of cocaine by rats. Behav Neurosci. 2004;118:1331–1339. doi: 10.1037/0735-7044.118.6.1331. [DOI] [PubMed] [Google Scholar]
- Mischel W, Grusec J. Waiting for rewards and punishments: effects of time and probability on choice. J Pers Soc Psychol. 1967;5:24–31. doi: 10.1037/h0024180. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001a;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001b;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47(Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Nogueira L, Kalivas PW, Lavin A. Long-term neuroadaptations produced by withdrawal from repeated cocaine treatment: role of dopaminergic receptors in modulating cortical excitability. J Neurosci. 2006;26:12308–12313. doi: 10.1523/JNEUROSCI.3206-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol Clin Exp Res. 2009;33:1294–1303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Krueger DD, Tronson NC, Nairn AC, Taylor JR. Orbitofrontal cortex and cognitive-motivational impairments in psychostimulant addiction: evidence from experiments in the non-human primate. Ann N Y Acad Sci. 2007;1121:610–638. doi: 10.1196/annals.1401.016. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004;173:98–104. doi: 10.1007/s00213-003-1702-9. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Hellemans KG, Paine TA. Alcohol-induced impulsivity in rats: an effect of cue salience? Psychopharmacology (Berl) 2006;184:221–228. doi: 10.1007/s00213-005-0215-0. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Crofts HS, McGuigan M, Tomic DL, Everitt BJ, Roberts AC. The role of the primate amygdala in conditioned reinforcement. J Neurosci. 2001;21:7770–7780. doi: 10.1523/JNEUROSCI.21-19-07770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pears A, Parkinson JA, Hopewell L, Everitt BJ, Roberts AC. Lesions of the orbitofrontal but not medial prefrontal cortex disrupt conditioned reinforcement in primates. J Neurosci. 2003;23:11189–11201. doi: 10.1523/JNEUROSCI.23-35-11189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self-administration and reinstatement of cocaine seeking behavior in male and female rats. Exp Clin Psychopharmacol. 2008;16:165–177. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Foerde K. Category learning and the memory systems debate. Neurosci Biobehav Rev. 2008;32:197–205. doi: 10.1016/j.neubiorev.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–814. [PubMed] [Google Scholar]
- Poulos CX, Parker JL, Le DA. Increased impulsivity after injected alcohol predicts later alcohol consumption in rats: evidence for “loss-of-control drinking” and marked individual differences. Behav Neurosci. 1998;112:1247–1257. doi: 10.1037//0735-7044.112.5.1247. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. J Exp Anal Behav. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivalan M, Ahmed SH, Dellu-Hagedorn F. Risk-prone individuals prefer the wrong options on a rat version of the iowa gambling task. Biol Psychiatry. 2009;66:743–749. doi: 10.1016/j.biopsych.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JFW, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Romer D, Betancourt L, Giannetta JM, Brodsky NL, Farah M, Hurt H. Executive cognitive functions and impulsivity as correlates of risk taking and problem behavior in preadolescents. Neuropsychologia. 2009;47:2916–2926. doi: 10.1016/j.neuropsychologia.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross MW, Hwang LY, Zack C, Bull L, Williams ML. Sexual risk behaviours and STIs in drug abuse treatment populations whose drug of choice is crack cocaine. Int J STD AIDS. 2002;13:769–774. doi: 10.1258/095646202320753736. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Beck LH. A continuous performance test of brain damage. J Consult Psychol. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- Schachar R, Logan GD, Robaey P, Chen S, Ickowicz A, Barr C. Restraint and cancellation: multiple inhibition deficits in attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2007;35:229–238. doi: 10.1007/s10802-006-9075-2. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seu E, Lang A, Rivera RJ, Jentsch JD. Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology (Berl) 2009;202:505–519. doi: 10.1007/s00213-008-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Soubrie P. Reconciling the role of central serotonin neurons in human and animal behavior. Behav Brain Sci. 1986;9:319–364. [Google Scholar]
- Spreux-Varoquaux O, Alvarez JC, Berlin I, Batista G, Despierre PG, Gilton A, Cremniter D. Differential abnormalities in plasma 5-HIAA and platelet serotonin concentrations in violent suicide attempters: relationships with impulsivity and depression. Life Sci. 2001;69:647–657. doi: 10.1016/s0024-3205(01)01158-4. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ. Difficulty overcoming learned non-reward during reversal learning in rats with ibotenic acid lesions of orbital prefrontal cortex. Ann N Y Acad Sci. 2007;1121:407–420. doi: 10.1196/annals.1401.010. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Horger BA. Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology (Berl) 1999;142:31–40. doi: 10.1007/s002130050859. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacol. 1986;90:390–397. doi: 10.1007/BF00179197. [DOI] [PubMed] [Google Scholar]
- Terman M, Terman JS. Latency differentiation of hits and false alarms in an operant-psychophysical test. J Exp Anal Behav. 1973;20:439–445. doi: 10.1901/jeab.1973.20-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A. Locating reward cue at response manipulandum (CAM) induces symptoms of drug abuse. Neurosci Biobehav Rev. 1996;20:505–535. doi: 10.1016/0149-7634(95)00023-2. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Quinn JJ, Taylor JR. Impulsivity, compulsivity, and habit: the role of orbitofrontal cortex revisited. Biol Psychiatry. 2008;63:253–255. doi: 10.1016/j.biopsych.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Robinson TE. Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice – mediation by enhanced incentive motivation? Eur J Neurosci. 2006;24:2345–2354. doi: 10.1111/j.1460-9568.2006.05117.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Suomi SJ, Chavanne TJ, Houser L, Hurley A, Cleveland A, Snoy PJ, Higley JD. Physiological correlates of aggression and impulsivity in free-ranging female primates. Neuropsychopharmacology. 2003;28:1045–1055. doi: 10.1038/sj.npp.1300171. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes Brain Behav. 2008;7:705–713. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson RT. Interaction of noise with knowledge of results and sleep deprivation. J Exp Psychol. 1963;66:332–337. doi: 10.1037/h0044161. [DOI] [PubMed] [Google Scholar]
- Winstanley CA. The orbitofrontal cortex, impulsivity and addiction: probing orbitofrontal dysfunction at the neural, neurochemical and molecular level. Ann N Y Acad Sci. 2007;1121:639–655. doi: 10.1196/annals.1401.024. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Bachtell RK, Theobald DE, Laali S, Green TA, Kumar A, Chakravarty S, Self DW, Nestler EJ. Increased impulsivity during withdrawal from cocaine self-administration: role for DeltaFosB in the orbitofrontal cortex. Cereb Cortex. 2009;19:435–444. doi: 10.1093/cercor/bhn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, LaPlant Q, Theobald DEH, Green TA, Bachtell RK, Perrotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW, Nestler EJ. Delta-FosB induction in orbitofrontal cortex mediates tolerance to cocaineinduced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles for basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004a;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004b;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Floresco SB, Winstanley CA. Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling and reward-related cues. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-1871-2. in press. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34:2329–2343. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]