Abstract
Gaucher disease, the inherited deficiency of the enzyme glucocerebrosidase, is the most common of the lysosomal storage disorders. Type 2 Gaucher disease, the most severe and progressive form, manifests either prenatally or in the first months of life, followed by death within the first years of life. The rarity of the many lysosomal storage disorders makes their diagnosis a challenge, especially in the newborn period when the focus is often on more prevalent illnesses. Thus, a heightened awareness of the presentation of these rare diseases is necessary to ensure their timely consideration. This review, designed to serve as a guide to physicians treating newborns and infants with Gaucher disease, discusses the presenting manifestations of Type 2 Gaucher disease, the diagnostic work-up, associated genotypes and suggestions for management. We also address the ethical concerns that may arise with this progressive and lethal disorder, since currently available treatments may prolong life, but do not impact the neurological manifestations of the disease.
Keywords: Gaucher disease, acute neuronopathic, infant, lysosomal storage disorder, hydrops fetalis, congenital ichthyosis, enzyme replacement therapy, palliative care
1. Introduction
Gaucher disease (GD) is an autosomal recessive lysosomal storage disorder (LSD) caused by a deficiency of the enzyme glucocerebrosidase (GCase). It is the most common of the more than 50 known LSDs, and is traditionally divided into three distinct types based on the presence and progression of neurological signs [1]. Type 1, non-neuronopathic GD, presents with vast clinical heterogeneity, ranging from asymptomatic individuals to patients with severely enlarged organs and bone involvement [2,3]. Type 3 GD is considered the chronic neuronopathic form and is likewise accompanied by a spectrum of manifestations, often with visceral involvement as well as impaired horizontal saccadic eye movements, and some patients may develop myoclonic epilepsy, ataxia, or dementia [2,3]. Type 2 GD, or acute neuronopathic GD, presents with neurological involvement within the first months of life and progresses rapidly, culminating in severe degeneration and death in infancy or early childhood [2,3].
While Type 2 GD is rare, its consideration is crucial for an accurate and timely diagnosis, appropriate management, genetic counseling and family planning [4]. It is encountered in all ethnicities. This work describes the signs and symptoms associated with Type 2 GD, management options, ethical concerns and how to establish the diagnosis. It is meant to serve as a guide for physicians and families of patients as they cope with a fatal diagnosis.
2. Phenotypes Associated with Type 2 GD
Patients with Type 2 GD invariably exhibit clinical signs in the first year of life, although the type and severity of manifestations can vary widely [5,6]. While newborns often appear normal at birth and develop neurological signs with a rapid decline in health within the first year [6], others present prenatally or as neonates. Although the visceral and neurological manifestations are fairly consistent, significant phenotypic heterogeneity is still observed. In the following sections we describe the key features reported.
2.1. Hydrops fetalis
Hydrops fetalis, characterized by an abnormal accumulation of fluid in multiple body areas, has been associated with several LSDs, including Type 2 GD [4]. While multiple disease processes are associated with hydrops, in over 25% of cases the cause is unknown. A portion of these undiagnosed cases may be due to LSDs that go unrecognized, leaving families unprepared regarding the risks for future pregnancies. Among patients with GD, cases involving hydrops tend to be perinatal lethal disorders and the pathophysiology is not well understood [6,7]. Generally, when hydrops is present in Type 2 GD, the fetus dies in utero, or is delivered prematurely and dies within several days of birth [7]. In cases of Type 2 GD without hydrops, fetuses generally reach full term [7].
2.2 Skin abnormalities
Newborns with Type 2 GD may present with congenital ichthyosis, an abnormal skin disorder resulting in shiny, almost cellophane-like skin. This is referred to as the collodion-baby phenotype [6,8]. The skin peels over the initial few weeks of life but then typically resolves while other symptoms worsen [6,9]. This pathology is thought to result from altered ratios of ceramides to glucosylceramides in the outermost layers of skin [10]. When severe, the skin must be treated aggressively with lubricants and fluids. Patients must be monitored vigilantly because of the risk for excessive loss of fluid and electrolytes.
2.3. Hepatosplenomegaly
Hepatosplenomegaly, enlargement of both the liver and spleen, is one of the characteristic signs observed among patients with all three types of GD [7]. Often a first diagnostic clue can be the discovery of an enlarged spleen in the newborn nursery. Untreated, the enlargement can be massive, with organs that extend to the pelvic brim, compromising feeding and breathing. However, some infants may not exhibit prominent organomegaly.
2.4. Thrombocytopenia and anemia
Thrombocytopenia is observed among all forms of GD and occurs in approximately 40% of patients with Type 2 GD [7], either transiently during the neonatal period or as the disease progresses. Anemia is also frequently observed in these patients [6].
2.5. Dysmorphology
Dysmorphic facial features, often associated with other specific LSDs but generally not GD, have been described in some infants with Type 2 GD, including low-set ears and a small, upturned nose with a flat nasal bridge [6,7]. Dysmorphic features often accompany the collodion-baby phenotype and might result from the presence of a thick membranous layer and decreased skin elasticity in utero.
2.6. Neurological Involvement
All patients with Type 2 GD experience a rapid neurological decline, but manifestations vary widely. Some patients present with arthrogyphosis (joint contractures), microcephaly (abnormally small head circumference) or hypokinesia (decreased bodily movement) [6,7]. More common neurologic signs include a hyperextended neck, spontaneous apnea, hypertonia, and poor suck and swallow reflexes [6-8]. The characteristic facies and neck extension observed are thought to be secondary to a cervico-facial dystonia. Strabismus is also commonly seen. Many patients go on to develop seizures, rigidity and opisthotonos as the disease progresses.
3. Establishing the Diagnosis of Gaucher Disease
The recommended diagnostic workup for Type 2 GD is outlined in Figure 1. The most reliable diagnostic methods are measurement of glucocerebrosidase enzymatic activity and direct sequencing of GBA1. Glucocerebrosidase enzymatic activity is usually included when ordering lysosomal panels, although it is important to assist the laboratory by providing appropriate clinical information. Also, unlike other lysosomal storage disorders, the enzymatic diagnosis of GD cannot be performed on plasma or RBCs, for only nucleated cells can be used. Enzymatic assays can be performed on leukocytes, fibroblasts, amniocytes or tissue samples such as liver or spleen. Type 2 GD is often difficult to distinguish from Types 1 and 3 based solely on enzymatic activity or genotype, although total absence of glucocerebrosidase is associated with early lethality.
Figure 1.
Establishing the diagnosis of Gaucher disease
Genetic testing for GD by direct sequencing of GBA1 can be used to determine disease and carrier status [11]. Over 300 mutations in GBA1 have been identified, and more continue to be discovered. Because there is a nearby pseudogene sharing 96% homology with GBA1 in the coding regions, genotyping can be challenging as several mutations originate from the pseudogene sequence, and it is essential to differentiate between the two sequences. Further complicating molecular diagnosis, many mutations are rare or private point mutations, insertions, deletions or splice-site changes that may not be detected in limited mutation screens. Some mutations appear to have arisen as a result of a recombination event that occurred between GBA1 and its homologous nearby pseudogene and are referred to as recombinant alleles. These alleles are relatively common among infants with Type 2 GD. Since recombinant alleles may result in the introduction of more than one alteration on the same allele, the entire sequence must be carefully analyzed. Generally, the enzyme assay is not reliable for heterozygote detection, and DNA tends to be far more accurate, especially in families where the GBA1 mutations are known.
Patients with Type 2 GD exhibit epidermal abnormalities regardless of whether ichthyosis is clinically evident [9,12]. This finding may have potential diagnostic importance. On electron microscopy, the skin ultrastructure reveals immature partially-processed lamellar bilayers. A study comparing 20 cases with Type 2 GD to other forms of GD demonstrated that these alterations were unique to Type 2 GD and could help differentiate between patients with Type 2 versus Type 3 GD. However, such evaluations are currently performed on a research-only basis [13].
While it is not a recommended method for diagnosis, many affected infants have been identified as a result of pathologic evaluations of biopsy specimen. The classic finding in bone marrow biopsies or liver, lung, and spleen samples is the presence of Gaucher cells, lipid engorged macrophages with a “wrinkled tissue” appearance of the cytoplasm. However, enzymatic or DNA confirmation is essential, as storage cells resembling Gaucher cells can be found in other conditions.
Clinicians should consider a diagnosis of GD in newborns with hepatosplenomegaly, hematological abnormalities, developmental delay, and failure to thrive. Among Ashkenazi Jewish families the carrier frequency is about 1 in 16; however neuronopathic forms of GD are relatively uncommon in this population [14].
4. Genotype/Phenotype Correlation
It is generally difficult to predict disease progression in GD based on the GBA1 genotype. Table 1 summarizes the genotypes and clinical features of 77 patients with Type 2 GD described in the literature. The nomenclature for each amino acid changes encountered in GBA1 has recently been revised to take into consideration the 39 amino acid leader sequence and thus mutation reports can be a source of some confusion. We have included both designations with the newer versions in parenthesis. It is evident that there are numerous genotypes and clinical phenotypes associated with Type 2 GD. However, certain generalizations can be made based on these data, notably the absence of one of the most common GBA1 mutations, Asn370Ser (Asn409Ser) [15]. Patients heterozygous or homozygous for this mutation do not manifest Type 2 GD. There has, however, been at least one report where Asn370Ser (Asn409Ser) was found on the same allele in cis with another GBA1 mutation, which can further confuse the picture [16]. In contrast, the mutation Leu444Pro (Leu483Pro) is frequently encountered in Type 2 GD, although it is rare to identify patients who are homozygous. When homozygosity for this mutation is reported in patients with Type 2 GD, further evaluation often shows that at least one Leu444Pro (Leu483Pro) allele is part of a recombinant allele with other mutations. Such alleles are null alleles, and homozygosity results in early lethality. Other null alleles like c.84insG or IVS2+1 are also found in Type 2 GD, often with a Leu444Pro (Leu483Pro) mutation on the second allele. Mutations involving an arginine such as Arg257Gly (Arg286Gly), Arg285His (Arg324His), Arg131Leu (Arg170Leu), Arg120Try (Arg159Try), Arg359X (Arg398X), and Arg463His (Arg463His) appear to be frequent. One allele with two GBA1 mutations, D409H+H255Q (Asp448His+His294Gln), is often found among patients with Type 2 GD of Greek or Albanian ancestry [17]. A relatively frequent GBA1 polymorphism E326K (E365K) is found in roughly 2-4% of the population and can also be encountered among patients with Type 2 GD along with a second GBA1 mutation on the same allele.
Table 1.
Genotypes and significant clinical features observed in patients with Type 2 Gaucher disease
| Alleles | Protein | cDNA | Age at diagnosis |
Age at death |
Hydrops | Ichthyosis | Hepatosplenomegaly | Seizures | Additional clinical information |
Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 55 bp del/Met416Val | p.Met455Val | c.1263del55/c.1363A>G | 8 months | 17 months | Yes | [12] | ||||
| 55bp del / Arg285His | p.Arg324His | c.1263del55/c.971G>A | At birth | Perinatal | Unknown | [13] | ||||
| 55bp del/Arg257Gln | p.Arg296Gln | c.1263del55/c.887G>A | At birth | 3 weeks | Yes | Yes | [13] | |||
| Ala190Glu/Gly325Arg | p.Ala229Glu p.Gly364Arg |
c.686C>A/c.1090G>A | 9 months | Unknown | Unknown | [13] | ||||
| Arg120Trp/Ser196Pro | p.Arg159Trp p.Ser235Pro |
c.475C>T/c.703T>C | At birth | 3 months | Yes | Yes | Yes | Laryngospasm | [70] | |
| Arg120Trp/Ser196Pro | p.Arg159Trp p.Ser235Pro |
c.475C>T/c.703T>C | At birth | 11 days | Yes | Yes | Yes (refractory) | [13] | ||
| Arg131Leu/Arg131Leu | p.Arg170Leu p.Arg170Leu |
c.509G>T/c.509G>T | 5 months | 7 months | Yes | Developmental regression | [13] | |||
| Arg131Leu/Arg131Leu | p.Arg170Leu p.Arg170Leu |
c.509G>T/c.509G>T | Prenatal | 9 months | Yes | Yes | [71] | |||
| Arg359X/Val398Phe | p.Arg398X p.Val437Phe |
c.1192C>T/c.1309G>T | At birth | 4 weeks | Yes | Facial dysmorphy (triangular face, malformed ears) | [72] | |||
| Arg463His/Arg463His | Arg502His Arg502His |
c.1505G>A/c.1505G>A | At birth | 1 month | Yes | Yes | Yes (myoclonic) | Respiratory failure | [73] | |
| Asp409His + His255Gln/Asp409His + His255Gln | Arg448His + His294Gln/Asp448His + His294Gln | c.[1342G>C; c.882T>G]/c.[1342G>C; c.882T>G] | 1 month | Unknown | Unknown | [74] | ||||
| Asp409His + His255Gln/Asp409His + His255Gln | Asp448His + His294Gln/Asp448His + His294Gln | c.[1342G>C; c.882T>G]/c.[1342G>C; c.882T>G] | 8 months | 2 years | Brainstem involvement; developmental regression | [74] | ||||
| c.533delC/c.533delC | c.533delC/c.533delC | Prenatal | 22 weeks gestation | Yes | Yes | External abnormalities (rocker bottom feet, flat nose) | [5,75] | |||
| c.84GG/Asp409His | Asp448His | c.84dupG/c.1342G>C | 5 months | Unknown | Abnormal eye saccades; delayed cognition | [74] | ||||
| Cys16Ser/Cys16Ser | Cys55Ser/Cys55Ser | c.164G>C c.164G>C |
Postmortem | At birth | Unknown | [76] | ||||
| Cys16Ser/Cys16Ser | Cys55Ser/Cys55Ser | c.164G>C c.164G>C |
Prenatal | 33 weeks gestation | 4th unsuccessful pregnancy for couple | [76] | ||||
| Gly202Arg/Gly202Arg | Gly241Arg/Gly241Arg | c.721G>A c.721G>A |
5 months | Unknown | Unknown | [13] | ||||
| Gly202Arg/Gly202Arg | Gly241Arg/Gly241Arg | c.721G>A c.721G>A |
6 months | Unknown | Unknown | [13] | ||||
| His311Arg/His311Arg | His350Arg/His350Arg | c.1049A>G c.1049A>G |
Prenatal | At birth | Yes | Yes | [72] | |||
| His311Arg/His311Arg | His350Arg/His350Arg | c.1049A>G c.1049A>G |
Prenatal | At birth | Yes | Yes | [72] | |||
| IVS2 + 1 G>A/Phe251Leu | Phe290Leu | c.115+1G>A/c.870C>A | 16 days | 1 month | Decreased spontaneous movement | [74] | ||||
| IVS2 + 1 G>A/Phe251Leu | Phe290Leu | c.115+1G>A/c.870C>A | At birth | 1 month | Yes | Yes | Myoclonic jerks/tremors | [77] | ||
| IVS2 + 1 G>A/RecNciI | c.115+1G>A/* | Prenatal | 45 days | Yes | Yes | Yes | [78] | |||
| IVS2 + 1 G>A/RecNciI | c.115+1G>A/* | Unknown | Unknown | Oculomotor apraxia; developmental regression | [74] | |||||
| Leu444Pro + Glu326Lys/Pro182Leu | Leu483Pro + Glu365Lys/Pro221Leu | c.[1448T>C; c.1093G>A]/c.662C>T | At birth | 25 days | Yes | Yes | [79] | |||
| Leu444Pro/Arg131Cys | Leu483Pro/Arg179Cys | c.1448T>C/c.508C>T | 8 months | 10.5 months | Yes | Strabismus | [80] | |||
| Leu444Pro/Arg257Gln | Leu483Pro/Arg296Gln | c.1448T>C/c.887G>A | Unknown | Unknown | Extensor posturing of trunk and neck; dysconjugate and crossed gaze | [74] | ||||
| Leu444Pro/Arg257Gln | Leu483Pro/Arg296Gln | c.1448T>C/c.887G>A | 9 months | 3 years | Yes (myoclonic) | [81] | ||||
| Leu444Pro/Arg257Gln | Leu483Pro/Arg296Gln | c.1448T>C/c.887G>A | 7 months | Unknown | Yes | Strabismus; bilateral hearing loss; failure to thrive | [77] | |||
| Leu444Pro/Asp380Asn | Leu483Pro/Asp419Asn | c.1448T>C/c.1255G>A | Unknown | Unknown | Unknown | [74] | ||||
| Leu444Pro/c.330delA | Leu483Pro | c.1448T>C/c.330delA | 3 months | Unknown | Unknown | [13] | ||||
| Leu444Pro/Gln414X | Leu483Pro/Gln453X | c.1448T>C/c.1357C>T | 9 months | 10 months | Apneic spells; developmental regression; ophthalmoplegia | [74] | ||||
| Leu444Pro/Gln414X | Leu483Pro/Gln453X | c.1448T>C/c.1357C>T | 2 months | 10 months | Unknown | [74] | ||||
| Leu444Pro/Gly202Arg | Leu483Pro/Gly241Arg | c.1448T>C/c.721G>A | 8 months | 2.5 years | Yes (tonic) | [81] | ||||
| Leu444Pro/Ile260Thr | Leu483Pro/Ile299Thr | c.1448T>C/c.896T>C | 6 months | 21 months | Strabismus; facial dysmorphy; respiratory difficulties | [79] | ||||
| Leu444Pro/IVS2 + 1 G>T | Leu483Pro | c.1448T>C/c.115+1G>T | 6 months | 9 months | Brainstem involvement; developmental regression | [74] | ||||
| Leu444Pro/IVS 2+1G>A | Leu483Pro | c.1448T>C/c.115+1G>A | 4 months | Unknown | Unknown | [13] | ||||
| Leu444Pro/IVS 2+1G>A | Leu483Pro | c.1448T>C/c.115+1G>A | 4 months | Unknown | Unknown | [13] | ||||
| Leu444Pro/IVS2 + 1G>A | Leu483Pro | c.1448T>C/c.115+1G>A | 6.5 months | 1 year | Strabismus; hypotonia; swallowing difficulties | [82] | ||||
| Leu444Pro/IVS2 + 1G>A | Leu483Pro | c.1448T>C/c.115+1G>A | At birth | 9 months | Yes | Progressive difficulty swallowing; bilateral brain stem dysfunction | [82] | |||
| Leu444Pro/IVS2 + 1 G>T | Leu483Pro | c.1448T>C/c.115+1G>T | Unknown | 8.5 months | Apnea; hypotonia; hyperflexia; esotropia | [74] | ||||
| Leu444Pro/Leu385Pro | Leu483Pro/Leu424Pro | c.1448T>C/c.1271T>C | 11 months | 18 months | Yes | Ophthalmoplegia | [12] | |||
| Leu444Pro/Leu444Pro + Ala456Pro | Leu483Pro/Leu483Pro + Ala495Pro | c.1448T>C/c.[1448T>C; c.1453G>C] | 7 months | 10 months | Yes | Strabismus | [82] | |||
| Leu444Pro/Phe213Ile | Leu483Pro/Phe252Ile | c.1448T>C/c.754T>A | 1 year | 28 months | Ophthalmoplegia | [12] | ||||
| Leu444Pro/Phe213Ile | Leu483Pro/Phe252Ile | c.1448T>C/c.754T>A | 11 months | Unknown | Bone involvement; ophthalmoplegia | [12] | ||||
| Leu444Pro/RecNciI | Leu483Pro | c.1448T>C* | 5 months | 1 year | Yes | Strabismus; facial paralysis; poor gag | [8] | |||
| Leu444Pro/RecNciI | Leu483Pro | c.1448T>C* | 1 year | 22 months | Abnormal tone | [12] | ||||
| Leu444Pro/RecNciI | Leu483Pro | c.1448T>C* | 8 months | 18 months | Yes | Ophthalmoplegia | [12] | |||
| Leu444Pro/RecNciI | Leu483Pro | c.1448T>C* | 1 month | 6 months | Unknown | [74] | ||||
| Leu444Pro/Thr323Ile | Leu483Pro/Thr362Ile | c.1448T>C/c.1085C>T | 6 months | Unknown | Hand tremors | Strabismus; brainstem involvement; clonus in feet | [74] | |||
| Leu444Pro/Tyr304Cys | Leu483Pro/Tyr343Cys | c.1448T>C/c.1028A>G | 1 year | Unknown | Unknown | [13] | ||||
| Leu444Pro/Tyr304Cys | Leu483Pro/Tyr343Cys | c.1448T>C/c.1028A>G | 1 year | 2 years | Yes (myoclonic) | [81] | ||||
| Leu444Pro/Tyr304Cys | Leu483Pro/Tyr343Cys | c.1448T>C/c.1028A>G | 1 year | Unknown | Unknown | [74] | ||||
| Leu444Pro/Tyr313His | Leu483Pro/Tyr352His | c.1448T>C/c.1054T>C | 7 months | 10 months | Oculomotor apraxia; developmental regression | [74] | ||||
| Leu444Pro/Unknown | Leu483Pro | c.1448T>C | 7 months | 11 months | Yes | Yes | Strabismus | [8] | ||
| Leu444Pro/Unknown | Leu483Pro | c.1448T>C | Unknown | Unknown | Unknown | [74] | ||||
| Leu444Pro/Unknown | Leu483Pro | c.1448T>C | 8 months | 2.5 years | Yes | Yes | Absent gag | [8] | ||
| Lys198Glu/Lys198Glu | Lys237Glu/Lys237Glu | c.709A>G/c.709A>G | 16 months | 2 years | Yes | Yes (myoclonic) | [81] | |||
| Lys198Glu/Lys198Glu | Lys237Glu/Lys237Glu | c.709A>G/c.709A>G | Unknown | Unknown | Myoclonic jerks | Hypotonia; gaze abnormalities | [74] | |||
| Phe213Ile/Asn382Lys | Phe252Ile/Asn421Lys | c.754T>A/c.1263C>A | 10 months | 18 months | Unknown | [12] | ||||
| Phe213Ile/Leu383Arg | Phe252Ile/Leu422Arg | c.754T>A/c.1265T>G | 7 months | 26 months | Yes | [12] | ||||
| Phe213Ile/Lys74X | Phe252Ile/Lys113X | c.754T>A/c.337A>T | Unknown | 10 months | Yes (myoclonic) | Strabismus; ophthalmoplegia; apneic spells | [74] | |||
| Phe259Leu/Asn188Lys | Phe298Leu/Asn227Lys | c.894C>A/c.681T>G | At birth | Perinatal | Opisthotonus | [13] | ||||
| Pro122Leu/Val375Leu | Pro161Leu/Val414Leu | c.482C>T/c.1240G>C | 1 year | Unknown | Yes | [12] | ||||
| RecA/IVS 10 + 2 | *c.1505+2T>G | Prenatal | Prenatal | Yes | Yes | Ascites; pleural effusions | [74] | |||
| RecA/IVS 10+2 T>G | c.1505+2T>G | Prenatal | Prenatal termination | Yes | [82] | |||||
| RecB/RecB | * | Prenatal | 1 day | Yes | Yes | Yes | Facial dysmorphy | [8] | ||
| RecB/RecB | * | Prenatal | Miscarriage | Yes | Thymic hypoplasia | [8] | ||||
| RecD/RecD | * | Prenatal | Perinatal | Yes | [13] | |||||
| RecD/RecD | * | Prenatal | 2 hours | Yes | Yes | Yes | [83] | |||
| RecD/RecD | * | Prenatal | 1 hour | Yes | Yes | Yes | Hypokinesia; facial dysmorphy | [84] | ||
| RecG + Leu444Pro/Arg257Gln | Leu483Pro/Arg296Gln | *c.1448T>C/c.887G>A | 6 months | Unknown | Unknown | [13] | ||||
| RecNciI/Glu41Leu | Glu80Leu | *c.238G>A | Several months | 18 months | Yes | Failure to thrive | [80] | |||
| RecNciI/RecNciI | * | Prenatal | At birth | Yes | Yes | Hyperextension of neck | [71] | |||
| Ser196Pro/Ser196Pro | Ser235Pro/Ser235Pro | c.703T>C c.703T>C |
At birth | Perinatal | Respiratory difficulty | [13] | ||||
| Trp184Arg/Asp409His | Trp223Arg/Asp448His | c.667T>C c.1342G>C |
5 months | Unknown | Opisthotonos; hypertonia; increased gag reflex | [74] | ||||
| Tyr363Cys/Unknown | Tyr402Cys | c.1205A>G | 5 months | 15 months | Yes | [12] | ||||
| Unknown/Unknown | At birth | 2 months | Yes | Hyperextension of the neck; hypertonia; poor suck/swallow | [85] | |||||
| Unknown/Unknown | At birth | 1 week | Yes | Flat nose | [85] |
Rec D: recombination at the end of exon 7; Rec G: recombination in intron 10-exon 11 or the 3’ untranslated region; Rec A: Recombination in intron 3; Rec NciI: Recombinant allele in exon 10 containing L444P, A456P, and V460V
5. Pathophysiology
It is still not known why only a subset of patients with GD develops aggressive neurodegeneration. Elucidating the etiology has additional relevance because mutations in GBA1 are a risk factor for developing Parkinson disease [18]. The deficiency of GCase in patients leads to the accumulation of glucosylceramide (GlcCer) and glucosylsphingosine (GlcSph) in many tissues, including the brain. Measurements of GlcCer and GlcSph in brain samples from patients with GD reveal that GlcSph is greatly elevated in neuronopathic GD, with the highest levels seen in Type 2 [19]. Recent work by Futerman and colleagues using a mouse model of neuronopathic GD found that the continued accumulation of GlcCer reaches a critical threshold that triggers a rapid cascade leading to neuroinflammation and neurodegeneration in certain brain regions [20,21].
The role of necroptosis in the pathogenesis of Type 2 GD has also been explored and considered as a potential therapeutic target based on work animal models [22]. Cell death occurs in a regulated manner via necrotic mechanisms, beginning with the formation of the necrosome, a multiprotein complex which includes receptor-interacting protein kinase-1 (RIPK1) and RIPK3 [23]. Necrosome formation can be initiated by ligand binding to death receptors and toll-like receptors, among others [23]. A caspase inhibitor must be present to prevent the complex from following a caspase-dependent apoptotic pathway [23]. Modification of RIPK1 and RIPK3 activate the kinases, beginning a downstream signaling cascade resulting in necroptotic cell death [23]. Active RIPK1 and RIPK3 levels were elevated in brain tissue of Gaucher mice generated by a conditional knock-out strategy. Furthermore, Ripk3−/− knock-out mice treated with the highly specific glucocerebrosidase inhibitor, conduritol-beta-epoxide, had fewer neurologic and systemic manifestations and greater longevity compared to similarly treated control mice [22]. These observations support the role of necroptosis in neuronopathic forms of GD, but further studies are needed in human tissues.
6. The Management of Patients with Type 2 Gaucher Disease
Management of Type 2 GD can be challenging for physicians as well as for the patients’ families. It is not the intent of this article to present rigid management guidelines, as many cultural, financial and ethical considerations come into play. Common management challenges and suggestions are also outlined in Table 2.
Table 2.
Managing common clinical features of Type 2 GD
| Clinical feature | Recommended evaluation | Management |
|---|---|---|
| Feeding difficulties and failure to thrive | Exam by a speech pathologist Video fluoroscopy to assess swallowing and aspirations Nutrition assessment |
Modification of bottle type / formula thickening Consider strict enteral feeding per nasogastric tube / gastric tube Individualized nutrition plan with sufficient macro and micronutrients |
| Gastrointestinal reflux | Clinical diagnosis Can be demonstrated on video fluoroscopy and pH meter |
Head elevation Proton pump inhibitors / H2 blockers |
| Laryngeal spasm/choking/apnea | Consider ENT evaluation | Treat GE reflux Atropine drops for excess salivation Consider strict enteral feeding Consider tracheostomy although may prolong survival Take precautions when performing anesthesia |
| Spasticity / abnormal movements | Neurologic evaluation | Consider benzodiazepines Consider EEG |
| Irritability | Nutrition assessment of intake | Provide sufficient macro- and micronutrients Gentle handling and maintenance of a calm and familiar environment Consider benzodiazepines |
| Fever | Evaluate for infection / recurrent aspirations | Antipyretics Consider empiric antibiotic treatment |
| Lung disease | Monitor for oxygen desaturations / respiratory distress on physical exam Chest X ray for evaluation of disease progression and pulmonary infections Video fluoroscopy for evaluation of aspirations Consider Pulmonology evaluation |
Treat GE reflux Consider strict enteral feeding Treat pulmonary infections Oxygen |
| Visceromegaly | Monitor CBC | Enzyme replacement therapy is generally not indicated |
| Bleeding diasthesis | Check CBC, PT/PTT Check liver function tests Consider testing coagulation factors |
Vitamin K Consider transfusion of platelets / fresh frozen plasma |
6.1. Failure to thrive and feeding difficulties
Failure to thrive is a common finding in patients with Type 2 GD and results from a combination of anorexia, nausea, hypotonia or hypertonia, vomiting and feeding difficulties [6]. Chewing difficulties, a weak suck, and/or problems with swallowing occur due to neurological involvement. In addition, the presence of abnormal neck tone and opisthotonos, trismus and/or the presence of laryngospasm can be contributing factors [24]. When the diagnosis is reached at an early stage, oral feeding may still be possible. A clinical evaluation of feeding should be performed by a speech pathologist, and infants should be monitored for weight gain, dehydration and/or signs of aspiration [25]. A video fluoroscopic evaluation of swallowing (modified barium scan) provides further information regarding the presence of aspiration, gastro-esophageal reflux and laryngeal penetration with different types of feedings [25] and can be used to adjust the type of bottle/nipple, formula thickening or recommend strict enteral feeding. It can also provide objective evidence for the extent of bulbar involvement [24,26]. The images can be used as an educational tool in discussions regarding the advantages and disadvantages of oral versus gavage feedings. In cases when it is decided to switch to enteral feedings, a nasogastric tube or gastric tube insertion with gastrostomy are two acceptable approaches. Decisions regarding the most appropriate approach should take into account potential anesthesia- related complications during a gastrostomy or gastric tube insertion procedure [27].
Assessment of macronutrient, micronutrient and fluid intake is important in these patients because their oral intake is usually low. An individualized nutrition plan should be constructed, considering the mode of feeding and energy requirements. Patients with Type 2 GD may not gain weight even with enteral or parenteral feeding because of increased resting energy expenditure [28].
6.2. Bulbar involvement and airway compromise
Brain stem degeneration leads to bulbar involvement, characterized by choking events, laryngeal spasm and apnea. These frightening episodes occur in a subset of patients and tend to occur with increased frequency as the disease progresses [29]. Laryngospasm can also lead to hypoxemia, which may accelerate the disease process. Treatment of gastro-esophageal reflux and strict enteral feeding can decrease the incidence of these events in early stages of the disease, but spontaneous episodes may occur as the disease worsens [29]. Excessive oral secretions may also contribute to respiratory symptoms, necessitating suction. Treatment with sublingually delivered ophthalmic atropine drops has been used with some success [30].
Whether to place a tracheotomy tube is one of the most challenging issues faced by physicians and families caring for children with Type 2 GD. A tracheostomy has been effectively used when the primary manifestation is laryngospasm and airway compromise. While in general patients with Type 2 GD who undergo tracheostomy placement tend to survive longer, consideration that this intervention may prolong suffering should be discussed with the family [31]. Extensive dialogue regarding realistic expectations is desirable prior to undertaking this measure. Parents should be counseled that the decision not to undergo tracheotomy can be very reasonable in many circumstances.
There is an increased risk for anesthesia-related complications due to increased secretions, gastro-esophageal reflux, poor control of laryngeal musculature and/or laryngeal spasm. These may be triggered by pre-anesthesia medications or manipulation of the airway [27]. Difficult airway access may require the need for smaller endotracheal tubes [27]. Pulmonary involvement in GD is another risk factor for anesthesia-related complications.
6.3. Neurological aspects
Neurological involvement can manifest as irritability and spasticity. Non-pharmacological treatments include gentle handling, maintaining a calm and familiar surrounding, physical therapy and proper positioning [32]. Pharmacological treatment with benzodiazepines can be useful for both irritability and spasticity. Caregivers should be educated about possible side effects including sedation, constipation, urinary retention, hypersalivation and weakness. Respiratory depression can be seen with benzodiazepine toxicity. Airway compromise due to bulbar involvement may limit their use. Other anti-spasticity drugs used include baclofen, dantrolene and tizanidine [33]. These medications can be offered in the setting of palliative therapy [32].
Seizures, sudden episodes of sensory disturbance, loss of consciousness, or convulsions, associated with abnormal electrical activity in the brain, can occur as the disease progresses. Electroencephalography (EEG) can identify abnormal brain activity in the absence of clinical signs. Findings on EEG may include slowing and disorganization of the background and/or epileptiform activity [Prows:1997bh; 33]. Myoclonic epilepsy and myclonus are often described although not universally, and are thought to be mainly of cortical origin [29,34].
Seizure control can be very challenging in patients with type 2 GD. Careful attention to the identification of treatable etiological factors such as hypoglycemia, hypocalcemia, hyponatremia, hypomagnesemia, hypoxia, intracranial hemorrhage, hyperammonemia, and/or infections is of critical importance. High quality neuroimaging and accurate EEG interpretation may help in optimizing patient care and outcomes [35]. Continuous EEG monitoring for the detection of subclinical seizures is recommended, but may not be possible due to the need for specialized training and access to equipment [36]. Periodic conventional EEG monitoring is more generally available. Antiepileptic treatment is generally guided by the particular epilepsy syndrome at presentation [37]. However, benzodiazepines are usually first-line therapy for status epilepticus although they can cause respiratory depression and/or cardiac dysrhythmia. Midazolam and lorazepam are the preferred agents. Second-line therapies include phenytoin and fosphenytoin. Phenytoin has been associated with local skin necrosis and hypotension. Both agents can cause cardiac dysrhythmias with more rapid administration. Phenobarbital is frequently used in infants, but carries the risk of hypotension, respiratory depression, and cardiac arrhythmia. Levetiracetam can also be considered. Valproate can be effective but patients must be monitored for potential side effects such as thrombocytopenia and hepatotoxicity [38].
6.4. Visceral and hematologic involvement
Visceral involvement presents as splenomegaly and is associated with anemia and thrombocytopenia. Liver enlargement is also seen, and can be accompanied by elevations in transaminases. Portal hypertension and liver dysfunction are rarely described in Type 2 GD, although neonatal cholestasis is occasionally seen and may precede other manifestations [39,40]. Splenectomies have been performed in these patients but are generally not recommended due to the risk of surgical complications. It is important to note that the absence of overt organomegaly is not sufficient to rule out the diagnosis of Type 2 GD.
The main hematological complication is a bleeding tendency secondary to the thrombocytopenia and abnormal platelet aggregation [41]. A decrease in clotting factors secondary to consumption by an enlarged spleen has also been described [42]. The complete blood cell count should be monitored regularly to identify the need for transfusions. Systemic disease can be minor or almost absent in comparison to the neurological deficits observed in patients with Type 2 GD.
Pulmonary involvement with interstitial lung disease is a common finding primarily resulting from recurrent aspirations [6] or from the infiltration of alveolar, interstitial, perivascular, and peribronchial spaces by lipid-laden macrophages [43]. Chest X-ray may show diffuse interstitial infiltration in both lung fields, and a ground glass pattern can be seen on chest CT. On lung biopsy, Gaucher cells can be seen [43]. Periodic monitoring of pulmonary involvement should include examination for evidence of respiratory distress, oxygen saturation measurements, and chest X-rays. Such testing can identify the need for supportive treatment with oxygen and/or antibiotics if there is evidence for aspiration pneumonia. Fever may be a sign of a pulmonary infection, but can also be present in the absence of infection due to inflammation, possibly mediated by activated glucosylceramide-laden macrophages [6]. Prolonged, unexplained fever has been associated with a worsening disease course [29].
7. Treatment
7.1. Enzyme replacement and substrate reduction
Enzyme replacement therapy (ERT) can ameliorate the visceromegaly and hematologic abnormalities in patients with Type 2 GD [28,44]. It is not clear whether ERT impacts pulmonary involvement, although when the primary cause of pulmonary involvement is recurrent aspirations secondary to neurological deterioration, ERT is not beneficial [28,44]. The recombinant enzyme does not cross the blood-brain barrier and there is no evidence that ERT has reversed, stabilized, or slowed the progression of neurological involvement [45]. Siblings were described where one received ERT after diagnosis at 7 months, and the subsequent sibling was treated from birth. While the sibling treated pre-symptomatically survived 6 months longer, the final outcome was the same [45]. It has been debated whether it is ethical to treat Type 2 GD patients with ERT because it is a very costly and inconvenient therapy that may only prolong suffering [46]. It is generally accepted that ERT should be discussed with families presenting both its potential merits and the limitations. Some physicians feel that ERT is not indicated for patients with Type 2 GD, although in recent years many infants have been started on the therapy [45]. It is sometimes deemed appropriate to treat with ERT until it is clear that the patient does not have Type 3 GD. In addition, it is reasonable to consider therapy for Type 2 GD patients in situations where it could be palliative, for example if reduction of organomegaly would alleviate pain, avoid surgery or facilitate other necessary interventions such as gastric tube placement.
Alternative methods of enzyme delivery have been investigated to treat the neurological deterioration in GD. Intraventricular delivery via a reservoir was attempted without success [47]. An animal study evaluated convection-enhanced delivery (CED) to restore GCase levels in the brains of rats and primates [48]. Enzymatic assays showed significant increases in GCase activity in the white matter, cortex, and pons of treated animals, and immunohistochemical staining confirmed GCase localization in neurons of the cortex [48]. One patient with Type 2 GD was treated with GCase via CED, and while successful delivery of enzyme was documented, there was continued disease progression [49]. Intravascular delivery after disruption of the blood-brain barrier has been investigated but not attempted in these patients [50].
Substrate reduction therapy with Miglustat, an inhibitor of glucosylceramide synthase, was not shown to be beneficial for neurological manifestations in Type 3 GD [51]. This treatment is not indicated for Type 2 GD.
7.2. Bone marrow and hematopoietic stem cell transplantation
Full engraftment of a hematopoietic stem cell transplantation (HSCT) has been performed in patients with Type 1 GD resulting in complete hematologic correction [52,53]. Ringden et al described four Swedish patients with Type 3 GD who underwent bone marrow transplantation between the ages of 2-9 years old [54]. Two patients had no cognitive decline at follow-up ten years later [54]. However, both developed epilepsy 14 and 22 years after allo-HSCT [55]. Thus, the procedure does not prevent development of neurological damage although it might affect the extent or rate of neurological deterioration. Published reports regarding the results of bone marrow transplantation in patients with Type 2GD are not available. The risks involved in transplanting a seriously ill infant must be seriously considered, given the strong possibility of continued neurological deterioration. Moreover, end-of-life issues may need to be addressed in distant centers, away from the support of home and family.
8. Genetic Counseling
Genetic counseling is an important aspect of the care for these patients and families. Parental feelings of guilt or blame can complicate the decision-making process as the child's condition deteriorates. Families need to be educated about risks and alternatives relevant to future family planning, including prenatal diagnosis, pre-implantation diagnosis, sperm or egg donation, as well as adoption. In addition, the genetics team should be aware that unusual mechanisms including uniparental disomy for chromosome 1 and germline or de novo mutations have been described in patients with GD [56,57]. Thus the recurrence risk is not always obvious.
9. Potential Future Therapies
9.1. Substrate reduction therapy
Inhibitors of glucosylceramide synthase have been explored as a means to attenuate glucosylceramide production and accumulation in various organs. The currently available therapy is not beneficial in neuropathic GD (Type 2 and 3) [51]. A novel glucosylceramide synthase inhibitor decreased substrate accumulation in the brains of a mouse model for neuronopathic GD [58]. This treatment modality might be beneficial for neuronopathic GD if given pre-symptomatically and before significant accumulation of substrate in the brain occurs, perhaps through newborn screening programs [59].
9.2. Chaperone therapy
Restoration of the mutant enzyme activity by small molecule compounds (chaperones) capable of crossing the blood-brain barrier is a new approach to treat LSDs [60], [[61]. Currently, there is an effort to identify candidate molecules [62,63]. However, this treatment will be helpful only for mutations in GBA1 that result in an unstable mutant protein and may not be appropriate for patients with null alleles. Induced pluripotent stem cells (iPSCs) generated from fibroblasts of patients with Type 2 GD may facilitate studies of candidate molecules. Gaucher iPSC lines show deficient GCase and were differentiated into macrophages that exhibit substrate accumulation [64]. This GD phenotype in macrophages was successfully reversed with a new molecular chaperone [65].
9.3. Gene therapy
Gene therapy is a potentially promising future therapeutic strategy for genetic diseases with enzymatic deficiencies like GD [66], although the effectiveness of gene therapy in CNS disease is not clear. Intracerebral injection of the vector carrying the wild-type gene may be a possible strategy [66].
9.4. Additional treatment targets
As we advance our understanding of the mechanisms involved in neurodegeneration, other potential targets will likely be identified. Insights from basic science research like the potential role RIPK3 [22] may provide new leads for future drug development.
10. Counseling Parents and Palliative Care
Facing the diagnosis of Type 2 GD in an infant is extremely difficult and can be emotionally overwhelming for parents, caregivers and health care providers. In many cases, the child appears normal at birth and during the first months of life until rapid deterioration begins. In the midst of these circumstances, parents and clinicians should work together to make important and complex decisions about the child's medical care. These decisions are based on a variety of considerations including medical information, values, cultural expectations, economic considerations and parental beliefs [67]. Studies have shown that parents of critically ill children need sensitivity, empathy, follow-up contact and information about counseling from healthcare providers [68]. This combination of education, support, and empathy can best be achieved by a multidisciplinary approach. Patient support groups or on-line chat rooms can also be helpful to some parents.
Type 2 GD is a progressive condition with an aggressive neurodegenerative course and is therefore particularly appropriate for palliative care. This can be a supportive resource for the parents as well as the child. When it is evident that supportive measures are the only options appropriate for continued care, health care providers can help the parents reengage with a new set of goals. The new goals can include managing the child's condition with the least amount of treatment-related pain or suffering, limiting exposure to invasive or extreme interventions, and maintaining the child's quality of life [67]. This approach might seem very reasonable for some parents and not to others, who choose to continue intensive interventions. Cultural and religious beliefs affect the parental decisions on palliative and end of life care and must be considered [68]. It is important to provide the parents with ongoing counseling by healthcare professionals during the different stages of the disease as well as after the child's death [69].
When possible, the subject of an autopsy should be raised with families. Such postmortem evaluations can help clinicians and researchers to better understand disease pathogenesis. Frozen stored tissue specimens are very useful for many studies that could ultimately impact the care of future children afflicted with this disorder.
Caring for a dying child can have a great impact on both parents and siblings. Appropriate support and therapy should be recommended as needed. Also, with the parents’ consent, education of employers, extended family members, and clergy as to the gravity of the illness can help the family get the needed support and time with the child. Engaging the services of hospice can also be very helpful.
11. Conclusion
Diagnosing and treating infants with Type 2 Gaucher disease represents a challenging paradigm for neonatal and genetic illnesses. An accurate and timely diagnosis of GD is critical for genetic counseling, patient management and family planning that can inform future pregnancies. However, the phenotypic heterogeneity observed in Type 2 GD patients makes the diagnosis a challenge for physicians unfamiliar with the disease. In general, Type 2 GD should be considered in the differential diagnosis of infants presenting with splenomegaly, failure to thrive, congenital ichythosis, hydrops fetalis and in families with recurrent fetal losses. Reliable methods of diagnosing GD include measurement of glucocerebrosidase enzymatic activity and direct sequencing of GBA1. Confirmation of the diagnosis of Type 2GD will direct treatment considerations and allow physicians to focus on critical clinical issues. Treating these infants is a challenge, and it is important to involve the medical team, family, and support networks early on, in order to identify goals of treatment and help to cope with this grave diagnosis.
Highlights.
Type 2 Gaucher disease is a rare neurodegenerative lysosomal disorder of infancy.
Diagnosis is made by measurement of enzymatic activity and direct sequencing of GBA1.
Patients with Type 2 Gaucher disease have multiple different genotypes.
Currently, there is no effective therapy except supportive and palliative treatments.
Management of patients is complex and requires a multidisciplinary approach.
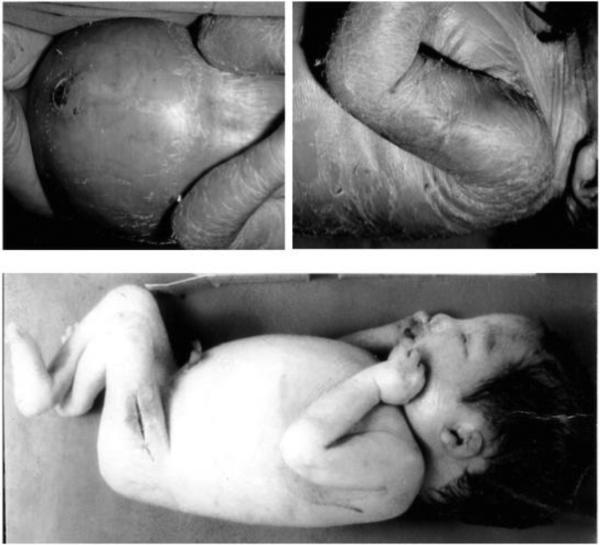
Figure 2.
Two patients with Type 2 Gaucher disease demonstrating the characteristic facies and strabismus. Top panel shows the first infant at age 6 months (left) and post-tracheotomy at 16 months (right). Lower panel shows the second child at age 8 months (left ) and after tracheotomy at age 2 years.
Figure 3.
Neonatal presentations of Gaucher disease demonstrating organomegaly (top left), hydrops (bottom) and peeling skin (top).
Acknowledgements
This work was supported by the Intramural Research Programs of the National Human Genome Research Institute, and the National Institutes of Health. The authors also greatly acknowledge the many patients, family members and referring physicians whose generous contributions enabled us to compose this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenbloom BE, Weinreb NJ. Gaucher disease: a comprehensive review. Crit Rev Oncog. 2013;18:163–175. doi: 10.1615/critrevoncog.2013006060. [DOI] [PubMed] [Google Scholar]
- 2.Sidransky E. Gaucher disease: complexity in a “simple” disorder. Mol Genet Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. doi:10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Sidransky E. Gaucher disease: insights from a rare mendelian disorder. Discov Med. 2012;14:273–281. [PMC free article] [PubMed] [Google Scholar]
- 4.Stone DL, Sidransky E. Hydrops fetalis: lysosomal storage disorders in extremis. Adv Pediatr. 1999;46:409–440. [PubMed] [Google Scholar]
- 5.Tayebi N, Stone DL, Sidransky E. Type 2 Gaucher disease: an expanding phenotype. Mol Genet Metab. 1999;68:209–219. doi: 10.1006/mgme.1999.2918. doi:10.1006/mgme.1999.2918. [DOI] [PubMed] [Google Scholar]
- 6.Gupta N, Oppenheim IM, Kauvar EF, Tayebi N, Sidransky E. Type 2 Gaucher disease: phenotypic variation and genotypic heterogeneity. Blood Cells Mol Dis. 2011;46:75–84. doi: 10.1016/j.bcmd.2010.08.012. doi:10.1016/j.bcmd.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mignot C, Gelot A, Bessières B, Daffos F, Voyer M, Menez F, et al. Perinatal-lethal Gaucher disease. Am. J. Med. Genet. A. 2003;120A:338–344. doi: 10.1002/ajmg.a.20117. doi:10.1002/ajmg.a.20117. [DOI] [PubMed] [Google Scholar]
- 8.Tayebi N, Reissner KJ, Lau EK, Stubblefield BK, Klineburgess AC, Martin BM, et al. Genotypic heterogeneity and phenotypic variation among patients with type 2 Gaucher's disease. Pediatr. Res. 1998;43:571–578. doi: 10.1203/00006450-199805000-00003. doi:10.1203/00006450-199805000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Sidransky E, Fartasch M, Lee RE, Metlay LA, Abella S, Zimran A, et al. Epidermal abnormalities may distinguish Type 2 from Type 1 and Type 3 of Gaucher disease. Pediatr. Res. 1996;39:134–141. doi: 10.1203/00006450-199601000-00020. doi:10.1203/00006450-199601000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Holleran WM, Ginns EI, Menon GK, Grundmann J-U, Fartasch M, McKinney CE, et al. Consequences of B-glucocerebrosidase deficiency in epidermis. J. Clin. Invest. 1994;93:1756–1764. doi: 10.1172/JCI117160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA) Hum Mutat. 2008;29:567–583. doi: 10.1002/humu.20676. doi:10.1002/humu.20676. [DOI] [PubMed] [Google Scholar]
- 12.Tang NLS, Zhang W, Grabowski GA, To KF, Choy FYM, Ma SL, et al. Novel mutations in type 2 Gaucher disease in Chinese and their functional characterization by heterologous expression. Hum Mutat. 2005;26:59–60. doi: 10.1002/humu.9348. doi:10.1002/humu.9348. [DOI] [PubMed] [Google Scholar]
- 13.Stone DL, Tayebi N, Orvisky E, Stubblefield B, Madike V, Sidransky E. Glucocerebrosidase gene mutations in patients with type 2 Gaucher disease. Hum Mutat. 2000;15:181–188. doi: 10.1002/(SICI)1098-1004(200002)15:2<181::AID-HUMU7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.Scott SA, Edelmann L, Liu L, Luo M, Desnick RJ, Kornreich R. Experience with carrier screening and prenatal diagnosis for 16 Ashkenazi Jewish genetic diseases. Hum Mutat. 2010;31:1240–1250. doi: 10.1002/humu.21327. doi:10.1002/humu.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koprivica V, Stone DL, Park JK, Callahan M, Frisch A, Cohen IJ, et al. Analysis and classification of 304 mutant alleles in patients with type 1 and type 3 Gaucher disease. Am J Hum Genet. 2000;66:1777–1786. doi: 10.1086/302925. doi:10.1086/302925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beutler E, Liebman H, Gelbart T, Stefanski E. Three Gaucher-Disease-Producing Mutations in a Patient with Gaucher Disease: Mechanism and Diagnostic Implications. Acta Haematologica. 2000;104:103–105. doi: 10.1159/000039760. doi:10.1159/000039760. [DOI] [PubMed] [Google Scholar]
- 17.Vithayathil J, Gibney G, Baxevanis AD, Stubblefield BK, Sidransky E, Tayebi N. Glucocerebrosidase mutation H255Q appears to be exclusively in cis with D409H: structural implications. Clin Genet. 2009;75:503–504. doi: 10.1111/j.1399-0004.2009.01163.x. doi:10.1111/j.1399-0004.2009.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N. Engl. J. Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. doi:10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orvisky E, Park JK, LaMarca ME, Ginns EI, Martin BM, Tayebi N, et al. Glucosylsphingosine accumulation in tissues from patients with Gaucher disease: correlation with phenotype and genotype. 2002;76:262–270. doi: 10.1016/s1096-7192(02)00117-8. [DOI] [PubMed] [Google Scholar]
- 20.Farfel-Becker T, Vitner EB, Pressey SNR, Eilam R, Cooper JD, Futerman AH. Spatial and temporal correlation between neuron loss and neuroinflammation in a mouse model of neuronopathic Gaucher disease. 2011;20:1375–1386. doi: 10.1093/hmg/ddr019. doi:10.1093/hmg/ddr019. [DOI] [PubMed] [Google Scholar]
- 21.Farfel-Becker T, Vitner EB, Kelly SL, Bame JR, Duan J, Shinder V, et al. Neuronal accumulation of glucosylceramide in a mouse model of neuronopathic Gaucher disease leads to neurodegeneration. Hum Mol Genet. 2014;23:843–854. doi: 10.1093/hmg/ddt468. doi:10.1093/hmg/ddt468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitner EB, Salomon R, Farfel-Becker T, Meshcheriakova A, Ali M, Klein AD, et al. RIPK3 as a potential therapeutic target for Gaucher's disease. Nat. Med. 2014;20:204–208. doi: 10.1038/nm.3449. doi:10.1038/nm.3449. [DOI] [PubMed] [Google Scholar]
- 23.Zhou W, Yuan J. Necroptosis in health and diseases. Seminars in Cell and Developmental Biology. 2014:1–10. doi: 10.1016/j.semcdb.2014.07.013. doi:10.1016/j.semcdb.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Tylki-Szymańska A, Vellodi A, El-Beshlawy A, Cole JA, Kolodny E. Neuronopathic Gaucher disease: demographic and clinical features of 131 patients enrolled in the International Collaborative Gaucher Group Neurological Outcomes Subregistry. J Inherit Metab Dis. 2010;33:339–346. doi: 10.1007/s10545-009-9009-6. doi:10.1007/s10545-009-9009-6. [DOI] [PubMed] [Google Scholar]
- 25.Newman LA, Keckley C, Petersen MC, Hamner A. Swallowing function and medical diagnoses in infants suspected of dysphagia. Pediatr. 2001;108:e106–e106. doi: 10.1542/peds.108.6.e106. doi:10.1542/peds.108.6.e106. [DOI] [PubMed] [Google Scholar]
- 26.Hanna R, McDonald MT, Sullivan JA, Mackey JF, Krishnamurthy V, Kishnani PS. Diagnostic and treatment challenges of neuronopathic Gaucher dsease: two cases with an intermediate phenotype. J Inherit Metab Dis. 2004:687–690. doi: 10.1023/b:boli.0000043027.80328.75. [DOI] [PubMed] [Google Scholar]
- 27.Tobias JD, Atwood R, Lowe S, Holcomb GW. Anesthetic considerations in the child with Gaucher disease. J Clin Anesth. 1993;5:150–153. doi: 10.1016/0952-8180(93)90144-4. [DOI] [PubMed] [Google Scholar]
- 28.Prows CA, Sanchez N, Daugherty C, Grabowski GA. Gaucher disease: enzyme therapy in the acute neuronopathic variant. Am. J. Med. Genet. 1997;71:16–21. doi:10.1002/(SICI)1096-8628(19970711)71:1<16::AID AJMG3>3.0.CO;2-O. [PubMed] [Google Scholar]
- 29.Mignot C, Doummar D, Maire I, De Villemeur TB. Type 2 Gaucher disease: 15 new cases and review of the literature. 2006;28:39–48. doi: 10.1016/j.braindev.2005.04.005. doi:10.1016/j.braindev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Rapoport A. Sublingual atropine drops for the treatment of pediatric sialorrhea. J Pain Symptom Manage. 2010;40:783–788. doi: 10.1016/j.jpainsymman.2010.02.007. doi:10.1016/j.jpainsymman.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Erikson A, Bembi B, Schiffmann R. Neuronopathic forms of Gaucher's disease. Baillieres Clin Hameatol. 1997;10:711–723. doi: 10.1016/s0950-3536(97)80035-2. [DOI] [PubMed] [Google Scholar]
- 32.Wusthoff CJ, Shellhaas RA, Licht DJ. Management of common neurologic symptoms in pediatric palliative care: seizures, agitation, and spasticity. Pediatr Clin N Am. 2007;54:709–733. doi: 10.1016/j.pcl.2007.06.004. doi:10.1016/j.pcl.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Chung C-Y, Chen C-L, Wong AM-K. Pharmacotherapy of Spasticity in Children With Cerebral Palsy. J Formos Med Assoc. 2011;110:215–222. doi: 10.1016/S0929-6646(11)60033-8. doi:10.1016/S0929-6646(11)60033-8. [DOI] [PubMed] [Google Scholar]
- 34.Park JK, Orvisky E, Tayebi N, Kaneski C, LaMarca ME, Stubblefield BK, et al. Myoclonic epilepsy in Gaucher disease: genotype-phenotype insights from a rare patient subgroup. Pediatr. Res. 2003;53:387–395. doi: 10.1203/01.PDR.0000049515.79882.94. doi:10.1203/01.PDR.0000049515.79882.94. [DOI] [PubMed] [Google Scholar]
- 35.Berg AT, Baca CB, Loddenkemper T, Dlugos D. Priorities in pediatric epilepsy research. Neurology. 2013:1166–1175. doi: 10.1212/WNL.0b013e3182a55fb9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glass HC. Neonatal Seizures. Clinics in Perinatology. 2014;41:177–190. doi: 10.1016/j.clp.2013.10.004. doi:10.1016/j.clp.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dulac O, Plecko B, Gataullina S, Wolf NI. Occasional seizures, epilepsy, and inborn errors of metabolism. The Lancet Neurology. 2014;13:727–739. doi: 10.1016/S1474-4422(14)70110-3. doi:10.1016/S1474-4422(14)70110-3. [DOI] [PubMed] [Google Scholar]
- 38.Szlam S, Meredith M. Shake, rattle, and roll: an update on pediatric seizures. Peidatr Emerg Care. 2013;29:1287–1294. doi: 10.1097/PEC.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 39.Elias AF, Johnson MR, Boitnott JK, Valle D. Neonatal Cholestasis as Initial Manifestation of Type 2 Gaucher Disease: A Continuum in the Spectrum of Early Onset Gaucher Disease. JIMD Rep. 2011;5:95–98. doi: 10.1007/8904_2011_104. doi:10.1007/8904_2011_104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbier C, Devisme L, Dobbelaere D, Noizet O, Nelken B, Gottrand F. Neonatal cholestasis and infantile Gaucher disease: a case report. Acta Pediatr. 2002:1399–1401. doi: 10.1111/j.1651-2227.2002.tb02841.x. [DOI] [PubMed] [Google Scholar]
- 41.Kelsey H, Christopoulos C, Gray AA, Machin SJ. Acquired pseudo-pseudo Bernard-Soulier syndrome complicating Gaucher's disease. J Clin Pathol. 1994:162–165. doi: 10.1136/jcp.47.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CE H, Levi M, Berends F, Aerts JM, van Oers MH. Coagulation abnormalities in type 1 Gaucher disease are due to low-grade activation and can bepartly restored by enzyme supplementation therapy. Br. J. Haematol. 1996:470–476. doi: 10.1046/j.1365-2141.1997.d01-2076.x. [DOI] [PubMed] [Google Scholar]
- 43.Gulhan B, Ozcelik U, Gurakan F, Gucer S, Orhan D, Cinel G, et al. Different features of lung involvement in Niemann-Pick disease and Gaucher disease. Resp Med. 2012;106:1278–1285. doi: 10.1016/j.rmed.2012.06.014. doi:10.1016/j.rmed.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Erikson A, Johansson K, Mansson JE, Scennerholm L. Enzyme replacement therapy of infantile Gaucher disease. Neuropediatrics. 1993:237–238. doi: 10.1055/s-2008-1071549. [DOI] [PubMed] [Google Scholar]
- 45.Vellodi A, Tylki-Szymanska A, Davies EH, Kolodny E, Bembi B, Collin-Histed T, et al. Management of neuronopathic Gaucher disease: revised recommendations. J Inherit Metab Dis. 2009;32:660–664. doi: 10.1007/s10545-009-1164-2. doi:10.1007/s10545-009-1164-2. [DOI] [PubMed] [Google Scholar]
- 46.Elstein D, Abrahamov A, Zimran A. Ethical considerations for enzyme replacement therapy in neuronopathic Gaucher disease. Clin Genet. 1998;54:179–184. doi: 10.1111/j.1399-0004.1998.tb04281.x. [DOI] [PubMed] [Google Scholar]
- 47.Bembi B, Ciana G, Zanatta M. Cerebrospinal-fluid infusion of alglucerase in the treatment for acute neuronopathic Gaucher's disease. Peidatr Res. 1995:A425. [Google Scholar]
- 48.Lonser RR, Walbridge S, Murray GJ, Aizenberg MR, Vortmeyer AO, Aerts JMFG, et al. Convection perfusion of glucocerebrosidase for neuronopathic Gaucher's disease. Ann Neurol. 2005;57:542–548. doi: 10.1002/ana.20444. doi:10.1002/ana.20444. [DOI] [PubMed] [Google Scholar]
- 49.Lonser RR, Schiffman R, Robison RA, Butman JA, Quezado Z, Walker ML, et al. Image-guided, direct convective delivery of glucocerebrosidase for neuronopathic Gaucher disease. Neurology. 2007;68:254–261. doi: 10.1212/01.wnl.0000247744.10990.e6. doi:10.1212/01.wnl.0000247744.10990.e6. [DOI] [PubMed] [Google Scholar]
- 50.Barranger JA, Rapoport SI, Fredericks WR, Pentchev PG, MacDermot KD, Steusing JK, et al. Modification of the blood-brain barrier: increased concentration and fate of enzymes entering the brain. Proc. Natl. Acad. Sci. U.S.a. 1979;76:481–485. doi: 10.1073/pnas.76.1.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiffmann R, FitzGibbon EJ, Harris C, DeVile C, Davies EH, Abel L, et al. Randomized, controlled trial of miglustat in Gaucher's disease type 3. Ann Neurol. 2008;64:514–522. doi: 10.1002/ana.21491. doi:10.1002/ana.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ito S, Pophali P, CO W, Koklanaris EK, Superata J, Fahle GA, et al. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplantation. 2013;48:1313–1316. doi: 10.1038/bmt.2013.49. doi:10.1038/bmt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ito S, Barrett AJ. Gauchers Disease—A Reappraisal of Hematopoietic Stem Cell Transplantation. Pediatr Hematol Oncol. 2013;30:61–70. doi: 10.3109/08880018.2012.762076. doi:10.3109/08880018.2012.762076. [DOI] [PubMed] [Google Scholar]
- 54.Ringdén O, Groth CG, Erikson A, Granqvist S, Mansson JE, Sparrelid E. Ten years' experience of bone marrow transplantation for Gaucher disease. Transplantation. 1995;59:864–870. [PubMed] [Google Scholar]
- 55.Machaczka M. Allogeneic Hematopoietic Stem Cell Transplantation for Treatment of Gaucher Disease. Pediatr Hematol Oncol. 2013;30:459–461. doi: 10.3109/08880018.2013.793757. doi:10.3109/08880018.2013.793757. [DOI] [PubMed] [Google Scholar]
- 56.Saranjam H, Chopra SS, Levy H, Stubblefield B, Maniwang E, Cohen IJ, et al. A germline or de novo mutation in two families with Gaucher disease: implications for recessive disorders. Eur. J. Hum. Genet. 2013;21:115–117. doi: 10.1038/ejhg.2012.105. doi:10.1038/ejhg.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benko WS, Hruska KS, Nagan N, Goker-Alpan O, Hart PS, Schiffmann R, et al. Uniparental disomoy of chromosome 1 causing concurrent Charcot-Marie-Tooth and Gaucher disesae type 3. Neurology. 2008;70:976–978. doi: 10.1212/01.wnl.0000305963.37449.32. doi:10.1212/01.wnl.0000305963.37449.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cabrera-Salazar MA, DeRiso M, Bercury SD, Li L, Lydon JT, Weber W, et al. Systemic Delivery of a Glucosylceramide Synthase Inhibitor Reduces CNS Substrates and Increases Lifespan in a Mouse Model of Type 2 Gaucher Disease. PLoS ONE. 2012;7:e43310. doi: 10.1371/journal.pone.0043310. doi:10.1371/journal.pone.0043310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang RY, Bodamer OA, Watson MS, Wilcox WR. Lysosomal storage diseases: diagnostic confirmation and management of presymptomatic individuals. Genet Med. 2011;13:457–484. doi: 10.1097/GIM.0b013e318211a7e1. doi:10.1097/GIM.0b013e318211a7e1. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki Y. Chaperone therapy update: Fabry disease, GM1-gangliosidosis and Gaucher disease. Brain Dev. 2013;35:515–523. doi: 10.1016/j.braindev.2012.12.002. doi:10.1016/j.braindev.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Giugliani R, Waldek S, Germain DP, Nicholls K, Bichet DG, Simosky JK, et al. A Phase 2 study of migalastat hydrochloride in females with Fabry disease: selection of population, safety and pharmacodynamic effects. Mol Genet Metab. 2013;109:86–92. doi: 10.1016/j.ymgme.2013.01.009. doi:10.1016/j.ymgme.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Joosten A, Decroocq C, de Sousa J, Schneider JP, Etame E, Bodlenner A, et al. A systematic investigation of Iminosugar click clusters as pharmacological chaperones for the treatment of Gaucher disease. ChemBioChem. 2014;15:309–319. doi: 10.1002/cbic.201300442. doi:10.1002/cbic.201300442. [DOI] [PubMed] [Google Scholar]
- 63.Schonemann W, Gallienne E, Ikeda-Obatake K, Asano N, Nakagawa S, Kato A, et al. Glucosylceramide mimics: highly potent GCase inhibitors and selective pharmacological chaperones for mutations associated with types 1 and 2 Gaucher disease. ChemMedChem. 2013;8:1805–1817. doi: 10.1002/cmdc.201300327. doi:10.1002/cmdc.201300327. [DOI] [PubMed] [Google Scholar]
- 64.Tiscornia G, Lorenzo Vivas E, Matalonga L, Berniakovich I, Barragan Monasterio M, Eguizabal C, et al. Neuronopathic Gaucher's disease: induced pluripotent stem cells for disease modelling and testing chaperone activity of small compounds. Hum Mol Genet. 2013;22:633–645. doi: 10.1093/hmg/dds471. doi:10.1093/hmg/dds471. [DOI] [PubMed] [Google Scholar]
- 65.Aflaki E, Stubblefield BK, Maniwang E, Lopez G, Moaven N, Goldin E, et al. Macrophage models of Gaucher disease for evaluating disease pathogenesis and candidate drugs. Science Translational Medicine. 2014;6:240ra73–240ra73. doi: 10.1126/scitranslmed.3008659. doi:10.1126/scitranslmed.3008659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parenti G, Pignata C, Pietro Vajro MC. Salerno, New strategies for the treatment of lysosomal storage diseases (Review) Int J Mol Med. 2012;31:11–20. doi: 10.3892/ijmm.2012.1187. doi:10.3892/ijmm.2012.1187. [DOI] [PubMed] [Google Scholar]
- 67.Hill DL, Miller V, Walter JK, Carroll KW, Morrison WE, Munson DA, et al. Regoaling: a conceptual model of how parents of children with serious illness change medical care goals. BMC Palliative Care. 2014;13:1–8. doi: 10.1186/1472-684X-13-9. doi:10.1186/1472-684X-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Youngblut JM, Brooten D. Perinatal and pediatric issues in palliative and end of-life care from the 2011 Summit on the Science of Compassion. Nursing Outlook. 2012;60:343–350. doi: 10.1016/j.outlook.2012.08.007. doi:10.1016/j.outlook.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Macdonald ME, Liben S, Carnevale FA, Rennick JE, Wolf SL, Meloche D, et al. Parental perspectives on hospital staff members“ acts of kindness and commemoration after a child”s death. Pediatr. 2005;116:884–890. doi: 10.1542/peds.2004-1980. doi:10.1542/peds.2004-1980. [DOI] [PubMed] [Google Scholar]
- 70.Lui K, Commens C, Choong R, Jaworski R. Collodion babies with Gaucher's disease. Arch. Dis. Child. 1988;63:854–856. doi: 10.1136/adc.63.7.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stone DL, Carey WF, Christodoulou J, Sillence D, Nelson P, Callahan M, et al. Type 2 Gaucher disease: the collodion baby phenotype revisited. Arch. Dis. Child. Fetal Neonatal Ed. 2000;82:F163–6. doi: 10.1136/fn.82.2.F163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stone DL, van Diggelen OP, de Klerk JB, Gaillard JL, Niermeijer MF, Willemsen R, et al. Is the perinatal lethal form of Gaucher disease more common than classic type 2 Gaucher disease? 1999;7:505–509. doi: 10.1038/sj.ejhg.5200315. doi:10.1038/sj.ejhg.5200315. [DOI] [PubMed] [Google Scholar]
- 73.Haverkaemper S, Marquardt T, Hausser I, Timme K, Kuehn T, Hertzberg C, et al. Congenital ichthyosis in severe type II Gaucher disease with a homozygous null mutation. Neonatology. 2011;100:194–197. doi: 10.1159/000324116. doi:10.1159/000324116. [DOI] [PubMed] [Google Scholar]
- 74.Chan A, Holleran WM, Ferguson T, Crumrine D, Goker-Alpan O, Schiffmann R, et al. Skin ultrastructural findings in type 2 Gaucher disease: diagnostic implications. Mol Genet Metab. 2011;104:631–636. doi: 10.1016/j.ymgme.2011.09.008. doi:10.1016/j.ymgme.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reissner K, Tayebi N, Stubblefield BK, Koprivica V, Blitzer M, Holleran W, et al. Type 2 Gaucher disease with hydrops fetalis in an Ashkenazi Jewish family resulting from a novel recombinant allele and a rare splice junction mutation in the glucocerebrosidase locus. Mol Genet Metab. 1998;63:281–288. doi: 10.1006/mgme.1998.2675. doi:10.1006/mgme.1998.2675. [DOI] [PubMed] [Google Scholar]
- 76.Church HJ, Cooper A, Stewart F, Thornton CM, Wraith JE. Homozygous loss of a cysteine residue in the glucocerebrosidase gene results in Gaucher's disease with a hydropic phenotype. Eur. J. Hum. Genet. 2004;12:975–978. doi: 10.1038/sj.ejhg.5201251. doi:10.1038/sj.ejhg.5201251. [DOI] [PubMed] [Google Scholar]
- 77.Zhao H, Keddache M, Bailey L, Arnold G, Grabowski G. Gaucher's disease: identification of novel mutant alleles and genotype-phenotype relationships. Clin Genet. 2003;64:57–64. doi: 10.1034/j.1399-0004.2003.00100.x. [DOI] [PubMed] [Google Scholar]
- 78.Michelakakis H, Dimitriou E, Moraitou M, Valari M, Yatrakou E, Mitsiadi V, et al. Perinatal lethal form of Gaucher disease. Clinical and molecular characterization of a Greek case. Blood Cells Mol Dis. 2010;44:82–83. doi: 10.1016/j.bcmd.2009.11.007. doi:10.1016/j.bcmd.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 79.Chabás A, Gort L, Díaz-Font A, Montfort M, Santamaría R, Cidrás M, et al. Perinatal lethal phenotype with generalized ichthyosis in a type 2 Gaucher disease patient with the [L444P;E326K]/P182L genotype: effect of the E326K change in neonatal and classic forms of the disease. Blood Cells Mol Dis. 2005;35:253–258. doi: 10.1016/j.bcmd.2005.04.007. doi:10.1016/j.bcmd.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 80.Sinclair G, Choy FY, Humphries L. A novel complex allele and two new point mutations in type 2 (acute neuronopathic) Gaucher disease. Blood Cells Mol Dis. 1998;24:420–427. doi: 10.1006/bcmd.1998.0210. doi:10.1006/bcmd.1998.0210. [DOI] [PubMed] [Google Scholar]
- 81.Goker-Alpan O, Schiffmann R, Park JK, Stubblefield BK, Tayebi N, Sidransky E. Phenotypic continuum in neuronopathic Gaucher disease: an intermediate phenotype between type 2 and type 3. J. Pediatr. 2003;143:273–276. doi: 10.1067/S0022-3476(03)00302-0. doi:10.1067/S0022-3476(03)00302-0. [DOI] [PubMed] [Google Scholar]
- 82.Aviner S, Garty B-Z, Rachmel A, Baris HN, Sidransky E, Shuffer A, et al. Type 2 Gaucher disease occurs in Ashkenazi Jews but is surprisingly rare. Blood Cells Mol Dis. 2009;43:294–297. doi: 10.1016/j.bcmd.2009.08.004. doi:10.1016/j.bcmd.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rowlands S, Murray H. Prenatal ultrasound findings in a fetus diagnosed with Gaucher's disease (type 2) at birth. Prenat. Diagn. 1997;17:765–769. doi: 10.1002/(sici)1097-0223(199708)17:8<765::aid-pd122>3.0.co;2-3. doi:10.1002/(SICI)1097-0223(199708)17:8<765::AID-PD122>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 84.Sidransky E, Tayebi N, Stubblefield BK, Eliason W, Klineburgess A, Pizzolato GP, et al. The clinical, molecular, and pathological characterisation of a family with two cases of lethal perinatal type 2 Gaucher disease. J. Med. Genet. 1996;33:132–136. doi: 10.1136/jmg.33.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sidransky E, Sherer DM, Ginns EI. Gaucher Disease in the neonate: a distinct Gaucher phenotype Is analogous to a mouse model created by targeted disruption of the glucocerebrosidase gene. Pediatr. Res. 1992;32:1–5. doi: 10.1203/00006450-199210000-00023. [DOI] [PubMed] [Google Scholar]