Abstract
The interaction of drugs with biologic targets is a critical area of research, particularly for the development of medications to treat substance use disorders. In addition to understanding these drug-target interactions, however, there is a need to understand more fully the psychosocial influences that moderate these interactions. The first section of this review introduces some examples from human behavioral pharmacology that illustrate the clinical importance of this research. The second section covers preclinical evidence to characterize some of the key individual differences that alter drug sensitivity and abuse vulnerability, related primarily to differences in response to novelty and impulsivity. Evidence is presented to indicate that critical neuropharmacological mechanisms associated with these individual differences involve integrated neurocircuits underlying stress, reward, and behavioral inhibitory processes. The third section covers social influences on drug abuse vulnerability, including effects experienced during infancy, adolescence, and young adulthood, such as maternal separation, housing conditions, and social interactions (defeat, play, and social rank). Some of the same neurocircuits involved in individual differences also are altered by social influences, although the precise neurochemical and cellular mechanisms involved remain to be elucidated fully. Finally, some speculation is offered about the implications of this research for the prevention and treatment of substance abuse.
I. Introduction
The interaction of drugs with biologic targets is a fundamental area of neuropharmacological research and has critical implications for the development of medications to treat various neurologic and neuropsychiatric disorders. However, basic principles of drug-target interactions do not provide a full understanding of drug action at the systems level because the targets are dynamic within and across individuals. That is, differences in drug action occur not only across individuals drawn from a single population but also across individuals exposed to different environmental histories. The current review examines relatively stable phenotypic differences and malleable social-based differences in the effects of abused drugs. Since the vast majority of literature in this area has examined either individual or social-based differences as main effects independent of each other, these factors are reviewed separately. However, recent work on gene × environment interactions (Caspi and Moffitt, 2006; Suomi, 2011) suggests that individual × social interactions need to be considered, even though little is known currently about these interactions.
In the case of individual differences, vulnerability to addiction, as defined by the risk for meeting the diagnostic criteria for substance use disorders (American Psychiatric Association, 2000), varies across individuals. That is, although many individuals experiment or initiate drug use early in life, most do not develop an abuse pattern that would meet the diagnostic criteria for substance use disorder, regardless of whether the drug is a stimulant, opiate, alcohol, or cannabis (Ellenbroek et al., 2005). Individual differences in vulnerability for abuse are thought to exist before the first drug experience, and they may relate, at least in part, to individual differences in sensitivity to drug reward (Haertzen et al., 1983). Individual differences in drug sensitivity are generally thought to be a consequence of differences in drug potency (Piazza et al., 2000).
In the case of social-based differences, psychosocial history and social circumstances encountered during the drug experience play prominent roles. Social interactions are among the basic needs that are essential for survival and reproductive success (Siviy and Panksepp, 2011), and these experiences are critical for the development of neural systems mediating reward and stress (Pedersen, 2004). Consequently, a history of neglect or deleterious social experiences can enhance sensitivity to rewarding and stressful events. Moreover, recent evidence shows the importance of social influence as a moderator of individual differences in response to drugs of abuse. In both humans and nonhuman animals, drugs that have an effect in one social context can be altered when administered in another social context (Gipson et al., 2011b; Varela and Pritchard, 2011).
In the current review, most cited studies involve highly controlled preclinical experiments that provide a mechanistic understanding of individual and social determinants for the rewarding effects of abused drugs. In these preclinical studies, we use the term reward as a general process that includes both Pavlovian and operant conditioning procedures, but we use the more specific term reinforcement when referring to operant responding. Before covering the preclinical literature, however, a brief presentation of some relevant clinical work is presented to set the stage for the more detailed review of preclinical studies. This initial section establishes the relevance of psychosocial factors in drug abuse vulnerability, but it is not intended to serve as a comprehensive review of all relevant studies. The review closes by offering some speculation about how this information may be useful for improving the development and implementation of preventive and treatment interventions. Future research directions are also suggested.
II. Human Behavioral Pharmacology
Studies of developmental trajectories of drug use provide clear evidence that initiation and escalation of drug use occur predominantly during adolescence and early adulthood. The most prominent view of this process is that drug use is a learned behavior influenced by the rewarding effects of abused drugs, with vulnerability being influenced by both individual differences and social factors occurring throughout development, as well as within the more proximal context of drug use (Swendsen and Le Moal, 2011). Whereas a comprehensive review of the role of various psychosocial factors on the acquisition of human drug-taking behavior is beyond the scope of this review, examples of well established individual differences and social factors that impact drug abuse vulnerability are described to establish the clinical relevance of basic preclinical research in this area.
A. Individual Differences
Studies examining vulnerability to drug abuse as a function of the degree of genetic relations among individuals (i.e., heritability studies) have established an important role of genetics (Koob and Le Moal, 2006). Vulnerability to the development of drug and alcohol dependence varies with the degree of shared inheritance (i.e., identical twins have higher concordance rates than fraternal twins, even when controlling for shared environmental influences). Recent molecular biology studies have begun to identify which genes contribute to vulnerability to drug abuse, as well as their mechanisms of action (Goldman et al., 2005), with genetic influences on the neurobiological processes mediating drug sensitivity playing a critical role in the acquisition of drug-taking behavior and abuse (Comer et al., 2010). However, it is equally important to acknowledge the independent and interactive role of environmental influences on drug abuse vulnerability (Swendsen and Le Moal, 2011). Recent developments in epigenetics, for example, have established mechanisms by which environmental experience can modify genetic expression (Rutter et al., 2006; Maze and Nestler, 2011).
1. Novelty Seeking
One of the most critical individual difference factors predicting drug use among humans is novelty seeking or sensation seeking (Kosten et al., 1994; Zuckerman, 1994; Wills et al., 1998; Ball, 2004). Zuckerman (1994) defines sensation seeking as a trait defined by the seeking of varied, novel, complex, and intense sensations and experiences and the willingness to take physical, social, legal, and financial risks for the sake of such experiences. Although drug use can increase sensation seeking (Ersche et al., 2010), longitudinal results also indicate a direct path leading from sensation seeking to initiation (Horvath et al., 2004). Adolescent sensation seekers are at increased risk for use of various drugs, including alcohol, tobacco, and marijuana (Martin et al., 2002, 2004; Sargent et al., 2010). Young adult high sensation seekers also are more sensitive to the effects of drugs (self-administration, positive subjective effects) than are low sensation seekers under controlled laboratory conditions (Stoops et al., 2007; Perkins et al., 2008; Fillmore et al., 2009; Kelly et al., 2009), suggesting that this facet is present before problem use. Neurobiological studies indicate that the underlying neurocircuitry associated with sensation seeking involves, at least in part, the nucleus accumbens. Human neuroimaging studies indicate that amphetamine produces the greatest dopamine release in the nucleus accumbens among high sensation seekers (Leyton et al., 2002), although this effect may be gender specific (Riccardi et al., 2006). When viewing highly arousing images, high sensation seekers show enhanced activation in regions involved in emotional induction and reward, as well as reduced activation of regions involved in emotional regulation (Joseph et al., 2009). Thus, brain systems altered by exposure to salient stimuli share a common link with drug reward, which may mediate the association between sensation seeking and drug use.
2. Impulsivity
Another individual difference factor predicting drug use among humans is impulsivity, defined broadly as the tendency to engage in premature, inappropriate, or maladaptive behavior without foresight (e.g., Dalley et al., 2011). Although impulsivity is a broadly defined biologically based trait that appears in most major theories of personality, it can be parsed into different facets (Whiteside and Lynam, 2001). Impulsivity reflects the balance of two independent behavioral processes: 1) approach or activation associated with reinforcement and 2) inhibition associated with punishment, with impulsive individuals exhibiting hyperactivation or hypoinhibition (e.g., Bechara, 2005). Neuroimaging studies have confirmed the interplay between reinforcement pathways (ventral striatum/nucleus accumbens and orbitofrontal, dorsal, and lateral regions of the prefrontal cortex) and inhibitory pathways (amygdala, insula, anterior cingulate, right inferior frontal gyrus, subthalamic nucleus, and supplementary motor areas of the prefrontal cortex) associated with impulsivity and drug abuse vulnerability (Nigg et al., 2006; Aragues et al., 2011; Dalley et al., 2011; Hommer et al., 2011). Impulsive individuals, determined using either personality- or performance-based criteria, initiate drug use at earlier ages, escalate to heavy use, and transition to abuse and dependence more quickly and are less likely to remain abstinent after treatment compared with nonimpulsive individuals (de Wit, 2009; Dick et al., 2010; Dalley et al., 2011). Longitudinal studies of early childhood temperament indicate that the association between impulsivity and vulnerability to drug abuse is present at an early age (Zucker et al., 2008; Chartier et al., 2010). Thus, individuals who are high in impulsivity are likely to engage in a variety of risky behaviors, including drug use, and are more sensitive to the reinforcing and other pharmacodynamic effects of drugs after initial use, thereby making them more inclined to continue and escalate drug use.
B. Social Influences
Social context impacts drug abuse vulnerability in at least two ways. First, social context associated with childhood development (e.g., family social context, peer relationships) influences drug abuse vulnerability. Second, the social context in which drug use occurs also influences the functional effects of drugs of abuse, thereby impacting directly the acquisition of drug-taking behavior and thus vulnerability to drug abuse.
1. Social Experiences during Development
Environmental experiences during early development impact the brain directly or modify the genetic developmental processes through gene-environment interactions (Zucker et al., 2008). Low prosocial family interaction, for example, as well as peer deviance and drug use, are risk factors for the initiation of alcohol use during childhood (Oxford et al., 2001; Rose et al., 2001). Other social context factors influencing alcohol use by children include alcohol use by parents and exposure to alcohol through the media. Peer influence on alcohol and drug use becomes more salient among older adolescents (ages 11–13). Peers influence use through three pathways: 1) direct modeling of use; 2) self-sustaining affiliation with like-minded peers; and 3) overestimation of peer drug use (Windle et al., 2008; Chartier et al., 2010). Adolescents reporting high rates of drug use also report lower levels of social support from family and peers, whereas adolescents with heavy alcohol use actually report higher social support from friends. Recent studies link social environment with drug abuse vulnerability through underlying neurobiological mechanisms in that dopamine D2/3 receptor binding efficacy, which is associated with the reinforcing effects of drugs, is correlated with both social status and perceived level of social support and inversely correlated with social detachment (e.g., Schneier et al., 2000; Martinez et al., 2010).
2. Social Context during Drug Use
Drug use is a learned behavior driven in large part by the functional effects of the drug. As mentioned earlier, the functional effect of a drug is not intrinsic to the pharmacology of the drug, but rather it reflects an interaction between pharmacology and the context in which drug is available (Hughes et al., 1988). The social environment can play a prominent role in modulating the functional effects of abused drugs. One conceptual model for social context as a determinant of the functional effects of drugs is based on the manner in which drug use sets the occasion for access to social opportunities (Falk, 1983). This model postulates that drug use becomes a requirement for affiliation with selective groups, and intoxication becomes a “time-out” from social rules, which permits individuals to engage in behaviors not otherwise acceptable. Subsequent studies provide support for this model. For example, clinical laboratory studies show that stimulant and sedative drugs increase verbal behavior and social interaction (Stitzer et al., 1981; Higgins and Stitzer, 1986; Ward et al., 1997), suggesting that the reinforcing effects of a drug are increased when there is a pharmacologically induced enhancement of social interaction (i.e., the cocktail-party phenomenon). This possibility has been examined by testing the effects of amphetamine, secobarbital, and marijuana on talking behavior and preference for social interaction among healthy volunteers (Heishman and Stitzer, 1989). Relative to placebo, both amphetamine and secobarbital increased rates of talking, as well as preference for social interaction (i.e., increased the reinforcing efficacy of social interaction). In comparison, marijuana had little effect on talking rate or preference for social interaction.
Drug-taking behavior can also be altered by the social consequences of drug use. In an early series of residential laboratory studies, Bigelow and colleagues demonstrated that time-out from social interaction resulted in decreased alcohol self-administration among heavy alcohol users (Griffiths et al., 1974, 1977). These early studies helped to establish the importance of social access as a primary motive for alcohol and drug use (Cooper, 1994). The possible role of negative reinforcement (e.g., drug modulation of negative socially induced mood states such as social anxiety) also is a likely determinant of drug abuse vulnerability (DeMartini and Carey, 2011). Despite these behavioral results, little is known currently about the neural factors that explain why social interaction alters the behavioral effects of abused drugs.
C. Summary
Clinical evidence demonstrates that individual differences present before the first drug experience modulate sensitivity to drug reward. These differences are thought to reflect both genetic and environmental determinants. Similarly, clinical evidence suggests that social influences can enhance drug use. However, there is limited information about the precise neural mechanisms underlying these individual and social differences in drug sensitivity in humans. In the next section, preclinical evidence is reviewed to address the neurobehavioral factors involved in individual differences and social influences in drug reward.
III. Preclinical Behavioral Neuropharmacology
Paralleling the work described in humans, there is no doubt that drug use in laboratory animals involves both genetic and environmental factors. Selective breeding, recombinant inbred lines, knock-out, knock-in, and gene silencing techniques are powerful tools for examining genetic heritability of individual differences in drug use. For example, selectively bred and recombinant inbred rats have been used to demonstrate that individual differences in response to novelty or preference for novelty are associated with individual differences in stimulant self-administration (Meyer et al., 2010; Cummings et al., 2011). Genetic influences also play a role in social behaviors, as illustrated by work showing the influence of serotonin (5-HT) transporter polymorphisms on social intrusion in nonhuman primates (Schwandt et al., 2010). The specific socially relevant genes and transgenic processes are extensive and are not covered in the current review. However, this work sets the stage for studying the neurobehavioral mechanisms involved in key individual differences and social influences underlying vulnerability to drug abuse. For each individual difference described in the next section, the following points are covered: 1) measurement of the individual difference, 2) relation of the individual difference to the psychostimulant and rewarding effects of abused drugs, and 3) the neurobiological mechanisms involved.
A. Individual Differences
1. Response to Novelty
The most reliable individual difference predicting the psychostimulant and reinforcing effect of drugs is the novelty “responder” test characterized initially by Piazza et al. (1989) and subsequently examined across many laboratories (Kabbaj, 2006). Rats are classified as high (HRs) or low responders (LRs) based on the amount of ambulatory activity recorded in an inescapable novel environment. The test is relatively brief, lasting 60 min or less, and rats are categorized as HR or LR based on a median split analysis. Since the initial report by Piazza et al. (1989), several studies have confirmed that HR rats show increased amphetamine-induced activity (Bevins et al., 1997) and amphetamine self-administration (Piazza et al., 1990, 2000; Pierre and Vezina, 1997; Klebaur et al., 2001a; Cain et al., 2005, 2006, 2008) compared with LR rats. The differences between HR and LR rats in amphetamine self-administration are similar for both males and females (Klebaur et al., 2001a), and these individual differences generalize to other stimulant drugs, including cocaine (Mantsch et al., 2001; Sell et al., 2005; Kabbaj, 2006; Belin et al., 2008, 2011; Walker et al., 2009) and methamphetamine (Bevins and Peterson, 2004; Gancarz et al., 2011). Nicotine self-administration also is greater in HR rats than in LR rats (Suto et al., 2001), although these individual differences may not occur in nicotine-induced hyperactivity (Coolon and Cain, 2009). Although individual differences have been reported for maintenance across various unit doses in cocaine self-administration (Piazza et al., 2000), they are most influential for acquisition at low unit doses (0.25 mg/kg per infusion; Mantsch et al., 2001), suggesting that HR/LR differences may be most closely associated with sensitivity to drug reinforcement. The HR/LR difference probably is due, at least in part, to genetic factors because inbred lines possessing the HR and LR phenotypes also display differences in the psychostimulant and reinforcing effects of stimulants (Gingras and Cools, 1997; Davis et al., 2008; Turner et al., 2008; Cummings et al., 2011).
A recently developed variation of the Piazza responder test was developed by Zahniser and colleagues (Gulley et al., 2003). In this test, rats are categorized as HR or LR based on their locomotor response to acute cocaine. This individual difference is linked to dopamine transporter (DAT) function (Sabeti et al., 2003; Briegleb et al., 2004) and cocaine CPP (Allen et al., 2007). However, in contrast to the HR/LR test, high cocaine responders do not differ from low cocaine responders in acquisition of cocaine self-administration (Mandt et al., 2008, 2012).
One apparent exception to the general finding that HR rats are more sensitive than LR rats to the locomotor and reinforcing effects of stimulants has been reported for methylphenidate. In the only study to date, Wooters et al. (2006) reported that male and female HR/LR rats do not differ in locomotor activity after acute methylphenidate during either adolescence or young adulthood. Although this conclusion from a single study requires confirmatory work, methylphenidate differs from cocaine and amphetamine by its greater potency at the dopamine and norepinephrine transporters relative to the 5-HT transporter (Han and Gu, 2006). This suggests the possibility that differences in 5-HT transporter function may play a role in the difference between HRs and LRs. Consistent with this possibility, tissue concentration of 5-HT is reduced in HR prefrontal cortex (Thiel et al., 1999). Further, using the conditioned place preference (CPP) paradigm in which rats are allowed to choose between two contexts paired previously with either drug or saline (Rossi and Reid, 1976), a 5-HT2C antagonist differentially affects cocaine reward in HR and LR rats (Capriles et al., 2012) .
Differences between HR and LR rats also occur among drugs outside the stimulant class. With opiates, HR rats show enhanced morphine locomotion and self-administration (Deroche et al., 1993; Kalinichev et al., 2004). These effects are modulated by social context, as the influence of social crowding on morphine hyperactivity is evident in HR rats but not LR rats (Xigeng et al., 2004). The locomotor effects of cannabinoids and alcohol also are enhanced in HR rats compared with LR rats (Galanopoulos et al., 2011). With alcohol, oral intake is enhanced in HR rats using an FR3 schedule of reinforcement (Nadal et al., 2002) but not using either a free-access two-bottle test or an FR1 schedule of reinforcement (Bisaga and Kostowski, 1993; Gingras and Cools, 1995; Bienkowski et al., 2001; Hayton et al., 2012), suggesting that HR rats are more motivated than are LR rats to earn alcohol under high-effort schedules. Other than alcohol, however, little is known about HR and LR differences in response to anxiolytic drugs. This is a notable gap in information because individual differences in anxiety (or novelty seeking) based on the elevated plus maze predict cocaine self-administration, with low-anxiety rats showing enhanced responding for cocaine (Bush and Vaccarino, 2007). Nonetheless, because both low-anxious and HR rats show an increased propensity to self-administer cocaine, these findings appear to rule out the possibility that the elevated activity in HR rats simply reflects enhanced anxiety in the inescapable novel environment.
In addition to abused drugs, individual differences in the novelty responder test are observed with nondrug reinforcers such as palatable food. Operant responding using sucrose pellet reinforcers is greater in HR rats than in LR rats (Dellu et al., 1996; Klebaur et al., 2001a; Cain et al., 2006), although this effect may not generalize to less palatable reinforcers such as a standard food diet (Gulley, 2007). The ability of individual differences to predict responding for both drug and palatable nondrug reinforcers opens the possibility that individual differences exist in learning generally, rather than drug sensitivity specifically (Mitchell et al., 2005). Alternatively, because both drug and nondrug reinforcers involve overlapping neurocircuitry (Kelley and Berridge, 2002), these individual differences may be associated with common neural systems. Although there is little information to address this issue directly, Xu et al. (2001) found that individual differences in performance in either the Morris water or Y maze are not associated with the magnitude of morphine CPP. Thus, individual differences in general learning do not account readily for HR and LR differences in behaviors reinforced by drug or palatable food.
In addition to food reinforcement, HR rats show greater responding for novel visual stimulation. For example, maintenance of amphetamine self-administration is disrupted by novel stimuli, and this disruption is greater in HR rats than it is in LR rats (Cain et al., 2004). HR rats also show more responding than LR rats to earn novel visual stimuli (cue light illumination; Gancarz et al., 2011). These latter results are important because drug self-administration studies often use cue light illumination to signal the drug infusion or a time-out period (no drug availability) after the infusion. The enhanced responding for visual stimuli observed in HR rats also may have implications for cue-elicited effects associated with drug, such as conditioned reinforcement and cue-induced relapse. Thus, it is important to determine whether the differences between HR and LR rats reflect primary reinforcement associated with the drug or the cue.
One somewhat puzzling feature of the novelty responder test is that it does not reliably predict drug-induced CPP. With cocaine CPP, HR rats have been reported to be less sensitive or equally sensitive compared with LR rats (Erb and Parker, 1994; Dellu et al., 1996; Kosten and Miserendino, 1998; Shimosato and Watanabe, 2003; Mathews et al., 2010). The lack of difference between HR and LR rats may be specific to cocaine, however, as HR rats are more sensitive to amphetamine and morphine CPP (Zheng et al., 2003, 2004; Pelloux et al., 2004). Thus, individual differences may predict only the direct primary reinforcing effect of cocaine as measured by self-administration but not the conditioned rewarding effect as measured by CPP (Bardo and Bevins, 2000).
Regarding the neural mechanisms mediating HR and LR differences (Fig. 1), a general view is that an ascending mesolimbic dopamine projection emanating from the midbrain ventral tegmental area to the nucleus accumbens via the medial forebrain bundle represents one important component of the neural circuitry, thus overlapping with drug reward-relevant circuitry (Wise and Rompre, 1989; Bardo et al., 1996; Berridge and Robinson, 1998; Kelley and Berridge, 2002). Although experimenter-delivered electrical stimulation of the medial forebrain bundle produces similar reward in HR and LR rats (Antoniou et al., 2004), basal firing of midbrain dopamine neurons is enhanced in HR rats, perhaps as a result of subsensitivity to impulse-regulating autoreceptors (Marinelli and White, 2000). HR rats also show more persistent mesoaccumbal impulse flow after withdrawal from cocaine self-administration (McCutcheon et al., 2009). Several downstream cellular changes also have been identified, including greater extracellular dopamine in nucleus accumbens and striatum (Piazza et al., 1991b; Hooks et al., 1992; Thiel et al., 1999), greater velocity of dopamine uptake in nucleus accumbens (Chefer et al., 2003), and greater mRNA levels for tyrosine hydroxylase and dopamine D1 receptors (Saigusa et al., 1999). Additionally, HR rats have a reduced density of accumbal dopamine D2 receptors (Hooks et al., 1994b), suggesting a decreased number of release-regulating autoreceptors or a compensatory downregulation of postsynaptic receptors in response to increased presynaptic dopamine release.
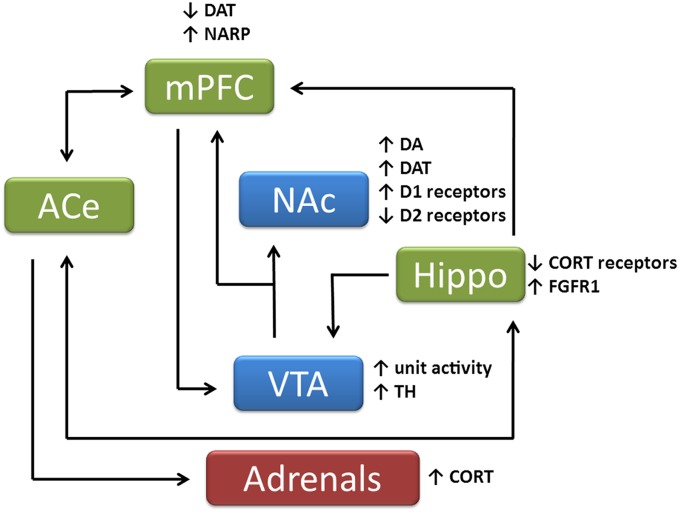
Fig. 1.
Schematic of brain changes in HR rats compared with LR rats. Regions in blue represent primarily reward-relevant central structures, regions in green represent primarily stress-related central structures, and region in red is a peripheral stress-related gland. Brain regions: ACe, central nucleus of amygdala; Hippo, hippocampus; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; VTA, ventral tegmental area. Cellular changes: CORT, corticosterone; DA, dopamine; DAT, dopamine transporter; NARP, neuronal activity-regulated pentraxin; FGFR1, fibroblast growth factor receptor 1; TH, tyrosine hydroxylase.
Individual differences in reward neurocircuitry also extend to the mesocortical dopamine system. HR rats have decreased dopamine transport function in the medial prefrontal cortex (Kosten et al., 2005b), which presumably results in increased basal extracellular dopamine, similar to the results obtained in nucleus accumbens. A role for the dopamine transporter also is implicated by HR rats showing greater locomotor activity after the DAT inhibitor GBR12909 compared with LR rats (Hooks et al., 1994a). The immediate early gene Narp is enhanced in prefrontal cortex of HR rats (Lu et al., 2002). Since prefrontal cortical regions are important for behavioral inhibition (Fuster, 2008), these cellular differences may be involved in the disinhibited responding observed in HR rats using a differential reinforcement of low rate (DRL) schedule (Stoffel and Cunningham, 2008). In a DRL schedule, reinforcement is contingent on the occurrence of a response separated from the preceding response by a fixed time (e.g., 10 s), thus providing a measure of inhibitory control during timed behavior (Hodos et al., 1962).
The prefrontal cortex interconnects with amygdala and hippocampal circuitry involved in affective disorders, fear conditioning, and output modulation of the hypothalamic-pituitary-adrenal (HPA) axis (Ressler and Mayberg, 2007; Rodrigues et al., 2009). Activation of the HPA axis involves triggering corticotrophin-releasing hormone (CRH) from the hypothalamus, which elicits release of adrenocorticotropic hormone (ACTH) from the pituitary and subsequently elevates corticosterone from the adrenals (Sapolsky et al., 2000). The neurocircuitry involving hippocampal-prefrontal-amygdala interconnections with the HPA axis may play a role in individual HR and LR differences. Whereas basal levels of corticosterone do not differ between HR and LR rats (Piazza et al., 1991a; Mantsch et al., 2001), HR rats have elevated corticosterone in response to novelty and after repeated cocaine relative to LR rats (Piazza et al., 1990; Dellu et al., 1996; Lucas et al., 1997). These results implicate a differential HPA sensitivity between HR and LR animals.
Blocking different components of the HPA axis can reduce the differences between HR and LR rats. For example, adrenalectomy reduces HR/LR differences in morphine-induced hyperactivity (Deroche et al., 1993). With regard to the hippocampal-prefrontal-amygdala circuitry, inactivation of the central nucleus of the amygdala with the GABAA agonist muscimol also reduces the individual differences in amphetamine self-administration and locomotor sensitization (Cain et al., 2008, 2009). Whereas direct blockade of hippocampal or prefrontal activity has not been examined directly in HR and LR rats, affinity of hippocampal type I and II corticosteroid receptors is decreased in HR rats (Maccari et al., 1991), likely because of a compensatory response to excessive levels of corticosterone. HR rats also have increased fibroblast growth factor expression in hippocampus compared with LR rats (Turner et al., 2008), although this difference is negated by repeated cocaine. This last study indicates that individual differences interact with cocaine to regulate gene expression in stress-relevant brain systems.
Since learning may be enhanced when glucocorticoids are elevated to some optimal level (de Quervain et al., 2009), novelty- or drug-induced elevations in corticosterone in HR rats may increase drug self-administration as a result of enhanced learning of the operant response. However, as indicated previously, HR rats do not show superior performance over LR rats when reinforced with a standard food diet (Gulley, 2007) or when tested in nonoperant tasks (Xu et al., 2001), suggesting that the increase in drug self-administration in HR rats does not simply reflect improved learning. Instead, corticosterone may have direct reinforcing effects alone (Piazza et al., 1991a, 1993), as well as potentiating the reinforcing effects of stimulant drugs (Piazza et al., 1991a; Goeders, 2002). The ability of corticosterone to potentiate drug reinforcement likely reflects an interaction with the mesolimbic dopamine system (Gilad et al., 1987; Piazza and Le Moal, 1997).
In summary, individual differences in activity in an inescapable novel environment is a reliable predictor of the stimulant and reinforcing effects of abuse drugs, with HR rats showing greater sensitivity than LR rats. This relation exists across each major drug class. HR rats also show greater sensitivity to the reinforcing effects of palatable food (sucrose) and novel visual stimuli. At least two overlapping neural systems play a role in the behavioral response to drugs in HR rats compared with LR rats: 1) the reward-relevant mesolimbic dopamine system and 2) the stress-relevant HPA system regulated by a hippocampal-prefrontal-amygdala loop.
2. Novelty Seeking
Although the novelty responder test used to define HR and LR animals has sometimes been referred to as a measure of “novelty seeking,” this is inappropriate because it is unclear whether activity in an inescapable novel environment reflects either exploratory or escape behavior. Instead, novelty seeking may be better measured as a preference for a novel context compared with a familiar context, a measure that is not correlated reliably with activity in the novelty responder test (Pelloux et al., 2004). Thus, novelty seeking should be defined by giving animals a choice to either approach or avoid novelty.
The most common method used to measure individual differences in novelty seeking in rats is the novelty place preference test. Rats are first habituated to one distinct context of a CPP apparatus by being placed in a side compartment for one or more sessions, typically lasting 30 min each. Rats then are given free-choice access to the entire apparatus and are categorized as either high or low novelty seekers based on their preference for the novel compartment relative to the familiar compartment (Hughes, 1968; Parker, 1992; Bardo et al., 1993). A variation of this method is to allow rats a choice between novel or familiar objects. This can be accomplished using either two objects (one novel and one familiar; Ennaceur and Aggleton, 1997) or multiple objects (one novel object and several familiar objects; Nicholls et al., 1992). Regardless of whether a two-object or a multiple-object test is used, it is critical that rats are habituated to the context in which the objects are presented. Thus, the only novel feature on the test day is a single object.
In mice, a holeboard test also can be used to measure individual differences in novelty seeking. In this test, mice are placed in an open-field apparatus that has a series of holes in the floor. The number of head dips is used to measure novelty seeking (File and Wardill, 1975; Kliethermes and Crabbe, 2006). Although this test has some features reminiscent of the novelty responder test (i.e., it is inescapable), it is interpreted typically as a measure of novelty seeking, and thus it is included here.
Operant conditioning procedures also are useful for measuring individual differences in novelty seeking. Some classic work has shown that primates and rodents will emit an operant response to obtain access to novel stimuli (Marx et al., 1955; Fiske and Maddi, 1961). More recently, operant conditioning measures of novelty seeking have been used to predict individual differences in sucrose self-administration in mice (Olsen and Winder, 2010). This measure of novelty seeking overlaps to some extent with the novelty responder test, as HR rats respond more for a novel visual reinforcer compared with LR rats (Gancarz et al., 2011).
Compared with the novelty responder test of Piazza et al. (1989), the predictive relation between individual differences in novelty seeking and drug reward is weaker (Table 1). Some reports show that high novelty seekers are more sensitive than low novelty seekers to the locomotor and reinforcing effects of amphetamine (Bevins et al., 1997; Cain et al., 2005, 2006) and oral nicotine self-administration (Abreu-Villaca et al., 2006). However, other reports show no significant relation between novelty seeking and stimulant activity induced by amphetamine or methylphenidate (Klebaur and Bardo, 1999; Wooters et al., 2006) or self-administration of amphetamine (Klebaur et al., 2001a; Marusich et al., 2011), and the effects with oral alcohol self-administration are mixed (Bienkowski et al., 2001; Johansson and Hansen, 2002). The effects with CPP are also mixed, as individual differences in novelty seeking predict amphetamine CPP in some studies (Klebaur and Bardo, 1999; Robinet et al., 1998), but not in all studies (Pelloux et al., 2004). Given the range of methodological differences among studies, it is difficult to ascertain the critical variables that may explain these mixed findings. However, most of these studies examined only acquisition and/or maintenance of behavior. In contrast, recent evidence indicates that novelty seeking may be a better predictor of compulsive self-administration (persistence of responding in the absence of drug) compared with acquisition of self-administration (Belin et al., 2011). “Compulsivity” is defined operationally by various procedures, including 1) persistent responding when a cue signaling the drug is no longer available; 2) high progressive ratio (PR) breakpoints, determined by increasing the response requirement after each reinforcer delivery until there is a cessation of responding; and 3) resistance to punishment (Belin et al., 2011). To the extent that compulsivity models dysfunctional drug abuse, these results suggest that novelty seeking may be useful for identifying individuals at greatest risk.
TABLE 1.
Representative studies showing relation between novelty seeking and drug reward
| Reference | Animal | Predictor Variable | Outcome Variable | Drug | Results |
|---|---|---|---|---|---|
| Stimulants | |||||
| Abreu-Villaca et al. (2006) | Female and male C57/BL/6 mice | Head dips in hole board | 2-Bottle choice | Nicotine (0.01 mg/ml p.o.) | HiNS > LoNS |
| Belin et al. (2011) | Male Sprague-Dawley rats | Novel place preference | SA on FR1 | Cocaine (0.8 mg/kg i.v.) | HiNS =LoNS (acquisition) HiNS > LoNS (compulsivity) |
| Cain, et al. (2005) | Male Sprague-Dawley rats | Novel place and novel object preference | SA on FR5 | Amphetamine (0.01–0.1 mg/kg i.v.) | HiNS > LoNS (regression Analysis) |
| Klebaur and Bardo (1999) | Male Sprague-Dawley rats | Novel object preference | CPP | Amphetamine (1–3 mg/kg s.c.) | HiNS > LoNS (1 mg/kg) |
| Klebaur et al. (2001a) | Female and male Sprague-Dawley rats | Novel place and novel object preference | SA on FR5 | Amphetamine (0.03–0.16 mg/kg i.v.) | HiNS =LoNS |
| Marusich et al. (2011) | Male Sprague-Dawley rats | Novel place preference | SA on FR5 and PR | Amphetamine (0.0056–0.1 mg/kg i.v.) | HiNS > LoNS (linear mixed model) |
| Pelloux et al. (2004) | Male Wistar rats | Novel place preference | CPP and SA | Amphetamine CPP (1.25–5 mg/kg i.p.) Amphetamine SA (10-50 mg/ml p.o.) | HiNS =LoNS (CPP) HiNS > LoNS (SA of 15 mg/ml) |
| Robinet et al. (1998) | Male Sprague-Dawley rats | Novel place preference | CPP | Amphetamine (0.4 mg/kg s.c.) | HiNS > LoNS |
| Vidal-Infer et al. (2012) | Female and male OF1 mice | Novel place preference | CPP | Cocaine (1 mg/kg i.p.) | HiNS > LoNS |
| Opiates | |||||
| Pelloux et al. (2006) | Male Wistar rats | Novel place preference | CPP and 2-bottle choice | Morphine CPP (1.25–5 mg/kg i.p.) Morphine 2-bottle (25-50 mg/ml p.o.) | HiNS > LoNS (CPP with 5 mg/kg) HiNS > LoNS (choice of 25 mg/ml) |
| Alcohol | |||||
| Bienkowski et al. (2001) | Male Wistar rats | Novel object preference | SA on FR1 | Alcohol (8% p.o.) | HiNS =LoNS |
HiNS, high novelty seeker; LoNS, low novelty seeker; SA, self-administration.
Genetic models of novelty seeking yield mixed effects on drug self-administration. In one study, mice bred selectively for novelty seeking using the holeboard test showed no differences in response to amphetamine activity, CPP, or oral self-administration of methamphetamine or alcohol (Kliethermes et al., 2007). In contrast, another study using inbred rat strains showed a relation between novelty seeking using the place preference test and i.v. amphetamine self-administration (Meyer et al., 2010); no strain-dependent differences were observed using the novelty responder test. These discrepant findings do not likely relate to the different routes of administration, as novelty seeking in the place preference test also predicts oral amphetamine self-administration in rats (Pelloux et al., 2004). Instead, the discrepant results more likely reflect a difference in species (mouse versus rat) and/or novelty test (holeboard versus place preference) used between studies.
Further insight regarding the differences between the novelty responder and novelty seeking tests were revealed by Cain et al. (2005). In that report, a large number of rats (n =165) were tested in both the novelty responder and novelty place preference tests before being trained in amphetamine self-administration across different FR schedules of reinforcement; the large sample size afforded the opportunity to apply analytic techniques normally reserved for large-sample human studies. A hierarchical regression analysis of these preclinical results indicated that the novelty responder test was a better predictor than the novelty seeking test for acquisition of amphetamine self-administration. However, when each variable was entered sequentially into the regression, novelty seeking significantly improved the predictive power of the novelty responder test. These latter results indicate that novelty seeking is not redundant with the novelty responder test and that individual differences in novelty seeking contribute to drug self-administration.
Individual differences in novelty seeking also play a role in the relation between reward cues and drug self-administration. Rats showing the greatest approach to food-associated cues (“sign trackers”) show the greatest novelty seeking (Beckmann et al., 2011) and cocaine self-administration (Beckmann et al., 2011; Saunders and Robinson, 2011). In contrast, no relation is evident between sign tracking and the HR/LR test, suggesting that novelty seeking may mediate uniquely the relation between approach to reward-related cues and cocaine reinforcement.
From a neurobehavioral perspective, one reason why novelty seeking may be a weaker predictor of drug self-administration than the HR/LR novelty responder test is that it is mediated by only a portion of the circuitry outlined in Fig. 1. Specifically, novelty seeking may differ from the novelty responder test because it involves primarily the mesolimbic dopamine system (Bardo et al., 1996), rather than the stress axis that modulates drug reward (Piazza and Le Moal, 1997). Consistent with this, inescapable novelty, but not free-choice novelty, elevates levels of corticosterone (Misslin et al., 1982).
Considerable evidence indicates that free-choice approach to novelty activates directly the reward-relevant mesocorticolimbic dopamine circuitry. Novelty place preference is blocked by dopamine antagonists (Misslin et al., 1984; Bardo et al., 1993) and by depleting dopamine levels in nucleus accumbens and forebrain with the neurotoxin 6-hydroxydopamine (Pierce et al., 1990). When rats enter a novel compartment, there is a rapid, transient surge in extracellular accumbal dopamine measured by in vivo voltammetry (Rebec et al., 1997a, 1997b), as well as a novelty-induced response in accumbal single-unit electrophysiological activity (Wood and Rebec, 2004). Thus, high novelty seekers may show enhanced drug reward as a result of greater activation of mesocorticolimbic dopamine systems.
Monoamines other than dopamine also play a role in novelty seeking. For example, accumulating evidence indicates that norepinephrine activity in the hippocampus is involved in novelty signal detection (Knight, 1996). Although no direct neural connections have been found between the ventral tegmental area and the hippocampus, the hippocampus sends projections to the medial prefrontal cortex, nucleus accumbens, amygdala, and septal area, which relay input to the ventral tegmental area (Floresco and Grace, 2003; Lisman and Grace, 2005; Luo et al., 2011). Similar to the novelty-induced increase in mesocorticolimbic dopamine, exposure to novel environmental stimuli increases the concentration of extracellular norepinephrine in the frontal cortex assessed by in vivo microdialysis (Feenstra et al., 2000), which presumably reflects an increased impulse flow of neurons in the locus coeruleus. In any case, these results indicate that the ventral tegmental area acquires information about stimulus novelty via circuitry involving the prefrontal cortex and hippocampus, sites for memory storage and novelty detection.
Both 5-HT and glutamate systems are also implicated in novelty seeking. The 5-HT1A agonist 8-OHDPAT produces a dose-dependent decrease in novel object exploration in rats, whereas the 5-HT1A antagonist WAY-100635 produces an increase in novel object exploration (Carey et al., 2008). It is important to note that these 5-HT-mediated effects are obtained without any change in locomotor activity, thus demonstrating that the effects are specific for approach to novelty rather than general exploratory behavior. Similarly, a role for glutamate has been illustrated in rats conditioned to approach an environmental context paired previously with a novel object (Bevins and Bardo, 1999). The noncompetitive NMDA antagonist MK-801 given during the conditioning phase blocks this effect, thus implicating a role of NMDA receptors in learning produced by stimulus novelty. Given the importance of glutamate-dopamine interactions in drug reinforcement and addiction (Kalivas, 2009), a more complete investigation of glutamatergic systems mediating novelty seeking is warranted.
In summary, although individual differences in novelty seeking measured in a free-choice preference test are not as strongly predictive of drug self-administration as individual differences in inescapable novelty (HR/LR test), they predict drug self-administration using a large sample size and may be especially predictive of compulsive drug self-administration. However, based on a lack of information, it is unclear whether these conclusions generalize beyond the stimulant class. In any case, in contrast to the two overlapping neural systems (reward and stress) involved in inescapable novelty, the reward-relevant mesolimbic dopamine system is associated primarily with individual differences in novelty seeking.
3. Impulsivity
Impulsivity is a broad psychologic construct that appears in virtually all major theories of personality (Whiteside and Lynam, 2001), being incorporated into various psychiatric diagnoses such as anxiety and bipolar mood disorders, as well as conduct disorder, attention deficit hyperactivity disorder, and substance use disorders (American Psychiatric Association, 2000). With preclinical models, although the term disinhibition may be more appropriate because it connotes a task-specific deficit or loss of an active neurobehavioral process (inhibition), we use the more commonly used broad term impulsivity. Individual differences among laboratory animals in impulsivity are measured by a host of behavioral tasks, including delay discounting, fixed consecutive number, five-choice serial reaction time task (5-CSRTT), go/no-go, DRL, and stop-signal reaction time. A comprehensive coverage of these tasks is provided in several excellent reviews (Evenden, 1999; Winstanley et al., 2010; Dalley et al., 2011). It is important to note that there is little relation in performance among these various tasks (Anker et al., 2009; Marusich et al., 2011), suggesting that each task measures a different facet of impulsivity. One nomenclature is to parse impulsivity tasks into three broad categories: 1) impulsive choice, which is primarily decision making when choosing between a small immediate reward and a larger delayed reward; 2) impulsive action, which is primarily motoric; and 3) impulsive reflection, which is premature responding before adequate sensory processing (Dalley et al., 2011). Regardless of the category, individual differences in impulsivity do not correlate with individual differences in either the novelty responder test or novelty seeking (Bardo et al., 2006; Marusich et al., 2011; Molander et al., 2011), thus implicating dissociable neurobehavioral systems.
Among the various tasks, those categorized as measures of impulsive choice using a delay of reward are linked most closely to drug self-administration (Table 2). Perhaps the best example is the delay discounting task, which allows animals to choose between an immediate small reward and a delayed large reward, with impulsivity being defined as a preference for the small immediate reward (Rodriguez and Logue, 1988). When screened initially on delay discounting, rats that are high in impulsivity show faster acquisition, escalation, and reinstatement of self-administration with cocaine (Perry et al., 2005, 2008; Anker et al., 2009), methylphenidate (Marusich and Bardo, 2009), nicotine (Diergaarde et al., 2008, 2012), morphine (Garcia-Lecumberri et al., 2011), and alcohol (Poulos et al., 1995). The predictive effect of delay discounting generalizes to amphetamine CPP (Yates et al., 2012), indicating that lever pressing is not a prerequisite to show a relation between individual differences in impulsive choice and drug reward. In contrast, this last study did not find any relation between impulsive choice and amphetamine-stimulated activity. Similarly, rats that are high or low in impulsive choice do not differ in cocaine-induced hyperactivity (Perry et al., 2005), and mice that are high in impulsivity are less sensitive to the hyperactivity produced by acute alcohol (Mitchell et al., 2006). Thus, impulsive choice specifically predicts the reinforcing effect of abused drugs rather than a nonspecific alteration in ongoing behavior.
TABLE 2.
Representative studies showing relation between impulsivity and drug reward
| Reference | Animal | Predictor Variable | Outcome Variable | Drug | Results |
|---|---|---|---|---|---|
| Stimulants | |||||
| Anker et al. (2009) | Female Wister rats | Delay discounting | SA on FR1 and PR | Cocaine (0.2–0.8 mg/kg i.v.) | HiI > LoI (escalation) |
| Bird and Schenk (2012) | Male Sprague-Dawley rats | 5-CSRTT | SA on FR5 | MDMA (1.0 mg/kg i.v.) | HiI =LoI (acquisition) HiI > LoI(reinstatement) |
| Broos et al. (2012) | Male Wister rats | Delay discounting | SA on FR1 | Cocaine (0.015–0.5 mg/kg i.v.) | HiI > LoI (resistance to extinction) |
| Dalley et al. (2007) | Male Lister rats | 5-CSRTT | SA on FR1 | Cocaine (0.25 mg/kg i.v.) | HiI =LoI (acquisition) HiI > LoI (escalation) |
| Diergaarde et al. (2008) | Male Wistar rats | 5-CSRTT and delay discounting | SA on FR1-25 | Nicotine (0.04 mg/kg i.v.) | HiI > LoI w/ 5-CSRTT (acquisition) HiI > LoI w/ delay discounting (reinstatement) |
| Marusich and Bardo (2009) | Male Sprague-Dawley rats | Delay discounting | SA on FR5 | Methylphenidate (0.03–1.0 mg/kg i.v.) | HiI > LoI (maintenance of 0.1 mg/kg) |
| Marusich et al. (2011) | Male Sprague-Dawley rats | Delay discounting and cued go/no-go | SA on FR5 and PR | Amphetamine (0.0056–0.1 mg/kg i.v.) | HiI < LoI w/ delay discounting (linear mixed model) HiI =LoI w/ cued go/no-go |
| Perry et al. (2005) | Female Wistar rats | Delay discounting | SA on FR1 | Cocaine (0.2 mg/kg i.v.) | HiI > LoI (acquisition) |
| Perry et al. (2008) | Female and Male Wister rats | Delay discounting | SA on FR1 | Cocaine (0.2 mg/kg i.v.) | HiI > LoI (acquisition in female and male) (reinstatement in female) |
| Yates et al. (2012) | Male Sprague-Dawley rats | Delay discounting | CPP | Amphetamine (0.1-1.5 mg/kg s.c.) | HiI > LoI (0.5 and 1.5 mg/kg) |
| Opiates | |||||
| McNamara et al. (2010) | Male Lister rats | 5-CSRTT | SA on FR1 | Heroin (0.04 mg/kg i.v.) | HiI =LoI (acquisition and escalation) |
| Schippers et al. (2012) | Female Wistar rats | Delay discounting | SA on FR4 and PR | Heroin (0.1 mg/kg i.v.) | HiI =LoI |
| Alcohol | |||||
| Poulos et al. (1995) | Male N/NIH rats | Delay discounting | 2-Bottle choice | Alcohol (3%–12% p.o.) | HiI > LoI (maintenance of 12%) |
HiI, high impulsive; LoI, low impulsive; SA, self-administration.
In addition to delay discounting, individual differences in 5-CSRTT performance predict stimulant self-administration (Dalley et al., 2007; Diergaarde et al., 2008). 5-CSRTT involves detection of five visual targets to earn food, with premature responding to a target being punished by a time-out period (food omission), thus defining impulsivity (Robbins, 2002). In contrast to delay discounting, 5-CSRTT is a better predictor of compulsive drug intake than regulated intake (Dalley et al., 2011). For example, with 5-CSRTT performance, rats that are high in impulsivity display greater cocaine seeking than rats that are low in impulsivity, even when responding is punished (Belin et al., 2008; Economidou et al., 2009). This conclusion may not generalize beyond stimulants, however, because individual differences in 5-CSRTT performance do not predict heroin self-administration (McNamara et al., 2010; Schippers et al., 2012).
Lesion studies show that several brain regions are associated with individual differences in impulsivity, although the role of these regions varies across tasks. In general, dopamine-mediated sensorimotor systems involving the nucleus accumbens core and neostriatum are implicated most strongly across various facets of impulsivity. For example, impulsive choice measured by delay of reward, impulsive action measured by stop-signal reaction time, and impulsive reflection measured by 5-CSRTT and DRL are each increased with damage to nucleus accumbens core or dorsal striatum but not the nucleus accumbens shell (Cardinal et al., 2001; Eagle and Robbins, 2003; Pothuizen et al., 2005). The neostriatal brain system is also implicated in habit formation after repeated drug exposure (Belin et al., 2009; Goldstein et al., 2009; Balleine and O’Doherty, 2010). However, the task-dependent facets of impulsivity also are dissociable based on various lesion studies showing that 1) impulsive choice is increased by damage to medial prefrontal cortex and basolateral amygdala (Weissenborn et al., 1997; Winstanley et al., 2004; Gill et al., 2010); 2) impulsive action is increased by damage to the orbitofrontal cortex and subthalamic nucleus (Eagle et al., 2008); and 3) impulsive reflection is increased by damage to anterior cingulate cortex, infralimbic cortex, nucleus basalis magnocellularis, and hippocampus (Muir et al., 1996; Bannerman et al., 1999; Chudasama et al., 2003; Harati et al., 2008). Although some discrepancies can be found in the literature, this evidence supports the general conclusion that dopamine-rich accumbal core and dorsal striatum systems involved in different forms of behavioral activation are modulated by multiple top-down cortical inputs (Dalley et al., 2011).
It is uncommon to find lesion sites that decrease impulsivity. In one exception, damage to the orbitofrontal cortex decreases impulsivity in a delay discounting task, although this effect may depend on individual differences in baseline impulsivity (Zeeb et al., 2010). This finding contrasts with other results showing that an orbitofrontal cortex lesion increases impulsivity in delay discounting (Mobini et al., 2002). However, orbitofrontal cortex is not a homogeneous structure, and the extent of the lesion site into medial and lateral boundaries may provide an explanation for these discrepant reports (Mar et al., 2011).
Given the lesion results showing roles of nucleus accumbens core and striatal terminal regions, it is not surprising that dopamine is implicated in impulsivity. Positron emission tomography (PET) scans reveal that rats showing high impulsivity on 5-CSRTT have greater [18F]fallypride binding in nucleus accumbens than rats showing low impulsivity, indicating that high impulsivity is associated with greater D2-like dopamine receptor availability (Dalley et al., 2007). Dopamine release may also be altered, as electrically evoked dopamine release is attenuated in accumbal tissue slices obtained from rats that are high in impulsivity based on either delay of reward or 5-CSRTT performance (Diergaarde et al., 2008). Dopamine in the medial prefrontal cortex is also involved in impulsive choice, as electrically evoked dopamine release is attenuated in this region in rats that are high in impulsivity based on a delay of reward task (Diergaarde et al., 2008).
In addition to mesocorticolimbic dopamine, in vivo microdialysis results reveal an increase in 5-HT in orbitofrontal cortex in rats performing the delay discounting task relative to yoked controls (Winstanley et al., 2006). These results are generally consistent with work from nonhuman primates showing an inverse relationship between impulsivity and 5-HT metabolites in cerebrospinal fluid (Westergaard et al., 2003). In rats, 5-HT2A antagonists and 5-HT2C agonists also attenuate impulsivity measured by either delay discounting or 5-CSRTT tasks (Paterson et al., 2012; Homberg, 2012). More work is needed to identify the specific brain regions involved in impulsivity modulated by 5-HT systems, as well as other neurotransmitter systems such as glutamate and the endocannabinoids (Pattij and Vanderschuren, 2008).
In summary, impulsivity is a broad term that has multiple facets that can be measured by a host of behavioral tests. Among the various tests, individual differences in impulsive choice measured by delay discounting are perhaps the most reliable predictor of self-administration of stimulants, opiates, and alcohol. This relation also occurs with stimulant CPP but not with hyperactivity. Brain microinjection, lesion, and microdialysis studies have revealed intricate dopamine and 5-HT neural systems involved in various facets of impulsivity. Among the critical brain regions, impulsive choice involves the nucleus accumbens core, neostriatum, medial prefrontal cortex, and basolateral amygdala.
4. Other Individual Differences
Individual differences in consumption of highly palatable tastes (e.g., saccharin/sucrose) also predict various responses to abused drugs (Carroll et al., 2008). High sucrose consumers are more sensitive than low consumers to the locomotor stimulant effect of amphetamine (Sills and Vaccarino, 1994). In contrast, high consumers are less sensitive to the locomotor stimulant effect of morphine (Sills and Vaccarino, 1998). These latter results may be explained by the biphasic effect of morphine, which is characterized by an initial depression, followed by rebound hyperactivity (Vasko and Domino, 1978). Perhaps high sucrose consumers are more sensitive to the depressant phase, as opposed to being less sensitive to the hyperactive phase. In any case, high sweet consumers also self-administer more cocaine and alcohol compared with low consumers (Bell et al., 1994; Gosnell, 2000; Carroll et al., 2002) and are more impulsive on a delay discounting task using food as a reinforcer (Perry et al., 2007). Thus, overlapping neurobehavioral mechanisms exist in preference for palatable tastes, drug intake, and impulsive choice.
Finally, individual differences in wheel-running activity also are related to stimulant self-administration in rats. High wheel runners self-administer more cocaine than low wheel runners (Larson and Carroll, 2005), although this difference does not generalize to cocaine-induced locomotion. As with sucrose/saccharin preference, however, the neural mechanisms linking wheel-running behavior to drug reinforcement are largely unknown.
B. Social Influences
Social context associated with childhood development (e.g., family social context, peer relations) and the social context at the time of drug use influence sensitivity to abused drugs, thereby impacting directly the acquisition of drug-taking behavior. Early life social experiences are critical to development, including the development of reward and stress systems. Consequently, a history of neglect or deleterious social experiences can affect these systems and result in increased vulnerability for abuse. Several animal models have been used to capture various aspects of psychosocial history and its impact on individual vulnerability to drug abuse. These include maternal separation and rearing conditions to capture early life stress and various social and enriched living conditions that provide opportunity for social interactions, including social hierarchies. Many of these topics have been reviewed previously (Miczek et al., 2008) and are updated and further detailed here. In some cases, the animal model or the observed social influences vary depending on sex, perhaps because males and females have different social roles and interactions with each other. Social interactions likely engage neural circuits involved in drug-taking and drug-seeking behaviors in ways that are unique from other environmental stimuli, thus highlighting the importance of social context. For instance, unlike most other stressors, animals fail to habituate to social defeat stress (Nikulina et al., 2004; Engler et al., 2005; Barnum et al., 2007). Furthermore, some neuropeptide hormones mediate responses specifically to social stimuli and not nonsocial stimuli (Nishimori et al., 1996; Ferguson et al., 2000; Cushing and Kramer, 2005; Veenema and Neumann, 2008). Because psychosocial factors play a prominent role in human drug use and dependence, it is important to study such factors using laboratory animal models.
1. Maternal Separation
One animal model of early life social stress is the maternal separation (MS) model (Hofer, 1970; Smotherman et al., 1977). In the rodent version of this model, neonatal pups are separated from their dam for a period of time before weaning. Studies of rodent maternal separation have used a variety of experimental parameters that affect outcome, including the duration and number of separations from the dam, the preweanling period when the separations occur, whether pups are separated as a litter or individually, litter composition, and the age when testing occurs later in life. Maternal separation has been described using different terminology across laboratories, as reviewed previously (Moffett et al., 2007); this review uses the MS notation followed by a number that corresponds to the minutes during which the separation occurs unless otherwise specified (e.g., MS360 denotes maternal separation for a 360-min period). Studies using this model also differ in terms of the control groups used, with many including animal facility-reared controls (AFR) that are left undisturbed except for custodial cage changes, nonhandled controls (NH) that are not handled or in some cases are handled by the experimenters only for cage changes, or MS0 that are briefly handled and returned to the dam each time other MS groups are separated from the dam for a specified period. For comparison across studies, these group notations will be used, even though in some cases they are not the same or may even conflict with group notation used in the references cited.
Effects of maternal separation on drug abuse later in life occur across different pharmacological classes (Table 3). In general, both increases and decreases in sensitivity to drug reward occur depending on separation length, with separations of ≥60 min generally increasing sensitivity, and brief separations of ≤15 min (handling controls) decreasing sensitivity. Interestingly, minimal separation that occurs in AFR and NH conditions is sometimes less protective against drug abuse-related behaviors than MS15. One explanation for the less-than-optimal protection in AFR and NH groups is that having the dam and pups in the same vicinity continuously may be stressful because dams in a natural setting typically leave the nest completely for brief periods (Moffett et al., 2007). Another explanation is that brief (AFR or NH groups) and longer (MS15 group) separations differentially alter maternal behaviors toward the pups once they are returned (Marmendal et al., 2004; Francis and Kuhar, 2008; Der-Avakian and Markou, 2010).
TABLE 3.
Representative studies showing relation between maternal separation and drug reward
| Reference | Animal | Maternal Separation (Short ≤15 min Long ≥60 min) | Outcome Variable | Drug | Results |
|---|---|---|---|---|---|
| Stimulants | |||||
| Campbell and Spear (1999) | Female and male Sprague-Dawley rats | Short | CPP | Amphetamine (1–5 mg/kg i.p.) | ↓ CPP |
| Flagel et al. (2003) | Female and male Sprague-Dawley rats | Short | SA on FR1 | Cocaine (0.125–0.5 mg/kg i.v.) | ↑ SA (females) ↓ SA (males) |
| Kosten et al. (2000) | Male Sprague-Dawley rats | Long | SA on FR1 | Cocaine (0.0625–0.5 mg/kg i.v.) | ↑ SA (acquisition) |
| Moffet et al. (2006) | Female and male Long-Evans rats | Short and long | SA on FR1 | Cocaine (0.0625–1.0 mg/kg i.v.) | ↓ SA (short separation) ↑ SA (long separation) |
| Vazquez et al. (2006) | Male Long-Evans rats | Long | 2-Bottle choice | Amphetamine (25 mg/liter p.o.) Cocaine (100 mg/liter p.o.) | ↑ Preference ↔ Preference |
| Opiates | |||||
| Michaels and Holtzman (2008) | Female and male Long-Evans rats | Long | CPP | Morphine (3–10 mg/kg s.c.) | ↑ CPP |
| Vazquez et al. (2006) | Female and male Long-Evans rats | Long | 2-Bottle choice | Morphine (25 mg/liter p.o.) | ↑ Preference |
| Alcohol | |||||
| Barr et al. (2004a) | Female Rhesus macaques | Long | Voluntary consumption | Alcohol (8.4% p.o.) | ↑ Consumption |
| Cruz et al. (2008) | CFW Male mice | Long | SA on FR3, PR and 3-bottle choice | Alcohol (6–10% p.o.) | ↑ SA (on FR3 and 3-bottle choice) |
| Gustafsson et al. (2005) | Female Wistar rats | Short and long | 2-Bottle choice | Alcohol (8% p.o.) | ↔ Preference |
| Huot et al. (2001) | Male Long-Evans rats | Long | 2-Bottle choice | Alcohol (8% p.o.) | ↑ Preference |
| Roman et al. (2003) | Male alcohol-preferring rats | Short | Voluntary consumption | Alcohol (2–10% p.o.) | ↓ Consumption |
SA, self-administration; ↑, increase; ↓, decrease; ↔, no difference.
The effects of long periods of maternal separation (≥60 min) on locomotor activity produced by acute stimulant administration later in life have been mixed. Most studies show an increase (Zimmerberg and Shartrand, 1992; Kehoe et al., 1996, 1998; Pryce et al., 2001; Brake et al., 2004; Marin and Planeta, 2004; Kikusui et al., 2005) or no effect (Lehmann et al., 1998; Li et al., 2003; Marmendal et al., 2004; Kosten et al., 2005a,c; Hensleigh et al., 2011) compared with stimulant effects in controls, whereas others show a decrease (Matthews et al., 1996a; Moffett et al., 2006). Factors that likely account for these differences are drug history before acute stimulant administration, doses of stimulant tested, and sex. Effects of repeated stimulant administration are also mixed, although most studies have found that maternal separation either decreases (Matthews et al., 1996b; Li et al., 2003) or has no effect (Weiss et al., 2001; Planeta and Marin, 2002; Brake et al., 2004; Muhammad and Kolb, 2011) on locomotor sensitization, whereas one study found enhanced sensitization (Kikusui et al., 2005). The age at which animals are tested for sensitization may contribute to differences observed across these studies as Weiss et al. (2001) and Kikusui et al. (2005) tested animals during adolescence, and in fact, the latter study found that the sensitized response did not persist into adulthood in female mice, although it did persist in male mice. Another complicating factor is that a previous history of saline injections can sensitize rats to acute amphetamine (Brake et al., 2004), potentially obscuring drug-sensitization effects.
Prolonged maternal separation increases stimulant reward, although not all studies support this conclusion (Faure et al., 2009), and the effect appears more reliable in males than in females. In males, maternal separation increases intake of oral amphetamine (Vazquez et al. (2006) and i.v. cocaine (Kosten et al., 2000, 2004; Zhang et al., 2005; Moffett et al., 2006), although one study using a 24-h discrete trial reinforcement schedule found no effect of maternal separation in cocaine self-administration in either male or female rats (Lynch et al., 2005). In females, increases in cocaine self-administration are obtained (Matthews et al., 1999; Kosten et al., 2006; Moffett et al., 2006); however, decreases in intake also have been reported (Kosten et al., 2004; Matthews et al., 1999). This sex dependency is corroborated by other evidence showing that maternal separation increases amphetamine reward as measured by intracranial brain stimulation threshold in male rats (Der-Avakian and Markou, 2010) but not in female rats (Matthews and Robbins, 2003). In maternally separated females, the D2 antagonist raclopride increases brain stimulation reward threshold (Matthews and Robbins, 2003), suggesting that females have enhanced anhedonia in adulthood after prolonged maternal separation. The reason for the discrepancies across studies using female rats is unclear, but it likely relates to hormonal fluctuations during the estrous cycle, which influence stimulant self-administration and stimulant seeking in female rats (Roth et al., 2004; Fuchs et al., 2005; Kippin et al., 2005; Jackson et al., 2006; Feltenstein and See, 2007).
There is some support for a protective effect of brief maternal separation on stimulant reward. Campbell and Spear (1999) found that brief neonatal isolation attenuates amphetamine CPP during early adulthood. With cocaine self-administration, MS15 male and female rats fail to acquire cocaine self-administration with a unit dose (0.0625 mg/kg per infusion) that supports self-administration in controls and MS180 rats (Moffett et al., 2006). Further, a protective effect of brief maternal separation against cocaine self-administration is obtained in MS10 males receiving the separation during the first postnatal week, whereas MS10 females separated briefly during the second, but not the first, postnatal week self-administer more cocaine (Flagel et al., 2003). Thus, sex- and time-dependent differences exist in the protective effect of brief maternal separation.
Other models of neonatal experience have examined vulnerability to stimulant behavioral effects. Neonatal sibling deprivation during the first 2–3 weeks of life attenuates the locomotor and rewarding effects of cocaine in male rats relative to controls reared in a litter of four males and four females (Li et al., 2008). Although no differences were reported in females, only one conditioning regimen was examined, and thus it is possible that sensitivity of females to stimulants may be affected under different conditioning parameters. In any case, this sex-dependent effect is interesting in light of other results showing increased vulnerability to cocaine self-administration in male rats, but not female rats, subjected to neonatal stress from cross-fostering (Vathy et al., 2007). Increases in locomotor activity to amphetamine also occur in male rats artificially reared in isolation with low levels of maternal-like stimulation, but not with high levels of stimulation (Lovic et al., 2006). These findings corroborate the general conclusion that males are more susceptible than females to stimulant effects after the loss of normal social interactions during the neonatal period.
Prolonged maternal separation also enhances the abuse-related effects of opiates. Locomotor sensitization to repeated morphine is enhanced by maternal separation in rats (Kalinichev et al., 2002). Maternal separation also increases morphine CPP (Vazquez et al., 2005b, 2007; Michaels and Holtzman, 2008). Morphine CPP is enhanced in a model comparing artificial rearing to mother rearing in male rats (Lomanowska et al., 2006), and neonatally isolated rats consume more morphine than controls when given access via a two-bottle choice between morphine and water (Vazquez et al., 2005b). Interestingly, conditioned place aversion produced by a κ-receptor agonist, spiradoline, is attenuated by maternal separation in males but not in females (Michaels and Holtzman, 2008). These results suggest that, at least in males, early life stress may enhance opiate abuse vulnerability by increasing reward sensitivity, as well as by decreasing the aversive effect produced at κ-opiate receptors.
Surprisingly, there is little information about the effect of maternal separation on opiate self-administration. Using brain stimulation reward, neither morphine nor naltrexone alters reward threshold after maternal separation (Michaels et al., 2007). However, basal stimulation response rates are attenuated by maternal separation (Michaels et al., 2007), suggesting that maternal separation may produce anhedonia during adulthood, thus exacerbating drug self-administration.
Maternal separation alters alcohol reward as measured by voluntary alcohol consumption later in life, although the precise conditions required for this outcome are unclear. In general, studies reveal a U-shaped time-dependent effect in which MS60-360 and control (NH or AFR) groups show greater alcohol intake relative to MS15 in both rats (Hilakivi-Clarke et al., 1991; Ploj et al., 2003a; Roman et al., 2003; Jaworski et al., 2005) and mice (Cruz et al., 2008). However, Huot et al. (2001) failed to observe a difference between male MS15 and AFR controls, although MS180 males did exhibit enhanced alcohol intake relative to AFR controls. These last findings suggest that the detrimental effects of prolonged maternal separation are greater than the protective effects of brief separation.
A number of studies failed to observe significant effects of maternal separation on alcohol intake in rodents (Marmendal et al., 2004; Roman et al., 2004, 2005; Gustafsson et al., 2005, 2007; Gustafsson and Nylander, 2006; Marmendal et al., 2006; Advani et al., 2007; Daoura and Nylander, 2011; Oreland et al., 2011). Although the reason for the discrepancy is not clear, because of the myriad of procedural differences across studies, several tentative conclusions may be drawn. First, the time of test is important, as rats are more sensitive to maternal separation effects on alcohol intake when tested in adulthood rather than adolescence (Daoura et al., 2011). Since baseline alcohol intake is greater in adolescent rats than in adults, this difference may mask maternal separation effects in adolescents. Second, sex differences likely play a role because two studies examining female rats failed to observe effects of prolonged maternal separation on alcohol consumption (Roman et al., 2004; Gustafsson et al., 2005), although a protective effect of brief maternal separation was observed in females (Hilakivi-Clarke et al., 1991). In a study of alcohol-preferring rats, no effect of maternal separation was observed in females (Roman and Nylander, 2005). Third, alcohol concentration is important because preference varies for different concentrations across different maternal separation conditions in male rats, with MS15 rats preferring 5% alcohol and MS360 rats preferring 20% alcohol (Gustafsson et al., 2007).
Altered vulnerability to alcohol use later in life occurs in nonhuman primates using a model comparing peer-reared to mother-reared rhesus monkeys. These studies report an increase in alcohol consumption in peer-reared monkeys (Barr et al., 2004b; Higley et al., 1991, 1996; Fahlke et al., 2000; Zhang and Kosten, 2007; Newman et al., 2009). Although this effect has been observed in both male and female monkeys, there are likely sex differences in the genes that are involved. Female peer-reared monkeys with the long/short allele for the 5-HT transporter gene promoter exhibit higher alcohol intake than peer-reared females with the long/long allele, as well as higher alcohol intake than maternal-reared females regardless of genotype (Hall and Degenhardt, 2009). This finding suggests that neither peer-rearing nor genotype alone influences alcohol intake, but both are risk factors that interact such that the environmental peer-rearing factor increases vulnerability in individuals with the long/short genotype. Similarly, vulnerability to cocaine abuse is associated with a genotype x maternal rearing interaction in mice (van der Veen et al., 2008). This study showed that cross fostering two different strains of mice, C57BL/6J and DBA/2J, with two other strains of mice that exhibit high (C3H/HeN strain) versus low (AKR stain) maternal licking resulted in a maternal influence on the acquisition of cocaine self-administration. The latter mice fostered by AKR dams self-administered less cocaine relative to those fostered by C3H/HeN dams. Again, these findings suggest that genotype makes a difference only under certain environmental rearing conditions.
As with other abuse-related psychosocial differences, dopamine systems play a key role in the effects of maternal separation (Meaney et al., 2002). Prolonged maternal separation increases extracellular dopamine levels in striatum after both K+ and amphetamine challenge (Hall et al., 1999), as well as increasing total tissue levels of dopamine (Matthews et al., 2001; Brake et al., 2004). Maternal separation also reduces DAT levels in striatum (Meaney et al., 2002), which may contribute to enhanced dopamine responses. In contrast, brief maternal separation decreases tail pinch-induced dopamine release compared with controls; dopamine D3 receptor binding and mRNA are also decreased compared with controls (Brake et al., 2004).
5-HT systems also are linked to maternal separation-induced changes in sensitivity to stress and abused drugs (Miczek et al., 2008; Valentino et al., 2010). In rats, maternal separation decreases 5-HT functioning in amygdala, including reduced 5-HT levels, 5-HT1A autoreceptors, and 5-HT transporters (Vicentic et al., 2006; Oreland et al., 2009). In monkeys, peer-reared adults have decreased 5-HT transporters measured by PET in amygdala and related structures compared with maternal-reared adults (Ichise et al., 2006). In addition, peer-reared monkeys have decreased cerebrospinal fluid (CSF) levels of the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA), which is negatively correlated with alcohol consumption (Higley et al., 1996; Ichise et al., 2006). The decrease in limbic 5-HT function after maternal separation may disinhibit dopamine reward processes (De Deurwaerdere et al., 2004; O’Dell and Parsons, 2004).
Since maternal separation is a powerful stressor in neonates, it is not surprising that the HPA axis is implicated as a key neurobiological factor associated with the effects of maternal separation and abuse-related behaviors. Maternal separation alters the HPA axis, with reports of either hypersensitivity marked by elevated basal and stress-induced blood corticosterone measured later in life (Higley et al., 1991; Plotsky and Meaney, 1993; Vazquez et al., 2005a) or hyposensitivity marked by lower basal and stress-induced blood corticosterone levels (Greisen et al., 2005; Kim et al., 2005; Roman et al., 2006); however, these latter studies assayed blood after testing animals in different behavioral tasks such as the elevated plus maze. Despite these discrepant findings, prolonged maternal separation blunts the negative feedback of glucocorticoids on the HPA axis (Ladd et al., 2004) and produces a constellation of behavioral changes reflecting increased anxiety and depressive symptoms (Zhang et al., 2004; Faturi et al., 2010). Even more subtle early life stress, such as being reared by a mother who engages in low levels of licking and grooming her offspring, engenders hypersensitivity and slower negative feedback of the HPA axis (Liu et al., 1997), thus attesting to the long-lasting influence of early life mother-infant interactions.
In relation to drug abuse–related behaviors, early life levels of corticosterone are positively correlated with alcohol intake later in life. Peer-reared monkeys have higher cortisol levels than mother-reared monkeys, regardless of sex (Fahlke et al., 2000), and consume more alcohol than mother-reared monkeys (Higley et al., 1991). In addition to cortisol, peer-reared monkeys have elevated blood levels of ACTH and CSF levels of the norepinephrine metabolite 3-methoxy-4-hydroxyphenylglycol (Higley et al., 1991). The effects of maternal separation on the HPA axis are sex dependent, as the alcohol-induced elevation in ACTH is greater in peer-reared female monkeys compared with peer-reared males (Barr et al., 2004a).
As another component of the stress system, μ-opioids are implicated in the effects of maternal separation on drug abuse-related behaviors. When separated from the dam, rat pups exhibit increased distress calling, and on return they exhibit increased approach to the dam; these behaviors are decreased and increased, respectively, by μ-opioid agonists and antagonists (Carden et al., 1991; Agmo et al., 1997). μ-Opiate receptor knock-out mice fail to display preference for mother-associated cues or vocalizations in response to separation from their mothers (Moles et al., 2004). Food-deprivation stress during adulthood in MS180 female rats is associated with decreased cocaine amphetamine-related transcript (CART) and pro-opiomelanocortin (Yoo et al., 2011), the latter being the precursor for μ-peptide β-endorphin and ACTH. These peptides are decreased in the arcuate nucleus of the hypothalamus, which is involved in feeding behavior (Lopaschuk et al., 2010). These findings suggest that maternal separation produces a long-lasting dysregulation of the endogenous opioid component of the stress system.
Central δ-opioid systems also are altered by maternal separation. Met-enkephalin and preproenkephalin mRNA levels decrease in the nucleus accumbens (Vazquez et al., 2005b), and this change is coupled with a decrease in enkephalin metabolic enzymes (Irazusta et al., 1999) and δ-opioid receptors (Ploj et al., 2003b) in amygdala. In contrast, met-enkephlin-Arg6-Phe7 (MEAP), a marker of proenkephalin, is increased in the prefrontal cortex, nucleus accumbens, hypothalamus, and periaqueductal gray (Ploj et al., 2001, 2003b). After a history of alcohol self-administration, MEAP is decreased in medial prefrontal cortex and periaqueductal gray area, although increases in MEAP are evident in the striatum and hypothalamus (Gustafsson et al., 2007). In addition, a history of alcohol self-administration is linked to increases in MEAP in the hypothalamus, striatum, and amygdala (Gustafsson et al., 2005, 2007), suggesting an overlap of δ-opioid-mediated effects of maternal separation and alcohol exposure.
κ-Opioid systems also play a role in maternal separation. Dynorphin B, a marker of prodynorphin, is increased in several regions, with more widespread effects observed with brief maternal separation (i.e., pituitary gland, striatum, periaqueductal gray, hippocampus, substantia nigra, medulla) and more restricted effects observed with prolonged maternal separation (Ploj et al., 1999, 2003b). Findings in the hypothalamus have been mixed, likely the result of sex differences, as maternally separated females exhibit a decrease in dynorphin B (Gustafsson et al., 2005), whereas maternally separated males exhibit an increase in dynorphin B (Ploj et al., 1999, 2003b). Additionally, with brief maternal separation, dynorphin B is decreased in the frontal cortex and amygdala (Ploj et al., 1999, 2001, 2003b). However, opposite effects are observed in animals after alcohol self-administration. For instance, in frontal cortex, hypothalamus, and substantia nigra, alcohol-experienced MS360 rats exhibit an increase in dynorphin B, whereas MS360 nondrinkers show no change (Gustafsson et al., 2005, 2007). Taken together, endogenous opioid-mediated modulation of the HPA stress axis during maternal separation appears especially susceptible to long-lasting changes important for mediating alcohol effects, although the implications of opioid modulation of the HPA axis on other abused drugs are less certain.
In summary, there is a vast literature on the neurobehavioral effects of maternal separation during development on later sensitivity to abused drugs. Some inconsistencies have been reported, likely based on how and when the maternal separation is applied, as well as the methods used to measure drug sensitivity. One overall conclusion from these studies is that brief separation (≤15 min per day) reduces drug sensitivity, whereas prolonged separation (≥ 60 min per day) potentiates drug sensitivity. In cases where effects are observed, these effects are relatively long-term, and they tend to generalize across drug classes. The long-term behavioral effects of prolonged maternal separation involve dopamine and 5-HT systems, as well as enhanced sensitivity of the HPA axis. The altered HPA axis may be due to long-term changes in μ-, δ-, and κ-opioid receptor systems.
2. Housing Conditions
Environmental enrichment is a manipulation in which animals are exposed to housing conditions that differ in the amount of stimulus novelty and opportunity to engage in social activity (Bennett et al., 1969). In the typical procedure, rodents are housed in either an enriched condition (EC) with novel objects and conspecifics or an isolated condition (IC) without objects or conspecifics. To determine the relative contribution of novel objects and conspecifics, a separate group is often housed in a social condition (SC) with conspecifics, but without novel objects. Some studies have varied the number of conspecifics with or without novel objects (Zakharova et al., 2009). In general, neurobehavioral differences are greater when comparing EC and IC rodents, with SC rats falling intermediate between these two extreme groups. Although enrichment is often applied during the periadolescent period of development, it produces effects across the life span, and these effects are reversible (Renner and Rosenzweig, 1987). In the current review, IC animals are considered the treated group, and EC animals are considered the comparison group because IC rats are most vulnerable to drug self-administration, which is similar to animals that score high on the novelty responder, novelty seeking, and impulsivity tests described earlier.
The hyperactivity produced by acute stimulant treatment is blunted in IC rats relative to EC rats (Bowling et al., 1993; Bowling and Bardo, 1994; Bardo et al., 1995); one exception, however, is that nicotine-induced hyperactivity is increased in IC rats (Green et al., 2003). In contrast to acute treatment, repeated stimulant treatment, including nicotine, is increased in IC rats relative to EC rats (Bardo et al., 1995; Funk et al., 2005; Solinas et al., 2009; Wooters et al., 2011). One caveat in these findings is that IC rats have higher baseline activity than EC rats before any drug treatment, which can complicate interpretation of the findings (Smith et al., 2009). That is, similar to inherent differences in activity between HR and LR rats, the conclusions drawn for IC and EC rats may differ depending on whether drug-induced hyperactivity is expressed as an absolute value or as a percent change relative to the different baselines.
Similar to enhanced sensitization to repeated stimulants, IC rats show greater propensity to self-administer amphetamine and cocaine relative to EC rats (Bardo et al., 2001; Miczek et al., 2008; Green et al., 2010; Gipson et al., 2011a). This difference is greater at low unit doses than at high unit doses delivered on either FR or PR schedules. Although baseline differences in lever pressing between IC and EC rats need to be considered as described previously (Smith et al., 2009), evidence indicates that differential housing alters sensitivity to the reinforcing effects of abused drugs independent of baseline rates of lever pressing. For example, although IC rats have higher baseline (nonreinforced) rates of lever pressing, they acquire food-reinforced lever pressing at a slower rate than do EC rats (Bardo et al., 2001). It is possible that IC rats self-administer more drug in an attempt to compensate for a relative insensitivity to the reinforcing effect of stimulants, but this is unlikely because IC rats respond for lower unit doses on an FR schedule and show higher breakpoints on a PR schedule. Instead, isolation rearing likely enhances sensitivity to stimulant reinforcement.
In contrast to the effects on self-administration, isolation housing decreases amphetamine and cocaine CPP (Bowling and Bardo, 1994; Bardo et al., 1995). These findings seem paradoxical because isolation housing decreases stimulant CPP and increases stimulant self-administration. However, self-administration and CPP measure different aspects of reward, with self-administration measuring directly the reinforcing effect of the drug and CPP measuring the rewarding value of contextual cues associated with drug (Bardo and Bevins, 2000). This suggests that isolation housing may produce differential effects on the primary and secondary reinforcement associated with abused drugs. Alternatively, since isolation rearing increases impulsive lever pressing (Engler et al., 2005; Perry et al., 2008), this change may enhance the propensity to lever press for drug specifically, without altering the strength of CPP. Further, since isolation rearing also disrupts contextual fear conditioning (Weiss et al., 2004; Gresack et al., 2010), isolation-induced deficits in general Pavlovian learning processes inherent in the CPP paradigm cannot be ruled out.
In addition to the discrepancy between self-administration and CPP results using either amphetamine or cocaine, isolated housing does not produce consistent alterations in self-administration and CPP using other drugs. For example, neither methamphetamine- nor morphine-induced CPP is altered reliably by isolation housing (Smith et al., 2005; Thiriet et al., 2011), although one study found that SC mice display greater heroin CPP than EC mice (El Rawas et al., 2009). In the case of alcohol, isolation housing reliably increases free-access intake in both rats and rhesus monkeys (Kraemer and McKinney, 1985; Procopio-Souza et al., 2011), and similar results are obtained with diazepam (Wolffgramm and Heyne, 1991). Self-administration of oral alcohol also is higher in IC rats compared with EC rats (Bienkowski et al., 2001); when given a choice between response-contingent water or 10% alcohol, IC rats exhibit a preference for the alcohol lever over the water lever. Free-access intake and PR breakpoints also are higher in IC alcohol-preferring rats (Bisaga and Kostowski, 1993), although an isolation-induced increase is not obtained with standard outbred rats (Gingras and Cools, 1995), suggesting a gene x environment interaction. In sum, although isolation rearing increases self-administration of amphetamine, cocaine, and alcohol, this effect does not generalize to all drug classes and does not generalize to the reward accrued to drug-associated cues measured by CPP.
To determine the influence of conspecifics per se, several studies have compared IC and SC animals. Early work indicated that IC rats display greater self-administration and CPP relative to SC rats using cocaine or heroin, but not amphetamine (Schenk et al., 1983, 1985, 1988); however, more recent work indicates that IC rats also self-administer more amphetamine when a low unit dose (0.03 mg/kg per i.v. infusion) is used (Bardo et al., 2001). IC rats also display a leftward shift in the cocaine dose response curve compared with SC rats (Howes et al., 2000), indicating that isolation housing enhances sensitivity to stimulant reinforcement compared with social housing. However, the presence of novel objects also plays a role, as the behavioral responses to stimulants, opiates, and alcohol differ between SC and EC animals (Bardo et al., 2001; Green et al., 2003; Coolon and Cain, 2009; El Rawas et al., 2009; de Carvalho et al., 2010).
Isolation housing produces profound alterations in various neural systems (Renner and Rosenzweig, 1987). Since much of the early work in this area focused on learning and memory deficits, changes in cortical function have been examined extensively. IC rats have decreased cortical thickness compared with EC rats (Diamond et al., 1964), primarily as a result of decreases in astrocytic branching and in brain capillaries associated with a lowering of mitochondrial metabolic activity (Sirevaag and Greenough, 1991). Neuronal cytoarchitecture is altered, characterized by a decrease in the number and size of dendrites, as well as a decrease in dendritic branching and number of dendritic spines (Rosenzweig and Bennett, 1996; Diamond, 2001). Although these changes are most prominent in cortical regions, particularly the visual and auditory cortices, subcortical regions also are affected. For example, in the hippocampus, both nerve growth factor and brain-derived neurotropic factor (BDNF) are reduced by isolation housing (Torasdotter et al., 1998; Thiel et al., 2012). There also is a decrease in number of dendritic spines found on type I spiny neurons in striatum (Comery et al., 1996), as well as a decrease in dendritic arborization on spiny neurons in the nucleus accumbens (Kolb et al., 2003). These last results indicate that reward-relevant dopamine systems may not function optimally after isolation housing.
As shown in Fig. 2, isolation housing alters the dopamine systems involved in hyperactivity and drug reward. The brain circuitry implicated in the behavioral effects of isolation is remarkably similar to that described previously in HR rats (see Fig. 1), suggesting that isolated and HR rats share overlapping neural mechanisms. One exception to this conclusion is that DAT function in nucleus accumbens and medial prefrontal cortex differs between isolated and HR rats (see Figs. 1 and 2). Despite this, there is a similar increase in accumbal dopamine in both isolated and HR rats, which may explain the hyperactivity observed in both groups.
Fig. 2.
Schematic of brain changes in isolate-housed rats compared with socially enriched rats. Regions in blue represent primarily reward-relevant central structures, regions in green represent primarily stress-related central structures, and the region in red is a peripheral stress-related gland. Note that the overlap in circuitry depicted previously in Fig. 1 suggests that HR rats (Fig. 1) and isolated rats (this figure) share similar neural mechanisms. Brain regions: ACe, central nucleus of amygdala; Hippo, hippocampus; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; VTA, ventral tegmental area. Cellular changes: CAT, choline acetyltransferase; CORT, corticosterone; CREB, cAMP response element binding; DA, dopamine; NE, norepinephrine; NGF, nerve growth factor.
Although initial in vitro studies did not observe differences between IC and EC rats in electrically or amphetamine-stimulated dopamine release in striatal or accumbal tissue slices (Bowling et al., 1993; Bardo et al., 1995;), ex vivo and in vivo studies show differences in accumbal function (Jones et al., 1992). Isolation rearing increases dopamine in the nucleus accumbens, striatum, and medial prefrontal cortex (Bowling et al., 1993) and also increases the transcription factor cAMP response element-binding in the nucleus accumbens (Green et al., 2010), suggesting an isolation-induced overactivity of basal mesolimbic function. This overactivity may be due to a decrease in DAT protein in the nucleus accumbens of IC rats (Zakharova et al., 2009), thus potentiating transynaptic dopamine signaling. In contrast to the deficit in DAT functioning in the nucleus accumbens, IC rats have enhanced DAT function in the prefrontal cortex as is evident by an increase in the maximum velocity of [3H]dopamine uptake compared with EC rats (Zhu et al., 2004), an effect that involves protein kinase C (Wooters et al., 2011). In the prefrontal cortex, IC rats also have more DAT protein at the cell surface compared with EC rats (Zhu et al., 2005). As part of a complex neurocircuitry, the medial prefrontal cortex has direct inputs to the nucleus accumbens (Sesack et al., 1989), as well as indirect inputs into other structures within the mesocorticolimbic system (Tzschentke and Schmidt, 2000). Thus, the isolation-induced increase in dopamine reuptake via DAT in the prefrontal cortex may diminish the influence of this inhibitory cortical structure over limbic reward processes.
In addition to changes in basal dopamine function, isolation housing alters drug-induced changes in mesocorticolimbic function. After amphetamine, IC rats have decreased levels of the dopamine precursor dihydroxyphenylalanine (DOPA) in striatum and decreased levels of the dopamine metabolite dihydroxyphenylacetic acid (DOPAC) in striatum and the medial prefrontal cortex compared with either EC or SC rats (Bowling et al., 1993). In contrast, IC rats have increased amphetamine-stimulated levels of extracellular dopamine in striatum and the nucleus accumbens using in vivo microdialysis compared with SC rats (Jones et al., 1992), although this effect is not obtained under chloral hydrate anesthesia (Bardo et al., 1999). IC rats also show greater cocaine-stimulated release of accumbal dopamine and greater alterations in the immediate-early genes c-fos and zif-268 in striatum and the central nucleus of the amygdala and, in some cases, in the nucleus accumbens and frontal cortex (Howes et al., 2000; Solinas et al., 2009; Thiel et al., 2010). Housing-induced differences in neurochemical response to stimulant drugs do not likely reflect pharmacokinetic changes, as brain levels of [3H]amphetamine are similar in IC and EC rats after systemic injection (Bardo et al., 1999). Instead, although biosynthetic and metabolic processes are diminished, isolation housing likely enhances the readily releasable stores of dopamine from vesicular or nonvesicular presynaptic pools.
Similar to abuse-prone HR rats, described earlier, isolated animals have an overactive stress axis (Serra et al., 2005). Basal corticosterone is higher in IC than in EC rats, and the amphetamine-stimulated increase in corticosterone is greater in IC rats (Stairs et al., 2011). However, the ability of the glucocorticoid receptor antagonist RU-486 to reduce amphetamine self-administration is blunted in IC rats (Stairs et al., 2011), suggesting that chronic elevation of stress hormones may desensitize glucocorticoid receptors. Further, IC rats show reduced CB1 receptor mRNA levels in the hypothalamus and basolateral amygdala (El Rawas et al., 2011), suggesting that endocannabinoid systems may contribute to the enhanced response to stress in IC rats. Regardless of the precise mechanism, the isolation-induced increase in corticosterone would be expected to increase stimulant reinforcement (Piazza et al., 1991a; Goeders, 2002).
Beyond dopamine, other neurotransmitter systems are affected by isolation housing. In the nucleus accumbens, IC rats have decreased 5-HIAA and decreased levels of the acetylcholine synthetic enzyme choline acetyltransferase (Jones et al., 1991). 5-HT levels in the medial prefrontal cortex and hippocampus also are reduced by isolation housing (Brenes et al., 2008), whereas hippocampal norepinephrine is increased (Galani et al., 2007). Further, glutamate signaling is blunted by isolation housing. For example, IC rats have decreased glutamatergic tone mediated via mGluR2 receptors in the dorsal medial prefrontal cortex compared with EC rats (Melendez et al., 2004). Amphetamine-stimulated extracellular glutamate levels in the nucleus accumbens also are blunted in IC rats compared with EC rats (Rahman and Bardo, 2008). Pretreatment with MK-801, a noncompetitive NMDA receptor antagonist, prevents the acute amphetamine-induced increase in extracellular glutamate levels in the nucleus accumbens, thus implicating an accumbal glutamatergic mechanism in the environment-dependent effects of amphetamine.
In summary, isolate-housed animals are hypersensitive to abused drugs relative to animals raised in enriched or social housing conditions. Isolated rats self-administer lower doses of stimulants compared with EC rats. Isolation rearing also enhances the self-administration of alcohol. Although these effects indicate enhanced sensitivity to the primary reinforcing effect of abused drugs, they do not generalize to contextual conditioning measured by CPP. The isolation-induced changes in sensitivity to abused drugs are associated with overactive dopamine and HPA systems. Recent evidence also indicates that isolation rearing blunts glutamate systems in the nucleus accumbens and frontal cortex.
3. Social Reward
Initiation of drug taking nearly always occurs in a social context in which peers provide social reward for the behavior, yet surprisingly little research has been conducted with animal models to investigate the influence of social interactions at the time of drug taking (i.e., social context). The presence of a nonthreatening conspecific is highly salient and rewarding, especially in adolescent rats (Vanderschuren et al., 1997; Spear, 2000); for instance, nonthreatening conspecifics 1) elicit approach (Panksepp et al., 1984), 2) elicit ultrasonic vocalizations thought to be indicative of positive affect (Burgdorf et al., 2008), 3) are positive reinforcers (Angermeier et al., 1959; Werner and Anderson, 1976; Evans et al., 1994), and 4) produce CPP (Calcagnetti and Schechter, 1992; Crowder and Hutto, 1992; Douglas et al., 2004). Research examining drug effects in a social context suggests that this is an important variable that influences sensitivity to the rewarding effects of drugs.
Social context (i.e., being with a conspecific at the time of the drug experience) alters stimulant reward. Social reward interacts synergistically with both cocaine and nicotine to produce CPP in adolescent male rats since conditioning parameters that fail to support social or drug reward when given alone can produce CPP when given together (Thiel et al., 2008, 2009). Social reward competes with drug reward in young male rats since pairing a conspecific with one compartment and cocaine with the other compartment fails to produce CPP, even though either reward alone produces CPP (Fritz et al., 2011b,d). After establishing cocaine CPP, pairing a conspecific with the previously saline-paired compartment shifts the preference away from the cocaine-paired compartment in favor of the social reward-paired compartment (Fritz et al., 2011b). Further, young adult male mice show social enhancement of methamphetamine CPP, but only when mice paired together are both under drug influence and not when one mouse is under drug influence and the other is not (Watanabe, 2011). This last finding suggests that sharing of the drug experience may be critical for drug-social reward synergistic interactions.
The degree to which social context influences drug effects and the degree to which drugs influence social behavior appears to be related for some drugs but not for others. For instance, 3,4-methylenedioxymethamphetamine (MDMA) increases social interactions in rats (Thompson et al., 2009), and MDMA-induced locomotor sensitization is greater in a social context than in a nonsocial context (Procopio-Souza et al., 2011). Under similar conditions, amphetamine has no effect on social interaction, and amphetamine-induced sensitization is not affected by social context (Procopio-Souza et al., 2011). In contrast, morphine increases social interactions, yet morphine-induced sensitization is attenuated in a social context (Procopio-Souza et al., 2011). Thus, the relationship between social context and drug effects varies depending on the drug.
Some abused drugs may decrease the effects of social interaction. For example, exposure to a conspecific or methylphenidate alone produces CPP, whereas no CPP is produced when these stimuli are combined (Trezza et al., 2009), suggesting an inhibitory interaction between social reward and methylphenidate. One interpretation of these findings is that methylphenidate decreases social reward, perhaps by reducing play behavior (Vanderschuren et al., 2008). However, nicotine and cocaine also reduce play behavior, yet enhance social CPP (Thiel et al., 2008, 2009), suggesting that a reduction in play does not necessarily result in an inhibitory interaction between all stimulants and social reward. Further research is needed to investigate whether drug x social reward interactions depend on the drug or environmental determinants that vary across studies.
Stimulant self-administration may be enhanced by the presence of a nonthreatening conspecific. Acquisition of nicotine self-administration paired with oral delivery of a sweet-scented solution is facilitated in male and female adolescent rats when a conspecific drinks the scented solution in an adjacent cage (Chen et al., 2011). In that experiment, the divider between the cages was perforated to allow the transfer of olfactory cues. In the absence of the conspecific, rats failed to increase licking on an active spout that resulted in solution + nicotine delivery compared with an inactive spout. The social facilitation of nicotine self-administration was blocked by the nicotinic receptor antagonist mecamylamine and was stronger in the presence of a familiar rat versus a novel rat, suggesting that social cues, rather than novel cues, drive the receptor-mediated facilitation. Similarly, a conspecific in an adjacent cage sharing a clear Plexiglas wall with the self-administration cage facilitates intake of a high (0.1 mg/kg per infusion), but not a low (0.01 mg/kg per infusion) unit dose of amphetamine (Gipson et al., 2011b). This latter effect is selective for amphetamine, as rats reinforced with sucrose pellets show a social-induced disruption of intake.
In addition to stimulant drugs, opiate-induced locomotor activity and reward are altered by social context. Morphine facilitates social interaction, including play behavior (Normansell and Panksepp, 1990), yet sensitization of morphine-induced locomotor activity is attenuated in mice that receive morphine in a social context (Procopio-Souza et al., 2011). Similarly, using a highly social strain of mice (C57BL/6J), morphine CPP is inhibited in male mice when conditioning occurs in a social context compared with an isolated context (Kennedy et al., 2012). No influence of social context is observed in male mice that are socially housed.
There is strong support for modulation of alcohol intake by social context, and in general, social context facilitates alcohol intake and preference. Exceptions where social context is protective against alcohol consumption are studies comparing chronic isolation versus social housing for an extended period, which show that chronic isolation stress increases alcohol consumption and preference for high concentrations of alcohol compared with social housing (Wolffgramm, 1990; Wolffgramm and Heyne, 1991; Ehlers et al., 2007). In contrast, studies have found social facilitation of drinking using pre-exposure to a demonstrator rat that had been exposed to alcohol (Hunt et al., 2001, 2000). Males are more sensitive than females to familiarity of social partners, with a familiar demonstrator rat being the best facilitator of drinking, followed by a familiar sober rat. Less drinking occurs with an unfamiliar demonstrator and sober rat. In contrast, females exhibit comparable enhancements of drinking after exposure to either familiar or unfamiliar demonstrator rats (Maldonado et al., 2008). Adolescent rats given access to alcohol (i.e., forced consumption) consume more alcohol when pair-housed rather than individually housed; however, when these groups are later given a choice between alcohol and water, there are no group differences in alcohol consumption (Thorsell et al., 2005). Using highly social prairie voles, alcohol preference is higher under duplex housing (i.e., living quarters separated by mesh separation barrier allowing some social contact) compared with single housing, whereas there is no difference across conditions for saccharin preference (Anacker et al., 2011a). Voles predetermined to be high drinkers decrease alcohol intake when paired with a low drinker, and the reduced drinking persists even if the previously high drinker is isolated (Anacker et al., 2011b). Thus, social influence can have protracted effects on alcohol intake.
Social context also modulates the effects of alcohol-associated cues. For instance, alcohol alone produces conditioned place aversion, but if alcohol is given in the presence of an intoxicated or sober conspecific, it fails to produce conditioned place aversion (Gauvin et al., 1994), suggesting that the social context attenuates the aversive effects of alcohol. When an alcohol scent is paired with either a conscious or unconscious rat, conditioned approach occurs only to the scent paired with the conscious rat (Fernandez-Vidal and Molina, 2004). Similarly, cues paired previously with social interaction facilitate subsequent alcohol intake (Tomie et al., 2004).
The facilitation of drug intake by social context can be mediated by reduced stress reactivity compared with that experienced in isolation. Blunted and delayed plasma corticosterone levels are observed in response to amphetamine in social versus isolate housed rats (Stairs et al., 2011). Blunted plasma corticosterone responses to nicotine also are observed in adolescent male rats tested in a social context relative to those tested in isolation (Pentkowski et al., 2011), indicating that stress-related social influences exist across different stimulants.
Neural processes associated with appetitive social interactions overlap with those implicated in drug abuse, including involvement of nucleus accumbens and amygdala (Burgdorf and Panksepp, 2006; Alcaro et al., 2007). Activation of accumbal shell, amygdala (central and basolateral nuclei), and ventral tegmental area as measured by expression of zif268 in response to a cocaine-conditioned environment is blunted by the presence of social reward–conditioned cues (Fritz et al., 2011b). Lesion experiments also suggest dissociable contributions of some of these regions to drug versus social reward. Under CPP conditions in which social interaction is paired with one compartment and cocaine is paired with the other compartment, lesions of accumbal core or basolateral amygdala produce a preference for the social compartment, whereas lesions of accumbal shell produce a preference for the cocaine compartment (Fritz et al., 2011a).
Although the influence of abused drugs on social behaviors has been examined extensively (e.g., for review, see Vanderschuren et al., 1997; Burgdorf and Panksepp, 2006), relatively little is known about the neurobiology of appetitive social context at the time of drug initiation on vulnerability to continued drug use. However, some recent studies demonstrate a relationship between dopamine, opiate, and oxytocin involvement in social behavior and drug abuse–related behaviors. In male prairie voles that have formed pair bonds with their mates, amphetamine fails to produce CPP under conditioning parameters that support amphetamine CPP in sexually naïve males that have not yet formed pair bonds (Liu et al., 2011), suggesting that pair bonding reduces sensitivity to amphetamine reward. Amphetamine also increases D1 receptor binding in the nucleus accumbens and produces CPP in sexually naïve voles, but it has the opposite effect of decreasing D1 receptor binding in pair-bonded voles that also fail to acquire CPP (Liu et al., 2011). Moreover, amphetamine CPP in sexually naïve voles is altered by intra-accumbens manipulations of D1, but not D2, receptors (Liu et al., 2011). As for opioid systems, Burgdorf et al. (2007) found that stimulation of brain reward pathways produces high-pitch (50 kHz) ultrasonic vocalizations and that there is a relationship between the ability of μ-opiate receptor agonist administration into the ventral tegmental area to support CPP and to elicit ultrasonic vocalizations. Finally, oxytocin systems play a role in various appetitive social behaviors, including pair bonding, mother-infant bonding, and social approach and recognition (Insel, 1992; Young et al., 2001; McGregor et al., 2008; Young et al., 2011). Male rats given oxytocin during early adolescence later exhibit enhanced social behavior, less anxiety-like behavior, and upregulated levels of oxytocin and oxytocin mRNA (Bowen et al., 2011). After oxytocin administration in adulthood, they also exhibit less alcohol drinking (Bowen et al., 2011), suggesting that upregulation of oxytocin may have a protective effect against alcohol intake (Bowen et al., 2011). Such systems are prime candidates for investigating the neural basis of social context at the time of drug initiation and on vulnerability to development of further drug use and dependence.
In summary, social contexts are rewarding alone, and they can alter the effects of abused drugs. In the presence of social interactions, sensitivity to the rewarding effects of abused drugs is enhanced using either CPP or self-administration, and these effects are observed with both stimulants and alcohol. In a choice situation, a social context can reduce the preference for a drug-paired context. Several neural systems appear altered, including the HPA axis and dopamine systems in nucleus accumbens and amygdala. Recent evidence suggests that social reward also is influenced by oxytocin systems.
4. Social Defeat
Social defeat (SD) is used to model the effects of physical and psychologic stress on drug abuse–related behaviors in animals. SD is typically investigated in rodents selected as either dominant or subordinate, or aggressive and nonaggressive, to facilitate fighting and defeat during encounters (Hilakivi-Clarke et al., 1991; Kudryavtseva et al., 1991; Marrow et al., 1999; Shimamoto et al., 2011). The resident intruder procedure is another common method for examining SD effects on drug abuse–related behavior (Yap and Miczek, 2007). In this procedure, a male rodent (the intruder) is placed in the cage of a resident male rodent that is pair-housed with a female rodent without the female present. The resident rodent attacks the intruder, and after meeting a criterion of defeat, the intruder is removed. Sometimes before or after SD, the intruder is placed into a smaller cage within the resident’s cage, which may have small openings that allow transmittance of social cues indicative of threat (i.e., vocalizations, smells). In addition to housing with a female to stimulate territorial aggression, the resident rat is often larger (Miczek and Mutschler, 1996), from a more aggressive strain (Kabbaj et al., 2001), or selected for aggressive behavior toward intruders (de Jong et al., 2005). SD has been studied in female rodents by using a lactating female as the resident and a nonlactating, less aggressive female as the intruder (Haney et al., 1995).
In general, SD enhances subsequent effects of stimulant drugs. For instance, acute or repeated SD typically cross sensitizes animals to cocaine- and amphetamine-induced locomotion (Nikulina et al., 1998; Marrow et al., 1999; Miczek et al., 1999b; Covington and Miczek, 2001; Nikulina et al., 2004; Boyson et al., 2011; de Jong et al., 2005; Yap et al., 2005; Yap and Miczek, 2007; Dietz et al., 2008; Quadros and Miczek, 2009). In rats bred for low responsivity to amphetamine-induced locomotion, SD cross-sensitizes these animals to acute amphetamine-induced hyperactivity such that these rats do not differ from rats bred for high responsivity to amphetamine (Dietz et al., 2008). These findings suggest that SD eliminates the individual differences normally observed between high versus low amphetamine responders. However, studies examining adolescent rats and hamsters found either no effect or decreased sensitivity to stimulant-induced locomotion after SD (Kabbaj et al., 2002; Trzcinska et al., 2002; Burke et al., 2011), suggesting that cross-sensitization with stimulants occurs when SD is experienced in adulthood but not when it is experienced during adolescence. The time course of cross-sensitization between SD and stimulants also differs across studies, with some studies finding persistent effects (Nikulina et al., 1998, 2004; Covington et al., 2005) and other studies finding only transient effects (de Jong et al., 2005). Further research directly comparing effects of SD in adolescent versus adult rodents on stimulant-induced locomotion tested at varying intervals is needed to address these issues.
Sensitivity to stimulant reward also is enhanced as a result of SD (Table 4). In contrast to the results with locomotor activity, SD enhances sensitivity to stimulant CPP in both adolescents and adults (McLaughlin et al., 2006; Burke et al., 2011). The effect observed in adolescents is specific to SD, as cross-sensitization is not observed in adolescent rats pre-exposed to foot shock stress (Burke et al., 2011). SD also enhances cocaine-primed reinstatement of extinguished cocaine CPP in mice (Ribeiro Do Couto et al., 2009).
TABLE 4.
Representative studies showing relation between social defeat and drug reward
| Reference | Animal | Predictor Variable | Outcome Variable | Drug | Results |
|---|---|---|---|---|---|
| Stimulants | |||||
| Boyson et al. (2011) | Male Long-Evans rats | Periodic SD (4 times) | SA on FR5 and PR | Cocaine (0.75 mg/kg i.v.) | ↑ SA (acquisition and binge) |
| Burke et al. (2011) | Male Sprague-Dawley rats | Daily SD (5 days) | CPP | Amphetamine (1 mg/kg i.p.) | ↑ CPP |
| Covington and Miczek (2001) | Male Long-Evans rats | Periodic SD (4 times) | SA on FR5 and PR | Cocaine (0.38–1.5 mg/kg i.v.) | ↔ SA (acquisition) ↑ SA (binge) |
| Haney et al. (1995) | Female and male Sprague-Dawley rats | Periodic SD (4 times) | SA on FR1 | Cocaine (0.32 mg/kg i.v.) | ↑ SA |
| McLaughlin et al. (2006) | Male C57Bl/6 mice | Daily SD (3 days) | CPP | Cocaine (15 mg/kg s.c.) | ↑ CPP |
| Quadros and Miczek (2009) | Male Long-Evans rats | Periodic SD (4 times) | SA on FR3 and PR | Cocaine (0.75 mg/kg i.v.) | ↔ SA (escalation) ↑ SA (breakpoint and binge) |
| Tidey and Miczek (1997) | Male Long-Evans rats | Daily SD (4 days) | SA on FR1 | Cocaine (0.75 mg/kg i.v.) | ↑ SA (acquisition) |
| Opiates | |||||
| Cruz et al. (2011) | Male Long-Evans rats | Periodic SD (4 times) | SA on FR3 and PR | Heroin (0.03 mg/kg i.v.) | ↔ SA |
| Alcohol | |||||
| Croft et al. (2005) | Male C57BL/10 mice | Daily SD (5 days) | 2-Bottle choice | Alcohol (8% p.o.) | ↑ Preference |
| Funk et al. (2005) | Male Wistar rats | Acute SD (before session) | SA on FR3 | Alcohol (12% p.o.) | ↓ SA (maintenance and extinction) ↔ SA (reinstatement) |
| van Erp et al. (2001) | Male Long-Evans rats | Daily SD (5 days) | SA on FR4 | Alcohol (10% p.o.) | ↓ SA |
SA, self-administration; ↑, increase; ↓, decrease; ↔, no change.
Several studies have found that SD enhances the stimulant self-administration. An increase in acquisition of cocaine self-administration occurs with continuous access to cocaine, with SD rats acquiring more rapidly than controls (Tidey and Miczek, 1997). With short access daily acquisition sessions, however, SD effects are not reliably observed early during acquisition (Haney et al., 1995; Covington and Miczek, 2001; Quadros and Miczek, 2009), which may be due to individual differences. Rats classified as high responders to amphetamine exhibit delayed self-administration acquisition on short access sessions after SD compared with nondefeated high responders (Kabbaj et al., 2001). During later acquisition sessions, however, rats classified as low responders exhibit an increase in cocaine intake after SD relative to nondefeated rats (Kabbaj et al., 2001). A similar increase in cocaine intake is observed during late acquisition sessions in nonclassified male and female rats that experience SD (Haney et al., 1995), indicating that the effect of SD is most prominent with extended self-administration training.
In animals that have acquired cocaine self-administration, SD increases cocaine intake during maintenance of self-administration (Boyson et al., 2011), and this effect is greater when low doses of cocaine (0.03–0.125 mg/kg per infusion) are available (Miczek and Mutschler, 1996). An increase in cocaine intake as a result of SD is observed consistently in animals given binge access (i.e., 24 h) to cocaine (Covington and Miczek, 2001, 2005; Covington et al., 2005; Quadros and Miczek, 2009; Boyson et al., 2011; Miczek et al., 2011b). This effect reflects a shortened inter-reinforcer interval at high doses of cocaine (>0.25 mg/kg per infusion) earned during the late portion of the binge, as well as a longer persistence of responding before stopping the binge. Most studies find that SD increases cocaine intake on a PR schedule of reinforcement in rats (Covington et al., 2005; Covington and Miczek, 2005; Covington et al., 2008; Quadros and Miczek, 2009), although this effect might not generalize to mice (Yap and Miczek, 2007).
Although relatively little is known about the effects of SD on opiate abuse–related behaviors, some results suggest that SD enhances sensitivity to opiates similar to its effects on stimulant-induced behaviors. For instance, SD cross-sensitizes rats to morphine- and heroin-induced hyperactivity (Stohr et al., 1999; Cruz et al., 2011), and acute SD reinstates extinguished morphine CPP (Ribeiro Do Couto et al., 2006). Animals subjected to SD also exhibit analgesia (Miczek et al., 1982), which is reversed by a κ-opiate receptor antagonist, demonstrating that opiate systems are affected by SD (McLaughlin et al., 2006). It is unclear whether SD increases the reinforcing effects of opiates, however, since intake of heroin is not altered, but intake of a speedball mixture of cocaine and heroin is increased (Cruz et al., 2011). Further research is needed to address more thoroughly the effects of SD on opiate self-administration.
The effects of SD on alcohol abuse–related behaviors are more complex than with other drugs, likely as a result of variation across studies in the duration between SD and drug treatment, as well as the influence of individual differences. Alcohol intake decreases when rats are tested immediately after SD or when the threat of SD is present for 6 h postdefeat (van Erp et al., 2001; Funk et al., 2005), although one study found no change when the threat of SD occurred continuously in the normal housing condition (Keeney and Hogg, 1999). The decrease in alcohol consumption is consistent with the general suppressant effects that stressors have on ongoing behavior in rats (e.g., Meerlo et al., 1996). In contrast, when access to alcohol is delayed 2 h post-SD, rats exhibit an increase in alcohol intake (Caldwell and Riccio, 2010), in contrast to the decrease in alcohol consumption immediately after SD. Also, Croft et al. (2005) found a delayed increase in alcohol preference in mice with a history of repeated SD that emerged about 2 weeks after the last defeat. This study used mice that had a low alcohol preference, and it is unclear whether an increase in preference also would be observed in high alcohol-preferring mice. Similarly, CRH knock-out mice lacking CRH1 receptors exhibit a delayed increase in alcohol consumption weeks after repeated SD episodes (Sillaber et al., 2002). Individual differences in the effects of SD on alcohol consumption involve genetic influences, as SD increases alcohol consumption in C57BL/J6 mice but not in CBA/Lac mice (Kudryavtseva et al., 1991). Individual differences in fighting experience also influence SD effects on consumption, as increases in alcohol intake are observed in mice that are defeated but not in mice that are successful in defeating another mouse (Kudryavtseva et al., 1991; Hilakivi-Clarke and Lister, 1992). Finally, Funk et al. (2005) found a transient increase in alcohol consumption in animals deprived of alcohol before SD, suggesting that alcohol deprivation interacts with SD effects on alcohol consumption.
Among the various mechanisms of action, the HPA axis has received considerable attention. SD increases plasma corticosterone (Pich et al., 1993; Ribiero Do Couto et al., 2006), in some cases for several hours (Koolhaas et al., 1997). SD also enhances corticosterone release in response to subsequent mild stressors, such as exposure to a novel environment. This effect is observed in both male and female rats, although SD females exhibit a higher initial increase in corticosterone than female controls, whereas SD males exhibit a longer lasting elevation of corticosterone relative to male controls (Haney et al., 1995). With repeated exposures to SD, corticosterone release does not habituate, unlike that typically observed with nonsocial stressors (Covington and Miczek, 2005). Enhanced activation of the HPA axis also is involved in SD-induced increases in cocaine self-administration, and this increase is blocked by a CRH1 receptor antagonist administered either systemically or into the ventral tegmental area (Boyson et al., 2011). By contrast, a blunted response of the HPA axis is observed in rats that experience SD during adolescence and then are tested for amphetamine-induced elevation in corticosterone as adults (Burke et al., 2010), suggesting that SD produces differential effects on the HPA axis across the life span.
The mesocorticolimbic dopamine system also is implicated in the SD-induced increase in stimulant abuse–related behaviors. SD or exposure to a cage where social defeat has been experienced previously increases dopamine in nucleus accumbens and enhances cocaine-induced increases in dopamine in the nucleus accumbens (Tidey and Miczek, 1997; Miczek et al., 1999a, 2011a). The enhancement of cocaine-induced dopamine may be related to the accelerated acquisition of cocaine self-administration and increased intake during a binge (Tidey and Miczek, 1997; Miczek et al., 2011a), perhaps because of an SD-induced elevation in BDNF in the ventral tegmental area (Miczek et al., 2011a). BDNF is thought to facilitate dopamine release (Altar et al., 1992; Cordeira et al., 2010), and BDNF in mesolimbic dopamine neurons has been linked to increased cocaine-taking and cocaine-seeking behaviors (Grimm et al., 2003; Graham et al., 2007).
The SD-induced changes in dopamine D2 receptors also are related to behavioral changes in response to stimulants. As mentioned previously, HR rats show greater sensitization to amphetamine and lower dopamine D2 receptor binding in striatum compared with LR rats. However, LR rats exposed to SD exhibit cross-sensitization to amphetamine and have D2 receptor levels similar to HR rats exposed to SD (Dietz et al., 2008). Thus, SD appears to reduce HR and LR individual differences in sensitivity to amphetamine, perhaps as a result of downregulation of striatal dopamine D2 receptors. In contrast, when SD is experienced during adolescence, amphetamine challenge later in life no longer downregulates dopamine D2 receptors (Burke et al., 2011), even though the SD treatment produces enhanced dopamine levels in nucleus accumbens tissue samples (Burke et al., 2010). Further research is needed to determine whether altered responses of mesocortical dopamine neurons play a role in the different behavioral outcomes of SD when experienced during adolescence versus adulthood.
Glutamate systems also are implicated in the effects of SD on stimulant abuse-related behaviors. For instance, SD cross-sensitization with amphetamine, but not amphetamine sensitization alone, is blocked by pretreatment with the glutamate mGluR5 receptor noncompetitive antagonist MPEP during induction (Yap et al., 2005), suggesting a dissociation between the mechanisms involved in SD and drug sensitization effects. SD cross-sensitization with amphetamine-induced locomotion and enhanced cocaine self-administration on a PR schedule or during binge access are blocked by either MK-801 or the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist AP-5 given during induction before SD episodes (Yap et al., 2005; Covington et al., 2008). These effects are mimicked by administration of AP-5 into the ventral tegmental area, suggesting that AMPA receptors are involved in SD-induced increases in cocaine intake (Covington et al., 2008). Further support for this idea is that SD increases AMPA GluR1 receptor subunits, but it has no effect on NMDA NR1 receptor subunits (Covington et al., 2008). Glutamate NMDA NR2 receptor subunit upregulation has been implicated in SD-induced increases in alcohol consumption in CRH1 receptor knock-out mice (Sillaber et al., 2002). Further research is needed to determine the role of NR2-containing NMDA receptors in SD-induced vulnerability to drug abuse–related behaviors.
The effect of SD on alcohol intake has been dissociated from its anxiogenic effects. Croft et al. (2005) examined two different anxiolytics given before SD and found that a cholecystokinin B receptor antagonist blocked SD effects on alcohol consumption, whereas the benzodiazepine receptor agonist diazepam had no effect.
Studies examining immediate early gene expression support a role for activity in mesocorticolimbic structures, as well as other brain regions. Amphetamine challenge increases zif268 mRNA in the medial amygdala of nonstressed rats but decreases it in SD rats (Covington et al., 2005). SD also increases zif268 mRNA relative to nonstressed controls in the central nucleus of the amygdala, and this effect is reversed by an amphetamine challenge. In the infralimbic prefrontal cortex, SD decreases zif268 mRNA, whereas amphetamine challenge has no effect. After an acute cocaine challenge before a single SD episode, the immediate early gene protein Fos and its related antigen (i.e., Fos-Li) are attenuated in the periaqueductal gray, dorsal raphe, and locus coeruleus compared with nonstressed, cocaine-challenged controls (Nikulina et al., 1998). This time point corresponds to the time of induction of cocaine sensitization. Interestingly, acute cocaine administration or SD increases Fos-Li in the periaqueductal gray, but when given together, a decrease is observed, suggesting that Fos likely plays a role in plasticity underlying interactions between these two stressors (Miczek et al., 1999b). When either cocaine or amphetamine challenges are given 7 days after SD, there is an increase in Fos-Li in the periaqueductal gray and locus coeruleus, and when given 17–70 days later, there is an increase in ventral tegmental area as well (Miczek et al., 1999b; Nikulina et al., 2004). There also is an increase in Fos-Li in the central amygdala 17–70 days after SD, regardless of amphetamine challenge (Nikulina et al., 2004). Collectively, these findings suggest that plasticity in response to SD within cell bodies of monoamine neurons, as well as within the defense-relevant periaqueductal gray, is likely involved in subsequent changes in responsivity to stimulants.
In summary, social defeat is a profound stressor that produces long-lasting effects of sensitivity to abused drugs, most notably with stimulants and alcohol. SD produces cross-sensitization to the locomotor and rewarding effects of stimulant drugs. In contrast, repeated SD reduces alcohol self-administration, indicating pharmacological specificity. The increase in stimulant reward likely reflects enhanced CRF1 function within the HPA axis, although alterations in dopamine and glutamate systems also are implicated.
5. Social Rank
Social dominance or subordination can be viewed as either a state or trait. In the previous section, social defeat was considered a state induced when an animal interacts with a dominant conspecific, thus leading to a transient alteration in drug effect in the defeated animal. In the present section, social dominance or subordination is considered a trait that alters drug effects across multiple domains. As a trait, social dominance generally increases resource allocation (food, water, mating opportunities) compared with social submission. In rodents, social dominance can be measured by various behaviors, including aggressive attacks and threatening postures relative to submissive rodents (Blanchard et al., 1993). With nonhuman primates, dominance also is measured by fighting and posturing, as well as increases in general activity, the amount of grooming received, and the latency to approach a novel object (Miczek and Gold, 1983; Czoty et al., 2010).
Social submission is a known risk trait for drug abuse in laboratory animals. In rats and mice, subordinate individuals consume more alcohol and diazepam than dominant individuals (Ellison and Potthoff, 1984; Hilakivi-Clarke and Lister, 1992; Pohorecky, 2006). The elevated intake in subordinate rats is not due to state-based defeat from dominant rats because the same relationship holds when rats are tested after long-term isolation (Wolffgramm and Heyne, 1991). Similarly, subordinate monkeys show greater activity and reinforcement with cocaine or amphetamine (Miczek and Gold, 1983; Morgan et al., 2000, 2002). The difference between subordinate and dominant monkeys is blunted by chronic cocaine self-administration, but the difference re-emerges with abstinence (Czoty et al., 2010), demonstrating the stability of this trait. Subordinate monkeys also consume more alcohol than dominants (McKenzie-Quirk and Miczek, 2008). However, consumption of sucrose fluid is not altered by dominance rank (McKenzie-Quirk and Miczek, 2008), demonstrating that the effect is specific to abused drugs rather than a nonspecific alteration in sensitivity to reinforcement.
Relatively little is known about the precise neural mechanisms underlying differences in social rank. Whereas state-based social defeat activates the HPA axis (Pich et al., 1993; Ribeiro Do Couto et al., 2006), trait-based differences between dominant and subordinate animals are not associated with serum corticosterone levels (Morgan et al., 2000; Stavisky et al., 2001; Ribeiro Do Couto et al., 2006; Czoty et al., 2009; Riddick et al., 2009). Platelet monoamine oxidase (MAO) levels are reduced in subordinate rhesus monkeys (Fahlke et al., 2002). Since MAO is the major enzyme that metabolizes monoamine neurotransmitters such as dopamine and 5-HT, these results suggest that the activity of monoamine brain systems may differ between dominant and subordinate individuals. However, this conclusion is speculative since platelet MAO activity is not a reliable index of MAO activity in brain (Young et al., 1986).
Tied more closely to brain function, CSF assays suggest that altered brain activity is associated with social rank. In monkeys, the CSF level of the dopamine metabolite homovanillic acid is reduced in subordinates (Kaplan et al., 2002), whereas 5-HIAA is increased (Howell et al., 2007; Riddick et al., 2009). These CSF metabolite differences between dominant and subordinate monkeys may be greater in males (Kaplan et al., 2002), although females also exhibit this relationship (Riddick et al., 2009). Regardless of any sex differences, the enhanced vulnerability for drug abuse in subordinate individuals may reflect enhanced dopamine activity (lower metabolism), combined with blunted 5-HT activity (higher metabolism). This conclusion is consistent with evidence in humans suggesting that low levels of impulsive aggression, which typifies subordinate behavior, is associated with the combined effect of dopamine hyperfunctioning and 5-HT hypofunctioning (Seo et al., 2008).
More direct analyses of postmortem monoamine neurochemistry also reveal region-specific alterations in brain function related to social rank. A study in birds revealed that steady-state levels of striatal dopamine are decreased in subordinates compared with dominants (McIntyre and Chew, 1983); however, this dominance-subordination difference is not observed in rodents (Blanchard et al., 1991). More important, indices of functional brain activity are altered by social rank in rodents. Consistent with the results from CSF sampling, subordinate rats show elevated 5-HIAA levels in several brain regions, including the preoptic area, basal hypothalamus, hippocampus, and amygdala, although no changes in DOPAC are evident (Blanchard et al., 1991). In contrast to the 5-HT–associated effects observed in rodents, PET evidence from monkeys shows a decrease in dopamine D2-like receptor availability in subordinates, with no change in 5-HT transporter availability (Morgan et al., 2002; Riddick et al., 2009). The decrease in D2 receptor availability may be related to the enhanced sensitivity of subordinates to the reinforcing effect of cocaine compared with dominants (Morgan et al., 2002). However, PET technology using D2 selective radiotracers such as [11C]raclopride or [18F]fluoroclebopride for imaging does not readily distinguish between receptor levels and alterations in dopamine release, nor does it readily distinguish between autoreceptors and heteroreceptors. In any case, an important aspect of these preclinical findings is that they parallel studies in humans showing that decreased D2 receptor availability is associated with drug reinforcement and abuse (Volkow et al., 1999, 2009).
In summary, social subordination enhances the sensitivity to abused drugs, including stimulants, benzodiazepines, and alcohol; however, little is known about social submission and dominance with opiates. The neural mechanisms of dominance and subordination have been explored primarily using nonhuman primates. These studies reveal that social subordination is associated with decreased D2 receptor availability.
IV. Implications for Prevention and Treatment of Drug Abuse
The current review focuses primarily on the basic neurobehavioral mechanisms involved in individual differences and social influences that alter response to abused drugs. Although a few studies have examined the interactive effects of individual differences and social influences, the vast majority of studies have examined individual and social-based differences separately, and thus these factors are reviewed separately here. The basic research findings covered may have important clinical implications for preventing and treating substance-use disorders among various populations. Although information is limited in this area, some examples illustrate how such findings may be applied in the field and clinic.
In the case of individual differences in facets such as novelty seeking and impulsivity, these traits may serve as useful targeting variables to identify those at greatest risk. To the extent that risk-related traits become stable early in life, they may be useful predictors of vulnerability to drug experimentation before the first drug experience. This would allow for maximizing prevention resources toward those individuals. Although it is difficult to capture and analyze experimentally the first drug experience in real time in humans, the first experience may have a profound influence on the subsequent abuse trajectory. For example, substance abusers report greater “liking” of the first experience compared with nonabusing experimenters, and this effect is observed across all drug classes (Haertzen et al., 1983). Although these results are retrospective, cross-sectional results support the idea that risk-related traits (e.g., sensation seeking) predict the reinforcing effects of amphetamine (Kelly et al., 2006). Thus, prevention efforts among these individuals should be oriented toward reducing or delaying early experimentation, such as through peer-refusal skills and providing alternative nondrug reinforcers.
Nondrug alternative reinforcers decrease drug self-administration using either choice procedures or noncontingent direct exposure to an alternative rewarding stimulus (Carroll et al., 1989; Woolverton and Anderson, 2006). In humans, prosocial behaviors such as physical activity and engaging in high sensation experiences may be effective in reducing drug use. These findings parallel those observed in rats (Klebaur et al., 2001b; Smith and Pitts, 2011), and results from monkeys suggest that enriching stimuli may decrease drug reinforcement (Nader et al., 2008). Not only do alternative appetitive stimuli reduce drug self-administration generally, the effectiveness of alternative stimuli may be greatest among individuals prone to addiction. For example, novel stimulation decreases amphetamine self-administration to a greater extent in HR rats than in LR rats (Cain et al., 2004). Targeting the application of novel, enriching, and physically invigorating stimulation toward those at highest risk may be especially effective in preventing or delaying the initiation and acquisition of drug use.
Some of the social experiences discussed in the current review also are relevant to prevention and treatment. Deviant peer influence is known play a key role in drug use in humans. In a controlled laboratory setting, the mere presence of peers increases the incidence of risky behavior among adolescents (Albert and Steinberg, 2011). Similarly, in laboratory animals, the presence of social peers produces a transient increase in cocaine self-administration (Gipson et al., 2011b). Thus, it is not surprising that the presence of social peers, combined with access to drugs, provides an especially risky environment for some individuals.
In contrast to this risky social situation, evidence suggests that social influences can also influence drug use, with the outcome to some extent dependent on whether a social encounter is positive or negative and whether it occurs in or outside of the drug taking context as reviewed elsewhere (Neisewander et al., 2012). Examples of social influences ameliorating desire for drug may involve the opportunity for social interaction during the later stages of drug abuse. As discussed earlier, cocaine CPP is reversed in rats that are given social interaction episodes after the establishment of CPP (Fritz et al., 2011b). Cocaine CPP established in isolate housed mice also is reversed by a period of living in enriched housing before the CPP test (Solinas et al., 2008). Similarly, when rats that have been trained to self-administer cocaine while isolate housed and are then are moved to enriched housing, they show an attenuation in cocaine-seeking behavior induced by drug-associated cues and stressors (Chauvet et al., 2009; Fritz et al., 2011c; Thiel et al., 2011). These social and environmental enrichment experiences also reverse immediate early gene expression throughout mesocorticolimbic circuits, suggesting that these experiences inhibit brain activation that motivates cocaine-seeking behavior (Thiel et al., 2010, 2011a; Chauvet et al., 2011; Fritz et al., 2011b). Translation of these results to humans will be important, especially given that drug treatment strategies often emphasize socially oriented professional and self-help support groups to promote abstinence.
Finally, despite the recent advances in our understanding of the neural mechanisms underlying individual differences and social influences in sensitivity to abused drugs, several notable gaps exist that may be addressed in future investigations. First, relatively few studies have examined individual differences or social influences on the sensitivity to tetrahydrocannabinol, perhaps because laboratory animal models of tetrahydrocannabinol reward are lacking. This is unfortunate because marijuana use during adolescence and young adulthood is a health concern (Hall and Degenhardt, 2009). Second, relative to monoamine and HPA systems, there is only limited information about the potential role of amino acid neurotransmitters such as GABA and glutamate in mediating individual differences and social influences. Given the important role of GABA and glutamate homeostasis in stimulant reward (Mansvelder and McGehee, 2000; Carroll et al., 2002; Kalivas, 2009), more information in this area is needed. Lastly, the vast majority of preclinical studies in this area have examined either individual differences or social influences as separate independent predictors of sensitivity to abused drugs. Although this approach has yielded predictive relations and key neural mechanisms, it does not capture the multifaceted nature of drug abuse vulnerability in humans. A major challenge will be to identify what combination of individual differences and different social contexts influences the neurobehavioral pharmacology of abused drugs. Moreover, the interactive effects of individual and social-based differences remain an understudied area.
Acknowledgments
The authors acknowledge E. Denehy for technical assistant in manuscript preparation.
Abbreviations
- ACTH
adrenocorticotropic hormone
- AFR
animal facility-reared
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BDNF
brain-derived neurotropic factor
- CPA
conditioned place aversion
- CPP
conditioned place preference
- CSF
cerebrospinal fluid
- CRH
corticotrophin-releasing hormone
- 5-CSRTT
five-choice serial reaction time task
- DAT
dopamine transporter
- DOPAC
3,4-dihydoxyphenylacetic acid
- DRL
differential reinforcement of low response rate
- EC
enriched condition
- FR
fixed ratio
- 5-HIAA
5-hydoxyindoleacetic acid
- HPA
hypothalamic-pituitary-adrenal
- HR
high responder
- 5-HT
serotonin
- IC
isolated condition
- LR
low responder
- MAO
monoamine oxidase
- MDMA
3,4-methylenedioxymethamphetamine
- MEAP
met-enkephlin-Arg6-Phe7
- MHPG
3-methoxy-4-hydroxyphenylglycol
- MS
maternal separation
- NH
nonhandled
- NMDA
N-methyl-d-aspartate
- PET
positron emission tomography
- PR
progressive ratio
- SC
social condition
- SD
social defeat
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Bardo, Neisewander, Kelly.
Footnotes
This work was supported in part by the National Institutes of Health [Grants P50-DA05312, R01-DA12964, UL1-RR033173] (to M.T.B.); National Institutes of Health [Grants DA11064, DA023957] to (J.L.N.); and National Institutes of Health [Grants P50-DA05312, UL1-RR033173] (to T.H.K.).
References
- Abreu-Villaça Y, Queiroz-Gomes FdoE, Dal Monte AP, Filgueiras CC, Manhães AC. (2006) Individual differences in novelty-seeking behavior but not in anxiety response to a new environment can predict nicotine consumption in adolescent C57BL/6 mice. Behav Brain Res 167:175–182 [DOI] [PubMed] [Google Scholar]
- Advani T, Hensler JG, Koek W. (2007) Effect of early rearing conditions on alcohol drinking and 5-HT1A receptor function in C57BL/6J mice. Int J Neuropsychopharmacol 10:595–607 [DOI] [PubMed] [Google Scholar]
- Agmo A, Barreau S, Lemaire V. (1997) Social motivation in recently weaned rats is modified by opiates. Dev Neurosci 19:505–520 [DOI] [PubMed] [Google Scholar]
- Albert D, Steinberg L. (2011) Peer influences on adolescent risk behavior, in Inhibitory Control and Drug Abuse Prevention (Bardo MT, Milich R, Fishbein DH, eds) pp 211–226, Springer, New York [Google Scholar]
- Alcaro A, Huber R, Panksepp J. (2007) Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Brain Res Rev 56:283–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RM, Everett CV, Nelson AM, Gulley JM, Zahniser NR. (2007) Low and high locomotor responsiveness to cocaine predicts intravenous cocaine conditioned place preference in male Sprague-Dawley rats. Pharmacol Biochem Behav 86:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Boylan CB, Jackson C, Hershenson S, Miller J, Wiegand SJ, Lindsay RM, Hyman C. (1992) Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc Natl Acad Sci USA 89:11347–11351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders, 4th ed, American Psychiatric Association, Washington, DC [Google Scholar]
- Anacker AM, Loftis JM, Kaur S, Ryabinin AE. (2011a) Prairie voles as a novel model of socially facilitated excessive drinking. Addict Biol 16:92–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AM, Loftis JM, Ryabinin AE. (2011b) Alcohol intake in prairie voles is influenced by the drinking level of a peer. Alcohol Clin Exp Res 35:1884–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angermeier WF, Schaul LT, James WT. (1959) Social conditioning in rats. J Comp Physiol Psychol 52:370–372 [DOI] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. (2009) Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav 93:343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou K, Papathanasiou G, Panagis G, Nomikos GG, Hyphantis T, Papadopoulou-Daifoti Z. (2004) Individual responses to novelty predict qualitative differences in d-amphetamine-induced open field but not reward-related behaviors in rats. Neuroscience 123:613–623 [DOI] [PubMed] [Google Scholar]
- Aragues M, Jurado R, Quinto R, Rubio G. (2011) Laboratory paradigms of impulsivity and alcohol dependence: a review. Eur Addict Res 17:64–71 [DOI] [PubMed] [Google Scholar]
- Ball SA. (2004) Personality Traits, Disorders, and Substance Abuse, Elsevier, New York [Google Scholar]
- Balleine BW, O’Doherty JP. (2010) Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35:48–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. (1999) Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci 113:1170–1188 [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. (2000) Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 153:31–43 [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bowling SL, Robinet PM, Rowlett JK, Lacy M, Mattingly BA. (1993) Role of dopamine D1 and D2 receptors in novelty-maintained place preference. Exp Clin Psychopharmacol 1:101–109 [Google Scholar]
- Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP. (1995) Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol Biochem Behav 51:397–405 [DOI] [PubMed] [Google Scholar]
- Bardo MT, Cain ME, Bylica KE. (2006) Effect of amphetamine on response inhibition in rats showing high or low response to novelty. Pharmacol Biochem Behav 85:98–104 [DOI] [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. (1996) Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res 77:23–43 [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. (2001) Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 155:278–284 [DOI] [PubMed] [Google Scholar]
- Bardo MT, Valone JM, Robinet PM, Shaw WB, Dwoskin LP. (1999) Environmental enrichment enhances the stimulant effect of intravenous amphetamine: Search for a cellular mechanism in the nucleus accumbens. Psychobiology 27:292–299 [Google Scholar]
- Barnum CJ, Blandino P, Jr, Deak T. (2007) Adaptation in the corticosterone and hyperthermic responses to stress following repeated stressor exposure. J Neuroendocrinol 19:632–642 [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Becker ML, Shannon C, Champoux M, Suomi SJ, Higley JD. (2004a) Early experience and sex interact to influence limbic-hypothalamic-pituitary-adrenal-axis function after acute alcohol administration in rhesus macaques (Macaca mulatta). Alcohol Clin Exp Res 28:1114–1119 [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Shannon BA, Champoux M, Lesch KP, Soumi SJ, Goldman D, Higley JD. (2004b) Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry 61:1146–1152 [DOI] [PubMed] [Google Scholar]
- Bechara A. (2005) Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci 8:1458–1463 [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Marusich JA, Gipson CD, Bardo MT. (2011) Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behav Brain Res 216:159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. (2011) High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacol 36:569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. (2009) Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res 199:89–102 [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science 320:1352–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SM, Gosnell BA, Krahn DD, Meisch RA. (1994) Ethanol reinforcement and its relationship to saccharin preference in Wistar rats. Alcohol 11:141–145 [DOI] [PubMed] [Google Scholar]
- Bennett EL, Rosenzweig MR, Diamond MC. (1969) Rat brain: effects of environmental enrichment on wet and dry weights. Science 163:825–826 [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28:309–369 [DOI] [PubMed] [Google Scholar]
- Bevins RA, Bardo MT. (1999) Conditioned increase in place preference by access to novel objects: antagonism by MK-801. Behav Brain Res 99:53–60 [DOI] [PubMed] [Google Scholar]
- Bevins RA, Klebaur JE, Bardo MT. (1997) Individual differences in response to novelty, amphetamine-induced activity and drug discrimination in rats. Behav Pharmacol 8:113–123 [PubMed] [Google Scholar]
- Bevins RA, Peterson JL. (2004) Individual differences in rats’ reactivity to novelty and the unconditioned and conditioned locomotor effects of methamphetamine. Pharmacol Biochem Behav 79:65–74 [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Koros E, Kostowski W. (2001) Novelty-seeking behaviour and operant oral ethanol self-administration in Wistar rats. Alcohol Alcohol 36:525–528 [DOI] [PubMed] [Google Scholar]
- Bird J, Schenk S. (2012) Contribution of impulsivity and novelty-seeking to the acquisition and maintenance of MDMA self-administration. Addict Biol DOI: 10.1111/j.1369–1600.2012.00477 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Kostowski W. (1993) Individual behavioral differences and ethanol consumption in Wistar rats. Physiol Behav 54:1125–1131 [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Cholvanich P, Blanchard RJ, Clow DW, Hammer RP, Jr, Rowlett JK, Bardo MT. (1991) Serotonin, but not dopamine, metabolites are increased in selected brain regions of subordinate male rats in a colony environment. Brain Res 568:61–66 [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. (1993) Subordination stress: behavioral, brain, and neuroendocrine correlates. Behav Brain Res 58:113–121 [DOI] [PubMed] [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS. (2011) Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PLoS ONE 6:e27237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SL, Bardo MT. (1994) Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol Biochem Behav 48:459–464 [DOI] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. (1993) The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology 32:885–893 [DOI] [PubMed] [Google Scholar]
- Boyson CO, Miguel TT, Quadros IM, Debold JF, Miczek KA. (2011) Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl) 218:257–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. (2004) Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci 19:1863–1874 [DOI] [PubMed] [Google Scholar]
- Brenes JC, Rodríguez O, Fornaguera J. (2008) Differential effect of environment enrichment and social isolation on depressive-like behavior, spontaneous activity and serotonin and norepinephrine concentration in prefrontal cortex and ventral striatum. Pharmacol Biochem Behav 89:85–93 [DOI] [PubMed] [Google Scholar]
- Briegleb SK, Gulley JM, Hoover BR, Zahniser NR. (2004) Individual differences in cocaine- and amphetamine-induced activation of male Sprague-Dawley rats: contribution of the dopamine transporter. Neuropsychopharmacology 29:2168–2179 [DOI] [PubMed] [Google Scholar]
- Broos N, Diergaarde L, Schoffelmeer AN, Pattij T, De Vries TJ. (2012) Trait impulsive choice predicts resistance to extinction and propensity to relapse to cocaine seeking: a bidirectional investigation. Neuropsychopharmacology 37:1377–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. (2008) Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol 122:357–367 [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. (2006) The neurobiology of positive emotions. Neurosci Biobehav Rev 30:173–187 [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. (2007) Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res 182:274–283 [DOI] [PubMed] [Google Scholar]
- Burke AR, Renner KJ, Forster GL, Watt MJ. (2010) Adolescent social defeat alters neural, endocrine and behavioral responses to amphetamine in adult male rats. Brain Res 1352:147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Watt MJ, Forster GL. (2011) Adolescent social defeat increases adult amphetamine conditioned place preference and alters D2 dopamine receptor expression. Neuroscience 197:269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DE, Vaccarino FJ. (2007) Individual differences in elevated plus-maze exploration predicted progressive-ratio cocaine self-administration break points in Wistar rats. Psychopharmacology (Berl) 194:211–219 [DOI] [PubMed] [Google Scholar]
- Cain ME, Coolon RA, Gill MJ. (2009) The contribution of the central nucleus of the amygdala to individual differences in amphetamine-induced hyperactivity. Behav Brain Res 202:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain ME, Denehy ED, Bardo MT. (2008) Individual differences in amphetamine self-administration: the role of the central nucleus of the amygdala. Neuropsychopharmacology 33:1149–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain ME, Dotson WF, Bardo MT. (2006) Individual differences in the effect of novel environmental stimuli prior to amphetamine self-administration in rats (Rattus norvegicus). Exp Clin Psychopharmacol 14:389–401 [DOI] [PubMed] [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. (2005) Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol 13:367–375 [DOI] [PubMed] [Google Scholar]
- Cain ME, Smith CM, Bardo MT. (2004) The effect of novelty on amphetamine self-administration in rats classified as high and low responders. Psychopharmacology (Berl) 176:129–138 [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. (1992) Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav 51:667–672 [DOI] [PubMed] [Google Scholar]
- Caldwell EE, Riccio DC. (2010) Alcohol self-administration in rats: modulation by temporal parameters related to repeated mild social defeat stress. Alcohol 44:265–274 [DOI] [PubMed] [Google Scholar]
- Campbell J, Spear LP. (1999) Effects of early handling on amphetamine-induced locomotor activation and conditioned place preference in the adult rat. Psychopharmacology (Berl) 143:183–189 [DOI] [PubMed] [Google Scholar]
- Capriles N, Watson S, Jr, Akil H. (2012) Individual differences in the improvement of cocaine-induced place preference response by the 5-HT(2C) receptor antagonist SB242084 in rats. Psychopharmacology (Berl) 220:731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden SE, Barr GA, Hofer MA. (1991) Differential effects of specific opioid receptor agonists on rat pup isolation calls. Brain Res Dev Brain Res 62:17–22 [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. (2001) Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 292:2499–2501 [DOI] [PubMed] [Google Scholar]
- Carey RJ, Shanahan A, Damianopoulos EN, Müller CP, Huston JP. (2008) Behavior selectively elicited by novel stimuli: modulation by the 5-HT1A agonist 8-OHDPAT and antagonist WAY-100635. Behav Pharmacol 19:361–364 [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Nygaard SL. (1989) A concurrently available nondrug reinforcer prevents the acquisition or decreases the maintenance of cocaine-reinforced behavior. Psychopharmacology (Berl) 97:23–29 [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. (2008) Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol 19:435–460 [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. (2002) Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 161:304–313 [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. (2006) Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci 7:583–590 [DOI] [PubMed] [Google Scholar]
- Chartier KG, Hesselbrock MN, Hesselbrock VM. (2010) Development and vulnerability factors in adolescent alcohol use. Child Adolesc Psychiatr Clin N Am 19:493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. (2009) Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology 34:2767–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Jaber M, Solinas M. (2011) Brain regions associated with the reversal of cocaine conditioned place preference by environmental enrichment. Neuroscience 184:88–96 [DOI] [PubMed] [Google Scholar]
- Chefer VI, Zakharova I, Shippenberg TS. (2003) Enhanced responsiveness to novelty and cocaine is associated with decreased basal dopamine uptake and release in the nucleus accumbens: quantitative microdialysis in rats under transient conditions. J Neurosci 23:3076–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Sharp BM, Matta SG, Wu QL. (2011) Social interaction promotes nicotine self-administration with olfactogustatory cues in adolescent rats. Neuropsychopharmacology 36:2629–2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. (2003) Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res 146:105–119 [DOI] [PubMed] [Google Scholar]
- Comer SD, Bickel WK, Yi R, de Wit H, Higgins ST, Wenger GR, Johanson CE, Kreek MJ. (2010) Human behavioral pharmacology, past, present, and future: symposium presented at the 50th annual meeting of the Behavioral Pharmacology Society. Behav Pharmacol 21:251–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comery TA, Stamoudis CX, Irwin SA, Greenough WT. (1996) Increased density of multiple-head dendritic spines on medium-sized spiny neurons of the striatum in rats reared in a complex environment. Neurobiol Learn Mem 66:93–96 [DOI] [PubMed] [Google Scholar]
- Coolon RA, Cain ME. (2009) Individual differences in response to novelty and the conditioned locomotor effects of nicotine. Behav Pharmacol 20:322–329 [DOI] [PubMed] [Google Scholar]
- Cooper ML. (1994) Motivations for alcohol use among adolescents: development and validation of a four-factor model. Psychol Assess 6:117–128 [Google Scholar]
- Cordeira JW, Frank L, Sena-Esteves M, Pothos EN, Rios M. (2010) Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J Neurosci 30:2533–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. (2005) Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology 30:310–321 [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. (2001) Repeated social-defeat stress, cocaine or morphine: effects on behavioral sensitization and intravenous cocaine self-administration “binges.” Psychopharmacology (Berl) 158:388–398 [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. (2005) Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 183:331–340 [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA. (2008) NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology (Berl) 197:203–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft AP, Brooks SP, Cole J, Little HJ. (2005) Social defeat increases alcohol preference of C57BL/10 strain mice: effect prevented by a CCKB antagonist. Psychopharmacology (Berl) 183:163–170 [DOI] [PubMed] [Google Scholar]
- Crowder WF, Hutto CW., Jr (1992) Operant place conditioning measures examined using two nondrug reinforcers. Pharmacol Biochem Behav 41:817–824 [DOI] [PubMed] [Google Scholar]
- Cruz FC, Quadros IM, Hogenelst K, Planeta CS, Miczek KA. (2011) Social defeat stress in rats: escalation of cocaine and “speedball” binge self-administration, but not heroin. Psychopharmacology (Berl) 215:165–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Quadros IM, Planeta CdaS, Miczek KA. (2008) Maternal separation stress in male mice: long-term increases in alcohol intake. Psychopharmacology (Berl) 201:459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB. (2011) Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing BS, Kramer KM. (2005) Mechanisms underlying epigenetic effects of early social experience: the role of neuropeptides and steroids. Neurosci Biobehav Rev 29:1089–1105 [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gage HD, Nader MA. (2010) Differences in D2 dopamine receptor availability and reaction to novelty in socially housed male monkeys during abstinence from cocaine. Psychopharmacology (Berl) 208:585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Nader MA. (2009) Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis). J Neuroendocrinol 21:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. (2011) Impulsivity, compulsivity, and top-down cognitive control. Neuron 69:680–694 [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, et al. (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315:1267–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoura L, Haaker J, Nylander I. (2011) Early environmental factors differentially affect voluntary ethanol consumption in adolescent and adult male rats. Alcohol Clin Exp Res 35:506–515 [DOI] [PubMed] [Google Scholar]
- Daoura L, Nylander I. (2011) The response to naltrexone in ethanol-drinking rats depends on early environmental experiences. Pharmacol Biochem Behav 99:626–633 [DOI] [PubMed] [Google Scholar]
- Davis BA, Clinton SM, Akil H, Becker JB. (2008) The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred high-responder and low-responder rats. Pharmacol Biochem Behav 90:331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho CR, Pandolfo P, Pamplona FA, Takahashi RN. (2010) Environmental enrichment reduces the impact of novelty and motivational properties of ethanol in spontaneously hypertensive rats. Behav Brain Res 208:231–236 [DOI] [PubMed] [Google Scholar]
- De Deurwaerdère P, Navailles S, Berg KA, Clarke WP, Spampinato U. (2004) Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci 24:3235–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JG, Wasilewski M, van der Vegt BJ, Buwalda B, Koolhaas JM. (2005) A single social defeat induces short-lasting behavioral sensitization to amphetamine. Physiol Behav 83:805–811 [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. (2009) Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol 30:358–370 [DOI] [PubMed] [Google Scholar]
- de Wit H. (2009) Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14:22–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. (1996) Novelty-seeking in rats: biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology 34:136–145 [DOI] [PubMed] [Google Scholar]
- DeMartini KS, Carey KB. (2011) The role of anxiety sensitivity and drinking motives in predicting alcohol use: a critical review. Clin Psychol Rev 31:169–177 [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. (2010) Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience 170:1189–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Le Moal M, Simon H. (1993) Individual differences in the psychomotor effects of morphine are predicted by reactivity to novelty and influenced by corticosterone secretion. Brain Res 623:341–344 [DOI] [PubMed] [Google Scholar]
- Diamond MC. (2001) Response of the brain to enrichment. An Acad Bras Cienc 73:211–220 [DOI] [PubMed] [Google Scholar]
- Diamond MC, Krech D, Rosenzweig MR. (1964) The effects of an enriched environment on the histology of the rat cerebral cortex. J Comp Neurol 123:111–120 [DOI] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. (2010) Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol 15:217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. (2008) Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry 63:301–308 [DOI] [PubMed] [Google Scholar]
- Diergaarde L, van Mourik Y, Pattij T, Schoffelmeer AN, De Vries TJ. (2012) Poor impulse control predicts inelastic demand for nicotine but not alcohol in rats. Addict Biol. 17:575–587 [DOI] [PubMed] [Google Scholar]
- Dietz DM, Dietz KC, Moore S, Ouimet CC, Kabbaj M. (2008) Repeated social defeat stress-induced sensitization to the locomotor activating effects of d-amphetamine: role of individual differences. Psychopharmacology (Berl) 198:51–62 [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. (2004) Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol 45:153–162 [DOI] [PubMed] [Google Scholar]
- Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW. (2008) Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb Cortex 18:178–188 [DOI] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. (2003) Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci 117:1302–1317 [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. (2009) High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry 65:851–856 [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Walker BM, Pian JP, Roth JL, Slawecki CJ. (2007) Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behav Neurosci 121:111–119 [DOI] [PubMed] [Google Scholar]
- El Rawas R, Thiriet N, Lardeux V, Jaber M, Solinas M. (2009) Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacology (Berl) 203:561–570 [DOI] [PubMed] [Google Scholar]
- El Rawas R, Thiriet N, Nader J, Lardeux V, Jaber M, Solinas M. (2011) Early exposure to environmental enrichment alters the expression of genes of the endocannabinoid system. Brain Res 1390:80–89 [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, van der Kam EL, van der Elst MC, Cools AR. (2005) Individual differences in drug dependence in rats: the role of genetic factors and life events. Eur J Pharmacol 526:251–258 [DOI] [PubMed] [Google Scholar]
- Ellison GD, Potthoff AD. (1984) Social models of drinking behavior in animals: the importance of individual differences. Recent Dev Alcohol 2:17–36 [DOI] [PubMed] [Google Scholar]
- Engler H, Engler A, Bailey MT, Sheridan JF. (2005) Tissue-specific alterations in the glucocorticoid sensitivity of immune cells following repeated social defeat in mice. J Neuroimmunol 163:110–119 [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Aggleton JP. (1997) The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behav Brain Res 88:181–193 [DOI] [PubMed] [Google Scholar]
- Erb SM, Parker LA. (1994) Individual differences in novelty-induced activity do not predict strength of amphetamine-induced place conditioning. Pharmacol Biochem Behav 48:581–586 [DOI] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. (2010) Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry 68:770–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Duvel A, Funk ML, Lehman B, Sparrow J, Watson NT, Neuringer A. (1994) Social reinforcement of operant behavior in rats: a methodological note. J Exp Anal Behav 62:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. (1999) Varieties of impulsivity. Psychopharmacology (Berl) 146:348–361 [DOI] [PubMed] [Google Scholar]
- Fahlke C, Garpenstrand H, Oreland L, Suomi SJ, Higley JD. (2002) Platelet monoamine oxidase activity in a nonhuman primate model of type 2 excessive alcohol consumption. Am J Psychiatry 159:2107. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, Higley JD. (2000) Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcohol Clin Exp Res 24:644–650 [PubMed] [Google Scholar]
- Falk JL. (1983) Drug dependence: myth or motive? Pharmacol Biochem Behav 19:385–391 [DOI] [PubMed] [Google Scholar]
- Faturi CB, Tiba PA, Kawakami SE, Catallani B, Kerstens M, Suchecki D. (2010) Disruptions of the mother-infant relationship and stress-related behaviours: altered corticosterone secretion does not explain everything. Neurosci Biobehav Rev 34:821–834 [DOI] [PubMed] [Google Scholar]
- Faure J, Stein DJ, Daniels W. (2009) Maternal separation fails to render animals more susceptible to methamphetamine-induced conditioned place preference. Metab Brain Dis 24:541–559 [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Botterblom MH, Mastenbroek S. (2000) Dopamine and noradrenaline efflux in the prefrontal cortex in the light and dark period: effects of novelty and handling and comparison to the nucleus accumbens. Neuroscience 100:741–748 [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. (2007) Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend 89:183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. (2000) Social amnesia in mice lacking the oxytocin gene. Nat Genet 25:284–288 [DOI] [PubMed] [Google Scholar]
- Fernández-Vidal JM, Molina JC. (2004) Socially mediated alcohol preferences in adolescent rats following interactions with an intoxicated peer. Pharmacol Biochem Behav 79:229–241 [DOI] [PubMed] [Google Scholar]
- File SE, Wardill AG. (1975) The reliability of the hole-board apparatus. Psychopharmacology (Berl) 44:47–51 [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Ostling EW, Martin CA, Kelly TH. (2009) Acute effects of alcohol on inhibitory control and information processing in high and low sensation-seekers. Drug Alcohol Depend 100:91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske DW, Maddi SR. (1961) Functions of Varied Experience, The Dorsey Press, Inc, Homewood, IL [Google Scholar]
- Flagel SB, Vázquez DM, Robinson TE. (2003) Manipulations during the second, but not the first, week of life increase susceptibility to cocaine self-administration in female rats. Neuropsychopharmacology 28:1741–1751 [DOI] [PubMed] [Google Scholar]
- Floresco SB, Grace AA. (2003) Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. J Neurosci 23:3930–3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Kuhar MJ. (2008) Frequency of maternal licking and grooming correlates negatively with vulnerability to cocaine and alcohol use in rats. Pharmacol Biochem Behav 90:497–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Klement S, Kummer K, Mayr MJ, Eggart V, Salti A, Bardo MT, Saria A, Zernig G. (2011a) Differential effects of accumbens core vs. shell lesions in a rat concurrent conditioned place preference paradigm for cocaine vs. social interaction. PLoS ONE 6:e26761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, Dechant G, Saria A, Zernig G. (2011b) Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict Biol 16:273–284 [DOI] [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, Dechant G, Saria A, Zernig G. (2011c) Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict Biol 16:273–284 [DOI] [PubMed] [Google Scholar]
- Fritz M, Klement S, El Rawas R, Saria A, Zernig G. (2011d) Sigma1 receptor antagonist BD1047 enhances reversal of conditioned place preference from cocaine to social interaction. Pharmacology 87:45–48 [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. (2005) Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 179:662–672 [DOI] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Lê AD. (2005) Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 183:341–349 [DOI] [PubMed] [Google Scholar]
- Fuster JM. (2008) The Prefrontal Cortex, Elsevier, Amsterdam [Google Scholar]
- Galani R, Berthel MC, Lazarus C, Majchrzak M, Barbelivien A, Kelche C, Cassel JC. (2007) The behavioral effects of enriched housing are not altered by serotonin depletion but enrichment alters hippocampal neurochemistry. Neurobiol Learn Mem 88:1–10 [DOI] [PubMed] [Google Scholar]
- Galanopoulos A, Polissidis A, Papadopoulou-Daifoti Z, Nomikos GG, Antoniou K. (2011) Δ(9)-THC and WIN55,212-2 affect brain tissue levels of excitatory amino acids in a phenotype-, compound-, dose-, and region-specific manner. Behav Brain Res 224:65–72 [DOI] [PubMed] [Google Scholar]
- Gancarz AM, San George MA, Ashrafioun L, Richards JB. (2011) Locomotor activity in a novel environment predicts both responding for a visual stimulus and self-administration of a low dose of methamphetamine in rats. Behav Processes 86:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Lecumberri C, Torres I, Martín S, Crespo JA, Miguéns M, Nicanor C, Higuera-Matas A, Ambrosio E. (2011) Strain differences in the dose-response relationship for morphine self-administration and impulsive choice between Lewis and Fischer 344 rats. J Psychopharmacol 25:783–791 [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Briscoe RJ, Goulden KL, Holloway FA. (1994) Aversive attributes of ethanol can be attenuated by dyadic social interaction in the rat. Alcohol 11:247–251 [DOI] [PubMed] [Google Scholar]
- Gilad GM, Rabey JM, Gilad VH. (1987) Presynaptic effects of glucocorticoids on dopaminergic and cholinergic synaptosomes: implications for rapid endocrine-neural interactions in stress. Life Sci 40:2401–2408 [DOI] [PubMed] [Google Scholar]
- Gill TM, Castaneda PJ, Janak PH. (2010) Dissociable roles of the medial prefrontal cortex and nucleus accumbens core in goal-directed actions for differential reward magnitude. Cereb Cortex 20:2884–2899 [DOI] [PubMed] [Google Scholar]
- Gingras MA, Cools AR. (1995) Differential ethanol intake in high and low responders to novelty. Behav Pharmacol 6:718–723 [PubMed] [Google Scholar]
- Gingras MA, Cools AR. (1997) Different behavioral effects of daily or intermittent dexamphetamine administration in Nijmegen high and low responders. Psychopharmacology (Berl) 132:188–194 [DOI] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. (2011a) Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology (Berl) 214:557–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Yates JR, Beckmann JS, Marusich JA, Zentall TR, Bardo MT. (2011b) Social facilitation of d-amphetamine self-administration in rats. Exp Clin Psychopharmacol 19:409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE. (2002) The HPA axis and cocaine reinforcement. Psychoneuroendocrinology 27:13–33 [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. (2005) The genetics of addictions: uncovering the genes. Nat Rev Genet 6:521–532 [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. (2009) The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci 13:372–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosnell BA. (2000) Sucrose intake predicts rate of acquisition of cocaine self-administration. Psychopharmacology (Berl) 149:286–292 [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. (2007) Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci 10:1029–1037 [DOI] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, Graham AR, Unterberg S, Graham DL, Vialou V, et al. (2010) Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry 67:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Cain ME, Thompson M, Bardo MT. (2003) Environmental enrichment decreases nicotine-induced hyperactivity in rats. Psychopharmacology (Berl) 170:235–241 [DOI] [PubMed] [Google Scholar]
- Greisen MH, Bolwig TG, Wörtwein G. (2005) Cholecystokinin tetrapeptide effects on HPA axis function and elevated plus maze behaviour in maternally separated and handled rats. Behav Brain Res 161:204–212 [DOI] [PubMed] [Google Scholar]
- Gresack JE, Risbrough VB, Scott CN, Coste S, Stenzel-Poore M, Geyer MA, Powell SB. (2010) Isolation rearing-induced deficits in contextual fear learning do not require CRF(2) receptors. Behav Brain Res 209:80–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R, Bigelow G, Liebson I. (1974) Suppression of ethanol self-administration in alcoholics by contingent time-out from social interactions. Behav Res Ther 12:327–334 [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow G, Liebson I. (1977) Comparison of social time-out and activity time-out procedures in supressing ethanol self-administration in alcoholics. Behav Res Ther 15:329–336 [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. (2003) Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci 23:742–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley JM. (2007) Individual differences in novelty- and cocaine-induced locomotor activity as predictors of food-reinforced operant behavior in two outbred rat strains. Pharmacol Biochem Behav 86:749–757 [DOI] [PubMed] [Google Scholar]
- Gulley JM, Hoover BR, Larson GA, Zahniser NR. (2003) Individual differences in cocaine-induced locomotor activity in rats: behavioral characteristics, cocaine pharmacokinetics, and the dopamine transporter. Neuropsychopharmacology 28:2089–2101 [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Nylander I. (2006) Time-dependent alterations in ethanol intake in male wistar rats exposed to short and prolonged daily maternal separation in a 4-bottle free-choice paradigm. Alcohol Clin Exp Res 30:2008–2016 [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Ploj K, Nylander I. (2005) Effects of maternal separation on voluntary ethanol intake and brain peptide systems in female Wistar rats. Pharmacol Biochem Behav 81:506–516 [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Zhou Q, Nylander I. (2007) Ethanol-induced effects on opioid peptides in adult male Wistar rats are dependent on early environmental factors. Neuroscience 146:1137–1149 [DOI] [PubMed] [Google Scholar]
- Haertzen CA, Kocher TR, Miyasato K. (1983) Reinforcements from the first drug experience can predict later drug habits and/or addiction: results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers, stimulants, marijuana, hallucinogens, heroin, opiates and cocaine. Drug Alcohol Depend 11:147–165 [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Robbins TW. (1999) Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse 32:37–43 [DOI] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. (2009) Adverse health effects of non-medical cannabis use. Lancet 374:1383–1391 [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH. (2006) Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol 6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. (1995) Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res 698:46–52 [DOI] [PubMed] [Google Scholar]
- Harati H, Barbelivien A, Cosquer B, Majchrzak M, Cassel JC. (2008) Selective cholinergic lesions in the rat nucleus basalis magnocellularis with limited damage in the medial septum specifically alter attention performance in the five-choice serial reaction time task. Neuroscience 153:72–83 [DOI] [PubMed] [Google Scholar]
- Hayton SJ, Mahoney MK, Olmstead MC. (2012) Behavioral traits predicting alcohol drinking in outbred rats: an investigation of anxiety, novelty seeking, and cognitive flexibility. Alcohol Clin Exp Res 36:594–603 [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML. (1989) Effect of d-amphetamine, secobarbital, and marijuana on choice behavior: social versus nonsocial options. Psychopharmacology (Berl) 99:156–162 [DOI] [PubMed] [Google Scholar]
- Hensleigh E, Smedley L, Pritchard LM. (2011) Sex, but not repeated maternal separation during the first postnatal week, influences novel object exploration and amphetamine sensitivity. Dev Psychobiol 53:132–140 [DOI] [PubMed] [Google Scholar]
- Higgins ST, Stitzer ML. (1986) Acute marijuana effects on social conversation. Psychopharmacology (Berl) 89:234–238 [DOI] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. (1991) Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Sci USA 88:7261–7265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. (1996) A nonhuman primate model of type II excessive alcohol consumption? Part 1. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcohol Clin Exp Res 20:629–642 [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Lister RG. (1992) Social status and voluntary alcohol consumption in mice: interaction with stress. Psychopharmacology (Berl) 108:276–282 [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke LA, Turkka J, Lister RG, Linnoila M. (1991) Effects of early postnatal handling on brain beta-adrenoceptors and behavior in tests related to stress. Brain Res 542:286–292 [DOI] [PubMed] [Google Scholar]
- Hodos W, Ross GS, Brady JV. (1962) Complex response patterns during temporally spaced responding. J Exp Anal Behav 5:473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA. (1970) Physiological responses of infant rats to separation from their mothers. Science 168:871–873 [DOI] [PubMed] [Google Scholar]
- Homberg JR. (2012) Serotonin and decision making processes. Neurosci Biobehav Rev 36:218–236 [DOI] [PubMed] [Google Scholar]
- Hommer DW, Bjork JM, Gilman JM. (2011) Imaging brain response to reward in addictive disorders. Ann N Y Acad Sci 1216:50–61 [DOI] [PubMed] [Google Scholar]
- Hooks MS, Colvin AC, Juncos JL, Justice JB., Jr (1992) Individual differences in basal and cocaine-stimulated extracellular dopamine in the nucleus accumbens using quantitative microdialysis. Brain Res 587:306–312 [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones DN, Holtzman SG, Juncos JL, Kalivas PW, Justice JB. (1994a) Individual differences in behavior following amphetamine, GBR-12909, or apomorphine but not SKF38393 or quinpirole. Psychopharmacology (Berl) 116:217–225 [DOI] [PubMed] [Google Scholar]
- Hooks MS, Juncos JL, Justice JB, Jr, Meiergerd SM, Povlock SL, Schenk JO, Kalivas PW. (1994b) Individual locomotor response to novelty predicts selective alterations in D1 and D2 receptors and mRNAs. J Neurosci 14:6144–6152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath LS, Milich R, Lynam D, Leukefeld C, Clayton R. (2004) Sensation seeking and substance use: a cross-lagged panel design. Individ Differ Res 2:175–183 [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. (2007) Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther 320:757–765 [DOI] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. (2000) Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berl) 151:55–63 [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. (1988) Behavioral “properties” of drugs. Psychopharmacology (Berl) 96:557. [DOI] [PubMed] [Google Scholar]
- Hughes RN. (1968) Behaviour of male and female rats with free choice of two environments differing in novelty. Anim Behav 16:92–96 [DOI] [PubMed] [Google Scholar]
- Hunt PS, Holloway JL, Scordalakes EM. (2001) Social interaction with an intoxicated sibling can result in increased intake of ethanol by periadolescent rats. Dev Psychobiol 38:101–109 [DOI] [PubMed] [Google Scholar]
- Hunt PS, Lant GM, Carroll CA. (2000) Enhanced intake of ethanol in preweanling rats following interactions with intoxicated siblings. Dev Psychobiol 37:90–99 [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. (2001) Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 158:366–373 [DOI] [PubMed] [Google Scholar]
- Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, Innis RB. (2006) Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. J Neurosci 26:4638–4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. (1992) Oxytocin—a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology 17:3–35 [DOI] [PubMed] [Google Scholar]
- Irazusta J, Tejedor-Real P, Varona A, Costela C, Gilbert-Rahola J, Casis L. (1999) Effect of neonatal handling on brain enkephalin-degrading peptidase activities. Neurochem Int 35:357–361 [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. (2006) Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology 31:129–138 [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Francis DD, Brommer CL, Morgan ET, Kuhar MJ. (2005) Effects of early maternal separation on ethanol intake, GABA receptors and metabolizing enzymes in adult rats. Psychopharmacology (Berl) 181:8–15 [DOI] [PubMed] [Google Scholar]
- Johansson AK, Hansen S. (2002) Novelty seeking and harm avoidance in relation to alcohol drinking in intact rats and following axon-sparing lesions to the amygdala and ventral striatum. Alcohol Alcohol 37:147–156 [DOI] [PubMed] [Google Scholar]
- Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW. (1992) Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol Biochem Behav 43:17–35 [DOI] [PubMed] [Google Scholar]
- Jones GH, Marsden CA, Robbins TW. (1991) Behavioural rigidity and rule-learning deficits following isolation-rearing in the rat: neurochemical correlates. Behav Brain Res 43:35–50 [DOI] [PubMed] [Google Scholar]
- Joseph JE, Liu X, Jiang Y, Lynam D, Kelly TH. (2009) Neural correlates of emotional reactivity in sensation seeking. Psychol Sci 20:215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M. (2006) Individual differences in vulnerability to drug abuse: the high responders/low responders model. CNS Neurol Disord Drug Targets 5:513–520 [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Isgor C, Watson SJ, Akil H. (2002) Stress during adolescence alters behavioral sensitization to amphetamine. Neuroscience 113:395–400 [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H. (2001) Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology (Berl) 158:382–387 [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Holtzman SG. (2002) Early neonatal experience of Long-Evans rats results in long-lasting changes in reactivity to a novel environment and morphine-induced sensitization and tolerance. Neuropsychopharmacology 27:518–533 [DOI] [PubMed] [Google Scholar]
- Kalinichev M, White DA, Holtzman SG. (2004) Individual differences in locomotor reactivity to a novel environment and sensitivity to opioid drugs in the rat. I. Expression of morphine-induced locomotor sensitization. Psychopharmacology (Berl) 177:61–67 [DOI] [PubMed] [Google Scholar]
- Kalivas PW. (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572 [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Fontenot MB, Mann JJ. (2002) Central nervous system monoamine correlates of social dominance in cynomolgus monkeys (Macaca fascicularis). Neuropsychopharmacology 26:431–443 [DOI] [PubMed] [Google Scholar]
- Keeney AJ, Hogg S. (1999) Behavioural consequences of repeated social defeat in the mouse: preliminary evaluation of a potential animal model of depression. Behav Pharmacol 10:753–764 [DOI] [PubMed] [Google Scholar]
- Kehoe P, Shoemaker WJ, Triano L, Callahan M, Rappolt G. (1998) Adult rats stressed as neonates show exaggerated behavioral responses to both pharmacological and environmental challenges. Behav Neurosci 112:116–125 [DOI] [PubMed] [Google Scholar]
- Kehoe P, Shoemaker WJ, Triano L, Hoffman J, Arons C. (1996) Repeated isolation in the neonatal rat produces alterations in behavior and ventral striatal dopamine release in the juvenile after amphetamine challenge. Behav Neurosci 110:1435–1444 [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. (2002) The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22:3306–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TH, Delzer TA, Martin CA, Harrington NG, Hays LR, Bardo MT. (2009) Performance and subjective effects of diazepam and d-amphetamine in high and low sensation seekers. Behav Pharmacol 20:505–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TH, Robbins G, Martin CA, Fillmore MT, Lane SD, Harrington NG, Rush CR. (2006) Individual differences in drug abuse vulnerability: d-amphetamine and sensation-seeking status. Psychopharmacology (Berl) 189:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BC, Panksepp JB, Runckel PA, Lahvis GP. (2012) Social influences on morphine-conditioned place preference in adolescent BALB/cJ and C57BL/6J mice. Psychopharmacology (Berl) 219:923–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Faccidomo S, Miczek KA. (2005) Repeated maternal separation: differences in cocaine-induced behavioral sensitization in adult male and female mice. Psychopharmacology (Berl) 178:202–210 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee JH, Choi SH, Lee YS, Jahng JW. (2005) Fasting-induced increases of arcuate NPY mRNA and plasma corticosterone are blunted in the rat experienced neonatal maternal separation. Neuropeptides 39:587–594 [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. (2005) Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 182:245–252 [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bardo MT. (1999) Individual differences in novelty seeking on the playground maze predict amphetamine conditioned place preference. Pharmacol Biochem Behav 63:131–136 [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bevins RA, Segar TM, Bardo MT. (2001a) Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol 12:267–275 [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Phillips SB, Kelly TH, Bardo MT. (2001b) Exposure to novel environmental stimuli decreases amphetamine self-administration in rats. Exp Clin Psychopharmacol 9:372–379 [PubMed] [Google Scholar]
- Kliethermes CL, Crabbe JC. (2006) Pharmacological and genetic influences on hole-board behaviors in mice. Pharmacol Biochem Behav 85:57–65 [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Kamens HM, Crabbe JC. (2007) Drug reward and intake in lines of mice selectively bred for divergent exploration of a hole board apparatus. Genes Brain Behav 6:608–618 [DOI] [PubMed] [Google Scholar]
- Knight R. (1996) Contribution of human hippocampal region to novelty detection. Nature 383:256–259 [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Söderpalm AH, Robinson TE. (2003) Environmental complexity has different effects on the structure of neurons in the prefrontal cortex versus the parietal cortex or nucleus accumbens. Synapse 48:149–153 [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. (2006) Neurobiology of Addiction, Elsevier, Amsterdam [Google Scholar]
- Koolhaas JM, Meerlo P, De Boer SF, Strubbe JH, Bohus B. (1997) The temporal dynamics of the stress response. Neurosci Biobehav Rev 21:775–782 [DOI] [PubMed] [Google Scholar]
- Kosten TA, Ball SA, Rounsaville BJ. (1994) A sibling study of sensation seeking and opiate addiction. J Nerv Ment Dis 182:284–289 [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ. (1998) Dissociation of novelty- and cocaine-conditioned locomotor activity from cocaine place conditioning. Pharmacol Biochem Behav 60:785–791 [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Kehoe P. (2000) Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res 875:44–50 [DOI] [PubMed] [Google Scholar]
- Kosten TA, Sanchez H, Jatlow PI, Kehoe P. (2005a) Neonatal isolation alters the estrous cycle interactions on the acute behavioral effects of cocaine. Psychoneuroendocrinology 30:753–761 [DOI] [PubMed] [Google Scholar]
- Kosten TA, Sanchez H, Zhang XY, Kehoe P. (2004) Neonatal isolation enhances acquisition of cocaine self-administration and food responding in female rats. Behav Brain Res 151:137–149 [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Kehoe P. (2005b) Neurochemical and behavioral responses to cocaine in adult male rats with neonatal isolation experience. J Pharmacol Exp Ther 314:661–667 [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Kehoe P. (2005c) Neurochemical and behavioral responses to cocaine in adult male rats with neonatal isolation experience. J Pharmacol Exp Ther 314:661–667 [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Kehoe P. (2006) Heightened cocaine and food self-administration in female rats with neonatal isolation experience. Neuropsychopharmacology 31:70–76 [DOI] [PubMed] [Google Scholar]
- Kraemer GW, McKinney WT. (1985) Social separation increases alcohol consumption in rhesus monkeys. Psychopharmacology (Berl) 86:182–189 [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Madorskaya IA, Bakshtanovskaya IV. (1991) Social success and voluntary ethanol consumption in mice of C57BL/6J and CBA/Lac strains. Physiol Behav 50:143–146 [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. (2004) Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biol Psychiatry 55:367–375 [DOI] [PubMed] [Google Scholar]
- Larson EB, Carroll ME. (2005) Wheel running as a predictor of cocaine self-administration and reinstatement in female rats. Pharmacol Biochem Behav 82:590–600 [DOI] [PubMed] [Google Scholar]
- Lehmann J, Stöhr T, Schuller J, Domeney A, Heidbreder C, Feldon J. (1998) Long-term effects of repeated maternal separation on three different latent inhibition paradigms. Pharmacol Biochem Behav 59:873–882 [DOI] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. (2002) Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology 27:1027–1035 [DOI] [PubMed] [Google Scholar]
- Li Y, Robinson TE, Bhatnagar S. (2003) Effects of maternal separation on behavioural sensitization produced by repeated cocaine administration in adulthood. Brain Res 960:42–47 [DOI] [PubMed] [Google Scholar]
- Li YQ, Wang XY, Zhai HF, Zheng YQ, Zhang XY, Kosten T, Lu L. (2008) Effects of early postnatal sibling deprivation on anxiety and vulnerability to cocaine in offspring rats. Psychopharmacology (Berl) 199:245–253 [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. (2005) The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46:703–713 [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. (1997) Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277:1659–1662 [DOI] [PubMed] [Google Scholar]
- Liu Y, Young KA, Curtis JT, Aragona BJ, Wang ZX. (2011) Social bonding decreases the rewarding properties of amphetamine through a dopamine D1 receptor-mediated mechanism. J Neurosci 31:7960–7966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomanowska AM, Rana SA, McCutcheon D, Parker LA, Wainwright PE. (2006) Artificial rearing alters the response of rats to natural and drug-mediated rewards. Dev Psychobiol 48:301–314 [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Ussher JR, Jaswal JS. (2010) Targeting intermediary metabolism in the hypothalamus as a mechanism to regulate appetite. Pharmacol Rev 62:237–264 [DOI] [PubMed] [Google Scholar]
- Lovic V, Fleming AS, Fletcher PJ. (2006) Early life tactile stimulation changes adult rat responsiveness to amphetamine. Pharmacol Biochem Behav 84:497–503 [DOI] [PubMed] [Google Scholar]
- Lu W, Marinelli M, Xu D, Worley PF, Wolf ME. (2002) Amphetamine and cocaine do not increase Narp expression in rat ventral tegmental area, nucleus accumbens or prefrontal cortex, but Narp may contribute to individual differences in responding to a novel environment. Eur J Neurosci 15:2027–2036 [DOI] [PubMed] [Google Scholar]
- Lucas LR, Schlussman SD, Ho A, McEwen BS, Kreek MJ. (1997) Novelty-induced locomoter activity in Long-Evans rats pre- and post-chronic ‘binge’-pattern cocaine treatment. Neurosci Lett 237:25–28 [DOI] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. (2011) Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science 333:353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Mangini LD, Taylor JR. (2005) Neonatal isolation stress potentiates cocaine seeking behavior in adult male and female rats. Neuropsychopharmacology 30:322–329 [DOI] [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Deminière JM, Angelucci L, Simon H, Le Moal M. (1991) Hippocampal type I and type II corticosteroid receptor affinities are reduced in rats predisposed to develop amphetamine self-administration. Brain Res 548:305–309 [DOI] [PubMed] [Google Scholar]
- Maldonado AM, Finkbeiner LM, Kirstein CL. (2008) Social interaction and partner familiarity differentially alter voluntary ethanol intake in adolescent male and female rats. Alcohol 42:641–648 [DOI] [PubMed] [Google Scholar]
- Mandt BH, Johnston NL, Zahniser NR, Allen RM. (2012) Acquisition of cocaine self-administration in male Sprague-Dawley rats: effects of cocaine dose but not initial locomotor response to cocaine. Psychopharmacology (Berl) 219:1089–1097 [Erratum in: Psychopharmacology (Berl) 2012:539. Dosage error in article text.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandt BH, Schenk S, Zahniser NR, Allen RM. (2008) Individual differences in cocaine-induced locomotor activity in male Sprague-Dawley rats and their acquisition of and motivation to self-administer cocaine. Psychopharmacology (Berl) 201:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. (2000) Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27:349–357 [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Ho A, Schlussman SD, Kreek MJ. (2001) Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 157:31–39 [DOI] [PubMed] [Google Scholar]
- Mar J, Arrospide A, Begiristain JM, Larrañaga I, Elosegui E, Oliva-Moreno J. (2011) The impact of acquired brain damage in terms of epidemiology, economics and loss in quality of life. BMC Neurol 11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin MT, Planeta CS. (2004) Maternal separation affects cocaine-induced locomotion and response to novelty in adolescent, but not in adult rats. Brain Res 1013:83–90 [DOI] [PubMed] [Google Scholar]
- Marinelli M, White FJ. (2000) Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci 20:8876–8885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmendal M, Eriksson CJ, Fahlke C. (2006) Early deprivation increases exploration and locomotion in adult male Wistar offspring. Pharmacol Biochem Behav 85:535–544 [DOI] [PubMed] [Google Scholar]
- Marmendal M, Roman E, Eriksson CJ, Nylander I, Fahlke C. (2004) Maternal separation alters maternal care, but has minor effects on behavior and brain opioid peptides in adult offspring. Dev Psychobiol 45:140–152 [DOI] [PubMed] [Google Scholar]
- Marrow LP, Overton PG, Brain PF, Clark D. (1999) Encounters with aggressive conspecifics enhance the locomotor-activating effects of cocaine in the rat. Addict Biol 4:437–441 [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli B, Himelreich K, Brenzel A, Bingcang CM, Omar H. (2004) Sensation seeking and symptoms of disruptive disorder: association with nicotine, alcohol, and marijuana use in early and mid-adolescence. Psychol Rep 94:1075–1082 [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, Omar HA. (2002) Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. J Am Acad Child Adolesc Psychiatry 41:1495–1502 [DOI] [PubMed] [Google Scholar]
- Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, Broft A, Van Heertum R, Kleber HD. (2010) Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol Psychiatry 67:275–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Bardo MT. (2009) Differences in impulsivity on a delay-discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behav Pharmacol 20:447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Darna M, Charnigo RJ, Dwoskin LP, Bardo MT. (2011) A multivariate assessment of individual differences in sensation seeking and impulsivity as predictors of amphetamine self-administration and prefrontal dopamine function in rats. Exp Clin Psychopharmacol 19:275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx MH, Henderson RL, Roberts CL. (1955) Positive reinforcement of the bar-pressing response by a light stimulus following dark operant pretests with no after effect. J Comp Physiol Psychol 48:73–76 [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Morrissey MD, McCormick CM. (2010) Individual differences in activity predict locomotor activity and conditioned place preference to amphetamine in both adolescent and adult rats. Pharmacol Biochem Behav 95:63–71 [DOI] [PubMed] [Google Scholar]
- Matthews K, Dalley JW, Matthews C, Tsai TH, Robbins TW. (2001) Periodic maternal separation of neonatal rats produces region- and gender-specific effects on biogenic amine content in postmortem adult brain. Synapse 40:1–10 [DOI] [PubMed] [Google Scholar]
- Matthews K, Hall FS, Wilkinson LS, Robbins TW. (1996a) Retarded acquisition and reduced expression of conditioned locomotor activity in adult rats following repeated early maternal separation: effects of prefeeding, d-amphetamine, dopamine antagonists and clonidine. Psychopharmacology (Berl) 126:75–84 [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW. (2003) Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev 27:45–55 [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW, Everitt BJ, Caine SB. (1999) Repeated neonatal maternal separation alters intravenous cocaine self-administration in adult rats. Psychopharmacology (Berl) 141:123–134 [DOI] [PubMed] [Google Scholar]
- Matthews K, Wilkinson LS, Robbins TW. (1996b) Repeated maternal separation of preweanling rats attenuates behavioral responses to primary and conditioned incentives in adulthood. Physiol Behav 59:99–107 [DOI] [PubMed] [Google Scholar]
- Maze I, Nestler EJ. (2011) The epigenetic landscape of addiction. Ann N Y Acad Sci 1216:99–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, White FJ, Marinelli M. (2009) Individual differences in dopamine cell neuroadaptations following cocaine self-administration. Biol Psychiatry 66:801–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Callaghan PD, Hunt GE. (2008) From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? Br J Pharmacol 154:358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre DC, Chew GL. (1983) Relation between social rank, submissive behavior, and brain catecholamine levels in ring-necked pheasants (Phasianus colchicus). Behav Neurosci 97:595–601 [DOI] [PubMed] [Google Scholar]
- McKenzie-Quirk SD, Miczek KA. (2008) Social rank and social separation as determinants of alcohol drinking in squirrel monkeys. Psychopharmacology (Berl) 201:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. (2006) Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology 31:1241–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara R, Dalley JW, Robbins TW, Everitt BJ, Belin D. (2010) Trait-like impulsivity does not predict escalation of heroin self-administration in the rat. Psychopharmacology (Berl) 212:453–464 [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, Gratton A. (2002) Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology 27:127–138 [DOI] [PubMed] [Google Scholar]
- Meerlo P, Overkamp GJF, Benning MA, Koolhaas JM, Van den Hoofdakker RH. (1996) Long-term changes in open field behaviour following a single social defeat in rats can be reversed by sleep deprivation. Physiol Behav 60:115–119 [DOI] [PubMed] [Google Scholar]
- Melendez RI, Gregory ML, Bardo MT, Kalivas PW. (2004) Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropsychopharmacology 29:1980–1987 [DOI] [PubMed] [Google Scholar]
- Meyer AC, Rahman S, Charnigo RJ, Dwoskin LP, Crabbe JC, Bardo MT. (2010) Genetics of novelty seeking, amphetamine self-administration and reinstatement using inbred rats. Genes Brain Behav 9:790–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels CC, Easterling KW, Holtzman SG. (2007) Maternal separation alters ICSS responding in adult male and female rats, but morphine and naltrexone have little affect on that behavior. Brain Res Bull 73:310–318 [DOI] [PubMed] [Google Scholar]
- Michaels CC, Holtzman SG. (2008) Early postnatal stress alters place conditioning to both mu- and kappa-opioid agonists. J Pharmacol Exp Ther 325:313–318 [DOI] [PubMed] [Google Scholar]
- Miczek KA, Gold LH. (1983) d-Amphetamine in squirrel monkeys of different social status: effects on social and agonistic behavior, locomotion, and stereotypies. Psychopharmacology (Berl) 81:183–190 [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH. (1996) Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology (Berl) 128:256–264 [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH, van Erp AM, Blank AD, McInerney SC. (1999a) d-Amphetamine “cue” generalizes to social defeat stress: behavioral sensitization and attenuated accumbens dopamine. Psychopharmacology (Berl) 147:190–199 [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina E, Kream RM, Carter G, Espejo EF. (1999b) Behavioral sensitization to cocaine after a brief social defeat stress: c-fos expression in the PAG. Psychopharmacology (Berl) 141:225–234 [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE., 3rd (2011a) Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci 31:9848–9857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Takahashi A, Covington HE, 3rd, Yap JJ, Boyson CO, Shimamoto A, de Almeida RMM. (2011b) Gene expression in aminergic and peptidergic cells during aggression and defeat: relevance to violence, depression and drug abuse. Behav Genet 41:787–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Shuster L. (1982) Opioid-like analgesia in defeated mice. Science 215:1520–1522 [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., 3rd (2008) Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther 120:102–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misslin R, Herzog F, Koch B, Ropartz P. (1982) Effects of isolation, handling and novelty on the pituitary-adrenal response in the mouse. Psychoneuroendocrinology 7:217–221 [DOI] [PubMed] [Google Scholar]
- Misslin R, Ropartz P, Jung L. (1984) Impairment of responses to novelty by apomorphine and its antagonism by neuroleptics in mice. Psychopharmacology (Berl) 82:113–117 [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Cunningham CL, Mark GP. (2005) Locomotor activity predicts acquisition of self-administration behavior but not cocaine intake. Behav Neurosci 119:464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH, Reeves JM, Li N, Phillips TJ. (2006) Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcohol Clin Exp Res 30:429–437 [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. (2002) Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 160:290–298 [DOI] [PubMed] [Google Scholar]
- Moffett MC, Harley J, Francis D, Sanghani SP, Davis WI, Kuhar MJ. (2006) Maternal separation and handling affects cocaine self-administration in both the treated pups as adults and the dams. J Pharmacol Exp Ther 317:1210–1218 [DOI] [PubMed] [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ. (2007) Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol 73:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander AC, Mar A, Norbury A, Steventon S, Moreno M, Caprioli D, Theobald DE, Belin D, Everitt BJ, Robbins TW, et al. (2011) High impulsivity predicting vulnerability to cocaine addiction in rats: some relationship with novelty preference but not novelty reactivity, anxiety or stress. Psychopharmacology (Berl) 215:721–731 [DOI] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D’Amato FR. (2004) Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science 304:1983–1986 [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. (2002) Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci 5:169–174 [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA. (2000) Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. Am J Primatol 52:115–131 [DOI] [PubMed] [Google Scholar]
- Muhammad A, Kolb B. (2011) Maternal separation altered behavior and neuronal spine density without influencing amphetamine sensitization. Behav Brain Res 223:7–16 [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. (1996) The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex 6:470–481 [DOI] [PubMed] [Google Scholar]
- Nadal R, Armario A, Janak PH. (2002) Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 162:333–338 [DOI] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Gould RW, Riddick NV. (2008) Review: positron emission tomography imaging studies of dopamine receptors in primate models of addiction. Philos Trans R Soc Lond B Biol Sci 363:3223–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Peartree NA, Pentkowski NS. (2012) Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacology (Berl) 224L33–56 [DOI] [PMC free article] [PubMed]
- Newman TK, Parker CC, Suomi SJ, Goldman D, Barr CS, Higley JD. (2009) DRD1 5’UTR variation, sex and early infant stress influence ethanol consumption in rhesus macaques. Genes Brain Behav 8:626–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls B, Springham A, Mellanby J. (1992) The playground maze: a new method for measuring directed exploration in the rat. J Neurosci Methods 43:171–180 [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. (2006) Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry 45:468–475 [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, 3rd, Ganschow L, Hammer RP, Jr, Miczek KA. (2004) Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: fos in the ventral tegmental area and amygdala. Neuroscience 123:857–865 [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Marchand JE, Kream RM, Miczek KA. (1998) Behavioral sensitization to cocaine after a brief social stress is accompanied by changes in fos expression in the murine brainstem. Brain Res 810:200–210 [DOI] [PubMed] [Google Scholar]
- Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. (1996) Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci USA 93:11699–11704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normansell L, Panksepp J. (1990) Effects of morphine and naloxone on play-rewarded spatial discrimination in juvenile rats. Dev Psychobiol 23:75–83 [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Parsons LH. (2004) Serotonin1B receptors in the ventral tegmental area modulate cocaine-induced increases in nucleus accumbens dopamine levels. J Pharmacol Exp Ther 311:711–719 [DOI] [PubMed] [Google Scholar]
- Olsen CM, Winder DG. (2010) Operant sensation seeking in the mouse. J Vis Exp 45:2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreland S, Pickering C, Gökturk C, Oreland L, Arborelius L, Nylander I. (2009) Two repeated maternal separation procedures differentially affect brain 5-hydroxytryptamine transporter and receptors in young and adult male and female rats. Brain Res 1305 (Suppl):S37–S49 [DOI] [PubMed] [Google Scholar]
- Oreland S, Raudkivi K, Oreland L, Harro J, Arborelius L, Nylander I. (2011) Ethanol-induced effects on the dopamine and serotonin systems in adult Wistar rats are dependent on early-life experiences. Brain Res 1405:57–68 [DOI] [PubMed] [Google Scholar]
- Oxford ML, Harachi TW, Catalano RF, Abbott RD. (2001) Preadolescent predictors of substance initiation: a test of both the direct and mediated effect of family social control factors on deviant peer associations and substance initiation. Am J Drug Alcohol Abuse 27:599–616 [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. (1984) The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev 8:465–492 [DOI] [PubMed] [Google Scholar]
- Parker LA. (1992) Place conditioning in a three- or four-choice apparatus: role of stimulus novelty in drug-induced place conditioning. Behav Neurosci 106:294–306 [DOI] [PubMed] [Google Scholar]
- Paterson NE, Wetzler C, Hackett A, Hanania T. (2012) Impulsive action and impulsive choice are mediated by distinct neuropharmacological substrates in rat. Int J Neuropsychopharmacol 15:1473–1487. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. (2008) The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci 29:192–199 [DOI] [PubMed] [Google Scholar]
- Pedersen CA. (2004) Biological aspects of social bonding and the roots of human violence. Ann N Y Acad Sci 1036:106–127 [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Costentin J, Duterte-Boucher D. (2004) Differential effects of novelty exposure on place preference conditioning to amphetamine and its oral consumption. Psychopharmacology (Berl) 171:277–285 [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Costentin J, Duterte-Boucher D. (2006) Novelty preference predicts place preference conditioning to morphine and its oral consumption in rats. Pharmacol Biochem Behav 84:43–50 [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Painter MR, Thiel KJ, Peartree NA, Cheung TH, Deviche P, Adams M, Alba J, Neisewander JL. (2011) Nicotine-induced plasma corticosterone is attenuated by social interactions in male and female adolescent rats. Pharmacol Biochem Behav 100:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Coddington SB, Jetton C, Karelitz JL, Scott JA, Wilson AS. (2008) Initial nicotine sensitivity in humans as a function of impulsivity. Psychopharmacology (Berl) 200:529–544 [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. (2005) Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 178:193–201 [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. (2007) Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol Biochem Behav 86:822–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. (2008) Impulsive choice as a predictor of acquisition of IV cocaine self- administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol 16:165–177 [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminière JM, Le Moal M, Simon H. (1989) Factors that predict individual vulnerability to amphetamine self-administration. Science 245:1511–1513 [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminière JM, Maccari S, Mormède P, Le Moal M, Simon H. (1990) Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav Pharmacol 1:339–345 [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. (2000) Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci 20:4226–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminière JM, Maccari S, Le Moal M, Simon H. (1993) Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci USA 90:11738–11742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. (1997) Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res Brain Res Rev 25:359–372 [DOI] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminière JM, Le Moal M, Mormède P, Simon H. (1991a) Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci USA 88:2088–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Rougé-Pont F, Deminière JM, Kharoubi M, Le Moal M, Simon H. (1991b) Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res 567:169–174 [DOI] [PubMed] [Google Scholar]
- Pich EM, Heinrichs SC, Rivier C, Miczek KA, Fisher DA, Koob GF. (1993) Blockade of pituitary-adrenal axis activation induced by peripheral immunoneutralization of corticotropin-releasing factor does not affect the behavioral response to social defeat stress in rats. Psychoneuroendocrinology 18:495–507 [DOI] [PubMed] [Google Scholar]
- Pierce RC, Crawford CA, Nonneman AJ, Mattingly BA, Bardo MT. (1990) Effect of forebrain dopamine depletion on novelty-induced place preference behavior in rats. Pharmacol Biochem Behav 36:321–325 [DOI] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. (1997) Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology (Berl) 129:277–284 [DOI] [PubMed] [Google Scholar]
- Planeta CS, Marin MT. (2002) Effect of cocaine on periadolescent rats with or without early maternal separation. Braz J Med BiolRes 35:1367–1371 [DOI] [PubMed] [Google Scholar]
- Ploj K, Pham TM, Bergström L, Mohammed AH, Henriksson BG, Nylander I. (1999) Neonatal handling in rats induces long-term effects on dynorphin peptides. Neuropeptides 33:468–474 [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Bergström L, Nylander I. (2001) Effects of neonatal handling on nociceptin/orphanin FQ and opioid peptide levels in female rats. Pharmacol Biochem Behav 69:173–179 [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. (2003a) Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience 121:787–799 [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. (2003b) Long-term effects of short and long periods of maternal separation on brain opioid peptide levels in male Wistar rats. Neuropeptides 37:149–156 [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. (1993) Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res 18:195–200 [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. (2006) Housing and rank status of male Long-Evans rats modify ethanol’s effect on open-field behaviors. Psychopharmacology (Berl) 185:289–297 [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Jongen-Rêlo AL, Feldon J, Yee BK. (2005) Double dissociation of the effects of selective nucleus accumbens core and shell lesions on impulsive-choice behaviour and salience learning in rats. Eur J Neurosci 22:2605–2616 [DOI] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. (1995) Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol 6:810–814 [PubMed] [Google Scholar]
- Procópio-Souza R, Fukushiro DF, Trombin TF, Wuo-Silva R, Zanlorenci LH, Lima AJ, Ribeiro LT, Correa JM, Marinho EA, Kameda SR, et al. (2011) Effects of group exposure on single injection-induced behavioral sensitization to drugs of abuse in mice. Drug Alcohol Depend 118:349–359 [DOI] [PubMed] [Google Scholar]
- Pryce CR, Bettschen D, Bahr NI, Feldon J. (2001) Comparison of the effects of infant handling, isolation, and nonhandling on acoustic startle, prepulse inhibition, locomotion, and HPA activity in the adult rat. Behav Neurosci 115:71–83 [DOI] [PubMed] [Google Scholar]
- Quadros IMH, Miczek KA. (2009) Two modes of intense cocaine bingeing: increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacology (Berl) 206:109–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Bardo MT. (2008) Environmental enrichment increases amphetamine-induced glutamate neurotransmission in the nucleus accumbens: a neurochemical study. Brain Res 1197:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Christensen JR, Guerra C, Bardo MT. (1997a) Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res 776:61–67 [DOI] [PubMed] [Google Scholar]
- Rebec GV, Grabner CP, Johnson M, Pierce RC, Bardo MT. (1997b) Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience 76:707–714 [DOI] [PubMed] [Google Scholar]
- Renner MJ, Rosenzweig MR. (1987) Enriched and Impoverished Environments: Effects on Brain and Behavior, Springer-Verlag, New York [Google Scholar]
- Ressler KJ, Mayberg HS. (2007) Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 10:1116–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Lluch J, Rodríguez-Arias M, Miñarro J. (2009) Social experiences affect reinstatement of cocaine-induced place preference in mice. Psychopharmacology (Berl) 207:485–498 [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodríguez-Arias M, Armario A, Miñarro J. (2006) Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology (Berl) 185:459–470 [DOI] [PubMed] [Google Scholar]
- Riccardi P, Zald D, Li R, Park S, Ansari MS, Dawant B, Anderson S, Woodward N, Schmidt D, Baldwin R, et al. (2006) Sex differences in amphetamine-induced displacement of [(18)F]fallypride in striatal and extrastriatal regions: a PET study. Am J Psychiatry 163:1639–1641 [DOI] [PubMed] [Google Scholar]
- Riddick NV, Czoty PW, Gage HD, Kaplan JR, Nader SH, Icenhower M, Pierre PJ, Bennett A, Garg PK, Garg S, et al. (2009) Behavioral and neurobiological characteristics influencing social hierarchy formation in female cynomolgus monkeys. Neuroscience 158:1257–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 163:362–380 [DOI] [PubMed] [Google Scholar]
- Robinet PM, Rowlett JK, Bardo MT. (1998) Individual differences in novelty-induced activity and the rewarding effects of novelty and amphetamine in rats. Behav Processes 44:1–9 [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. (2009) The influence of stress hormones on fear circuitry. Annu Rev Neurosci 32:289–313 [DOI] [PubMed] [Google Scholar]
- Rodriguez ML, Logue AW. (1988) Adjusting delay to reinforcement: comparing choice in pigeons and humans. J Exp Psychol Anim Behav Process 14:105–117 [PubMed] [Google Scholar]
- Roman E, Gustafsson L, Berg M, Nylander I. (2006) Behavioral profiles and stress-induced corticosteroid secretion in male Wistar rats subjected to short and prolonged periods of maternal separation. Horm Behav 50:736–747 [DOI] [PubMed] [Google Scholar]
- Roman E, Gustafsson L, Hyytiä P, Nylander I. (2005) Short and prolonged periods of maternal separation and voluntary ethanol intake in male and female ethanol-preferring AA and ethanol-avoiding ANA rats. Alcohol Clin Exp Res 29:591–601 [DOI] [PubMed] [Google Scholar]
- Roman E, Hyytiä P, Nylander I. (2003) Maternal separation alters acquisition of ethanol intake in male ethanol-preferring AA rats. Alcohol Clin Exp Res 27:31–37 [DOI] [PubMed] [Google Scholar]
- Roman E, Nylander I. (2005) The impact of emotional stress early in life on adult voluntary ethanol intake-results of maternal separation in rats. Stress 8:157–174 [DOI] [PubMed] [Google Scholar]
- Roman E, Ploj K, Nylander I. (2004) Maternal separation has no effect on voluntary ethanol intake in female Wistar rats. Alcohol 33:31–39 [DOI] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. (2001) Drinking or abstaining at age 14? A genetic epidemiological study. Alcohol Clin Exp Res 25:1594–1604 [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. (1996) Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res 78:57–65 [DOI] [PubMed] [Google Scholar]
- Rossi NA, Reid LD. (1976) Affective states associated with morphine injections. Physiol Psychol 4:269–274 [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. (2004) Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev 28:533–546 [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. (2006) Gene-environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry 47:226–261 [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. (2003) Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor-responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. J Pharmacol Exp Ther 305:180–190 [DOI] [PubMed] [Google Scholar]
- Saigusa T, Tuinstra T, Koshikawa N, Cools AR. (1999) High and low responders to novelty: effects of a catecholamine synthesis inhibitor on novelty-induced changes in behaviour and release of accumbal dopamine. Neuroscience 88:1153–1163 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89 [DOI] [PubMed] [Google Scholar]
- Sargent JD, Tanski S, Stoolmiller M, Hanewinkel R. (2010) Using sensation seeking to target adolescents for substance use interventions. Addiction 105:506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. (2011) Individual variation in the motivational properties of cocaine. Neuropsychopharmacology 36:1668–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Ellison F, Hunt T, Amit Z. (1985) An examination of heroin conditioning in preferred and nonpreferred environments and in differentially housed mature and immature rats. Pharmacol Biochem Behav 22:215–220 [DOI] [PubMed] [Google Scholar]
- Schenk S, Hunt T, Colle L, Amit Z. (1983) Isolation versus grouped housing in rats: differential effects of low doses of heroin in the place preference paradigm. Life Sci 32:1129–1134 [DOI] [PubMed] [Google Scholar]
- Schenk S, Robinson B, Amit Z. (1988) Housing conditions fail to affect the intravenous self-administration of amphetamine. Pharmacol Biochem Behav 31:59–62 [DOI] [PubMed] [Google Scholar]
- Schippers MC, Binnekade R, Schoffelmeer AN, Pattij T, De Vries TJ. (2012) Unidirectional relationship between heroin self-administration and impulsive decision-making in rats. Psychopharmacology (Berl) 219:443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier FR, Liebowitz MR, Abi-Dargham A, Zea-Ponce Y, Lin SH, Laruelle M. (2000) Low dopamine D(2) receptor binding potential in social phobia. Am J Psychiatry 157:457–459 [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Lindell SG, Sjöberg RL, Chisholm KL, Higley JD, Suomi SJ, Heilig M, Barr CS. (2010) Gene-environment interactions and response to social intrusion in male and female rhesus macaques. Biol Psychiatry 67:323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell SL, Dillon AM, Cunningham KA, Thomas ML. (2005) Estrous cycle influence on individual differences in the response to novelty and cocaine in female rats. Behav Brain Res 161:69–74 [DOI] [PubMed] [Google Scholar]
- Seo D, Patrick CJ, Kennealy PJ. (2008) Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress Violent Behav 13:383–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Floris I, Biggio G. (2005) Social isolation-induced changes in the hypothalamic-pituitary-adrenal axis in the rat. Stress 8:259–264 [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. (1989) Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 290:213–242 [DOI] [PubMed] [Google Scholar]
- Shimamoto A, Debold JF, Holly EN, Miczek KA. (2011) Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology (Berl) 218:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimosato K, Watanabe S. (2003) Concurrent evaluation of locomotor response to novelty and propensity toward cocaine conditioned place preference in mice. J Neurosci Methods 128:103–110 [DOI] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgänsberger W, Wurst W, Holsboer F, Spanagel R. (2002) Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science 296:931–933 [DOI] [PubMed] [Google Scholar]
- Sills TL, Vaccarino FJ. (1994) Individual differences in sugar intake predict the locomotor response to acute and repeated amphetamine administration. Psychopharmacology (Berl) 116:1–8 [DOI] [PubMed] [Google Scholar]
- Sills TL, Vaccarino FJ. (1998) Individual differences in the feeding and locomotor stimulatory effects of acute and repeated morphine treatments. Pharmacol Biochem Behav 60:293–303 [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. (1991) Plasticity of GFAP-immunoreactive astrocyte size and number in visual cortex of rats reared in complex environments. Brain Res 540:273–278 [DOI] [PubMed] [Google Scholar]
- Siviy SM, Panksepp J. (2011) In search of the neurobiological substrates for social playfulness in mammalian brains. Neurosci Biobehav Rev 35:1821–1830 [DOI] [PubMed] [Google Scholar]
- Smith MA, Chisholm KA, Bryant PA, Greene JL, McClean JM, Stoops WW, Yancey DL. (2005) Social and environmental influences on opioid sensitivity in rats: importance of an opioid’s relative efficacy at the mu-receptor. Psychopharmacology (Berl) 181:27–37 [DOI] [PubMed] [Google Scholar]
- Smith MA, Iordanou JC, Cohen MB, Cole KT, Gergans SR, Lyle MA, Schmidt KT. (2009) Effects of environmental enrichment on sensitivity to cocaine in female rats: importance of control rates of behavior. Behav Pharmacol 20:312–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pitts EG. (2011) Access to a running wheel inhibits the acquisition of cocaine self-administration. Pharmacol Biochem Behav 100:237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman WP, Brown CP, Levine S. (1977) Maternal responsiveness following differential pup treatment and mother-pup interactions. Horm Behav 8:242–253 [DOI] [PubMed] [Google Scholar]
- Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M. (2008) Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci USA 105:17145–17150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, El Rawas R, Lardeux V, Jaber M. (2009) Environmental enrichment during early stages of life reduces the behavioral, neurochemical, and molecular effects of cocaine. Neuropsychopharmacology 34:1102–1111 [DOI] [PubMed] [Google Scholar]
- Spear LP. (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463 [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Prendergast MA, Bardo MT. (2011) Environmental-induced differences in corticosterone and glucocorticoid receptor blockade of amphetamine self-administration in rats. Psychopharmacology (Berl) 218:293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavisky RC, Adams MR, Watson SL, Kaplan JR. (2001) Dominance, cortisol, and behavior in small groups of female cynomolgus monkeys (Macaca fascicularis). Horm Behav 39:232–238 [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Griffiths RR, Bigelow GE, Liebson I. (1981) Human social conversation: effects of ethanol, secobarbital and chlorpromazine. Pharmacol Biochem Behav 14:353–360 [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Cunningham KA. (2008) The relationship between the locomotor response to a novel environment and behavioral disinhibition in rats. Drug Alcohol Depend 92:69–78 [DOI] [PubMed] [Google Scholar]
- Stöhr T, Almeida OF, Landgraf R, Shippenberg TS, Holsboer F, Spanagel R. (1999) Stress- and corticosteroid-induced modulation of the locomotor response to morphine in rats. Behav Brain Res 103:85–93 [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Robbins CG, Martin CA, Rush CR, Kelly TH. (2007) The reinforcing, subject-rated, performance, and cardiovascular effects of d-amphetamine: influence of sensation-seeking status. Addict Behav 32:1177–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi SJ. (2011) Risk, resilience, and gene-environment interplay in primates. J Can Acad Child Adolesc Psychiatry 20:289–297 [PMC free article] [PubMed] [Google Scholar]
- Suto N, Austin JD, Vezina P. (2001) Locomotor response to novelty predicts a rat’s propensity to self-administer nicotine. Psychopharmacology (Berl) 158:175–180 [DOI] [PubMed] [Google Scholar]
- Swendsen J, Le Moal M. (2011) Individual vulnerability to addiction. Ann N Y Acad Sci 1216:73–85 [DOI] [PubMed] [Google Scholar]
- Thiel CM, Müller CP, Huston JP, Schwarting RK. (1999) High versus low reactivity to a novel environment: behavioural, pharmacological and neurochemical assessments. Neuroscience 93:243–251 [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Engelhardt B, Hood LE, Peartree NA, Neisewander JL. (2011) The interactive effects of environmental enrichment and extinction interventions in attenuating cue-elicited cocaine-seeking behavior in rats. Pharmacol Biochem Behav 97:595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. (2008) Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend 96:202–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Painter MR, Pentkowski NS, Mitroi D, Crawford CA, Neisewander JL. (2012) Environmental enrichment counters cocaine abstinence-induced stress and brain reactivity to cocaine cues but fails to prevent the incubation effect. Addict Biol 17:365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Pentkowski NS, Peartree NA, Painter MR, Neisewander JL. (2010) Environmental living conditions introduced during forced abstinence alter cocaine-seeking behavior and Fos protein expression. Neuroscience 171:1187–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. (2009) Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl) 204:391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiriet N, Gennequin B, Lardeux V, Chauvet C, Decressac M, Janet T, Jaber M, Solinas M. (2011) Environmental enrichment does not reduce the rewarding and neurotoxic effects of methamphetamine. Neurotox Res 19:172–182 [DOI] [PubMed] [Google Scholar]
- Thompson MR, Hunt GE, McGregor IS. (2009) Neural correlates of MDMA (“ecstasy”)-induced social interaction in rats. Soc Neurosci 4:60–72 [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Khoury A, Mathe AA, Ehlers CL. (2005) Effect of social isolation on ethanol consumption and substance P/neurokinin expression in Wistar rats. Alcohol 36:91–97 [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. (1997) Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 130:203–212 [DOI] [PubMed] [Google Scholar]
- Tomie A, Burger KM, Di Poce J, Pohorecky LA. (2004) Social opportunity and ethanol drinking in rats. Prog Neuropsychopharmacol Biol Psychiatry 28:1089–1097 [DOI] [PubMed] [Google Scholar]
- Torasdotter M, Metsis M, Henriksson BG, Winblad B, Mohammed AH. (1998) Environmental enrichment results in higher levels of nerve growth factor mRNA in the rat visual cortex and hippocampus. Behav Brain Res 93:83–90 [DOI] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Vanderschuren LJ. (2009) Conditioned place preference induced by social play behavior: parametrics, extinction, reinstatement and disruption by methylphenidate. Eur Neuropsychopharmacol 19:659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzcińska M, Bergh J, DeLeon K, Stellar JR, Melloni RH., Jr (2002) Social stress does not alter the expression of sensitization to cocaine. Physiol Behav 76:457–463 [DOI] [PubMed] [Google Scholar]
- Turner CA, Flagel SB, Clinton SM, Akil H, Watson SJ. (2008) Cocaine interacts with the novelty-seeking trait to modulate FGFR1 gene expression in the rat. Neurosci Lett 446:105–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. (2000) Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward. Crit Rev Neurobiol 14:131–142 [PubMed] [Google Scholar]
- Valentino RJ, Lucki I, Van Bockstaele E. (2010) Corticotropin-releasing factor in the dorsal raphe nucleus: linking stress coping and addiction. Brain Res 1314:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen R, Koehl M, Abrous DN, de Kloet ER, Piazza PV, Deroche-Gamonet V. (2008) Maternal environment influences cocaine intake in adulthood in a genotype-dependent manner. PLoS ONE 3:e2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp AM, Tachi N, Miczek KA. (2001) Short or continuous social stress: suppression of continuously available ethanol intake in subordinate rats. Behav Pharmacol 12:335–342 [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Trezza V, Griffioen-Roose S, Schiepers OJG, Van Leeuwen N, De Vries TJ, Schoffelmeer ANM. (2008) Methylphenidate disrupts social play behavior in adolescent rats. Neuropsychopharmacology 33:2946–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. (1997) The neurobiology of social play behavior in rats. Neurosci Biobehav Rev 21:309–326 [DOI] [PubMed] [Google Scholar]
- Varela A, Pritchard ME. (2011) Peer influence: use of alcohol, tobacco, and prescription medications. J Am Coll Health 59:751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasko MR, Domino EF. (1978) Tolerance development to the biphasic effects of morphine on locomotor activity and brain acetylcholine in the rat. J Pharmacol Exp Ther 207:848–858 [PubMed] [Google Scholar]
- Vathy I, Slamberová R, Liu X. (2007) Foster mother care but not prenatal morphine exposure enhances cocaine self-administration in young adult male and female rats. Dev Psychobiol 49:463–473 [DOI] [PubMed] [Google Scholar]
- Vazquez V, Farley S, Giros B, Daugé V. (2005a) Maternal deprivation increases behavioural reactivity to stressful situations in adulthood: suppression by the CCK2 antagonist L365,260. Psychopharmacology (Berl) 181:706–713 [DOI] [PubMed] [Google Scholar]
- Vazquez V, Giros B, Daugé V. (2006) Maternal deprivation specifically enhances vulnerability to opiate dependence. Behav Pharmacol 17:715–724 [DOI] [PubMed] [Google Scholar]
- Vazquez V, Penit-Soria J, Durand C, Besson MJ, Giros B and Dauge V (2005b) Maternal deprivation increases vulnerability to morphine dependence and disturbs the enkephalinergic system in adulthood. J Neurosci 25:4453–4462. [DOI] [PMC free article] [PubMed]
- Vazquez V, Weiss S, Giros B, Martres MP, Daugé V. (2007) Maternal deprivation and handling modify the effect of the dopamine D3 receptor agonist, BP 897 on morphine-conditioned place preference in rats. Psychopharmacology (Berl) 193:475–486 [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. (2008) Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res 170:261–276 [DOI] [PubMed] [Google Scholar]
- Vicentic A, Francis D, Moffett M, Lakatos A, Rogge G, Hubert GW, Harley J, Kuhar MJ. (2006) Maternal separation alters serotonergic transporter densities and serotonergic 1A receptors in rat brain. Neuroscience 140:355–365 [DOI] [PubMed] [Google Scholar]
- Vidal-Infer A, Arenas MC, Daza-Losada M, Aguilar MA, Miñarro J, Rodríguez-Arias M. (2012) High novelty-seeking predicts greater sensitivity to the conditioned rewarding effects of cocaine. Pharmacol Biochem Behav 102:124–132 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. (2009) Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 56 (Suppl 1):3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, Hitzemann R, Pappas NR. (1999) Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther 291:409–415 [PubMed] [Google Scholar]
- Walker QD, Schramm-Sapyta NL, Caster JM, Waller ST, Brooks MP, Kuhn CM. (2009) Novelty-induced locomotion is positively associated with cocaine ingestion in adolescent rats; anxiety is correlated in adults. Pharmacol Biochem Behav 91:398–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AS, Kelly TH, Foltin RW, Fischman MW. (1997) Effects of d-amphetamine on task performance and social behavior of humans in a residential laboratory. Exp Clin Psychopharmacol 5:130–136 [DOI] [PubMed] [Google Scholar]
- Watanabe S. (2011) Drug-social interactions in the reinforcing property of methamphetamine in mice. Behav Pharmacol 22:203–206 [DOI] [PubMed] [Google Scholar]
- Weiss IC, Domeney AM, Heidbreder CA, Moreau JL, Feldon J. (2001) Early social isolation, but not maternal separation, affects behavioral sensitization to amphetamine in male and female adult rats. Pharmacol Biochem Behav 70:397–409 [DOI] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Rêlo AL, Nanz-Bahr NI, Feldon J. (2004) Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res 152:279–295 [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Robbins TW, Everitt BJ. (1997) Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacology (Berl) 134:242–257 [DOI] [PubMed] [Google Scholar]
- Werner CM, Anderson DF. (1976) Opportunity for interaction as reinforcement in a T-maze. Pers Soc Psychol Bull 2:166–169 [Google Scholar]
- Westergaard GC, Suomi SJ, Chavanne TJ, Houser L, Hurley A, Cleveland A, Snoy PJ, Higley JD. (2003) Physiological correlates of aggression and impulsivity in free-ranging female primates. Neuropsychopharmacology 28:1045–1055 [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. (2001) The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers Individ Diff 30:669–689 [Google Scholar]
- Wills TA, Windle M, Cleary SD. (1998) Temperament and novelty seeking in adolescent substance use: convergence of dimensions of temperament with constructs from Cloninger’s theory. J Pers Soc Psychol 74:387–406 [DOI] [PubMed] [Google Scholar]
- Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, Smith GT, Giedd J, Dahl RE. (2008) Transitions into underage and problem drinking: developmental processes and mechanisms between 10 and 15 years of age. Pediatrics 121 (Suppl 4):S273–S289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. (2010) Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res 34:1306–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. (2004) Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci 24:4718–4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW. (2006) Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex 16:106–114 [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. (1989) Brain dopamine and reward. Annu Rev Psychol 40:191–225 [DOI] [PubMed] [Google Scholar]
- Wolffgramm J. (1990) Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology (Berl) 101:233–239 [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. (1991) Social behavior, dominance, and social deprivation of rats determine drug choice. Pharmacol Biochem Behav 38:389–399 [DOI] [PubMed] [Google Scholar]
- Wood DA, Rebec GV. (2004) Dissociation of core and shell single-unit activity in the nucleus accumbens in free-choice novelty. Behav Brain Res 152:59–66 [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Anderson KG. (2006) Effects of delay to reinforcement on the choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 186:99–106 [DOI] [PubMed] [Google Scholar]
- Wooters TE, Bardo MT, Dwoskin LP, Midde NM, Gomez AM, Mactutus CF, Booze RM, Zhu J. (2011) Effect of environmental enrichment on methylphenidate-induced locomotion and dopamine transporter dynamics. Behav Brain Res 219:98–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooters TE, Dwoskin LP, Bardo MT. (2006) Age and sex differences in the locomotor effect of repeated methylphenidate in rats classified as high or low novelty responders. Psychopharmacology (Berl) 188:18–27 [DOI] [PubMed] [Google Scholar]
- Xigeng Z, Yonghui L, Xiaojing L, Lin X, Dongmei W, Jie L, Xiaoyan Y, Nan S. (2004) Social crowding sensitizes high-responding rats to psychomotor-stimulant effects of morphine. Pharmacol Biochem Behav 79:213–218 [DOI] [PubMed] [Google Scholar]
- Xu N, Wang L, Wu C, Pei G. (2001) Spatial learning and morphine-rewarded place preference negatively correlates in mice. Pharmacol Biochem Behav 68:389–394 [DOI] [PubMed] [Google Scholar]
- Yap JJ, Covington HE, 3rd, Gale MC, Datta R, Miczek KA. (2005) Behavioral sensitization due to social defeat stress in mice: antagonism at mGluR5 and NMDA receptors. Psychopharmacology (Berl) 179:230–239 [DOI] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA. (2007) Social defeat stress, sensitization, and intravenous cocaine self-administration in mice. Psychopharmacology (Berl) 192:261–273 [DOI] [PubMed] [Google Scholar]
- Yates JR, Marusich JA, Gipson CD, Beckmann JS, Bardo MT. (2012) High impulsivity in rats predicts amphetamine conditioned place preference. Pharmacol Biochem Behav 100:370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SB, Ryu V, Park EY, Kim BT, Kang DW, Lee JH, Jahng JW. (2011) The arcuate NPY, POMC, and CART expressions responding to food deprivation are exaggerated in young female rats that experienced neonatal maternal separation. Neuropeptides 45:343–349 [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. (2011) The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol 32:53–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. (2001) Cellular mechanisms of social attachment. Horm Behav 40:133–138 [DOI] [PubMed] [Google Scholar]
- Young WF, Jr, Laws ER, Jr, Sharbrough FW, Weinshilboum RM. (1986) Human monoamine oxidase: lack of brain and platelet correlation. Arch Gen Psychiatry 43:604–609 [DOI] [PubMed] [Google Scholar]
- Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. (2009) Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience 163:890–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Floresco SB, Winstanley CA. (2010) Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology (Berl) 211:87–98 [DOI] [PubMed] [Google Scholar]
- Zhang TY, Parent C, Weaver I, Meaney MJ. (2004) Maternal programming of individual differences in defensive responses in the rat. Ann N Y Acad Sci 1032:85–103 [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. (2007) Previous exposure to cocaine enhances cocaine self-administration in an alpha 1-adrenergic receptor dependent manner. Neuropsychopharmacology 32:638–645 [DOI] [PubMed] [Google Scholar]
- Zhang XY, Sanchez H, Kehoe P, Kosten TA. (2005) Neonatal isolation enhances maintenance but not reinstatement of cocaine self-administration in adult male rats. Psychopharmacology (Berl) 177:391–399 [DOI] [PubMed] [Google Scholar]
- Zheng X, Ke X, Tan B, Luo X, Xu W, Yang X, Sui N. (2003) Susceptibility to morphine place conditioning: relationship with stress-induced locomotion and novelty-seeking behavior in juvenile and adult rats. Pharmacol Biochem Behav 75:929–935 [DOI] [PubMed] [Google Scholar]
- Zheng XG, Tan BP, Luo XJ, Xu W, Yang XY, Sui N. (2004) Novelty-seeking behavior and stress-induced locomotion in rats of juvenile period differentially related to morphine place conditioning in their adulthood. Behav Processes 65:15–23 [DOI] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. (2005) Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem 93:1434–1443 [DOI] [PubMed] [Google Scholar]
- Zhu J, Green T, Bardo MT, Dwoskin LP. (2004) Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res 148:107–117 [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Shartrand AM. (1992) Temperature-dependent effects of maternal separation on growth, activity, and amphetamine sensitivity in the rat. Dev Psychobiol 25:213–226 [DOI] [PubMed] [Google Scholar]
- Zucker RA, Donovan JE, Masten AS, Mattson ME, Moss HB. (2008) Early developmental processes and the continuity of risk for underage drinking and problem drinking. Pediatrics 121 (Suppl 4):S252–S272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. (1994) Behavioral Expressions and Biosocial Bases of Sensation Seeking, Cambridge University Press, Cambridge [Google Scholar]