Abstract
OBJECTIVES:
Treatments that prevent sepsis complications are needed. Circulating lipid and protein assemblies—lipoproteins play critical roles in clearing pathogens from the bloodstream. We investigated whether early inhibition of proprotein convertase subtilisin/kexin type 9 (PCSK9) may accelerate bloodstream clearance of immunogenic bacterial lipids and improve sepsis outcomes.
DESIGN:
Genetic and clinical epidemiology, and experimental models.
SETTING:
Human genetics cohorts, secondary analysis of a phase 3 randomized clinical trial enrolling patients with cardiovascular disease (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab [ODYSSEY OUTCOMES]; NCT01663402), and experimental murine models of sepsis.
PATIENTS OR SUBJECTS:
Nine human cohorts with sepsis (total n = 12,514) were assessed for an association between sepsis mortality and PCSK9 loss-of-function (LOF) variants. Incident or fatal sepsis rates were evaluated among 18,884 participants in a post hoc analysis of ODYSSEY OUTCOMES. C57BI/6J mice were used in Pseudomonas aeruginosa and Staphylococcus aureus bacteremia sepsis models, and in lipopolysaccharide-induced animal models.
INTERVENTIONS:
Observational human cohort studies used genetic PCSK9 LOF variants as instrumental variables. ODYSSEY OUTCOMES participants were randomized to alirocumab or placebo. Mice were administered alirocumab, a PCSK9 inhibitor, at 5 mg/kg or 25 mg/kg subcutaneously, or isotype-matched control, 48 hours prior to the induction of bacterial sepsis. Mice did not receive other treatments for sepsis.
MEASUREMENTS AND MAIN RESULTS:
Across human cohort studies, the effect estimate for 28-day mortality after sepsis diagnosis associated with genetic PCSK9 LOF was odds ratio = 0.86 (95% CI, 0.67–1.10; p = 0.24). A significant association was present in antibiotic-treated patients. In ODYSSEY OUTCOMES, sepsis frequency and mortality were infrequent and did not significantly differ by group, although both were numerically lower with alirocumab vs. placebo (relative risk of death from sepsis for alirocumab vs. placebo, 0.62; 95% CI, 0.32–1.20; p = 0.15). Mice treated with alirocumab had lower endotoxin levels and improved survival.
CONCLUSIONS:
PCSK9 inhibition may improve clinical outcomes in sepsis in preventive, pretreatment settings.
Keywords: endotoxins, lipid regulating agents, proprotein convertase subtilisin/kexin type 9, sepsis, septic shock
KEY POINTS
Question: What is the impact of early proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition on sepsis outcomes.
Findings: Human genetic cohort studies demonstrated a nonsignificant trend toward improved survival among carriers of PCSK9 loss-of-function genetic variants. In a post hoc analysis of a randomized clinical trial, alirocumab-treated patients reported nonsignificantly fewer incident and fatal sepsis vs. placebo-treated patients. In murine models of sepsis, PCSK9 inhibition with alirocumab significantly reduced circulating levels of bacterial endotoxins and improved survival.
Meaning: Taken together, although not all results reached statistical significance, data from these analyses suggest that PCSK9 inhibitor pretreatment may possibly improve sepsis outcomes.
Sepsis is a life-threatening dysregulated host response to infection (1). In 2017, the worldwide estimated annual incidence of sepsis was 48.9 million cases, with a reported 11 million sepsis-related deaths, representing approximately 20% of all global deaths (2). No drugs have been approved specifically for the prevention or treatment of sepsis, and antibiotics and supportive care remain the mainstay of treatment (3). A challenge in the management of sepsis is that patients often present beyond a window where treatments may provide benefit. Accordingly, new approaches based on preventive treatments—targeted at those at risk for sepsis—may present strategies to improve clinical outcomes, although such a hypothesis remains untested.
Sepsis is accelerated by the immune response to bacterial cell wall lipid components, including the endotoxin lipopolysaccharide (LPS), and hence accelerating endotoxin clearance from the bloodstream may reduce sepsis severity and improve clinical outcomes. Endotoxins are cleared from the bloodstream via their incorporation into constitutively circulating lipoproteins, including low-density lipoprotein (LDL) and other lipoproteins, which are in turn cleared from the circulation by hepatic LDL receptors (LDLRs) (4). Proprotein convertase subtilisin/kexin type 9 (PCSK9) binds to the LDLR, promotes its lysosomal degradation, and prevents its recycling to the hepatocyte surface. Inhibiting PCSK9 increases hepatocyte LDLR availability and in turn LDL clearance; it may therefore accelerate the removal of pathogenic endotoxins from the bloodstream (5–8), preventing or mitigating the severity of sepsis. Supporting this hypothesis, PCSK9 loss-of-function (LOF) genetic variants have been associated with increased survival in modest-sized cohorts of patients with sepsis (9). PCSK9-deficient mice and mice treated with antibodies against PCSK9 may have better outcomes in sepsis models (4), although this has not been consistently observed (10). No data on the use of clinically available PCSK9 inhibitors and septic outcomes are available.
Accordingly, we evaluated the potential role for preventive PCSK9 inhibition in sepsis, hypothesizing primarily that it may improve sepsis survival as well as, secondarily, reducing the frequency of sepsis. We first evaluated whether PCSK9 LOF genetic variants were associated with improved sepsis survival in a meta-analysis of nine cohorts. Second, in a post hoc analysis of a large, placebo-controlled cardiovascular outcomes trial, we evaluated whether long-term treatment with the PCSK9 inhibitor alirocumab was associated with reduced occurrence and number of deaths. Third, we evaluated whether pretreatment with pharmacologic PCSK9 inhibition may accelerate endotoxin clearance from the bloodstream and improve survival in mice following experimental induction of sepsis.
MATERIALS AND METHODS
Patient Cohorts
The association between sepsis mortality and the LOF variant R46L (rs11591147) in PCSK9 was tested separately in nine cohorts, totaling 3236 patients with sepsis who died (cases) and 9274 with sepsis who survived (controls), with further details below: DiscovEHR, a unique cohort dataset as a result of collaboration between Geisinger Health System (GHS) and Regeneron Genetics Center (RGC), Regeneron Pharmaceuticals, Inc. (11), partnership GENetics of SEPsis (12), St. Paul’s Hospital (Vancouver, BC, Canada) (4), Vasopressin and Septic Shock Trial (4, 13), the U.K. Biobank (UKB) (14), the Trøndelag Health Study/Norwegian University of Science and Technology (15), Copenhagen and Estonian Biobank, and Kaiser Permanente Resource for Genetic Epidemiology Research on Adult Health and Aging (16, 17). All cohort studies were approved by their relevant research ethics review boards, and informed consent was collected from all participants. The study analyzed sepsis based on the Third International Consensus Definitions for Sepsis (Sepsis 3) framework (1). Studies were stratified on the basis of their sepsis ascertainment. Tier 1 studies defined sepsis clinically or using International Classification of Diseases, 10th Revision (ICD-10) codes R65.2, R65.20, and R65.21 (suspected or confirmed infection and acute organ failure). Tier 2 studies defined sepsis using ICD-10 codes A40/A41 (suspected or confirmed infection and additional greater than or equal to 1 ICD-10 codes for organ failure [cardiogenic shock: I50 or R57.0; liver failure: K72; acute kidney failure: N17; or bone marrow failure D69] at the time of sepsis hospitalization). Association results were combined across cohorts using an inverse-variance weighted fixed-effects meta-analysis. Mortality was defined as death within 28 days of admission to the hospital.
The primary genetic instrument, PCSK9 variant p.Arg46Leu (rs11591147, alternative allele frequency [AAF] 0.012), a missense variant (18), was chosen based on relative frequency and availability in the above cohorts. Several other PCSK9 variants were evaluated for their association with 28-day mortality, including LOF variant p.Ala53Val (rs11583680), LOF variant p.Val474Ile (rs562556), and gain-of-function variant p.Gly670Glu (rs505151). In the UKB, the LDLR intronic variant in the LDLR (rs6511720; AAF 0.12), which increases expression of the LDLR and lowers LDL cholesterol (LDL-C) (19), was examined for a possibly consistent and additive effect with PCSK9 rs11591147.
Further details for each patient cohort are provided in the Additional Text S1 (http://links.lww.com/CCX/B264).
Secondary Analyses of a Randomized Clinical Trial
The Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab (ODYSSEY OUTCOMES; ClinicalTrials.gov Identifier: NCT01663402) trial was a randomized, parallel-group, double-blind, placebo-controlled clinical trial that evaluated the effect of alirocumab on cardiovascular outcomes in patients recently (4–52 wk) hospitalized for an acute coronary syndrome (20). Details on the study protocol and analysis of sepsis are provided in Additional Text S1 (http://links.lww.com/CCX/B264).
Experimental Models of Sepsis
Details of bacterial strains and culture, bacteremia sepsis models, and LPS-induced animal models are presented in Additional Text S1 (http://links.lww.com/CCX/B264).
An overview of the study approaches (as well as the key findings) are shown schematically in Figure S1 (http://links.lww.com/CCX/B264).
Ethics Approval and Consent to Participate
For all patient cohort studies and the ODYSSEY OUTCOMES trial, informed consent was collected from all participants prior to participation (20). The studies were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All mouse studies were overseen and approved by Regeneron’s Institutional Animal Care and Use Committee (IACUC) and were performed in accordance with relevant guidelines under approved Institutional Animal Care & Use Committee protocols 314, 323, and 467 (no study specific approval number available).
RESULTS
PCSK9 LOF Genetic Variants and Sepsis Mortality
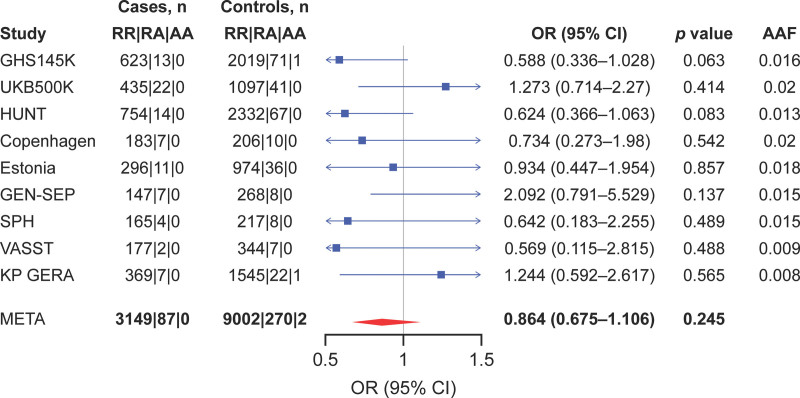
Nine human cohorts (12,514 patients) with sepsis were analyzed. Overall, 28-day mortality was 25.9% (range, 23.3–46.8%) (Table S1, http://links.lww.com/CCX/B264). Meta-analysis did not show a significant association between the PCSK9 partial LOF variant rs11591147 and lower 28-day mortality (odds ratio [OR], 0.86; 95% CI, 0.67–1.10; p = 0.245; Fig. 1), although the effect estimate favored lower 28-day mortality. Restricting to the five cohorts that used ICD codes which are potentially more specific for sepsis revealed similar results (OR, 0.86; 95% CI, 0.59–1.26; p = 0.450; Fig. S2, http://links.lww.com/CCX/B264).
Figure 1.
Meta-analysis of the association between the proprotein convertase subtilisin/kexin type 9 (PCSK9) loss-of-function variant rs11591147 (R46L) and 28-d mortality in all cohorts of patients with sepsis. AA = Alternate/Alternate, AAF = alternate allele fraction, GEN-SEP = GENetics of SEPsis, GHS = Geisinger Health System, HUNT = Trøndelag Health Study, KP GERA = Kaiser Permanente Resource for Genetic Epidemiology Research on Adult Health and Aging, META = meta-analysis, OR = odds ratio, RA = Reference/Alternate, RR = Reference/Reference, SPH = St. Paul’s Hospital, UKB = U.K. Biobank, VASST = Vasopressin and Septic Shock Trial.
Additional analyses were performed in the GHS, where more detailed data were available. First, the association between other PCSK9 variants and 28-day survival was examined, including: LOF variant p.Ala53Val (rs11583680) (OR, 0.81; 95% CI, 0.65–1.00; p = 0.046); LOF variant p.Val474Ile (rs562556) (OR, 1.10; 95% CI, 0.92–1.33; p = 0.291); and gain-of-function variant p.Gly670Glu (rs505151) (OR, 1.16; 95% CI, 0.82–1.64; p = 0.402) (Table S2, http://links.lww.com/CCX/B264). Sensitivity analyses of the association between the primary instrumental variable, rs11591147, and sepsis survival were performed in GHS participants (Table S3, http://links.lww.com/CCX/B264); this included the subset of patients receiving antibiotics, in whom rs11591147 was associated with increased survival (OR, 0.36; 95% CI, 0.16–0.82; p = 0.02). Finally, given that statins also upregulate LDLR, we evaluated the association between statin treatment and sepsis mortality. Among 8412 patients who survived sepsis, 3992 (47.5%) were taking a statin, whereas among 4638 patients who did not survive sepsis, 1911 (41.2%) were taking a statin. These differences corresponded to an adjusted (for age, number of inpatient visits in the previous 12 mo, cardiovascular risk, malignancy, and hepatic disease) (OR, 0.60; 95% CI, 0.55–0.65; p = 1.4 × 10–36).
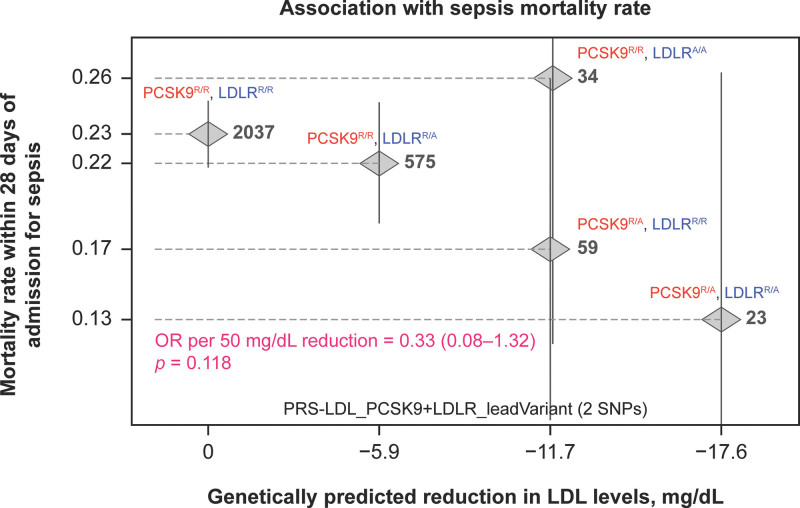
Additionally, given that the putative beneficial effects of PCSK9 inhibition may be mediated by an increase in LDLR availability, in the GHS, we examined the relationship between LDLR variant rs6511720, which increases LDLR and lowers LDL-C (19), and 28-day death from sepsis in an additive genetic model with rs11591147. A trend toward dose-dependent reduction in LDL-C was observed (Fig. S3, http://links.lww.com/CCX/B264) in parallel with a dose-dependent reduction in fatal sepsis (p = 0.12; Fig. 2).
Figure 2.
Relationship between sepsis mortality in an additive genetic model incorporating proprotein convertase subtilisin/kexin type 9 (PCSK9) loss-of-function (rs11591147) and low-density lipoprotein (LDL) receptor (LDLR) gain-of-function (rs6511720) variants; both of which would be expected to increase LDLR availability to remove bacterial endotoxin from the bloodstream. Data are from 2731 participants in the U.K. Biobank. A dose-dependent reduction in sepsis mortality was observed. AA = Alternate/Alternate, OR = odds ratio, PRS = polygenic risk score, RA = Reference/Alternate, RR = Reference/Reference, SNP = single nucleotide polymorphism.
Effect of Alirocumab on Incident and Fatal Sepsis
The ODYSSEY OUTCOMES trial compared the effect of alirocumab or placebo on cardiovascular outcomes in patients with recent acute coronary syndrome (20). Among 18,884 participants who received at least one dose of assigned study medication, 1172 had at least one investigator-reported treatment-emergent serious adverse event due to an infection broadly or sepsis explicitly. The full list of Medical Dictionary of Regulatory Activities terms used in the initial search query to identify potential sepsis events among investigator reports is provided in Table S4 (http://links.lww.com/CCX/B264). These reports were then evaluated by an independent expert blinded to treatment allocation and adjudicated as definite sepsis (n = 51), probable sepsis (n = 35), or not sepsis (n = 1086). Median (interquartile range) follow-up was 2.8 years (2.3–3.4 yr). In the alirocumab arm, 37 of 9450 participants developed definite or probable sepsis (frequency per 100 patient-years, 0.16; 95% CI, 0.11–0.22), compared with 49 of 9434 participants in the placebo arm (frequency per 100 patient-years, 0.20; 95% CI, 0.15–0.26; hazard ratio, 0.80; 95% CI, 0.52–1.22; p = 0.30) (Table 1). The relative hazard for death from sepsis among patients randomized to alirocumab (frequency per 100 patient-years, 0.15; 95% CI, 0.07–0.23) compared with placebo (0.25 [0.15–0.36]) was 0.62 (95% CI, 0.32–1.20; p = 0.15).
TABLE 1.
Frequency of Septic Events in the Alirocumab Versus Placebo Arms of the Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab Trial
| Parameter | Sepsis Frequency | Sepsis-Related Mortality | Relative Risk of Incident Sepsisa | Relative Risk of Death From Sepsisa | ||
|---|---|---|---|---|---|---|
| Placebo (n = 9434) | Alirocumab (n = 9450) | Placebo (n = 9434) | Alirocumab (n = 9450) | Hazard Ratio (95% CI)b | ||
| n (%) (95% CI)c | 49 (0.52) (0.37–0.66) | 37 (0.39) (0.27–0.52) | 24 (0.25) (0.15–0.36) | 14 (0.15) (0.07–0.23) | 0.80 (0.52–1.22; p = 0.30) | 0.62 (0.32–1.20; p = 0.15) |
| Frequency rate per 100 patient-years (95% CI) | 0.20 (0.15–0.26) | 0.16 (0.11–0.22) | 0.10 (0.06–0.15) | 0.06 (0.03–0.10) | ||
Relative risk for alirocumab compared with placebo.
Hazard ratio from Cox proportional hazards models with p value from log-rank test.
One patient in the alirocumab arm with a first sepsis episode ≥ 30 d after last treatment was reallocated to the placebo arm.
Events were collocated as treatment-emergent adverse events and adjudicated as probably or definite sepsis.
Prophylactic Alirocumab Administration in Mouse Models of Sepsis
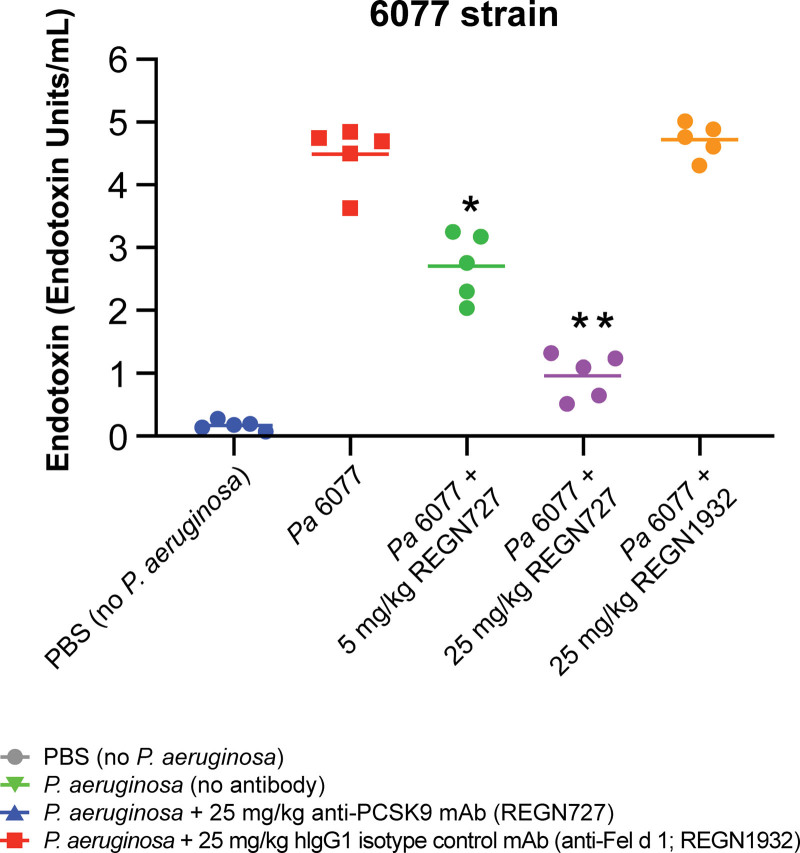
We evaluated whether PCSK9 inhibition accelerates endotoxin clearance in mice. Male C57BL/6J mice were administered 5 mg/kg or 25 mg/kg of alirocumab subcutaneously 48 hours prior to the induction of sepsis with three strains of live Pseudomonas aeruginosa intraperitoneal inoculation. Mice did not receive other treatments for sepsis, including antibiotics or IV fluids. A significant dose-dependent reduction in serum LPS concentrations at 16 hours after live P. aeruginosa infection was observed in all mice infected with the three P. aeruginosa strains and treated with alirocumab, compared with animals treated with vehicle or isotype control (Fig. 3; and Fig. S4 A and B, http://links.lww.com/CCX/B264). Alirocumab, compared with vehicle and isotype control, delayed death by 6–10 hours in P. aeruginosa infection, resulting in a modest increase in survival (range of 10–30% across P. aeruginosa strains) (Fig. S4C–E, http://links.lww.com/CCX/B264). Targeted replication of the P. aeruginosa PA14 strain inoculation model was performed in 48 female mice, showing consistent findings (Fig. S5, http://links.lww.com/CCX/B264).
Figure 3.
Effects of anti-proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibody (mAb) on lipopolysaccharide (LPS) levels and survival in a live bacteria-induced sepsis model due to Pseudomonas aeruginosa. C57Bl/6J mice were given a single subcutaneous injection of the indicated doses of human immunoglobulin G1 (hIgG1) Regeneron (REGN) monoclonal antibodies (mAbs) 48 hr before intraperitoneal injection of live P. aeruginosa (Pa) strains PAK (at 1.5 × 107–2.5 × 107 colony-forming units [CFUs]/mouse; n = 60), PA14 (at 5 × 107–7 × 107 CFU/mouse; n = 30), or 6077 (at 2.5 × 106–7 × 106 CFU/mouse; n = 40), in phosphate-buffered saline (PBS). LPS was measured in serum 16 hr after infection in mice (n = 5 per group), and survival was monitored for up to 96 hr. LPS was lower at 16 hr among mice receiving anti-PCSK9 mAb in strain 6077. *p < 0.001 by unpaired t test; **p < 0.0001 by unpaired t test. mAb to cat allergen, Fel d 1 is used as a control in this experiment, PA14 = P. aeruginosa strain 14, PAK = P. aeruginosa strain K.
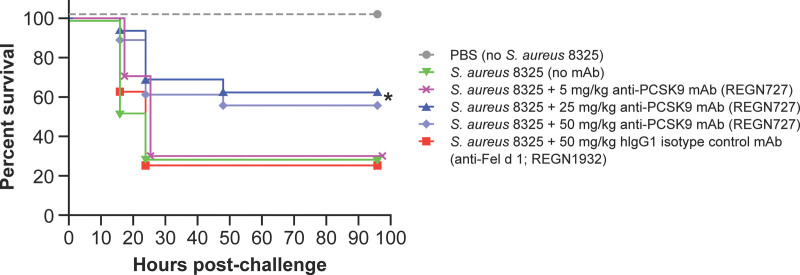
Modest protection against Staphylococcus aureus-induced sepsis was observed (Fig. 4), with a 4–6-hour delay in death and a 60% increase in survival of mice treated with alirocumab compared with mice treated with isotype control (plog-rank = 0.03). Death was delayed by 4–6 hours with prophylactic administration of alirocumab, compared with vehicle and isotype control, for Escherichia coli LPS intoxication mouse sepsis models (plog-rank < 0.001) (Fig. S6, http://links.lww.com/CCX/B264).
Figure 4.
Effects of anti-proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibody (mAb) on survival in live bacteria-induced sepsis models due to Staphylococcus aureus infection lipopolysaccharide administration. In the S. aureus infection sepsis mode, C57Bl/6J mice (n = 17/group) were given a single subcutaneous injection of the indicated doses of human immunoglobulin G1 (hIgG1) Regeneron (REGN) monoclonal antibodies (mAbs) 48 hr before intraperitoneal injection of live S. aureus 8325-4 strain bacteria (approximately 8 × 107 colony-forming units /mouse) in phosphate-buffered saline (PBS). Mice were monitored for survival and clinical signs for 96 hr and outcomes were compared by log-rank (Mantel-Cox) test. *p = 0.0328. mAb to cat allergen, Fel d 1 is used as a control in this experiment.
DISCUSSION
This study evaluated whether pharmacologic or genetic PCSK9 inhibition may improve sepsis outcomes. Human genetics suggested fewer deaths among PCSK9 LOF carriers, but this difference was not significant. However, significantly lower sepsis mortality was observed in a subset of PCSK9 LOF variant carriers with confirmed antibiotic use. A post hoc analysis of a large, randomized, placebo-controlled clinical trial suggested lower incident and fatal sepsis among patients assigned to alirocumab, although this difference was not statistically significant. Experimental models suggested that pharmacologic PCSK9 inhibitor pretreatment lowers circulating bacterial endotoxin, and improves survival in mice not receiving other treatments for sepsis. Combined, these results suggest that PCSK9 inhibition when provided prior to the onset of sepsis may improve sepsis outcomes.
Mortality from bacterial sepsis remains high (2) amidst dozens of neutral therapeutic clinical trials to date (3). Immunomodulatory therapies have proven ineffective, reflecting the observation that many patients downregulate key immune and inflammatory pathways during infection (21–23). Promising strategies to prevent sepsis or mitigate its severity may be those that accelerate clearance of immunogenic bacterial endotoxins, which contribute to the maladaptive host response that defines sepsis (5). Given that endogenous clearance of LPS is mediated by transit of lipoproteins through the bloodstream to the liver (24), strategies that accelerate this process may speed LPS clearance and improve clinical outcomes. PCSK9 inhibition upregulates hepatic LDLR, accelerating lipid clearance and possibly speeding endotoxin removal (5).
Walley et al (4) demonstrated an association between PCSK9 LOF genetic variants and increased survival in two cohorts of patients with sepsis comprising 960 patients. PCSK9 knockout mice demonstrated an attenuated response to LPS, and pharmacological PCSK9 inhibition enhanced survival of mice with polymicrobial peritonitis. Septic mice over-expressing PCSK9 demonstrated worse organ failure and higher levels of plasma interleukin-6 (IL-6) and thrombin-antithrombin complexes, whereas mice under-expressing PCSK9 demonstrated reduced bacterial load, organ failure, inflammatory markers, and sepsis severity (25). In contrast, using an endotoxin model of sepsis, differences in survival with administration of PCSK9 antibodies, or with PCSK9 knockdown were not observed (10). Our findings support that PCSK9 inhibition may accelerate bacterial endotoxin removal from the bloodstream and delay mortality. However, it is worth noting that large trials of other strategies for accelerating endotoxin removal—such as via polymyxin B hemoperfusion (26)—have not demonstrated improved clinical outcomes. The modest protection observed against S. aureus-induced murine sepsis with alirocumab may also suggest benefits on Gram-positive bacterial cell wall products, such as lipoteichoic acid (LTA), although the levels of LTA were not evaluated. Another experimental study observed beneficial effects of PCSK9 inhibition on LTA and outcomes in Gram-positive infection (8). Importantly, mice in our study did not receive antibiotics or other treatments for sepsis, and the findings may differ in the setting of antibiotic administration. The observed delays in sepsis mortality may translate clinically into a longer window for effective antimicrobial therapy (27). Antibiotic therapy may lead to a transient increase in circulating bacterial degradation products, such as endotoxins, which may enhance the benefit of PCSK9 inhibition (28), and better supportive care may overall improve survival. In contrast, antibiotics may improve survival sufficiently so as to attenuate the potential benefit of PCSK9 inhibition. Interestingly, in human genetics studies reported herein, sepsis mortality was more clearly reduced in the subset of patients confirmed to be treated with antibiotics in a sensitivity analysis in the GHS database where these data were available. Our genetic analysis of nine human cohorts with sepsis is distinguished by its large aggregate sample size. Our analysis supports that PCSK9 inhibition may be beneficial in slowing the clinical course of sepsis, potentially providing a longer window for effective antimicrobial therapy. Although the prespecified outcomes of the ODYSSEY OUTCOMES trial were cardiovascular, more than 45,000 patient-years of placebo-controlled observation allowed for a post hoc, blindly adjudicated assessment of the efficacy of alirocumab in 86 cases of incident sepsis and 38 cases of fatal sepsis showing lower incident and fatal sepsis with patients given alirocumab compared with placebo. Overall, although some associations were nonsignificant, all results were directionally concordant across experimental, human genetic, and observational studies and taken together may support a potential role for PCSK9 inhibition to improve sepsis outcomes.
A previous study observed that 37% of survivors without a PCSK9 LOF variant died or experienced infection-related readmission within 1 year of index hospital discharge, as opposed to 19% of carriers of PCSK9 LOF alleles (p = 0.02), an effect that was consistent after adjustment and across two modest-sized cohorts of sepsis survivors (29). These frequencies are consistent with other studies which suggest that, in the year after hospital discharge for sepsis, up to 60% of sepsis survivors have at least one rehospitalization, most commonly for infection, and that approximately one in six sepsis survivors dies (30). Such late morbidity is associated with significant healthcare costs (31). In the study by Genga et al (29), PCSK9 LOF carriers had accelerated decline of neutrophil counts, perhaps suggesting that a late benefit is mediated by enhanced resolution of initial infection. Thus, it is plausible that PCSK9 inhibitor pretreatment may represent a target for secondary prevention of recurrent infection and associated mortality among sepsis survivors. That sepsis survivors also experience a 50–70% increased relative risk of late cardiovascular events compared with nonseptic controls (32) may further encourage a secondary prevention strategy with established cardiovascular benefit. In our study, prior use of statins—which also upregulate LDLR—was associated with a 40% lower mortality from sepsis. In contrast, clinical trials do not indicate a benefit of de novo statin initiation in sepsis (33), supporting that treatment prior to the onset of sepsis may be required to allow effective LDLR upregulation in time to improve endotoxin clearance. A recent exploratory trial in 60 patients demonstrated that PCSK9 inhibition, compared with placebo, reduced the occurrence of death or need for intubation and IL-6 levels in patients with severe COVID-19 (34). These findings may support favorable effects of PCSK9 inhibitors on the host response to severe infection.
Our study combined diverse data and study types to evaluate an unprecedented sample size for human genetic studies in sepsis but has several potential limitations. Our genetic analysis of sepsis outcomes for PCSK9 and LDLR variants relied on ICD codes to identify patients with sepsis, recognizing the imperfect sensitivity and specificity of this approach. We observed similar results when using a potentially more specific ascertainment of sepsis. In the cohort with the most detailed clinical data (GHS), with analysis restricted to patients receiving antibiotics, a significant association of PCSK9 LOF variants with lower sepsis mortality was observed. Numerous factors influence mortality as an outcome in critically ill patients beyond biology (35, 36); this may challenge biologic association studies. Incident sepsis and septic mortality was ascertained in ODYSSEY OUTCOMES through routine trial treatment-emergent serious adverse event collection; although these events were subsequently adjudicated, they were not prespecified outcomes in the trial. As the population was not selected as one at-risk for sepsis, sepsis frequency was low and associations may be underpowered. A total of 97.5% of participants in the ODYSSEY OUTCOMES trial received background statin therapy; as noted, statins also upregulate the LDLR, and this may have both reduced infection rates overall, as well as attenuated a potential treatment effect associated with alirocumab. A further potential limitation may be that primarily live bacterial inoculation models of experimental sepsis were used; others have previously observed divergent results using other models (10), although with a Gram-negative LPS model, we also observed consistent results as with live inoculation models. Additional replication of murine models using female mice should be undertaken.
CONCLUSIONS
The findings from these analyses support the hypothesis that PCSK9 inhibitor pretreatment may improve sepsis outcomes. Benefit may be realized with the application of PCSK9 inhibitors as preventive treatments, possible among at-risk patients such as sepsis survivors. Future studies, including prospective, randomized clinical trials, may be warranted.
ACKNOWLEDGMENTS
We thank all patients and participating members of the GENetics of SEPsis and Kaiser Permanente Research Program on Genes, Environment, and Health studies. The Estonian Biobank thanks all participants and staff for their contribution to this research; the analytical work of the Estonian Biobank was carried out in part in the High-Performance Computing Center of the University of Tartu. We acknowledge the work of the Estonian Biobank Research Team: Andres Metspalu, Mari Nelis, Reedik Mägi, and Tõnu Esko. Also, we thank Khanh K. Thai and Dr. Catherine A. Schaefer of the Kaiser Permanente Division of Research for their tireless efforts in data analysis and cohort development.
Supplementary Material
Footnotes
This study was funded by Regeneron Pharmaceuticals. The GENetics of SEPsis study was supported by Instituto de Salud Carlos III, Madrid, Spain. The work by Drs. Krebs and Milani was supported by the European Union through the European Regional Development Fund (Project No. 2014-2020.4.01.15-0012) and the Estonian Research Council (Grant No. PRG184). The Trøndelag Health Study (HUNT Study) is a collaboration between the HUNT Research Centre (Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology), Trøndelag County Council, the Central Norway Regional Health Authority, and the Norwegian Institute of Public Health.
This study includes a secondary analysis of the phase Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab trial which is registered on ClinicalTrials.gov (NCT01663402).
Dr. Lawler is funded by a National New Investigator Award from the Heart and Stroke Foundation of Canada; he receives unrelated funding from the Canadian Institutes for Health Research, the National Institutes of Health, the Peter Munk Cardiac Centre, the LifeArc Foundation, the Thistledown Foundation, the Ted Rogers Centre for Heart Research, and the Province of Ontario; he has received unrelated consulting fees from Novartis, CorEvitas, and Brigham and Women’s Hospital; and he has received unrelated royalties from McGraw-Hill Publishing. Dr. Hernandez-Beeftink (FI17/00177), Dr. Flores (PI17/00610, PI20/00876 and CB06/06/1088), and Dr. Villar (PI16/00049, PI19/00141 and CB06/06/1088) were funded by Instituto de Salud Carlos III, Madrid, Spain and cofinanced by the European Regional Development Funds, “A way of making Europe,” from the European Union. Dr. Nordestgaard reports consultancies or talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. Drs. Manvelian, Coppi, Damask, Cantor, Ferreira, Paulding, Banerjee, Li, Jorgensen, Attre, Lotta, Kyratsous, Sleeman, DelGizzi, Pordy, Horowitz, and Baras are employees of, and own stocks/stock options in, Regeneron Pharmaceuticals. Dr. Walley holds a Foundation grant from the Canadian Institutes for Health Research. Dr. Sleeman is an employee of and owns stocks/stock options in Sanofi. Dr. Steg reports grants and nonfinancial support (cochair of the Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab trial) from Sanofi; he received research grants and personal fees from Bayer (Steering Committee MARINER, grant for epidemiological study), Merck (speaker fees, grant for epidemiological studies; cochair of the SCORED trial; consulting, speaking), and Servier (Chair of the CLARIFY registry; grant for epidemiological research); he received personal fees from Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer, Idorsia, MyoKardia, Novo Nordisk, Novartis, Regeneron Pharmaceuticals, and AstraZeneca; and he has a European application number/patent number, issued on October 26, 2016 (no. 15712241.7), for a method for reducing cardiovascular risk, all royalties assigned to Sanofi. Dr. Schwartz reports research support to the University of Colorado from AstraZeneca, Resverlogix, Roche, Sanofi, Silence Therapeutics, and The Medicines Company; and he is coinventor of pending U.S. patent 62/806,313 (“Methods for Reducing Cardiovascular Risk”) assigned in full to the University of Colorado. Dr. Szarek reports serving as a consultant or on advisory boards (or both) for CiVi, Resverlogix, Baxter, Esperion, Lexicon, Sanofi, and Regeneron Pharmaceuticals. Dr. Goodman reports research grant support (e.g., steering committee or data and safety monitoring committee) and/or speaker/consulting honoraria (e.g., advisory boards) from Amgen, Anthos Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Daiichi-Sankyo/American Regent, Eli Lilly, Esperion, Ferring Pharmaceuticals, HLS Therapeutics, JAMP Pharma, Merck, Novartis, Novo Nordisk A/C, Pendopharm/Pharmascience, Pfizer, Regeneron Pharmaceuticals, Sanofi, Servier, and Valeo Pharma; and he received salary support/honoraria from the Heart and Stroke Foundation of Ontario/University of Toronto (Polo) Chair, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Cleveland Clinic Coordinating Centre for Clinical Research, Duke Clinical Research Institute, New York University Clinical Coordinating Centre, PERFUSE Research Institute, and the TIMI Study Group (Brigham Health). The remaining authors have disclosed that they do not have any potential conflicts of interest.
Drs. Lawler, Damask, Cantor, Ferreira, Paulding, Banerjee, Li, Carey, Krebs, Milani, Hveem, Damås, Solligård, Tybjærg-Hansen, Nordestgaard, Hernandez-Beeftink, Rogne, Flores, Villar, Walley, Liu, Fohner, Lotta, Horowitz, Baras, Martin, Schwartz, Szarek, and Goodman contributed to the data acquisition. All authors contributed to the study design or concept, contributed to the analysis and interpretation of the data, critically reviewed and edited the article, and approved the final version. All authors agreed to be personally accountable for their own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even if not personally involved, are appropriately investigated and resolved, and the resolution documented in the literature.
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, and statistical analysis plan) that support the methods and findings reported in this article. Individual anonymized participant data will be considered for sharing once the product and indication has been approved by major health authorities (e.g., U.S. Food and Drug Administration, European Medicines Agency, Pharmaceuticals and Medical Devices Agency etc.), if there is legal authority to share the data and there is not a reasonable likelihood of participant re-identification. Submit requests to https://vivli.org/.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd KE, Johnson SC, Agesa KM, et al. : Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020; 395:200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall JC: Why have clinical trials in sepsis failed? Trends Mol Med 2014; 20:195–203 [DOI] [PubMed] [Google Scholar]
- 4.Walley KR, Thain KR, Russell JA, et al. : PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med 2014; 6:258ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sklar MC, Dos Santos CC, Lawler PR: Proprotein convertase subtilisin/kexin type 9 inhibition and survival in sepsis: Causal inference through human genetics. Crit Care Med 2019; 47:489–491 [DOI] [PubMed] [Google Scholar]
- 6.Boyd JH, Fjell CD, Russell JA, et al. : Increased plasma pcsk9 levels are associated with reduced endotoxin clearance and the development of acute organ failures during sepsis. J Innate Immun 2016; 8:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grin PM, Dwivedi DJ, Chathely KM, et al. : Low-density lipoprotein (LDL)-dependent uptake of Gram-positive lipoteichoic acid and Gram-negative lipopolysaccharide occurs through LDL receptor. Sci Rep 2018; 8:10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung AKK, Genga KR, Topchiy E, et al. : Reduced proprotein convertase subtilisin/kexin 9 (PCSK9) function increases lipoteichoic acid clearance and improves outcomes in Gram positive septic shock patients. Sci Rep 2019; 9:10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walley KR, Boyd JH, Kong HJ, et al. : Low low-density lipoprotein levels are associated with, but do not causally contribute to, increased mortality in sepsis. Crit Care Med 2019; 47:463–466 [DOI] [PubMed] [Google Scholar]
- 10.Berger JM, Loza Valdes A, Gromada J, et al. : Inhibition of PCSK9 does not improve lipopolysaccharide-induced mortality in mice. J Lipid Res 2017; 58:1661–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewey FE, Murray MF, Overton JD, et al. : Distribution and clinical impact of functional variants in 50,726 whole-exome sequences from the DiscovEHR study. Science 2016; 354:aaf6814. [DOI] [PubMed] [Google Scholar]
- 12.Guillen-Guio B, Lorenzo-Salazar JM, Ma SF, et al. : Sepsis-associated acute respiratory distress syndrome in individuals of European ancestry: A genome-wide association study. Lancet Respir Med 2020; 8:258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell JA, Walley KR, Singer J, et al. ; VASST Investigators: Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008; 358:877–887 [DOI] [PubMed] [Google Scholar]
- 14.Bycroft C, Freeman C, Petkova D, et al. : The UK Biobank resource with deep phenotyping and genomic data. Nature 2018; 562:203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krokstad S, Langhammer A, Hveem K, et al. : Cohort profile: The HUNT Study, Norway. Int J Epidemiol 2013; 42:968–977 [DOI] [PubMed] [Google Scholar]
- 16.Banda Y, Kvale MN, Hoffmann TJ, et al. : Characterizing race/ethnicity and genetic ancestry for 100,000 subjects in the genetic epidemiology research on adult health and aging (GERA) cohort. Genetics 2015; 200:1285–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kvale MN, Hesselson S, Hoffmann TJ, et al. : Genotyping informatics and quality control for 100,000 subjects in the genetic epidemiology research on adult health and aging (GERA) cohort. Genetics 2015; 200:1051–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen JC, Boerwinkle E, Mosley TH, Jr, et al. : Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354:1264–1272 [DOI] [PubMed] [Google Scholar]
- 19.Fairoozy RH, White J, Palmen J, et al. : Identification of the functional variant(s) that explain the low-density lipoprotein receptor (LDLR) GWAS SNP rs6511720 association with lower LDL-C and risk of CHD. PLoS One 2016; 11:e0167676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz GG, Steg PG, Szarek M, et al. ; ODYSSEY OUTCOMES Committees and Investigators: Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018; 379:2097–2107 [DOI] [PubMed] [Google Scholar]
- 21.Davenport EE, Burnham KL, Radhakrishnan J, et al. : Genomic landscape of the individual host response and outcomes in sepsis: A prospective cohort study. Lancet Respir Med 2016; 4:259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnham KL, Davenport EE, Radhakrishnan J, et al. : Shared and distinct aspects of the sepsis transcriptomic response to fecal peritonitis and pneumonia. Am J Respir Crit Care Med 2017; 196:328–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawler PR, Fan E: Heterogeneity and phenotypic stratification in acute respiratory distress syndrome. Lancet Respir Med 2018; 6:651–653 [DOI] [PubMed] [Google Scholar]
- 24.Walley KR, Francis GA, Opal SM, et al. : The central role of proprotein convertase subtilisin/kexin type 9 in septic pathogen lipid transport and clearance. Am J Respir Crit Care Med 2015; 192:1275–1286 [DOI] [PubMed] [Google Scholar]
- 25.Dwivedi DJ, Grin PM, Khan M, et al. : Differential expression of PCSK9 modulates infection, inflammation, and coagulation in a murine model of sepsis. Shock 2016; 46:672–680 [DOI] [PubMed] [Google Scholar]
- 26.Dellinger RP, Bagshaw SM, Antonelli M, et al. ; EUPHRATES Trial Investigators: Effect of targeted polymyxin B hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: The EUPHRATES randomized clinical trial. JAMA 2018; 320:1455–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar A, Roberts D, Wood KE, et al. : Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596 [DOI] [PubMed] [Google Scholar]
- 28.Holzheimer RG: Antibiotic induced endotoxin release and clinical sepsis: A review. J Chemother 2001; 13 Spec No 1:159–172 [DOI] [PubMed] [Google Scholar]
- 29.Genga KR, Lo C, Cirstea MS, et al. : Impact of PCSK9 loss-of-function genotype on 1-year mortality and recurrent infection in sepsis survivors. EBioMedicine 2018; 38:257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shankar-Hari M, Rubenfeld GD: Understanding long-term outcomes following sepsis: Implications and challenges. Curr Infect Dis Rep 2016; 18:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H, Doig CJ, Ghali WA, et al. : Detailed cost analysis of care for survivors of severe sepsis. Crit Care Med 2004; 32:981–985 [DOI] [PubMed] [Google Scholar]
- 32.Kosyakovsky LB, Angriman F, Katz E, et al. : Association between sepsis survivorship and long-term cardiovascular outcomes in adults: A systematic review and meta-analysis. Intensive Care Med 2021; 47:931–942 [DOI] [PubMed] [Google Scholar]
- 33.Chen M, Ji M, Si X: The effects of statin therapy on mortality in patients with sepsis: A meta-analysis of randomized trials. Medicine (Baltimore) 2018; 97:e11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarese EP, Podhajski P, Gurbel PA, et al. : PCSK9 inhibition during the inflammatory stage of SARS-CoV-2 infection. J Am Coll Cardiol 2023; 81:224–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ospina-Tascon GA, Buchele GL, Vincent JL: Multicenter, randomized, controlled trials evaluating mortality in intensive care: Doomed to fail? Crit Care Med 2008; 36:1311–1322 [DOI] [PubMed] [Google Scholar]
- 36.Bibas L, Peretz-Larochelle M, Adhikari NK, et al. : Association of surrogate decision-making interventions for critically ill adults with patient, family, and resource use outcomes: A systematic review and meta-analysis. JAMA Netw Open 2019; 2:e197229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.