Abstract
Protein kinase B/AKT plays a central role in cancer. The serine/threonine kinase is overexpressed or constitutively active in many cancers and has been validated as a therapeutic target for cancer treatment. However, targeting the kinase activity has revealed itself to be a challenge due to non-selectivity of the compounds towards other kinases. This review summarizes other approaches scientists have developed to inhibit the activity and function of AKT. They consist of targeting the pleckstrin homology (PH) domain of AKT. Indeed, upon the generation of 3-phosphorylated phosphatidylinositol phosphates (PI3Ps) by PI3-kinase (PI3K), AKT translocates from the cytosol to the plasma membrane and binds to the PI3Ps via its PH domain. Thus, several analogs of PI3Ps (PI Analogs or PIAs), alkylphospholipids (APLs), such as edelfosine or inositol phosphates (IPs) have been described to inhibit the binding of the PH domain to PI3Ps. Recent allostetic inhibitors and small molecules that do not bind the kinase domain but affect the kinase activity of AKT, presumably by interacting with the PH domain, have been also identified. Finally, several drug screening studies spawned novel chemical scaffolds that bind the PH domain of AKT. Together, these approaches have been more or less sucessful in vitro and to some extent translated in preclinical studies. Several of these new AKT PH domain inhibitors exhibit promising anti-tumor activity in mouse models and some of them show synergy with ionizing radiation and chemotherapy. Early clinical trials have started and results will attest to the validity and efficacy of such approaches in the near future.
Keywords: AKT, pleckstrin homology domain, rational drug design, inhibitor, allosteric, kinase
INTRODUCTION
Activated phosphophatidylinositol-3 kinase (PI3K) signaling is perhaps the most prevalent signaling event in cancer [1, 2]. The pathway integrates and transduces signals from growth factors, inflammatory stimuli [1], non-receptor oncogenic protein-tyrosine kinases [3] and p21Ras [4]. PI3K activation leads to the increased formation of plasma membrane PI(3,4,5)P3 and PI(3,4)P2 (herein referred to as PI3K products), which results in the activation of pleckstrin homology (PH) domain effector proteins [5]. PH domain containing proteins represent a wide diverse group of kinases, guanine exchange factors, structural [6] and docking proteins [7]. Among these proteins is protein kinase B or AKT, which upon formation of these PI3K products, translocates from the cytosol to the plasma membrane bringing the protein into proximity with activating partners or protein substrates at the plasma membrane. The major mechanism for the oncogenic activity of PI3K is by preventing apoptosis through the downstream activation of AKT [2, 8] (Fig. 1A). The dual specificity tyrosine-threonine/PI3-phosphatase tumor suppressor protein PTEN [9, 10] prevents the accumulation of the PI3K products and, thus, attenuates PI3K/AKT signaling [11]. PTEN is absent in a large number of human cancers including advanced prostate, endometrial, renal, glial, melanoma, and small cell lung cancers [11]. The PI3K/AKT pathway is activated in a large number of human cancers, either through increased input from over-expressed or mutated receptor and non-receptor protein-tyrosine kinases, increased input from mutant Ras, mutant activated PI3K itself, deleted PTEN, in rare cases mutant AKT [12], or a combination of these effects. Thus, because the PI3K/AKT pathway is turned on in many human cancers of different types, it a very attractive target for cancer drug discovery [13].
Fig. 1. The PI3K/AKT survival pathway.
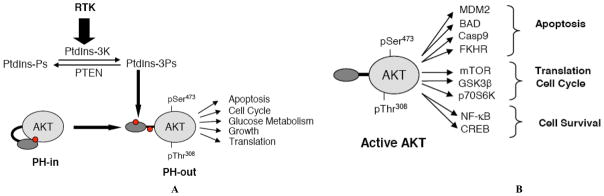
A, The cartoon represents the PI3K/AKT pathway. Upon activation of receptor tyrosine kinase (RTK), PI3K generates PI3Ps from PI at the inner leaflet of the plasma membrane. These PI3Ps binds the pleckstrin homology (PH) of AKT. In resting cells, AKT is in the cytosol in a closed conformation (PH-in). Upon binding the PI3Ps, the PH domain changes conformation, allowing AKT to be fully activated by phosphorylation on the threonine (Thr) 308 residue and the serine (Ser) 473 residue, resulting in the PH-out conformation. PTEN is the corresponding PI3 phosphatase which dephosphorylates PI3Ps. The red dot corresponds to the different AKT PH domain inhibitors that have been described to date: allosteric inhibitors which bind the PH-in conformation, sulfonamide based compounds which bind the PH domain within the PI3Ps binding pocket and compounds which inhibit AKT-TCL1 interaction at the hinge region.
B, Fully activated AKT phosphorylates a wide variety of substrates, involved in apoptosis, translation and cell cycle, and cell survival.
Over the years, a significant number of direct PI3K inhibitors have been developed and some are in early clinical development [13]. However, the involvement of PI3K on many aspects of normal physiological signaling as well as the lingering uncertainty of the role of different PI3K isoforms, which are not distinguished by most current inhibitors [13], has led to a search for other sites at which to inhibit the pathway. As one of the major direct downstream targets of PI3K, AKT plays a central role in promoting cancer cell proliferation and survival. AKT is a validated therapeutic target for cancer treatment. However, targeting the kinase activity has revealed itself to be a challenge due to non-selectivity of the compounds towards other kinases. Nearly all AKT inhibitors that have been developed to date bind to the ATP catalytic site [14]. Because of the similarity of the ATP pocket among serine/threonine kinases, particularly AGC family kinases to which AKT belongs, achieving target specificity has been extremely difficult [14]. The ATP-binding domain of AKT1 differs from that of PKA by only 13 amino acid residues, and none of these residues is located in the critical binding site [15, 16] and all the reported AKT ATP-pocket inhibitors to date also inhibit protein kinase A (PKA) [14]. In addition, because of the multifaceted activities of AKT and kinases in the AGC family, most of the non-selective AKT inhibitors have been shown to induce hyperglycemia and increased insulin levels [17, 18], systemic hypotension associated with inhibition of hERG channels, and femoral artery contraction [19]. Thus, alternative and novel approaches to targeting AKT, not involving the ATP-binding pocket have been developed in order to achieve a better therapeutic index. Nevertheless, it should be noted that targeting AKT may inevitably modulate insulin signaling and glucose homeostasis (as seen in AKT2 knockout mice [20]). Fortunately, these side effects associated with AKT inhibition may be clinically managed using anti-hyperglycemic drugs such as pioglitazone, a thiazolidinedione acting as ligand for the peroxisome proliferator-activated receptor-γ transcription factor [21] or a combination of fasting and low carbohydrate diet can potentially minimize AKT inhibition-induced hyperglycemia [22]. This review will summarize the recent progress made in the identification, characterization and development of novel AKT inhibitors targeting the PH domain of the protein.
1. AKT, THE MASTER KINASE: STRUCTURE AND FUNCTION
Discovered in 1991, AKT is a 56 kDa member of the AGC serine/threonine kinase family [23]. There are three mammalian genes giving AKT-1/α, AKT-2/β and AKT-3/γ, which share a high degree sequence homology in their catalytic and N-terminal PH domains but diverge in other regions of the protein [24]. AKT1 and AKT2 are ubiquitously expressed whereas AKT3 is found predominantly in brain, heart and kidney [25, 26]. All three isoforms undergo membrane recruitment through the N-terminal PH domain leading to phosphorylation and activation of AKT kinase activity [24, 27]. A second important regulatory domain is the C-terminal hydrophobic motif which may provide a docking site for PI-dependent kinase 1 (PDK1) [27] or the interaction with other proteins such as small G proteins (reviewed in [28]) or small protein regulators, such as TCL1. Of note, in a yeast screening of AKT to search for an interacting AKT partner, TCL1 was shown to interact with the PH domain of AKT [29–31]. TCL1 enhances AKT kinase activity, and therefore functions as an AKT kinase co-activator [31]. Both AKT interaction and dimerization of TCL1 are required for complete function of TCL1 in enhancing AKT kinase activity [32].
Transgenic mice with constitutively active AKT show increased tumor formation, including prostate tumors [33] and multifocal mammary tumors [34–36]. AKT1 is required for tumor formation in PTEN knockout mice and even haplodeficiency of AKT1 which reduces total AKT activity by only ~25%, is sufficient to inhibit tumor development [37]. There is considerable functional overlap between the different isoforms and adult mice lacking individual AKT isoforms are viable. Proof of principle for AKT as a novel anti-cancer drug target comes from the work of Cheng and collaborators who showed that ectopic expression of AKT induces malignant transformation and promotes cell survival [38]. The full activity of AKT in promoting cell survival relies on phosphorylation of a variety of targets to either prevent the expression of death genes or to induce cell survival [39] (Fig. 1B). Promoters of apoptosis that are inhibited by AKT include the forkhead transcription factor family members, FKHR, FHHRL1 and AFX [39], the pro-apoptotic Bcl-2 family member Bad [40], the apoptosis signaling kinase-1 (ASK-1) that transduces stress signals to the JNK and p38 MAP kinase pathways [41], and procaspase-9, the initiator of the caspase cell death cascade [42]. Targets that AKT activates to promote cell survival, are NF-κB [43] and the cyclic AMP response element binding protein (CREB) [44]. AKT also phosphorylates mTOR leading to the activation of p70S6kinase [45] and directly phosphorylates GSK3 [46] contributing to cyclin D accumulation of cell cycle entry [47].
2. PLECKSTRIN HOMOLOGY DOMAIN
The PH domain is a 100- to 120-amino acid, highly conserved, three dimensional superfold found in over 500 human proteins [28] and is the 11th most common domain in the human proteome [48]. Crystal structures and nuclear magnetic resonance structures of several PH domains show a highly conserved three dimensional organization although sequence identities are only 7% to 23%. The core of each PH domain consists of seven β-strands and a C-terminal α-helix. PH domains can bind to phosphotyrosine and polyproline sequences, Gβγ subunits of heterotrimeric G proteins, and phosphoinositides (PI). Thus PH domains are capable of protein-protein interactions and in most cases the protein interaction surface does not overlap with the lipid-binding region within the PH domain [49, 50]. Many PH domains have been shown to bind Gβγ, some with dissociation constants on the order of 20 to 50 nM [51, 52]. While for the majority of PH domain proteins PI binding is weak and non-specific, a subclass of approximately 40 PH domain proteins shows high affinity for PI [53]. Based on their differential in vitro affinities for phosphorylated phosphoinositides, PH domains can be sub-divided into four groups (reviewed in [54]). Group 1 includes PI(3,4,5)P3-binding PH domains such as BTK, GRP, ARNO, SOS, TIAM1, GAP and Vav proteins. Group 2 contains members that have high affinities for PI(4,5)P2 and PI(3,4,5)P3 in vitro. Preferential binding is observed for PI(4,5)P2 in vivo since PI(4,5)P2 is much more abundant than PI(3,4,5)P3. PH domains from this group include PLCδ, βARK, RasGAP, OSBP, DAGKδ, IRS-1 and others. AKT and PDK1 are found in group 3 and their PH domains bind PI(3,4)P2 as well as PI(3,4,5)P3. Finally, group 4, which includes dynamin and the C-terminal PH domain of TIAM1, exhibits relatively low affinity for the phosphoinositides. These PI-binding PH domain proteins are important components of signal transduction pathways.
3. STRUCTURE AND FUNCTION OF THE PH DOMAIN OF AKT
As early as 1998 a model of the PH domain of AKT was published [55]. Later, in 2001 and in collaboration with Kozikowski’s group, we performed molecular modeling studies of the AKT PH domain and its interaction with PI [56]. The homology model for the AKT PH domain was built based on the sequence alignment and similarities with spectrin-β (1BTN.pdb), PLCδ1 (1MAI.pdb) and BTK (1BTW.pdb). Two crystal structures at 1.4Å [57] and at 0.98Å [58] of the PH domain of AKT1 bound with the inositol head group of PI(3,4,5)P3 (that is inositol(1,3,4,5)P4) were later published in 2002 and 2003 and confirmed the exactitude of our model as well as the validity of molecular modeling techniques. The structure of the PH domain exhibits a fold of seven β-strands and one α–helix at the C-terminal portion of the protein. Variable loops (VL) 1–3 located between β1-β2, β3-β4 and β6-β7, respectively, define the PI3K products binding pocket. As predicted in our model, the positively charged residues Lys14, Arg25 and Arg86 were shown to interact with the 3- and 4-phosphate groups of the phosphoinositol head while the Arg48 residue binds the 1-phosphate group. The 5-phosphate group did not exhibit any interaction within the binding pocket explaining the observations that AKT can interact with both PI(3,4,5)P3 and PI(3,4)P2 with similar affinity [59, 60]. A second cluster of basic residues was identified and defined as Arg15, Lys20, Arg67 and Arg69. These amino acids are not involved in the binding with Ins(1,3,4,5)P4 but could be involved in stabilizing the PH domain at the plasma membrane by interacting with negatively charged lipids [61]. In agreement with a role of these residues in the activation of AKT, a mutation of Arg15Ala impaired the platelet-derived growth factor-stimulated AKT activation [55].
Binding of AKT PH domain to PI(3,4,5)P3 leads to a change in conformation of AKT [58]. The mechanism of AKT PH domain interaction with PI(3,4,5)P3 was recently reviewed [62]. Briefly, changes in the conformation of the PI binding pocket were detected in the unbound AKT1 PH domain (Apo form) resolved at 1.65 Å [58]. A shift in the position of VL3 of 7.4 Å was detected and a hydrophobic residue at the top of VL3 (Trp80) was found to be solvent exposed in the Apo form. This finding indicated that Trp80 might be interacting with another domain of AKT in the context of the full-length protein or with another protein. This specific point was recently confirmed using an allosteric inhibitor of the AKT PH domain [62] (details below). The binding of AKT PH domain to Ins(1,3,4,5)P4 also induced the re-ordering of the VL2 from a flexible structure to an α-helix upon a 7.6 Å shift, creating a patch of solvent exposed acidic residues (Asp44, Asp46, Glu49 and Glu40) whose purpose has not yet been elucidated. Interestingly, (Glu40Lys) mutation was shown to induce an increased AKT basal activity [55] and it seems likely to be due to an increase in the affinity of the PH domain for the membrane. Thus the change in conformation of AKT [63] allows the separation of the PH domain from the kinase domain leading to the closing of the cavity and the formation of a hydrophobic groove that is conserved in other AGC kinases [64]. In the inactive conformation of AKT, this motif would bind to Trp80 through the cavity. In the active conformer of AKT, it is buried inside a channel in the hydrophobic groove [64] (Fig. 1A).
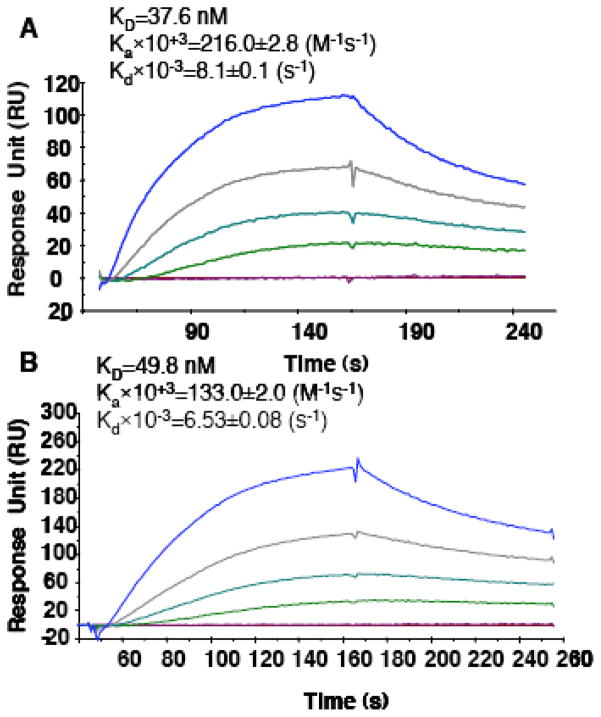
The movement of AKT from the cytoplasm to the plasma membrane allows the protein to be fully activated by phosphorylation on Thr308 in the activation loop and Ser473 in the hydrophobic motif on all three AKT isoforms [62, 65]. The kinase responsible for Thr308 phosphorylation is PDK1 [12] while the identity of the Ser473 kinase is unclear and more than 50 potential candidate kinases have been reported including rictor-mTOR [66], DNA-PK [67] and AKT itself [68]. When compiling the results of the recently published dynamic studies [63], it has been proposed that PDK1 and AKT would be in constant equilibrium between an associated and a dissociated form in the cytoplasm prior to stimulation. To date, there is no clear evidence for the role of dimerization for AKT. Although the experimental conditions used for obtaining crystals of the PH domain of AKT did not reveal the existence of dimers of AKT [57], the possible formation of dimers has been suggested [69]. Early experiments on the mechanisms of AKT activation indeed showed that the PH domain of AKT binds to itself and mediates the formation of protein complexes [69, 70]. Indeed, the process of dimerization in general plays a role in regulating the stability and/or activity of a protein. This is the case, for example, for RhoA (ROCK) [71] and pleckstrin [72], two other PH-domain containing proteins. Pertinently, we have obtained preliminary results that support the fact that the PH domain of AKT is able to form dimers (Fig. 2A) as well as heterodimers with the PH domain of PDK1 (Fig. 2B) in vitro using surface plasmon resonance (SPR) spectroscopy. We were able to detect a PH domain-PH domain interaction characterized by a nanomolar affinity between the PH domains of AKT and PDK1 which would confirm the interactions between the two proteins observed by Calleja et al. [62].
Fig. 2. Homo-dimers of AKT PH domains and hetero-dimers of AKT/PDK1 PH domains.
A, The figure represents the sensorgrams for the interaction between the PH domain of AKT with increasing concentration of the untagged PH domain of AKT. The PH domain of AKT is coated on the CM5 chip using the GST tag and anti-GST antibodies. Increasing concentrations of untagged AKT PH domain is then flowed through (curves from bottom to top correspond to 0, 1, 10, 25, 50, 100nM of AKT PH domain). The affinity was calculated with a KD of 37.6 nM. The homo dimers can be displaced by increasing concentrations of PI(3,4,5)P3 with a Ki of 1.7 μM or PH-427 with a Ki of 2.8 μM (data not shown).
B, the figure represents the heterodimerization between PDK and AKT PH domains. Increasing concentrations of untagged PH domain of AKT is flowed through over the GST-PDK surface (curves from bottom to top correspond to 0, 1, 10, 25, 50, 100 nM of AKT PH domain). The interaction occurs with an affinity (KD)of 49.8 nM. The hetero dimers can be displaced by increasing concentrations of PI(3,4,5)P3 with a Ki of 1.7 μM or PH-427 with a Ki of 3.2 μM (data not shown).
4. INHIBITORS OF THE PH DOMAIN OF AKT
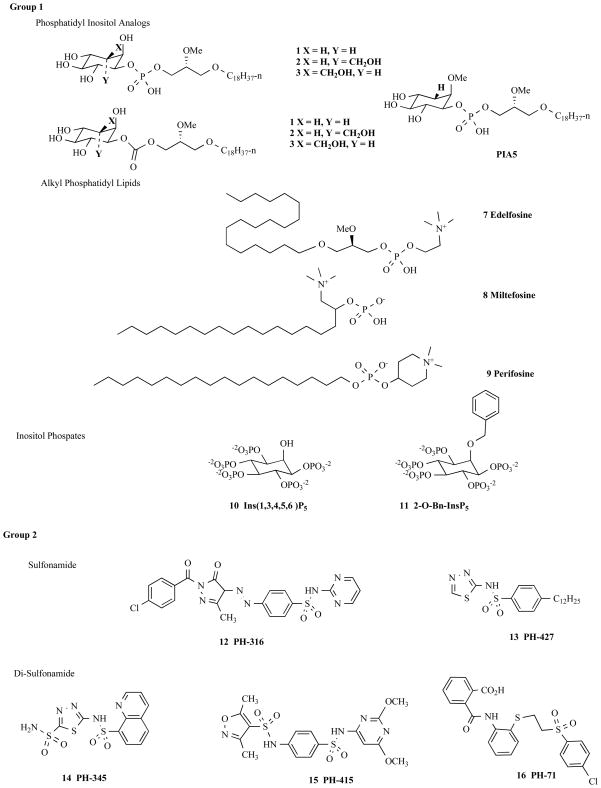
Using inhibition of AKT PH domain/PI-Ps interactions to regulate the translocation of the protein in the cell represents a novel approach in cancer drug discovery. This domain is druggable by small molecule inhibitors and below is a summary of compounds which were identified as PH domain inhibitors for AKT. Over the past 20 years, there have been an increasing number of studies describing the identification, characterization and further development of novel compounds that inhibit AKT via binding to the PH domain of AKT. These compounds can be classified into five categories (Fig. 3):1) phosphatidylinositol ether lipid analogs (PIAs), alkyl-phospholipids (APLs), and inositol phosphates (IPs); 2) sulfonamides; 3) purine/pyrimidine analogs; 4) allosteric compounds that interact only within the PH domain via Trp80; and 5) other inhibitors. The structures of these inhibitors are shown in Fig. 3 and Table 1 contains information regarding binding of the inhibitors to the PH domain.
Fig. 3.
Structure of several AKT PH domain inhibitors.
Table 1.
Binding Affinities of Known AKT PH Domain Inhibitors
| Compounds | KD (μM) Ki (μM) (PI(3,4,5)P3 control)* |
Reference(s) |
|---|---|---|
| 1 | 5.04±0.48 1.59 ± 0.17 |
[96, 121] |
| 7 | ND >50.0 |
[96, 97] |
| 9 | ND >50.0; 10.0 |
[96, 97] |
| 12 | 0.37±0.04 ND |
[121] |
| 13 | 40.8 ± 2.5 (3.08 ± 0.49)* 2.67 ± 0.37 (0.52 ± 0.1)* |
[96] |
| 17 | ND >50.0 0.7 (0.048)* ND |
[96] [131] |
| 18 | ND | [136] |
| 20 | ND 48.35±1.45 |
[96] |
| 21 | 8.0±3.1 ND |
[140] |
Note: Kis for reported AKT PH domain inhibitors. Kis were measured as the concentration of compound that displaces 50% of the PH domain bound to lipid vesicles enriched in PI(3,4,5)P3 in the absence of drug. Values are the mean of four determinations ± SE. ND, not determined.
for values determined for PI(3,4,5)P3 control.
4.1. Phosphatidyl-Inositol Ether Lipid Analogs (PIAs), Alkyl-Phospho-Lipids (APLs), and Other Inositol Phosphate (IP) Derivatives
4.1.1. Phosphatidylinositol Ether Lipid Analog (PIAs)
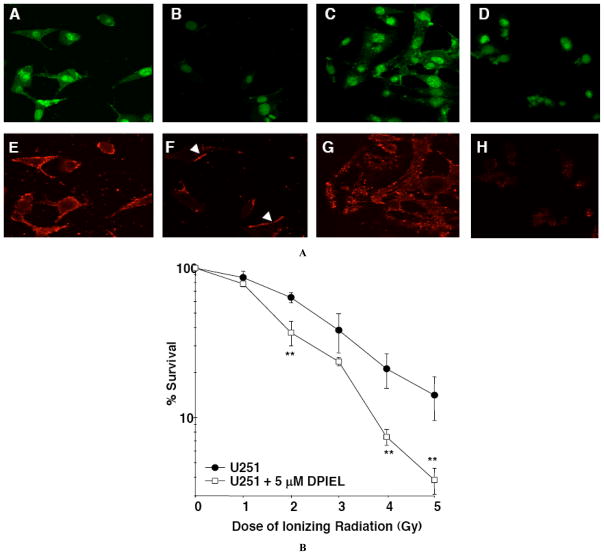
Phosphatidylinositol analogs (PIAs) were originally designed to prevent AKT translocation to the plasma membrane and activation. The feasibility of this novel approach was first suggested by the demonstration that D-3-deoxy-myo-inositols inhibited growth of transformed cells [73]. D-3-deoxy-phosphatidyl-myo-inositols (DPIs) that cannot be phosphorylated on the 3-position of the myo-inositol group were first synthesized by Kozikowski et al. jointly at Georgetown University and The University of Arizona. In an effort to increase the stability to phospholipase, a series of 1-O-octadecyl-3-deoxy- or 3-hydroxymethyl-phosphatidylinositol ether lipids and related carbonate analogs were synthesized [74–76]. The activity of the DPIs is summarized in the review [77]. These compounds were for the first time demonstrated to act as inhibitors of the AKT PH domain by our group in 2003 [78]: 1-[(R)-2,3-bis(hexade-canoyloxy)propyl hydrogen phosphate]) (DPI), its ether lipid derivative DPI 1-[(R)-2-methoxy-3-octadecyloxypropyl hydrogen phosphate] (DPIEL, 1), and its carbonate derivative DPI 1-[(R)-2-methoxy-3-octadecyloxypropyl carbonate] (DCIEL, 4) (Fig. 3A, compounds 1 and 4). The most active compound was D-3-deoxy-phosphatidyl-myo-inositol 1-[(R)-2-methoxy-3-octadecyloxypropyl hydrogen phosphate] (DPIEL). Using an ELISA-based assay, we showed that the DPIs bind to the PH domain of AKT, trapping it in the cytoplasm (Fig. 4A) and thus preventing AKT activation. We found using immunohistochemistry that DPIEL blocks the translocation of AKT1 from the cytoplasm to the plasma membrane in NIH3T3 cells where it normally binds to PI3Ps in the inner leaflet of the membrane (Fig. 4A). Interestingly, at that time, we noticed a much dimmer staining for pSer473AKT in the presence of DPIEL(Fig. 4H). Because AKT has been suggested to oligomerize through a PH domain interaction [69] (and see below) by binding to the PH domain, DPIEL may prevent the oligomerization of AKT. Another mechanism could be that DPIEL is in the plasma membrane as described similarly for alkyl-phospholipids [79] (see below). Upon its translocation, AKT binds to DPIEL at the membrane. The binding of DPIEL to the PH domain of AKT would induce a conformational change preventing AKT phosphorylation and thus inducing the release of AKT back into the cytosol. Such a mechanism could also explain the diffuse immunohistochemical staining observed as AKT is shuttling from the cytosol to the plasma membrane without complete activation.
Fig. 4. DPIEL inhibits AKT translocation and radiosensitizes glioma cells.
A, Immunohistochemistry was performed on DPIEL (20 μM) and wortmannin (1 μM)-treated control and PDGF (50 ng/ml)-stimulated NIH3T3 cells. Panels A–D show nuclear staining using YOYO-1. Panels E–H show the staining with a goat anti-AKT1 antibody and anti-goat IgG secondary antibody coupled to Alexa Fluor 568. In control cells AKT is located in the cytoplasm (panels A and E). Upon PDGF stimulation, AKT relocates to the plasma membrane (arrows in panel F). In DPIEL-treated cells, AKT translocation does not occur upon PDGF stimulation(panels D and H). Wortmannin-treated cells exhibited similar staining as with control non-stimulated cells (panels C and G).
B, To account for radiation mortality, increasing numbers of U251 glioma cells (102 to 10 × 104) were plated into 6-well plates and cultured for 16 hours. All irradiated cells were pre-incubated with 5 μM DPIEL for 2 hours and then given 0, 1 to 5 Gy of XRT (delivered by a 10 MeV Siemens linear accelerator at a dose rate of 3 Gy/min). Cells were allowed to form colonies for 10–12 days and then stained with crystal violet. The graph represents the average of three separate experiments done in triplicate. Values means are the ± SD; **, p<0.05; *, p<0.1.
DPIEL inhibits AKT phosphorylation with an IC50 of 1.5 μM and the phosphorylation of the downstream target BAD in PDGF-stimulated NIH3T3 cells [75]. DPIEL does not inhibit myristoylated-AKT, a constitutively active membrane-bound AKT expressed in NIH3T3 cells, and cell growth is not inhibited, unlike in wild-type NIH3T3 cells [78]. It should be noted that DPIEL does not affect other kinases (PKC or PDK1) and does not bind to other PH domains such as SOS, BTK, or IRS1 ([78] and personal communication). DPIEL shows good anti-proliferative activity against various human cancer cell lines with IC50 values in the micromolar range. Molecular modeling and docking studies show that DPIEL binds with much higher affinity to the AKT PH domain as compared to DPI and DCIEL. DPIEL has demonstrated in vivo efficacy against early human MCF-7 breast cancer (T/C 20%, 10 days, 75 mg/kg/day, i.p.) and HT-29 colon cancer xenografts in mice (5 days, 150 mg/kg/day, i.p.). DPIEL administered i.p. to mice at 150 mg/kg inhibited AKT activation in HT-29 human tumor xenografts up to 78% at 10 hours with recovery to 34% at 48 hours [80]. DPIEL is well tolerated in mice and rats with no hemolysis and no hematological toxicity.
Thus, DPIEL provided, for the first time, proof of principle that small molecule agents can bind to the PH domain of AKT and inhibit AKT survival signaling. Although DPIEL did not have good drug-like properties [81], it provided a pharmacological probe to help us understand the binding of small molecules to the PH domain of AKT and was the springboard for our rational design of small molecule inhibitors. Interestingly, we showed that DPIEL radiosensitizes U-251 glioma cells in preliminary clonogenic assays (Fig. 4B). As described for alkyl-phospholipids (see below), DPIEL synergizes with ionizing radiation.
Several other ether lipid analogs were concomitantly developed (see below). Although DCIEL didn’t show any anti-tumor activity in mice [78], a derivative of DCIEL that is hydroxymethylated on the 2-position of the inositol ring [75] exhibited some activity in leukemia models (Fig. 3A, compound 5). Martelli et al. demonstrated a reduction of the resistance of human leukemia cells to chemotherapeutic drugs and ionizing radiation [82].
4.1.2. Alkylphospholipids (APLs)
4.1.2.1. General Mechanism of Action of APLs
Alkylphospholipids (APLs), such as edelfosine (7), miltefosine (8) and perifosine (9), are a group of structurally related lipids that were shown in the late 1970s to exert anti-tumor activities in vitro and in vivo in a selective way [83–85] (Fig. 3A). Structurally based on the scaffold of lysophosphatidylcholine, APLs have a long hydrocarbon chain that allows easy partitioning into the plasma membrane of cells and thus accumulation into cell membranes (reviewed in [86]). Due to this property, APLs affect lipid metabolism and lipid-dependent signal transduction such as lipid rafts signaling leading to apoptosis. The effects of APLs has been shown to be most effective in metabolically active, proliferating cells, such as cancer cells, but not in quiescent normal cells [86]. A recent review from van Blitterswijk and Verheij describes the general mechanisms of APL cellular uptake and action [86]. APLs have been shown to mainly inhibit phosphatidylcholine synthesis, the MAP-kinase/ERK proliferative and PI3kinase/AKT survival pathways, as well as to stimulate the stress-activated protein kinase/JNK pathway, which may also lead to apoptosis in cancer cells [86].
APLs are most promising in combination with conventional cancer therapies (see below, “The future of AKT PH domain inhibitors” section) [87]. For example, APLs increase cancer cell sensitivity to radiotherapy in vitro and in vivo. Indeed, APLs exhibit radiosensitizing properties in vitro (reviewed in [88]) and in the late 1990s, edelfosine and miltefosine were demonstrated as radiosensitizers and to reduce clonogenic survival after ionizing radiation in KB squamous cell carcinoma cell line [87]. Other combination strategies with perifosine are discussed in the recent review by Gills and Dennis [89]. Over 13 trials were initiated using perifosine in combination with sorafenib, sunitinib, paclitaxel, docetaxel, lenalinomide or gemcitabine [89]. Most of the results of these trials show promising activity, with partial or minimal response and stable disease observed in myeloma patients treated with perifosine and dexamethasone [90].
4.1.2.2. Example of an Interesting APL: Perifosine
In the late 1990s, Engel’s group reported the anti-tumor activity of a novel APL analog of miltefosine with a heterocycle [91] (Fig. 3A, compound 9). This new compound named D-21266 (aka perifosine) showed a significantly improved gastrointestinal tract tolerance [92] and inhibited cancer cell growth with IC50 ranging from 0.2 μM to 19.9 μM as compared to its parent compound, miltefosine with IC50 ranging from 2.3 μM to 18.7 μM. In vivo, oral doses of perifosine exhibited antitumor effects in a DMBA-induced mammary carcinoma model in Sprague-Dawley rats [91]. It is later, in 2001, that van Blitterswijk and coworkers from the Netherlands showed for the first time that perifosine inhibits AKT phosphorylation in A431 cells [93]. Two years later, the same group published an extended study demonstrating that several APLs, including miltefosine, edelfosine and perifosine, inhibited the PI3K/AKT pathway in a dose-dependent manner [94]. Later that year, another report demonstrated the effects of perifosine on AKT phosphorylation and activation in PC-3 prostate cancer cells [95]. Although a potential mechanism was proposed, i.e. perifosine itself physically occupies the PH domain or interferes with the binding of PI(3,4,5)P3 to the PH domain of AKT; we were not able to show direct binding of the compound to purified PH domain of AKT using SPR spectroscopy [96], thus favoring the hypothesis that perifosine and other APLs exert their effects through the modification of the plasma membrane. Another report which consisted of an abstract presented at the AACR meeting in 2007 claimed that in vitro, using SPR, perifosine inhibited the binding of the AKT-PH domain to artificial membranes containing 3% (mole/mole) PI(3,4)P2. The IC50 for this inhibition was ~10 μM [97]. Clearly, different techniques (competitive binding assay using lipid vesicles as compared to artificial membranes) may be at the origin of the discrepancy and it remains to be clearly demonstrated as to whether perifosine and/or other APLs directly bind to the PI(3,4,5)P3 binding pocket of the AKT PH domain, thereby inhibiting the kinase activation.
In all, several studies are in agreement that perifosine inhibits AKT by preventing its translocation from the cytosol to the inner leaflet of the plasma membrane [95, 98]. Perifosine inhibits AKT in vivo against myeloma, prostate cancer and glioma xenografts in immune-compromised mice (reviewed in [99]). Interestingly, in PC-3 and DU-145 xenografts, a good correlation was observed among AKT inhibition, tumor growth inhibition and cumulative dose of perifosine [100]. In A431 and BT474 xenografts, perifosine was ineffective in inhibiting tumor growth and did not inhibit AKT. Pharmacokinetic analysis of [14C]-perifosine in blood plasma of female BALB/C nude mice bearing subcutaneous tumors, after a single oral dose of 40 mg/kg, showed a Cmax of 5.7 μg/ml, 22 hours after administration and a t1/2 of 137 ± 20 hours [101]. The compound is stable after 168 hours following administration and is not metabolized as compared to the other APL, miltefosine which is a substrate for phospholipase yielding choline, phosphocholine and 1,2-diacylphosphocholine [102].
Taken all together, and regardless of its mechanism of action, perifosine has been tested in clinical trials starting in the mid 2000s [101]. Compared to other APLs, in pre-clinical work [101] as well as in clinical studies [103, 104], perifosine behaves similarly and the drug accumulates in the small intestine creating gastrointestinal toxicity after oral intake. Gills and Dennis have recently reviewed the clinical history of perifosine [89]. More than 20 trials have been initiated in a variety of tumors and single-agent activity with perifosine has been observed only in sarcoma and Waldenstrom macroglobulinemia patients with disappointing results in prostate cancer, breast cancer, melanoma, pancreatic cancer, and head and neck cancers (reviewed in [89]). However, as pointed out by Gills and Dennis, only two trials evaluated the effects of perifosine on AKT inhibition in patient tissues. Clearly more studies validating perifosine as an AKT inhibitor, with the appropriate choice of patients and biomarkers are needed.
4.1.3. Other Phosphatidylinositol Ether Lipid Analogs (PIAs)
Similar to our studies [78], molecular modeling was used to guide the synthesis of other phosphatidylinositol ether lipid analogs (PIAs) designed to inhibit the PH domain of AKT [105]. Several alkylated and dehydroxylated at position 2 on the inositol ring were synthesized [106] (Fig. 3A, compounds 2, 3, 5, and 6). These PIAs, which have structures based on the structure of DPIEL (see above), inhibit AKT in cancer cells with IC50s around 2–4 μM, for the most active, and selectively induce apoptosis in cells with high levels of constitutively active AKT [105]. However, most of them were not tested in vivo for antitumor properties in xenografts. Two years later, PIAs were shown to be somewhat effective in vivo in a hollow fiber assay [107] but no pharmacokinetic or pharmacodynamic studies were performed to truly asses the efficacy of this series of compounds in vivo. In 2008 PIA5 was tested in vivo in nude mice bearing LKB1-mutant H157 non-small cell lung cancer xenografts [108] (see Fig. 3A for structure of PIA5). The compound was given i.p at 90 mg/kg for 5 days. PIA5 caused a 55% tumor growth inhibition. No corresponding pharmacodynamic study was performed in this model. However, using another animal model, PC-3 xenograft-AMPK activation was measured by immunohistochemistry and immunoblotting techniques. Quantification of the staining obtained for pThr172AMPK showed that PIA5 increased AMPK phosphorylation. Finally, taken together several observations suggest that PIAs might have other targets in addition to AKT [107] similar to APLs. In a recent study, PIAs were screened against a panel of purified kinases and it was shown that active PIAs activate a single isoform of p38, p38α, in vitro and in vivo [109]. p38α activation is independent of AKT inhibition and occurs directly through mechanisms probably based on structural features of p38α as well as indirectly through mechanisms involving MKK3/6 [109]. Structural similarities between DPIEL and PIAs raise the possibility that poor bioavailability and toxicity will also be characteristic of PIA administration in vivo, which would preclude development of PIAs as drugs. Additional toxicological and pharmacokinetic experiments would address these issues, but no other reports on the PIA series have been made since their synthesis in 2004 aside from those mentioned above investigating mechanisms of action, but not pharmacologic properties.
4.1.4. Inositol Phosphates (IPs) and Other Inositol Derivatives
Another strategy, different from the one described above, i.e. not based on the inability of PI-P to be phosphorylated by PI3 kinase, has been undertaken by Falasca et al. [110]. It is based on the hypothesis that specific exogenous inositol polyphosphates compete with PI(3,4,5)P3 binding to the AKT PH domain, and thus prevent AKT recruitment to the plasma membrane and activation [110]. Over the years, this group has demonstrated the following: 1) Ins (1,3,4,5,6)pentakisphosphate (IP5) (Fig. 3A, compound 10) and Ins (1,4,5,6) tetrakisphosphate (IP4) enter small cell lung cancer (SCLC) cells [111], inhibit IGF-induced tritiated thymidine incorporation in the MCF-7 human breast cancer cell line, and inhibit the ability to form colonies in agarose [111]. 2) 50 μM of IP4 inhibits growth factor-induced translocation of AKT-GFP in COS-7 cells [111]. 3) 50 μM of IP5 inhibits 50% pSer473-AKT and the downstream target pSer21/9-GSK3 in SKOV3 ovarian cancer cells, and enhances the pro-apoptotic effect of cisplatin [112]. IP5 also induces apoptosis in SKOV3 ovarian, SCLC-H69 lung and SKBR-3 breast cancer cells. Finally, IP5 sensitizes these cells to cisplatin and etoposide. 4) IP5 inhibits FGF-induced AKT phosphorylation, thereby blocking FGF2-mediated survival, migration and capillary tube formation of human umbilical vein endothelial cells (HUVEC) [113]. For the first time, it has also been demonstrated that IP5 exhibits some anti-tumor activity in SKOV3 xenografts in nude mice and showed anti-angiogenic effects with no apparent toxicity [113]. Using Western blots, a reduction in pSer473-AKT and pThr308-AKT was observed in the tumors following 12 days of treatment. Of note, it was also shown that Ins(1,2,3,4,5,6)hexakisphosphate (IP6) did not have an effect on SKOV3 tumor growth. In general, inositol polyphosphates are not likely to be drug-like molecules used for the treatment of cancers. However, as pointed out by the authors of this study, the inositols (especially phytic acid also known as IP6) are naturally occurring substances present in legume and grain fibers IP6 and its dephosphorylated metabolite (IP5) are believed to exhibit chemopreventive effects in colon cancer, prostate, hepatocarcinoma and other cancers [114–116].
Another report from the same group describes the effects of a novel IP5 analog, the 2-O-benzy-myo-inositol1,3,4,5,6-pentakisphosphate (2-O-Bn-InsP5) (Fig. 3A, compound 11) as a novel PDK1 inhibitor [117] based on the work of Mills et al. [118] who described the crystallization of the AKT PH domain with a derivative of IP4, Bz(1,2,3,4)P4. Interestingly, rather than affecting AKT, the compound strongly inhibits PDK1 activity in vitro with an IC50 in the low nanomolar range and exhibits a 50% anti-tumor activity in a PC-3 prostate cancer xenograft in nude mice at 50 mg/kg after 14 days [117]. The authors hypothesized that the effects of the compounds on AKT inhibition in vitro and in vivo, mainly at the Thr308 site, can result from a combination of a direct effect on PDK1 kinase activity and effects on both AKT and PDK1 recruitment to the plasma membrane. It remains unclear whether the compound binds directly to the PH domain(s) of PDK1 and/or AKT. In light of the newly discovered mechanisms of AKT and PDK1 interaction and regulation [119], one could hypothesize that the compound inhibits PDK1/AKT dimers or PDK1/PDK1 homodimers. Finally, a very recent study from John Hopkins University School of Medicine demonstrated that the endogenous diphosphoinositol pentakisphosphate (5-PP-[1,2,3,4,6]IP5), designated IP7, generated by the corresponding inositolphosphate-6-kinase1 (IP6K1), inhibits AKT by binding directly to the PH domain, thereby blocking the phosphorylation on Thr308 and activation by PDK1. IP7 behaves as an endogenous negative regulator of AKT in cells. The IC50 of IP7 for PI3Ps-induced AKT inhibition is ~1 μM in vitro. IP7 blocks AKT translocation in mouse embryonic fibroblasts [120].
In light of the structural similarities of the phosphates group on IP7 and the sulfonamide groups in inhibitors that we have developed to target the PH domain of AKT (see below), one can postulate that disulfonamide-containing compounds could mimic these phosphate groups. We have identified several of these types of compounds (Fig. 3A, compounds 14, 15 and 16) and are in the process of establishing their activity and selectivity for the PH domains of AKT and PDK1 (Table 2, personal communication; and see supplement information in [121]).
Table 2.
Characteristics of Sulfonamides as Novel AKT PH Domain Inhibitors
| Compounds | KD (μM) Ki (μM) |
pAKT inhibition (IC50; μM) | Cell growth (IC50; μM) | Anti-tumor activity |
|---|---|---|---|---|
| 13 | 40.8±2.5 3.3±0.4 |
6.3±0.9 | 5.4.0±2.5 | Yes@ T/C% 27.6±15.7 (p=0.01) |
| 16 | 4.6±1.7 9.7±1.3 |
5.0 | >100.0 | yes* T/C% 45.7±18.2 (p=0.06) |
| 14 | 1.8±0.3 Ki>50 |
50.0 | >100.0 | ND |
| 15 | 6.3±1.2 Ki>50 |
1.0 | 3.1 | Yes |
Binding affinities (K) were measured using SPR spectrometry in a direct binding assay (KD) or competition assay using PI(3,4,5)P3-enriched liposomes (Ki). Cell growth and pAKT inhibition were measured as described previuosly [122]. Antitumor activity at doses of 150–200 mg/kg p.o. for 5 to 10 days.
MCF-7 breast, # MiaPaCa-2 pancreatic, and @ BxPC-3 pancreatic s.c. xenografts.
ND, not determined.
4.2. Sulfonamides
4.2.1. Diazo-Sulpho-Amido Inhibitors of AKT
In 2008, the crystal structure for the PH domain of AKT1 was made available and our group used the crystal structure of Ins(1,3,4,5)P4 with the AKT1 PH domain as a pharmacophore for a three dimensional query search to identify compounds that would bind to the PH domain of AKT1. A virtual library of approximately 300,000 compounds from several databases was searched. The molecule selected as a lead was NSC348900 (herein PH-316, Fig. 3A, compound 12), with a predicted binding affinity (KD) to the AKT1 PH domain of 1.2 μM, which was three times better than the lipid-based compound we identified in earlier studies, DPIEL, as an AKT PH domain binding molecule with a KD of 4.0 μM. The predicted binding of PH-316 to the PH domain binding pocket of AKT1 showed hydrogen bonding interactions with both side chains and backbone of protein residues. In particular, the sulfonamide group interacts with Arg86 while similar hydrogen bonding interactions are involved with the diazopyrazolyl group and Arg23. These two arginine residues are strongly involved in the interaction with the phosphate head groups of the substrate Ins(1,3,4,5)P4, supporting the discovery of the molecule as an AKT1 PH domain inhibitor. PH-316 was also shown to bind weakly to IRS-1 but not to PDK1 using SPR studies and an ELISA-based assay (Table 1). The compound inhibited pSer473AKT with an IC50 2- to 10-fold higher than required to bind to the AKT1 PH domain. The phosphorylation of PDK1 and downstream target PKC were not decreased by PH-316. PH-316 induced apoptosis in HT-29 cells [121]. However, metabolism predictions suggested that the azo- (-N=N-) linkage in the compound and its derivatives might be susceptible to metabolic reduction. Stability in vitro showed relatively rapid breakdown of the compounds with half-lives of 1 hour to 2 hours. Although PH-316 did not show toxicity in animals up to 250 mg/kg, the poor solubility of the compound and the potential metabolism of the diazo group limit the plasma concentrations that can be achieved, thus preventing effective in vivo inhibition of AKT and possible anti-tumor activity [121]. Of note, a small inhibition of tumor pSer473AKT was measured 4 hours after a single injection dose and a significant decrease in total AKT was shown at 24 hours. Taken together, these results demonstrated the compound as the first small molecule non-phosphoinositide selectively targeting the PH domain of AKT [121].
4.2.2. 4-Dodecyl-N-(1,3,4-thiadiazol-2-yl)benzenesulfonamide
An in silico screen was conducted in collaboration of OpenEye Scientific Software (Santa Fe, NM) of a database of approximately 2.3 million unique available compounds assembled from vendor databases. From 60 hits identified, four distinct active scaffolds were identified with IC50 ranging from 1 μM to 50 μM in a cellular AKT inhibition assay [122] (Fig. 3A, compounds 12, 14, 15 and 16). To further improve the potency of the hits, several computational approaches were employed to study binding to the PH domain of AKT as well as ADMET properties. According to the docking studies, the sulfonyl moiety in these four compounds acts as a hydrogen bond acceptor interacting with residues Arg23, Arg25 and Lys14. Hydrogen bonding interactions were also observed between the nitrogen atoms in the thiadiazolyl group and residue Glu17. The sulfonyl group interacted with the protein by mimicking the 3-position phosphate of Ins(1,3,4,5)P4 ligand. Following structural modifications guided by in silico docking studies [123], a lead compound, #27 (herein PH-427, Fig. 3A, compound 13) showed dose-dependent inhibition of AKT and of the downstream targets GSK3β and p70S6K. The IC50 was measured to be 8.6±0.8 μM and 6.3±0.9 μM in BxPC-3 and Panc-1 pancreatic cancer cell lines, respectively. Using a competitive binding assay and SPR, the compound exhibited a Ki in the low micromolar range (Table 1). Anti-tumor activity was shown against BxPC-3 pancreatic cancer xenografts in scid mice with PH-427 administered at a dose of 125 mg/kg i.p., twice a day for 5 days. PH-427 showed significant anti-tumor activity with cessation of tumor growth and even regression during the course of treatment. Tumor growth resumed at its original rate when the drug was removed. A single dose of PH-427 of 125 mg/kg ip caused significant inhibition of tumor AKT measured as pSer473-AKT with up to 70% inhibition at 6 hours and 50% inhibition at 12 hours which returned to untreated levels by 24 hours [122]. These results correlated well with the plasma concentration determined at the same times following the single dose. Thus, for the first time a small molecule compound designed as an AKT PH domain binder inhibited AKT activity in cells and exhibited significant antitumor activity in vivo.
The binding affinity of PH-427 to the PH domain of AKT is shown in Table 1 in comparison to other AKT inhibitors that had been reported by others to bind to the PH domain of AKT, namely the alkyl-phospholipids perifosine and edelfosine, and the nucleoside triciribine. SPR measurement showed only weak binding to the domain by perifosine (Ki of 56 μM), while edelfosine and triciribine (see below) did not bind at all (Ki>100 μM). Another agent, MK-2206, recently reported to be an allosteric inhibitor of AKT (see below), was confirmed by our SPR measurements, to bind only weakly to the PH domain of AKT (Table 1). Significantly, except for PH-427, none of these compounds were found to bind to the PH domain of PDK1 [96].
The in vivo molecular pharmacology of PH-427 and some analogs with different alkyl chain lengths were also investigated [96]. Our group showed an oral formulation of PH-427 exhibits antitumor activity against a number of human tumor xenografts, particularly those with PIK3CA mutations. PH-427 administration resulted in up to 80% inhibition of tumor growth in the most sensitive tumors. Tumors with a K-Ras mutation were insensitive to PH-427, while in the absence of a K-Ras mutation tumor growth was reduced by 50%. The pattern of inhibition in different tumors is similar to that caused by the PI3K inhibitor PX-866 [124] suggesting that the main action of PH-427 is to inhibit the PI3K/PDK1/AKT signaling pathway. Finally, PH-427 increased the antitumor activity of both paclitaxel and gemcitabine in a Panc-1 tumor xenograft model, and that of erlotinib in a non small cell lung (NSCL) cancer model. When given over 5 days PH-427 caused no weight loss or change in blood chemistry. In summary, PH-427 was described as a novel PH domain binding inhibitor of AKT signaling that has anti-tumor activity in human tumor xenografts.
We have recently shown that PH-427 prevents UVB-induced AKT activation in HaCaT skin cancer cells [125] (and manuscript in preparation). Similar to PH-316, we noticed a decrease in total AKT expression following 4 hours to 8 hours exposure with PH-427 in HaCaT cells. These results led to ongoing studies aimed at developing PH-427 as a topical agent for skin cancer prevention.
4.3. Purine/Pyrimidine Analogues
Several new compounds were recently described by one group to behave as AKT inhibitors that prevent AKT translocation to the plasma membrane. These new compounds were shown recently to bind directly to the PH domain of AKT.
4.3.1. Triciribine (Tricyclic Dinucleoside, NSC 154020, TCN, AKT/PKB Signaling Inhibitor-2, API-2); Triciribine Phosphate (NSC 280594)
Triciribine (TCN) is a purine analog that was first identified as an inhibitor of DNA synthesis [126] (Fig. 3B, compound 17). TCN is a synthetic small molecule tricyclic nucleoside [127]. Previous studies have shown that TCN inhibits DNA synthesis and has antitumor and antiviral activity [126, 128]. Later, it was demonstrated that TCN inhibits AKT phosphorylation and had strong anti-tumor activity in tumors with high concentrations of AKT [129]. TCN inhibited AKT signaling and induced apoptosis and cell growth arrest only in cancer cells with elevated levels of AKT [129]. Similar observations were made in vivo using s.c. implanted AKT-overexpressing cells (OVCAR3, OVCAR8, and PANC-1) into the right flank and cells that express low levels of AKT (OVCAR5 and COLO357) into the left flank of nude mice. When the tumors reached an average size of about 100–150 mm3, the animals were treated i.p. with either vehicle or TCN (1 mg/kg/day) [129]. TCN inhibited OVCAR3, OVCAR8, and PANC-1 tumor growth by 90, 88, and 80%, respectively. In contrast, TCN had little effect on the growth of OVCAR5 and COLO357 cells in nude mice. At a dose of 1 mg/kg/day, TCN had no effect on blood glucose level, body weight, activity, and food intake of mice. In treated tumor samples, AKT activity was inhibited by TCN without a change of total AKT content. The authors of this study indicated that TCN selectively inhibited the growth of tumors with elevated levels of AKT and that TCN was selective for AKT but did not inhibit PI3K [129]. TCN was also shown to inhibit the phosphorylation of all three AKT isoforms in vitro and to inhibit the growth of tumor cells overexpressing AKT in mouse xenograft models [130]. Interestingly, in this study the authors noted that the compound did not inhibit constitutively active AKT (myristoylated-AKT) suggesting that TCN did not inhibit AKT directly in vitro and that it did not function as an ATP competitor nor as a substrate competitor that binds to the active site of AKT. Three years later the mechanism of action for TCN was published and TCN was shown to inhibit AKT by interacting with the PH domain of the protein kinase [131]. In fact, once inside the cells TCN is known to be converted to TCN-P, the active metabolite of TCN, by adenosine kinase [132]. We (Table 1 and [96]) and others [131] confirmed that only TCN-P, and not TCN, binds to the PH domain of AKT. As shown with our inhibitors, the authors used SPR to demonstrate the direct binding of the compound to the PH domain of AKT (Table 1). We note some discrepancies regarding controls and the binding affinity of the “natural” ligand PI(3,4,5)P3. A value of 0.048 μM is reported by Berndt et al; we reported 0.52±0.11 μM, which corresponds to the previously reported value of 0.40±0.03 μM published by Frech et al., [59]. However, it should also be noted that the experimental protocols and techniques vary greatly among these published results.
Because TCN-P is a known anti-cancer agent, a number of phase I and II clinical trials of TCN have been conducted in patients with advanced tumors from the mid-1980s to mid-1990s (reviewed in [133]). However, in light of the novel mechanism of action revealed for TCN-P, an open-label phase I dose-escalation study of TCN-P monohydrate (TCN-PM) mono-therapy was undertaken between late 2006 and early 2008, restricted to subjects whose tumors had evidence of activated (hyper-phosphorylated) AKT [133]. Pharmacokinetics and pharmacodynamics were performed as well. Preliminary data suggested that treatment with TCN-PM inhibited tumor pAKT at doses that were tolerable. A modest decrease in pAKT was demonstrated in tumor samples taken 24 hours following the second dose of TCN-P. Early studies had already shown that TCN exhibited some side effects, which included hepatotoxicity, hypertriglyceridemia, thrombocytopenia, and hyperglycemia [134, 135]. It is not clear whether the hyperglycemic effect of TCN is related to the inhibition of AKT activation. In this later study, mild thrombocytopenia was observed as well. However, the trial was terminated in early 2008 due to the lack of activity and decreased patient accrual.
4.3.2. API-1 (NSC 177223 – Pyrido[2,3-d]pyrimidines)
Recently, an analog of the antibiotic sangivamycin, herein called API-1, was identified as a novel AKT inhibitor [136] (Fig. 3B, compound 18). Out of 2300 compounds from the NCI-DTP Open Chemical Repository, only 32 compounds inhibited the growth the NIH3T3-AKT2 transformed cells but not empty vector LXSN-transfected NIH3T3 cells. TCN (also known as API-2) was also identified during this screen and characterized as an AKT inhibitor [129]. API-1 binds to the PH domain of AKT as determined by pull down assay using GST-PH, GST-kinase and GST-CT fusion proteins. It also inhibits IGF1-induced translocation of AKT in Hela cells. API-1 inhibits pSer473AKT phosphorylation with an IC50 of ~0.8 μM in OVCAR3 cells which express high endogenous levels of pAKT. An interesting feature of the compound is that it inhibits constitutively active AKT and naturally occurring mutant AKT1-E17K, but no further rationale nor any mechanistic explanation was provided [136].
4.4. Allosteric Compounds
Scientists at Merck have developed a series of AKT inhibitors that bind to both the PH domain and the kinase domain (not at the ATP site) forming a stabilized “closed” complex that is not capable of having any kinase activity or being activated by PDK1 [15]. The functionalized tricyclic quinoxaline (inhibitor VIII, Fig. 3B, compound 19) has IC50s of 58 nM, 210 nM and 2119 nM against AKT1, AKT2 and AKT3, respectively [14, 137]. Recently, this inhibitor was successfully used to show that a PH domain-induced cavity was present in the inactive PKB “PH-in” conformer [62]. The compound interacted with Trp80 within the PH domain of AKT. A derivative compound of this series, MK-2206 (Fig. 3B, compound 20) is in early clinical trial, but it has to be administered orally four times a day. MK-2206, a highly selective non-ATP competitive allosteric AKT inhibitor, exhibits a nanomolar IC50 and broad preclinical antitumor activity (reviewed in [77]). MK-2206 treatment abolished ionizing radiation-induced AKT phosphorylation. Moreover, treatment with MK-2206 also increased the radiosensitivity of U87MG cells [138]. MK-2206 is generally well tolerated at doses up to 60 mg QOD with plasma concentrations that portend activity in preclinical models. Pharmacokinetic and pharmacodynamic data suggest a substantial and maintained target inhibition at 60 mg [139].
4.5. Other Novel AKT PH Domain Inhibitors
4.5.1. Tirucallic Acids Are Novel PH Domain Inhibitors
Tirucallic acids (TAs) are tetracyclic triterpenoids that can be purified from the oleogum resin of Boswellia carterii [140]. In a recent study published in March 2010, researchers at Ulm University in Germany discovered that the TAs of frankincense inhibited the growth of prostate cancer in mice [140]. PC-3 cells became resistant to TAs upon overexpression of PTEN. TAs did not inhibit active AKT1 lacking the PH domain. Thus, the mechanism of action of the TAs was determined through the inhibition of AKT, via binding to the PH domain. Interestingly, phosphorylation of PDK1, another kinase containing a PH domain was only negligibly affected by the TAs, indicating some selectivity toward AKT. In fact the authors demonstrated that TA derivatives, in particular the 3-β-acetoxy-tirucallic acid (βATA; Fig. 3B, compound 21), binds to active recombinant AKT with a KD of 8.0±3.1 μM as measured by SPR (Table 1). It remains to be determined whether the compounds bind within the PI3Ps binding pocket of the PH domain of AKT because the authors have used full length AKT in their assay. Nevertheless, the molecular modeling studies predicted βATA binding to the PH domain within the PdtIns-3,4,5P3 pocket defined by Gly16, Glu17, Lys20 and Ile75 residues. βATA induces apoptosis in prostate cancer cells that have high phosphorylated AKT, LnCaP and PC-3 cells. Finally, βATA inhibits the growth of prostate cancer xenografts following a daily i.p. injection of 10 μmol/kg in a PVP micro suspension delivery method. No pharmacokinetic or pharmacodynamic studies were presented in this study. Further data are necessary to warrant describing the TAs as potential therapeutics targeting the PH domain of AKT for the treatment of cancer with activated AKT.
4.5.2. PITenins (PITs)
Miao et al. reported in a very recent study a novel class of selective non-phosphoinositide PI2P inhibitors termed PITenins (PITs) [141]. The authors developed a novel high-throughput fluorescence polarization (FP)-binding assay by using recombinant PH domains and a fluorescent NBD-labeled PI(3,4,5)P3 molecule. They screened over 50,000 small molecules using this assay and identified two distinct inhibitors of PI(3,4,5)P3/PH domain binding which were termed PIT-1 and PIT-2 (Fig. 3B, compounds 22 and 23). The IC50 was calculated to be around 30 μM and the direct binding of these compounds to the PH domain of AKT was measured to be around 40 μM using SPR. However, one can note the fast association and fast dissociation rates of the compounds as compared to sulfonamide-based compounds. Taken together, these types of compounds may not have the required pharmacological properties to have strong efficacy in animal studies. In U87 cells, PIT-1 inhibits AKT phosphorylation (at both Ser and Thr residues) at concentrations between 25 μM and 100 μM. The authors pointed out the poor solubility of the parent compound and thus used a polyethylene glycol-phospho-ethanolamine mixed micelle formulation as a method of i.v. delivery of the analog compound (termed DM-PIT-1, DM for di-methyl) for their in vivo studies. Using this compound delivered i.v. for 8 days, good anti-tumor properties were observed with a 95% tumor growth inhibition for DM-PIT-1M (micelle) as compared to 58% for DM-PIT-1. Interestingly, the authors also reported a small structure-activity relationship study for this series of compounds with an increased selectivity of the compounds for the PH domain of AKT over PDK1 and GRP1. The activity of the more selective compound in cells was weakly increased for AKT inhibition and the in vivo efficacy of these compounds was not shown.
4.5.3. Peptide Mimetics (AKT-in)
The activity of AKT can be inhibited by a peptide that mimics the AKT-binding domain of TCL1 and that binds the PH domain of AKT [142]. In an attempt to develop AKT-specific inhibitors, the authors have generated a peptide spanning the AKT binding sequences of TCL1, named “AKT-in” (AKT inhibitor, NH2-AVTDHPDRLWAWEKF-COOH), which interacted with AKT, but lacked the ability for oligomerization. Interaction of AKT-in with the AKT PH domain prevented phosphoinositide binding, and hence inhibited membrane translocation and activation of AKT. As revealed by NMR chemical mapping, the AKT-in peptide bound the AKT-PH in a surface similar to that recognized by TCL1 proteins. AKT-in inhibited not only cellular proliferation and anti-apoptosis in vitro, but also in vivo tumor growth [142].
The design of other inhibitory peptides that could interact with AKT-PH domains based on the corresponding amino acid sequences of the others members of the TCL1 family {[8–22 (GVPPGRLWIQRPG) in TCL1B: 5–19 (GAPPDHLWVHQEG) in MTCP1]} suggested that neither TCL1B nor MTCP1 peptides showed significant affinity to the AKT-PH domain. Further dynamic modification of the design of inhibitory molecules will be required. As noted by the authors, the combined use of structure/activity relationship methodology and of chemical fragment libraries to develop chemical compounds to manipulate the suppressive function of AKT-in might help to design more potent AKT inhibitors with minimal off-target activities for therapeutic use [143].
5. FUTURE OF AKT PH DOMAIN INHIBITORS
5.1. More Mechanistic Studies Are Needed to Ascertain of the Mode of Action of AKT PH Domain Inhibitors
It has been reported previously that Thr34 of the AKT PH domain is phosphorylated by PKCζ and that this phosphorylation prevents AKT translocation to the plasma membrane and activation [144]. Thr residue is located on the surface of the AKT PH domain that is not directly involved in PI3Ps binding and which corresponds to the βγ-binding interface of the GRK2-PH domain [50]. Thus, one could hypothesize that inhibitors of the PH domain may affect other unsuspected downstream targets via the inhibition of PH domain interaction with other protein partners. This issue should be addressed properly when testing novel AKT PH domain inhibitors as well as to clearly define their interaction within the PH domain.
Another point is that we and others have clearly suggested the possibility that small molecule inhibitors are able to bind several other pockets within the PH domain. Our group has identified four potential hydrophobic binding pockets: The biggest one with the highest rank is the PI3Ps binding pocket. The hydrophobic pocket 2 is near Trp80 and close to the α-helix of the protein. Pocket 3 is close to the PI3Ps binding site with which it shares several residues such as Arg15, Glu17, Lys20, and Arg86. Another potential binding pocket (Pocket 4) is around loop (β23) and (β34), with residue Val4, Leu28, Lys30, Thr34, Tyr38, Arg48, Glu49, and Asn54. These findings were in agreement with the observations that some of our compounds (e.g., PH-415, PH-345, and PH-71; Fig. 3B) possess strong binding (KD values) and pAKT inhibition, but could not displace PI3Ps binding (Table 2), suggesting other binding mechanisms. Although these in silico predictions need confirmation with X-ray crystallography and site directed mutagenesis coupled to SPR studies for further validation, they strongly suggest the existence of other binding pockets that could potentially be targeted.
5.2. Pan-PH Domain Inhibitors or Selectivity AKT PH Domain Inhibitors?
Other PH domain-containing proteins are involved in the PI3K/AKT survival pathway. We have tested for selectivity of our inhibitors against the PH domains of PDK1, IRS1 and GAB1, three other PH domain-containing proteins. Preliminary studies confirmed that we have non-selective PH domain inhibitors as well as more selective PH domain inhibitors (PH-427 derivatives [96]). It is becoming necessary to further investigate the selectivity of the PH domain inhibitors in order to evaluate their potential off target effects. Because PH domains are highly conserved three dimensional structures that bind PI-Ps with several degrees of affinity, PH domains can be grouped into four sub-classes according to their affinity for PI-phosphates. It is conceivable that selectivity could be achieved for PH domains from different subgroups but that selectivity may remain a challenge for distinguishing PH domains within the same group. Allosteric inhibitors, such as the MK-2206, have been reported PH domain dependent and exhibit selectivity for the individual AKT isozymes. Of note, the PH domain of PDK1 could represent an attractive drug target and a dual inhibitor for AKT and PDK would be useful for the treatment of cancers with an activated PI3K/AKT pathway. Through our in silico screening approach and SPR in vitro screen, we have identified and validated several dual inhibitors of PDK1 and AKT PH domain function. Indeed, although PH-427 was originally developed as an inhibitor of the PH domain of AKT [122], new results suggest that it is also an inhibitor of PDK1 [96]. Our SPR studies demonstrate that PH-427 binds to the expressed PH domain of PDK1 with an affinity similar to that of binding to the PH domain of AKT (Ki for PDK1, 5.2 μM; Ki for AKT, 2.7 μM) [96]. We have shown that the plasma membrane translocation of both the AKT and PDK1 PH domains was inhibited by PH-427. In vivo studies showed that PH-427 produced an inhibition of pSer473AKT and pSer241PDK1 concentrations in tumors after 8–12 hours, and after 4 8- hours respectively, with a maintained inhibition only for PDK1 for 24 hours. These results strongly suggest the possibility for PH-427 to behave as a dual AKT/PDK1 inhibitor.
5.3. Identification of Biomarkers for AKT PH Domain Inhibitors
The transition of any approach targeting AKT from preclinical compounds to useful therapeutics will depend in part upon identifying “off target” effects that could contribute to cellular responses as well as biomarkers of the responses. Screening of the PIAs in cancer cell lines showed that 5 of 25 analogues inhibited AKT and preferentially induced apoptosis in cancer cell lines with high levels of endogenous AKT activity [105]. When these compounds were screened in the NCI-60 cell line panel, cytotoxicity correlated with levels of phosphorylated AKT but not total AKT, but other molecular markers were also identified that had higher correlations with activity [107]. In addition, these studies showed that PIAs induced more apoptosis than other pathway inhibitors despite similar levels of AKT inhibition, which suggested that other targets of PIAs might contribute to the cytotoxicity of these compounds. Off targets identification of PIAs was the objective of the study by Gills et al. [109]. The authors screened PIAs against a panel of purified kinases and showed that active PIAs activate a single isoform of p38, p38α, in vitro and in vivo [109]. The authors concluded that because p38a activation occurs in cancer cells after chemotherapy and in normal cells during inflammatory processes, activation of p38a by PIAs could contribute to the efficacy and/or toxicity of PIAs. Although PIAs are not undergoing clinical trials, studies assessing specificity and allowing the identification of “off targets” are of utmost importance and should be considered early on during the pre-clinical development of AKT PH domain inhibitors. In light of the recent trials with perifosine [133, 145], better biomarkers and techniques for the evaluation of such biomarkers are necessary.
5.4. Potential Clinical Application of Known AKT PH Domain Inhibitors
Several of the AKT PH domain inhibitors have already been tested in vitro and some in vivo in combination with either ionizing radiation-or other chemotherapy-targeted therapies. Several strategies are summarized below:
Combination to overcome trastuzumab resistance caused by PTEN deficiency. Trastuzumab is a monoclonal antibody directed against HER-2 which has revolutionized the management of both early and advanced breast cancer. However, trastuzumab resistance occurs via the increase in PTEN phosphatase activity. PTEN is required for the antitumor activity of trastuzumab and PTEN loss predicted poor clinical response to trastuzumab-based chemotherapy in patients [146]. Because PTEN opposes the actions of PI3K, a strategy to overcome trastuzumab resistance using TCN was successful in vitro and in vivo and demonstrated some clinically applicable strategy [130].
Combination to increase sensitivity if the drug to chemotherapy. We [96] and others [105, 147] have demonstrated that ALP and PIAs can synergize with chemotherapy in vitro and in vivo. Synergy with ether lipids has also been observed after combination therapy with conventional chemotherapy drugs, such as doxorubicin, cisplatin, vinblastine, and mitomycin C [148].
Combination with ionizing radiation. Synergy of ether phospholipid analogs (edelfosine and perifosine) and ionizing radiation in human carcinoma cell lines was proposed in the late nineties [87]. The authors demonstrated that the effects were supra-additive. These results led to the design of a clinical phase I study testing the combination of perifosine with fractionated radiation therapy [149].
CONCLUSIONS
In spite of unique techniques in molecular modeling, drug screening and testing coupled to an increasing knowledge regarding AKT activation, no targeted AKT therapeutics have reached the market yet. Novel approaches targeting unconventional domains, such as the pleckstrin homology (PH) domain of AKT, have lead to the discovery of promising compounds and identified innovative scaffolds that deserve further attention. In the race of the development of AKT inhibitors, these novel molecules that bind the PH domain of AKT have a bright future as they do not have the inherent toxicities of the more conventional kinase inhibitors. Combination therapies (ionizing radiation and chemotherapy) also appear to be the best route for the therapeutic application of novel AKT PH domain inhibitors. However, although the PH domain of AKT represents a druggable site for the inhibition of the function of the protein, it remains a necessity 1) to test for selectivity of the compounds toward other PH domain-containing proteins; 2) to establish biomarkers for the inhibition of AKT; and 3) to clearly delineate the mechanism of action of such compounds in view of their future transition to successful clinical studies.
Acknowledgments
This work was supported in part by the National Cancer Institute CA139503 grant to EJM. The author would like to thank Dr. Song Zuohe for performing the SPR studies.
ABBREVIATIONS
- βATA
3-β-acetoxy-tirucallic acid
- APL
alkyl-phospholipid
- IP
inositol phosphates
- PDK1
phosphatidylinositol dependent kinase
- PH
pleckstrin homology
- PIA
phosphatidylinositol analog
- PKA
protein kinase A
- PI3K
phosphatidylinositol-3-kinase
- SPR
surface plasmon resonance
- TCN
triciribine
Footnotes
CONFLICT OF INTEREST
EJM has financial interests in PHusis Therapeutics, Inc (Houtson, TX).
References
- 1.Hawkins PT, Anderson KE, Davidson K, Stephens LR. Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans. 2006;34:647–662. doi: 10.1042/BST0340647. [DOI] [PubMed] [Google Scholar]
- 2.Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter CL, Cantley LC. Phosphoinositide kinases. Biochemistry. 1990;29:11147–11156. doi: 10.1021/bi00503a001. [DOI] [PubMed] [Google Scholar]
- 4.Sjolander A, Yamamoto K, Huber BE, Lapetina EG. Association of p21ras with phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 1991;88:7908–7912. doi: 10.1073/pnas.88.18.7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maffucci T, Falasca M. Specificity in pleckstrin homology (PH) domain membrane targeting: a role for a phosphoinositide-protein co-operative mechanism. FEBS Lett. 2001;506:173–179. doi: 10.1016/s0014-5793(01)02909-x. [DOI] [PubMed] [Google Scholar]
- 6.Lemmon MA, Ferguson KM, Abrams CS. Pleckstrin homology domains and the cytoskeleton. FEBS Lett. 2002;513:71–76. doi: 10.1016/s0014-5793(01)03243-4. [DOI] [PubMed] [Google Scholar]
- 7.Ingley E, Hemmings BA. Pleckstrin homology (PH) domains in signal transduction. J Cell Biochem. 1994;56:436–443. doi: 10.1002/jcb.240560403. [DOI] [PubMed] [Google Scholar]
- 8.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 9.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 10.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci US A. 1998;95:15587–15591. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci US A. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 13.Ihle NT, Powis G. Take your PIK: phosphatidylinositol 3-kinase inhibitors race through the clinic and toward cancer therapy. Mol Can Ther. 2009;8:1–9. doi: 10.1158/1535-7163.MCT-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, Hartman GD, Huff JR, Huber HE, Duggan ME. Allosteric AKT (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005;15:761–764. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Li T, Zhu GD, Gong J, Claibone A, Dalton C, Luo Y, Johnson EF, Shi Y, Liu X, Klinghofer V, Bauch JL, Marsh KC, Bouska JJ, Arries S, De Jong R, Oltersdorf T, Stoll VS, Jakob CG, Rosenberg SH, Giranda VL. Discovery of trans-3,4′-bispyridinylethylenes as potent and novel inhibitors of protein kinase B (PKB/AKT) for the treatment of cancer: Synthesis and biological evaluation. Bioorg Med Chem Lett. 2006;16:1679–1685. doi: 10.1016/j.bmcl.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Kumar CC, Madison V. AKT crystal structure and AKT-specific inhibitors. Oncogene. 2005;24:7493–7501. doi: 10.1038/sj.onc.1209087. [DOI] [PubMed] [Google Scholar]
- 17.Luo Y, Shoemaker AR, Liu X, Woods KW, Thomas SA, de Jong R, Han EK, Li T, Stoll VS, Powlas JA, Oleksijew A, Mitten MJ, Shi Y, Guan R, McGonigal TP, Klinghofer V, Johnson EF, Leverson JD, Bouska JJ, Mamo M, Smith RA, Gramling-Evans EE, Zinker BA, Mika AK, Nguyen PT, Oltersdorf T, Rosenberg SH, Li Q, Giranda VL. Potent and selective inhibitors of AKT kinases slow the progress of tumors in vivo. Mol Can Ther. 2005;4:977–986. doi: 10.1158/1535-7163.MCT-05-0005. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman K, Holmes FA, Fraschini G, Esparza L, Frye D, Raber MN, Newman RA, Hortobagyi GN. Phase I-II study: triciribine (tricyclic nucleoside phosphate) for metastatic breast cancer. Can Chem Pharmacol. 1996;37:254–258. doi: 10.1007/BF00688325. [DOI] [PubMed] [Google Scholar]
- 19.Zhu GD, Gandhi VB, Gong J, Thomas S, Woods KW, Song X, Li T, Diebold RB, Luo Y, Liu X, Guan R, Klinghofer V, Johnson EF, Bouska J, Olson A, Marsh KC, Stoll VS, Mamo M, Polakowski J, Campbell TJ, Martin RL, Gintant GA, Penning TD, Li Q, Rosenberg SH, Giranda VL. Syntheses of potent, selective, and orally bioavailable indazole-pyridine series of protein kinase B/AKT inhibitors with reduced hypotension. J Med Chem. 2007;50:2990–3003. doi: 10.1021/jm0701019. [DOI] [PubMed] [Google Scholar]
- 20.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase AKT2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 21.Cheng AY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. Can Med Ass J. 2005;172:213–226. doi: 10.1503/cmaj.1031414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crouthamel MC, Kahana JA, Korenchuk S, Zhang SY, Sundaresan G, Eberwein DJ, Brown KK, Kumar R. Mechanism and management of AKT inhibitor-induced hyperglycemia. Clin Can Res. 2009;15:217–225. doi: 10.1158/1078-0432.CCR-08-1253. [DOI] [PubMed] [Google Scholar]
- 23.Marte BM, Downward J. PKB/AKT: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 24.Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-AKT): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masure S, Haefner B, Wesselink JJ, Hoefnagel E, Mortier E, Verhasselt P, Tuytelaars A, Gordon R, Richardson A. Molecular cloning, expression and characterization of the human serine/threonine kinase AKT-3. Eur J Biochem. 1999;265:353–360. doi: 10.1046/j.1432-1327.1999.00774.x. [DOI] [PubMed] [Google Scholar]
- 26.Nakatani K, Thompson DA, Barthel A, Sakaue H, Liu W, Weigel RJ, Roth RA. Up-regulation of AKT3 in estrogen receptor-deficient breast cancers and androgen-independent prostate cancer lines. J Biol Chem. 1999;274:21528–21532. doi: 10.1074/jbc.274.31.21528. [DOI] [PubMed] [Google Scholar]
- 27.Scheid MP, Woodgett JR. Unravelling the activation mechanisms of protein kinase B/AKT. FEBS Lett. 2003;546:108–112. doi: 10.1016/s0014-5793(03)00562-3. [DOI] [PubMed] [Google Scholar]
- 28.Rebecchi MJ, Scarlata S. Pleckstrin homology domains: a common fold with diverse functions. Ann Rev Biophys Biomol Struct. 1998;27:503–528. doi: 10.1146/annurev.biophys.27.1.503. [DOI] [PubMed] [Google Scholar]
- 29.Laine J, Kunstle G, Obata T, Sha M, Noguchi M. The protooncogene TCL1 is an AKT kinase coactivator. Mol Cell. 2000;6:395–407. doi: 10.1016/s1097-2765(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 30.Auguin D, Barthe P, Auge-Senegas MT, Stern MH, Noguchi M, Roumestand C. Solution structure and backbone dynamics of the pleckstrin homology domain of the human protein kinase B (PKB/AKT). Interaction with inositol phosphates. J Biomol NMR. 2004;28:137–155. doi: 10.1023/B:JNMR.0000013836.62154.c2. [DOI] [PubMed] [Google Scholar]
- 31.Auguin D, Barthe P, Royer C, Stern MH, Noguchi M, Arold ST, Roumestand C. Structural basis for the co-activation of protein kinase B by T-cell leukemia-1 (TCL1) family proto-oncoproteins. JBiol Chem. 2004;279:35890–35902. doi: 10.1074/jbc.M400364200. [DOI] [PubMed] [Google Scholar]
- 32.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 33.Skeen JE, Bhaskar PT, Chen CC, Chen WS, Peng XD, Nogueira V, Hahn-Windgassen A, Kiyokawa H, Hay N. AKT deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Can Cell. 2006;10:269–280. doi: 10.1016/j.ccr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 34.Schwertfeger KL, Richert MM, Anderson SM. Mammary gland involution is delayed by activated AKT in transgenic mice. Mol Endocrinol. 2001;15:867–881. doi: 10.1210/mend.15.6.0663. [DOI] [PubMed] [Google Scholar]
- 35.Malstrom S, Tili E, Kappes D, Ceci JD, Tsichlis PN. Tumor induction by an Lck-MyrAKT transgene is delayed by mechanisms controlling the size of the thymus. Proc Natl Acad Sci US A. 2001;98:14967–14972. doi: 10.1073/pnas.231467698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ackler S, Ahmad S, Tobias C, Johnson MD, Glazer RI. Delayed mammary gland involution in MMTV-AKT1 transgenic mice. Oncogene. 2002;21:198–206. doi: 10.1038/sj.onc.1205052. [DOI] [PubMed] [Google Scholar]
- 37.Chen ML, Xu PZ, Peng XD, Chen WS, Guzman G, Yang X, Di Cristofano A, Pandolfi PP, Hay N. The deficiency of AKT1 is sufficient to suppress tumor development in Pten+/− mice. Genes Dev. 2006;20:1569–1574. doi: 10.1101/gad.1395006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci US A. 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholson KM, Anderson NG. The protein kinase B/AKT signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 40.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. AKT phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 41.Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. AKT phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 43.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the AKT serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 44.Du K, Montminy M. CREB is a regulatory target for the protein kinase AKT/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 45.Chung J, Grammer TC, Lemon KP, Kazlauskas A, Blenis J. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994;370:71–75. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- 46.van Weeren PC, de Bruyn KM, de Vries-Smits AM, van Lint J, Burgering BM. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J Biol Chem. 1998;273:13150–13156. doi: 10.1074/jbc.273.21.13150. [DOI] [PubMed] [Google Scholar]
- 47.Kerbel RS, Yu J, Tran J, Man S, Viloria-Petit A, Klement G, Coomber BL, Rak J. Possible mechanisms of acquired resistance to anti-angiogenic drugs: implications for the use of combination therapy approaches. Can Met Rev. 2001;20:79–86. doi: 10.1023/a:1013172910858. [DOI] [PubMed] [Google Scholar]
- 48.Lemmon MA. Pleckstrin homology (PH) domains and phosphoinositides. Biochem Soc Symp. 2007:81–93. doi: 10.1042/BSS0740081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carman CV, Barak LS, Chen C, Liu-Chen LY, Onorato JJ, Kennedy SP, Caron MG, Benovic JL. Mutational analysis of Gbetagamma and phospholipid interaction with G protein-coupled receptor kinase 2. J Biol Chem. 2000;275:10443–10452. doi: 10.1074/jbc.275.14.10443. [DOI] [PubMed] [Google Scholar]
- 50.Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gbetagamma. Science. 2003;300:1256–1262. doi: 10.1126/science.1082348. [DOI] [PubMed] [Google Scholar]
- 51.Mahadevan D, Thanki N, Singh J, McPhie P, Zangrilli D, Wang LM, Guerrero C, LeVine H, 3rd, Humblet C, Saldanha J, et al. Structural studies on the PH domains of Db1, Sos1, IRS-1, and beta ARK1 and their differential binding to G beta gamma subunits. Biochemistry. 1995;34:9111–9117. doi: 10.1021/bi00028a021. [DOI] [PubMed] [Google Scholar]
- 52.Touhara K, Inglese J, Pitcher JA, Shaw G, Lefkowitz RJ. Binding of G protein beta gamma-subunits to pleckstrin homology domains. J Biol Chem. 1994;269:10217–10220. [PubMed] [Google Scholar]
- 53.Park WS, Heo WD, Whalen JH, O’Rourke NA, Bryan HM, Meyer T, Teruel MN. Comprehensive identification of PIP3-regulated PH domains from C. elegans to H. sapiens by model prediction and live imaging. Mol Cell. 2008;30:381–392. doi: 10.1016/j.molcel.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurley JH, Misra S. Signaling and subcellular targeting by membrane-binding domains. Ann Rev Biophys Biomol Struct. 2000;29:49–79. doi: 10.1146/annurev.biophys.29.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. AKT activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 56.Rong SB, Hu Y, Enyedy I, Powis G, Meuillet EJ, Wu X, Wang R, Wang S, Kozikowski AP. Molecular modeling studies of the AKT PH domain and its interaction with phosphoinositides. J Med Chem. 2001;44:898–908. doi: 10.1021/jm000493i. [DOI] [PubMed] [Google Scholar]
- 57.Thomas CC, Deak M, Alessi DR, van Aalten DM. High-resolution structure of the pleckstrin homology domain of protein kinase b/AKT bound to phosphatidylinositol (3,4,5)-trisphosphate. Curr Biol. 2002;12:1256–1262. doi: 10.1016/s0960-9822(02)00972-7. [DOI] [PubMed] [Google Scholar]
- 58.Milburn CC, Deak M, Kelly SM, Price NC, Alessi DR, Van Aalten DM. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem J. 2003;375:531–538. doi: 10.1042/BJ20031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frech M, Andjelkovic M, Ingley E, Reddy KK, Falck JR, Hemmings BA. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem. 1997;272:8474–8481. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- 60.James SR, Downes CP, Gigg R, Grove SJ, Holmes AB, Alessi DR. Specific binding of the AKT-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem J. 1996;315(Pt 3):709–713. doi: 10.1042/bj3150709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gambhir A, Hangyas-Mihalyne G, Zaitseva I, Cafiso DS, Wang J, Murray D, Pentyala SN, Smith SO, McLaughlin S. Electrostatic sequestration of PIP2 on phospholipid membranes by basic/aromatic regions of proteins. Biophys J. 2004;86:2188–2207. doi: 10.1016/S0006-3495(04)74278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calleja V, Laguerre M, Parker PJ, Larijani B. Role of a novel PH-kinase domain interface in PKB/AKT regulation: structural mechanism for allosteric inhibition. PLoS Biol. 2009;7:e17. doi: 10.1371/journal.pbio.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calleja V, Laguerre M, Larijani B. 3-D structure and dynamics of protein kinase B-new mechanism for the allosteric regulation of an AGC kinase. J Chem Biol. 2009;2:11–25. doi: 10.1007/s12154-009-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang J, Cron P, Good VM, Thompson V, Hemmings BA, Barford D. Crystal structure of an activated AKT/protein kinase B ternary complex with GSK3-peptide and AMP-PNP. Nat Struct Biol. 2002;9:940–944. doi: 10.1038/nsb870. [DOI] [PubMed] [Google Scholar]
- 65.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 66.Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for AKT/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 67.Feng J, Park J, Cron P, Hess D, Hemmings BA. Identification of a PKB/AKT hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem. 2004;279:41189–41196. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- 68.Toker A, Newton AC. AKT/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J Biol Chem. 2000;275:8271–8274. doi: 10.1074/jbc.275.12.8271. [DOI] [PubMed] [Google Scholar]
- 69.Datta K, Franke TF, Chan TO, Makris A, Yang SI, Kaplan DR, Morrison DK, Golemis EA, Tsichlis PN. AH/PH domain-mediated interaction between AKT molecules and its potential role in AKT regulation. Mol Cell Biol. 1995;15:2304–2310. doi: 10.1128/mcb.15.4.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Ann Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 71.Jacobs M, Hayakawa K, Swenson L, Bellon S, Fleming M, Taslimi P, Doran J. The structure of dimeric ROCK I reveals the mechanism for ligand selectivity. J Biol Chem. 2006;281:260–268. doi: 10.1074/jbc.M508847200. [DOI] [PubMed] [Google Scholar]
- 72.Fushman D, Cahill S, Cowburn D. The main-chain dynamics of the dynamin pleckstrin homology (PH) domain in solution: analysis of 15N relaxation with monomer/dimer equilibration. J Mol Biol. 1997;266:173–194. doi: 10.1006/jmbi.1996.0771. [DOI] [PubMed] [Google Scholar]
- 73.Powis G, Aksoy IA, Melder DC, Aksoy S, Eichinger H, Fauq AH, Kozikowski AP. D-3-deoxy-3-substituted myo-inositol analogues as inhibitors of cell growth. Can Chemother Pharmacol. 1991;29:95–104. doi: 10.1007/BF00687317. [DOI] [PubMed] [Google Scholar]
- 74.Hu Y, Meuillet EJ, Berggren M, Powis G, Kozikowski AP. 3-Deoxy-3-substituted-D-myo-inositol imidazolyl ether lipid phosphates and carbonate as inhibitors of the phosphatidylinositol 3-kinase pathway and cancer cell growth. Bioorg Med Chem Lett. 2001;11:173–176. doi: 10.1016/s0960-894x(00)00640-5. [DOI] [PubMed] [Google Scholar]
- 75.Hu Y, Qiao L, Wang S, Rong SB, Meuillet EJ, Berggren M, Gallegos A, Powis G, Kozikowski AP. 3-(Hydroxymethyl)-bearing phosphatidylinositol ether lipid analogues and carbonate surrogates block PI3-K, AKT, and cancer cell growth. J Med Chem. 2000;43:3045–3051. doi: 10.1021/jm000117y. [DOI] [PubMed] [Google Scholar]
- 76.Qiao L, Nan F, Kunkel M, Gallegos A, Powis G, Kozikowski AP. 3-Deoxy-D-myo-inositol 1-phosphate, 1-phosphonate, and ether lipid analogues as inhibitors of phosphatidylinositol-3-kinase signaling and cancer cell growth. J Med Chem. 1998;41:3303–3306. doi: 10.1021/jm980254j. [DOI] [PubMed] [Google Scholar]
- 77.Li Q. Recent progress in computer-aided diagnosis of lung nodules on thin-section CT. Comput Med Imaging Graph. 2007;31:248–257. doi: 10.1016/j.compmedimag.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meuillet EJ, Mahadevan D, Vankayalapati H, Berggren M, Williams R, Coon A, Kozikowski AP, Powis G. Specific inhibition of the AKT1 pleckstrin homology domain by D-3-deoxy-phosphatidyl-myo-inositol analogues. Mol Can Ther. 2003;2:389–399. [PubMed] [Google Scholar]
- 79.Berkovic D. Cytotoxic etherphospholipid analogues. Gen Pharmacol. 1998;31:511–517. doi: 10.1016/s0306-3623(98)00081-0. [DOI] [PubMed] [Google Scholar]
- 80.Meuillet EJ, Ihle N, Baker AF, Gard JM, Stamper C, Williams R, Coon A, Mahadevan D, George BL, Kirkpatrick L, Powis G. In vivo molecular pharmacology and antitumor activity of the targeted AKT inhibitor PX-316. Oncol Res. 2004;14:513–527. doi: 10.3727/0965040042380487. [DOI] [PubMed] [Google Scholar]
- 81.Egorin M. Plasma pharmacokinetics and bioavailability for the phosphatidylinositide-3-kinase signaling inhibitor, OMDPI (NSC 710297) in CDF2F1 mice. Proc Am Assoc Cancer Res. 2002:604. [Google Scholar]
- 82.Martelli AM, Tazzari PL, Tabellini G, Bortul R, Billi AM, Manzoli L, Ruggeri A, Conte R, Cocco L. A new selective AKT pharmacological inhibitor reduces resistance to chemotherapeutic drugs, TRAIL, all-trans-retinoic acid, and ionizing radiation of human leukemia cells. Leukemia. 2003;17:1794–1805. doi: 10.1038/sj.leu.2403044. [DOI] [PubMed] [Google Scholar]
- 83.Modolell M, Andreesen R, Pahlke W, Brugger U, Munder PG. Disturbance of phospholipid metabolism during the selective destruction of tumor cells induced by alkyl-lysophospholipids. Cancer Res. 1979;39:4681–4686. [PubMed] [Google Scholar]
- 84.Andreesen R, Modolell M, Munder PG. Selective sensitivity of chronic myelogenous leukemia cell populations to alkyl-lysophospholipids. Blood. 1979;54:519–523. [PubMed] [Google Scholar]
- 85.Andreesen R, Modolell M, Weltzien HU, Eibl H, Common HH, Lohr GW, Munder PG. Selective destruction of human leukemic cells by alkyl-lysophospholipids. Cancer Res. 1978;38:3894–3899. [PubMed] [Google Scholar]
- 86.van Blitterswijk WJ, Verheij M. Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Curr Pharm Des. 2008;14:2061–2074. doi: 10.2174/138161208785294636. [DOI] [PubMed] [Google Scholar]
- 87.Berkovic D, Grundel O, Berkovic K, Wildfang I, Hess CF, Schmoll HJ. Synergistic cytotoxic effects of ether phospholipid analogues and ionizing radiation in human carcinoma cells. Radiother Oncol. 1997;43:293–301. doi: 10.1016/s0167-8140(97)01909-9. [DOI] [PubMed] [Google Scholar]
- 88.Vink SR, van Blitterswijk WJ, Schellens JH, Verheij M. Rationale and clinical application of alkylphospholipid analogues in combination with radiotherapy. Cancer Treat Rev. 2007;33:191–202. doi: 10.1016/j.ctrv.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 89.Gills JJ, Dennis PA. Perifosine: update on a novel AKT inhibitor. Curr Oncol Rep. 2009;11:102–110. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, San Miguel JF, Cavenagh JD, Anderson KC. Safety and efficacy of bortezomib in high-risk and elderly patients with relapsed multiple myeloma. Br J Haematol. 2007;137:429–435. doi: 10.1111/j.1365-2141.2007.06585.x. [DOI] [PubMed] [Google Scholar]
- 91.Hilgard P, Klenner T, Stekar J, Nossner G, Kutscher B, Engel J. D-21266; a new heterocyclic alkylphospholipid with antitumour activity. Eur J Cancer. 1997;33:442–446. doi: 10.1016/s0959-8049(97)89020-x. [DOI] [PubMed] [Google Scholar]
- 92.Stekar J, Hilgard P, Voegeli R, Maurer HR, Engel J, Kutscher B, Nossner G, Schumacher W. Antineoplastic activity and tolerability of a novel heterocyclic alkylphospholipid, D-20133. Cancer Chemother Pharmacol. 1993;32:437–444. doi: 10.1007/BF00685887. [DOI] [PubMed] [Google Scholar]
- 93.Ruiter GA, Verheij M, Zerp SF, van Blitterswijk WJ. Alkyl-lysophospholipids as anticancer agents and enhancers of radiation-induced apoptosis. Int J Radiat Oncol Biol Phys. 2001;49:415–419. doi: 10.1016/s0360-3016(00)01476-0. [DOI] [PubMed] [Google Scholar]
- 94.Ruiter GA, Zerp SF, Bartelink H, van Blitterswijk WJ, Verheij M. Anti-cancer alkyl-lysophospholipids inhibit the phosphatidylinositol 3-kinase-AKT/PKB survival pathway. Anticancer Drugs. 2003;14:167–173. doi: 10.1097/00001813-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 95.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Can Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- 96.Meuillet EJ, Zuohe S, Lemos R, Ihle N, Kingston J, Watkins R, Moses SA, Zhang S, Du-Cuny L, Herbst R, Jacoby JJ, Zhou LL, Ahad AM, Mash EA, Kirkpatrick DL, Powis G. Molecular pharmacology and antitumor activity of PHT-427, a novel AKT/phosphatidylinositide-dependent protein kinase 1 pleckstrin homology domain inhibitor. Mol Can Ther. 2010;9:706–717. doi: 10.1158/1535-7163.MCT-09-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Poradosu E. Perifosine selectively inhibits binding of AKT PH domain to PtdIns(3,4)P2. Proc Am Assoc Cancer Res. 2007:1645. [Google Scholar]
- 98.Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, Podar K, Munshi NC, Chauhan D, Richardson PG, Anderson KC. Perifosine, an oral bioactive novel alkylphospholipid, inhibits AKT and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/AKT/mTOR pathway: effective combinations and clinical considerations. Drug Resist, Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hennessy BT, Lu Y, Poradosu E, Yu Q, Yu S, Hall H, Carey MS, Ravoori M, Gonzalez-Angulo AM, Birch R, Henderson IC, Kundra V, Mills GB. Pharmacodynamic markers of perifosine efficacy. Clin Cancer Res. 2007;13:7421–7431. doi: 10.1158/1078-0432.CCR-07-0760. [DOI] [PubMed] [Google Scholar]
- 101.Vink SR, Schellens JH, van Blitterswijk WJ, Verheij M. Tumor and normal tissue pharmacokinetics of perifosine, an oral anti-cancer alkylphospholipid. Invest New Drugs. 2005;23:279–286. doi: 10.1007/s10637-005-1436-0. [DOI] [PubMed] [Google Scholar]
- 102.Breiser A, Kim DJ, Fleer EA, Damenz W, Drube A, Berger M, Nagel GA, Eibl H, Unger C. Distribution and metabolism of hexadecylphosphocholine in mice. Lipids. 1987;22:925–926. doi: 10.1007/BF02535556. [DOI] [PubMed] [Google Scholar]
- 103.Crul M, Rosing H, de Klerk GJ, Dubbelman R, Traiser M, Reichert S, Knebel NG, Schellens JH, Beijnen JH, ten Bokkel Huinink WW. Phase I and pharmacological study of daily oral administration of perifosine (D-21266) in patients with advanced solid tumours. Eur J Cancer. 2002;38:1615–1621. doi: 10.1016/s0959-8049(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 104.Van Ummersen L, Binger K, Volkman J, Marnocha R, Tutsch K, Kolesar J, Arzoomanian R, Alberti D, Wilding G. A phase I trial of perifosine (NSC 639966) on a loading dose/maintenance dose schedule in patients with advanced cancer. Clin Cancer Res. 2004;10:7450–7456. doi: 10.1158/1078-0432.CCR-03-0406. [DOI] [PubMed] [Google Scholar]
- 105.Castillo SS, Brognard J, Petukhov PA, Zhang C, Tsurutani J, Granville CA, Li M, Jung M, West KA, Gills JG, Kozikowski AP, Dennis PA. Preferential inhibition of AKT and killing of AKT-dependent cancer cells by rationally designed phosphatidylinositol ether lipid analogues. Cancer Res. 2004;64:2782–2792. doi: 10.1158/0008-5472.can-03-1530. [DOI] [PubMed] [Google Scholar]
- 106.Kozikowski AP, Sun H, Brognard J, Dennis PA. Novel PI analogues selectively block activation of the pro-survival serine/threonine kinase AKT. J Am Chem Soc. 2003;125:1144–1145. doi: 10.1021/ja0285159. [DOI] [PubMed] [Google Scholar]
- 107.Gills JJ, Holbeck S, Hollingshead M, Hewitt SM, Kozikowski AP, Dennis PA. Spectrum of activity and molecular correlates of response to phosphatidylinositol ether lipid analogues, novel lipid-based inhibitors of AKT. Mol Can Ther. 2006;5:713–722. doi: 10.1158/1535-7163.MCT-05-0484. [DOI] [PubMed] [Google Scholar]
- 108.Memmott RM, Gills JJ, Hollingshead M, Powers MC, Chen Z, Kemp B, Kozikowski A, Dennis PA. Phosphatidylinositol ether lipid analogues induce AMP-activated protein kinase-dependent death in LKB1-mutant non small cell lung cancer cells. Cancer Res. 2008;68:580–588. doi: 10.1158/0008-5472.CAN-07-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gills JJ, Castillo SS, Zhang C, Petukhov PA, Memmott RM, Hollingshead M, Warfel N, Han J, Kozikowski AP, Dennis PA. Phosphatidylinositol ether lipid analogues that inhibit AKT also independently activate the stress kinase, p38alpha, through MKK3/6-independent and -dependent mechanisms. J Biol Chem. 2007;282:27020–27029. doi: 10.1074/jbc.M701108200. [DOI] [PubMed] [Google Scholar]
- 110.Berrie CP, Falasca M. Patterns within protein/polyphosphoinositide interactions provide specific targets for therapeutic intervention. FASEB J. 2000;14:2618–2622. doi: 10.1096/fj.00-0096hyp. [DOI] [PubMed] [Google Scholar]
- 111.Razzini G, Berrie CP, Vignati S, Broggini M, Mascetta G, Brancaccio A, Falasca M. Novel functional PI 3-kinase antagonists inhibit cell growth and tumorigenicity in human cancer cell lines. FASEB J. 2000;14:1179–1187. doi: 10.1096/fasebj.14.9.1179. [DOI] [PubMed] [Google Scholar]
- 112.Piccolo E, Vignati S, Maffucci T, Innominato PF, Riley AM, Potter BV, Pandolfi PP, Broggini M, Iacobelli S, Innocenti P, Falasca M. Inositol pentakisphosphate promotes apoptosis through the PI 3-K/AKT pathway. Oncogene. 2004;23:1754–1765. doi: 10.1038/sj.onc.1207296. [DOI] [PubMed] [Google Scholar]
- 113.Maffucci T, Piccolo E, Cumashi A, Iezzi M, Riley AM, Saiardi A, Godage HY, Rossi C, Broggini M, Iacobelli S, Potter BV, Innocenti P, Falasca M. Inhibition of the phosphatidylinositol 3-kinase/AKT pathway by inositol pentakisphosphate results in antiangiogenic and antitumor effects. Cancer Res. 2005;65:8339–8349. doi: 10.1158/0008-5472.CAN-05-0121. [DOI] [PubMed] [Google Scholar]
- 114.Jenab M, Thompson LU. Phytic acid in wheat bran affects colon morphology, cell differentiation and apoptosis. Carcinogenesis. 2000;21:1547–1552. [PubMed] [Google Scholar]
- 115.Raina K, Rajamanickam S, Singh RP, Agarwal R. Chemopreventive efficacy of inositol hexaphosphate against prostate tumor growth and progression in TRAMP mice. Clin Cancer Res. 2008;14:3177–3184. doi: 10.1158/1078-0432.CCR-07-5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee HJ, Lee SA, Choi H. Dietary administration of inositol and/or inositol-6-phosphate prevents chemically-induced rat hepatocarcinogenesis. Asian Pac J Cancer Prev. 2005;6:41–47. [PubMed] [Google Scholar]
- 117.Falasca M, Chiozzotto D, Godage HY, Mazzoletti M, Riley AM, Previdi S, Potter BV, Broggini M, Maffucci T. A novel inhibitor of the PI3K/AKT pathway based on the structure of inositol 1,3,4,5,6-pentakisphosphate. Br J Cancer. 2010;102:104–114. doi: 10.1038/sj.bjc.6605408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mills SJ, Komander D, Trusselle MN, Safrany ST, van Aalten DM, Potter BV. Novel inositol phospholipid headgroup surrogate crystallized in the pleckstrin homology domain of protein kinase Balpha. ACS Chem Biol. 2007;2:242–246. doi: 10.1021/cb700019r. [DOI] [PubMed] [Google Scholar]
- 119.Masters TA, Calleja V, Armoogum DA, Marsh RJ, Applebee CJ, Laguerre M, Bain AJ, Larijani B. Regulation of 3-phosphoinositide-dependent protein kinase 1 activity by homodimerization in live cells. Sci Signal. 2010;3:ra78. doi: 10.1126/scisignal.2000738. [DOI] [PubMed] [Google Scholar]
- 120.Chakraborty A, Koldobskiy MA, Bello NT, Maxwell M, Potter JJ, Juluri KR, Maag D, Kim S, Huang AS, Dailey MJ, Saleh M, Snowman AM, Moran TH, Mezey E, Snyder SH. Inositol pyrophosphates inhibit AKT signaling, thereby regulating insulin sensitivity and weight gain. Cell. 2010;143:897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mahadevan D, Powis G, Mash EA, George B, Gokhale VM, Zhang S, Shakalya K, Du-Cuny L, Berggren M, Ali MA, Jana U, Ihle N, Moses S, Franklin C, Narayan S, Shirahatti N, Meuillet EJ. Discovery of a novel class of AKT pleckstrin homology domain inhibitors. Mol Can Ther. 2008;7:2621–2632. doi: 10.1158/1535-7163.MCT-07-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moses SA, Ali MA, Zuohe S, Du-Cuny L, Zhou LL, Lemos R, Ihle N, Skillman AG, Zhang S, Mash EA, Powis G, Meuillet EJ. In vitro and in vivo activity of novel small-molecule inhibitors targeting the pleckstrin homology domain of protein kinase B/AKT. Cancer Res. 2009;69:5073–5081. doi: 10.1158/0008-5472.CAN-08-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Du-Cuny L, Song Z, Moses S, Powis G, Mash EA, Meuillet EJ, Zhang S. Computational modeling of novel inhibitors targeting the AKT pleckstrin homology domain. Bioorg Med Chem. 2009;17:6983–6992. doi: 10.1016/j.bmc.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ihle NT, Lemos R, Jr, Wipf P, Yacoub A, Mitchell C, Siwak D, Mills GB, Dent P, Kirkpatrick DL, Powis G. Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumor activity of the inhibitor PX-866 whereas oncogenic Ras is a dominant predictor for resistance. Cancer Res. 2009;69:143–150. doi: 10.1158/0008-5472.CAN-07-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Casanova A, Moses S, Morgan S, Mash E, Powis G, Meuillet E. A novel inhibitor of AKT prevents UVB-induced signaling in human skin cancers. Proc Am Assoc Cancer Res. 2009:3709. [Google Scholar]
- 126.Wotring LL, Townsend LB, Jones LM, Borysko KZ, Gildersleeve DL, Parker WB. Dual mechanisms of inhibition of DNA synthesis by triciribine. Cancer Res. 1990;50:4891–4899. [PubMed] [Google Scholar]
- 127.Schweinsberg PD, Smith RG, Loo TL. Identification of the metabolites of an antitumor tricyclic nucleoside (NSC-154020) Biochem Pharmacol. 1981;30:2521–2526. doi: 10.1016/0006-2952(81)90577-3. [DOI] [PubMed] [Google Scholar]
- 128.Ptak RG, Borysko KZ, Porcari AR, Buthod JL, Holland LE, Shipman C, Jr, Townsend LB, Drach JC. Phosphorylation of triciribine is necessary for activity against HIV type 1. AIDS Res Hum Retroviruses. 1998;14:1315–1322. doi: 10.1089/aid.1998.14.1315. [DOI] [PubMed] [Google Scholar]
- 129.Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, Hamilton AD, Polokoff M, Nicosia SV, Herlyn M, Sebti SM, Cheng JQ. AKT/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of AKT signaling with antitumor activity in cancer cells overexpressing AKT. Cancer Res. 2004;64:4394–4399. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 130.Lu CH, Wyszomierski SL, Tseng LM, Sun MH, Lan KH, Neal CL, Mills GB, Hortobagyi GN, Esteva FJ, Yu D. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13:5883–5888. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- 131.Berndt N, Yang H, Trinczek B, Betzi S, Zhang Z, Wu B, Lawrence NJ, Pellecchia M, Schonbrunn E, Cheng JQ, Sebti SM. The AKT activation inhibitor TCN-P inhibits AKT phosphorylation by binding to the PH domain of AKT and blocking its recruitment to the plasma membrane. Cell Death Differ. 2010;17:1795–1804. doi: 10.1038/cdd.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wotring LL, Crabtree GW, Edwards NL, Parks RE, Jr, Townsend LB. Mechanism of activation of triciribine phosphate (TCN-P) as a prodrug form of TCN. Cancer Treat Rep. 1986;70:491–497. [PubMed] [Google Scholar]
- 133.Garrett CR, Coppola D, Wenham RM, Cubitt CL, Neuger AM, Frost TJ, Lush RM, Sullivan DM, Cheng JQ, Sebti SM. Phase I pharmacokinetic and pharmacodynamic study of triciribine phosphate monohydrate, a small-molecule inhibitor of AKT phosphorylation, in adult subjects with solid tumors containing activated AKT. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9479-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Feun LG, Blessing JA, Barrett RJ, Hanjani P. A phase II trial of tricyclic nucleoside phosphate in patients with advanced squamous cell carcinoma of the cervix. A Gynecologic Oncology Group Study. Am J Clin Oncol. 1993;16:506–508. doi: 10.1097/00000421-199312000-00010. [DOI] [PubMed] [Google Scholar]
- 135.Feun LG, Savaraj N, Bodey GP, Lu K, Yap BS, Ajani JA, Burgess MA, Benjamin RS, McKelvey E, Krakoff I. Phase I study of tricyclic nucleoside phosphate using a five-day continuous infusion schedule. Cancer Res. 1984;44:3608–3612. [PubMed] [Google Scholar]
- 136.Kim D, Sun M, He L, Zhou QH, Chen J, Sun XM, Bepler G, Sebti SM, Cheng JQ. A small molecule inhibits AKT through direct binding to AKT and preventing AKT membrane translocation. J Biol Chem. 285:8383–8394. doi: 10.1074/jbc.M109.094060. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 137.Barnett SF, Bilodeau MT, Lindsley CW. The AKT/PKB family of protein kinases: a review of small molecule inhibitors and progress towards target validation. Curr Top Med Chem. 2005;5:109–125. doi: 10.2174/1568026053507714. [DOI] [PubMed] [Google Scholar]
- 138.Li HF, Kim JS, Waldman T. Radiation-induced AKT activation modulates radioresistance in human glioblastoma cells. Radiat Oncol. 2009;4:43. doi: 10.1186/1748-717X-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tolcher A. A Phase I study of MK-2206; an oral potent allosteric AKT inhibitor (AKTi), in patients (pts) with advanced solid tumor (ST) J Clin Oncol. 2009;27:15s. [Google Scholar]
- 140.Estrada AC, Syrovets T, Pitterle K, Lunov O, Buchele B, Schimana-Pfeifer J, Schmidt T, Morad SA, Simmet T. Tirucallic acids are novel pleckstrin homology domain-dependent AKT inhibitors inducing apoptosis in prostate cancer cells. Mol Pharmacol. 77:378–387. doi: 10.1124/mol.109.060475. [DOI] [PubMed] [Google Scholar]
- 141.Miao B, Skidan I, Yang J, Lugovskoy A, Reibarkh M, Long K, Brazell T, Durugkar KA, Maki J, Ramana CV, Schaffhausen B, Wagner G, Torchilin V, Yuan J, Degterev A. Small molecule inhibition of phosphatidylinositol-3,4,5-triphosphate (PIP3) binding to pleckstrin homology domains. Proc Natl Acad Sci US A. 2010;107:20126–20131. doi: 10.1073/pnas.1004522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hiromura M, Okada F, Obata T, Auguin D, Shibata T, Roumestand C, Noguchi M. Inhibition of AKT kinase activity by a peptide spanning the betaA strand of the proto-oncogene TCL1. J Biol Chem. 2004;279:53407–53418. doi: 10.1074/jbc.M403775200. [DOI] [PubMed] [Google Scholar]
- 143.Noguchi M, Ropars V, Roumestand C, Suizu F. Proto-oncogene TCL1: more than just a coactivator for AKT. FASEB J. 2007;21:2273–2284. doi: 10.1096/fj.06-7684com. [DOI] [PubMed] [Google Scholar]
- 144.Powell DJ, Hajduch E, Kular G, Hundal HS. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/AKT by a PKCzeta-dependent mechanism. Mol Cell Biol. 2003;23:7794–7808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ashida N, Arai H, Yamasaki M, Kita T. Distinct signaling pathways for MCP-1-dependent integrin activation and chemotaxis. J Biol Chem. 2001;276:16555–16560. doi: 10.1074/jbc.M009068200. [DOI] [PubMed] [Google Scholar]
- 146.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, Hortobagyi GN, Hung MC, Yu D. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 147.Tabellini G, Tazzari PL, Bortul R, Billi AM, Conte R, Manzoli L, Cocco L, Martelli AM. Novel 2′-substituted, 3′-deoxy-phosphatidyl-myo-inositol analogues reduce drug resistance in human leukaemia cell lines with an activated phosphoinositide 3-kinase/AKT pathway. Br J Haematol. 2004;126:574–582. doi: 10.1111/j.1365-2141.2004.05073.x. [DOI] [PubMed] [Google Scholar]
- 148.Principe P, Braquet P. Advances in either phospholipids treatment of cancer. Crit Rev Oncol Hematol. 1995;18:155–178. doi: 10.1016/1040-8428(94)00118-d. [DOI] [PubMed] [Google Scholar]
- 149.Vink SR, Schellens JH, Beijnen JH, Sindermann H, Engel J, Dubbelman R, Moppi G, Hillebrand MJ, Bartelink H, Verheij M. Phase I and pharmacokinetic study of combined treatment with perifosine and radiation in patients with advanced solid tumours. Radiother Oncol. 2006;80:207–213. doi: 10.1016/j.radonc.2006.07.032. [DOI] [PubMed] [Google Scholar]